Salinity stress shapes root development through control of mRNA stability and requires the partially redundant function of SnRK2 protein kinases to modulate transcript levels.
Abstract
SNF1-RELATED PROTEIN KINASES 2 (SnRK2) are important components of early osmotic and salt stress signaling pathways in plants. The Arabidopsis (Arabidopsis thaliana) SnRK2 family comprises the abscisic acid (ABA)–activated protein kinases SnRK2.2, SnRK2.3, SnRK2.6, SnRK2.7, and SnRK2.8, and the ABA-independent subclass 1 protein kinases SnRK2.1, SnRK2.4, SnRK2.5, SnRK2.9, and SnRK2.10. ABA-independent SnRK2s act at the posttranscriptional level via phosphorylation of VARICOSE (VCS), a member of the mRNA decapping complex, that catalyzes the first step of 5′mRNA decay. Here, we identified VCS and VARICOSE RELATED (VCR) as interactors and phosphorylation targets of SnRK2.5, SnRK2.6, and SnRK2.10. All three protein kinases phosphorylated Ser-645 and Ser-1156 of VCS, whereas SnRK2.6 and SnRK2.10 also phosphorylated VCS Ser-692 and Ser-680 of VCR. We showed that subclass 1 SnRK2s, VCS, and 5′ EXORIBONUCLEASE 4 (XRN4) are involved in regulating root growth under control conditions as well as modulating root system architecture in response to salt stress. Our results suggest interesting patterns of redundancy within subclass 1 SnRK2 protein kinases, with SnRK2.1, SnRK2.5, and SnRK2.9 controlling root growth under nonstress conditions and SnRK2.4 and SnRK2.10 acting mostly in response to salinity. We propose that subclass 1 SnRK2s function in root development under salt stress by affecting the transcript levels of aquaporins, as well as CYP79B2, an enzyme involved in auxin biosynthesis.
Soil salinity is one of the biggest constraints of modern agriculture, severely affecting crop productivity (Fita et al., 2015). Plant acclimation to saline conditions relies on early activation of signaling cascades, which trigger protective mechanisms. Crucial components of salt and osmotic signaling pathways are protein kinases (Boudsocq and Laurière, 2005). One group of protein kinases recognized as pivotal regulators of responses to osmotic stress is the plant-specific SnRK2 family (SNF1-RELATED PROTEIN KINASE 2). Except for SnRK2.9, all other nine Arabidopsis (Arabidopsis thaliana) SnRK2 protein kinases have been shown to have an increased kinase activity upon treatment with either abscisic acid (ABA), osmotic stress, or salt stress, whereas differential responsiveness was observed for individual SnRK2 protein kinases (Boudsocq et al., 2004). The Arabidopsis SnRK2 protein kinase subfamily comprises three groups: subfamily 1 includes the ABA-independent kinases (SnRK2.1/SRK2G, SnRK2.4/SRK2A, SnRK2.5/SRK2H, SnRK2.9/SRK2J, SnRK2.10/SRK2B), group 2 consists of those involved in drought responses (SnRK2.7/SRK2F and SnRK2.8/SRK2C), and group 3 kinases are strongly activated by ABA (SnRK2.2/SRK2D, SnRK2.3/SRK2I, SnRK2.6/SRK2E/OST1; Kulik et al., 2011). Members of the plant SnRK2 subfamily have also been identified in tobacco (Kelner et al., 2004), rice (Kobayashi et al., 2004), sorghum (Li et al., 2010), maize (Huai et al., 2008), wheat (Zhang et al., 2016), bean (Li and Assmann, 1996), soybean (Monks et al., 2001), and tomato (Yang et al., 2015).
Activity of SnRK2 protein kinases relies on their autophosphorylation; however activation by another protein kinase acting upstream has been also proposed (Boudsocq et al., 2007; Fujii et al., 2009). Two crucial residues, Ser-171 and Ser-175, have been found to be phosphorylated independently in the ABA-dependent SnRK2.6 protein, whereas activation of ABA-independent SnRK2.10 relied on sequential phosphorylation of Ser-154 followed by phosphorylation of Ser-158 (Vlad et al., 2010). Members of the ABA-dependent subclass 3 have been most extensively studied so far. SnRK2.2, SnRK2.3, and SnRK2.6 are components of the core ABA signaling pathway (Cutler et al., 2010). Several protein phosphatases from the PP2C clade A family have been shown to act as negative regulators of ABA-dependent SnRK2 kinases (Merlot et al., 2001; Ma et al., 2009; Park et al., 2009; Nishimura et al., 2010). In the absence of ABA, PP2Cs dephosphorylate SnRK2 to maintain their inactive state. ABA triggers the interaction of PP2Cs with ABA receptors PYR/PYL/RCAR (PYRABACTIN RESISTANCE 1/PYR1-LIKE/REGULATORY COMPONENT OF ABA RECEPTOR), thus releasing SnRK2 from their inhibited state (Merlot et al., 2001; Ma et al., 2009; Park et al., 2009; Nishimura et al., 2010). Members of subclass 2, SnRK2.7 and SnRK2.8, interacted with PP2Cs in vitro, whereas interactions in planta with one of the PP2Cs, ABI1 (ABSCISIC ACID INSENSITIVE 1), have been recently shown for SnRK2.8 and also the subclass 1 isoform SnRK2.4, but not for SnRK2.10 (Vlad et al., 2010; Umezawa et al., 2013; Krzywińska et al., 2016). SnRK2.4 and SnRK2.10 can be de-activated by members of the phosphoprotein phosphatase family (Krzywińska et al., 2016). Activity of all ten SnRK2 protein kinases is negatively regulated by SCS (SnRK2-INTERACTING CALCIUM SENSOR; Bucholc et al., 2011). SnRK2.4 and SnRK2.10 have been shown to bind to the phospholipid second messenger phosphatidic acid; however, the effect of this interaction on protein kinase activity remains unknown (Testerink et al., 2004; Julkowska et al., 2015).
Several downstream targets have been identified for subclass 2 and 3 SnRK2s. SnRK2.6 phosphorylates the anion channel SLAC1, the potassium channel KAT1, Atrboh NADPH oxidases, and aquaporin PIP2;1, thereby mediating ABA-dependent stomatal closure. The snrk2.6 knock-out mutant is impaired in closing stomata in low humidity conditions and has a wilting phenotype (Mustilli et al., 2002; Yoshida et al., 2002; Geiger et al., 2009; Lee et al., 2009; Sato et al., 2009; Sirichandra et al., 2009; Grondin et al., 2015). SnRK2.6, as well as SnRK2.2 and SnRK2.3, can phosphorylate ABA RESPONSIVE ELEMENTS-BINDING FACTORS AREB1, AREB2, and ABF3, bZIP transcription factors that bind to ABA-responsive elements in promoters of ABA-dependent genes (Furihata et al., 2006; Fujii et al., 2007). The snrk2.2/snrk2.3/snrk2.6 triple mutant is insensitive to ABA and has low tolerance to drought, confirming the role of SnRK2 subclass 3 kinases as major regulators of ABA responses (Fujii and Zhu, 2009; Fujita et al., 2009). SnRK2 subclass 3 and/or kinase(s) downstream of this group of SnRK2s can also phosphorylate mitogen-activated protein kinases MPK1, MPK2, and MPK6, another class of ABA-activated protein kinases (Droillard et al., 2002; Umezawa et al., 2013; Wang et al., 2013). A recent phosphoproteomics study identified many putative SnRK2 subclass 3 targets that are involved in DNA and RNA binding and microRNA regulation, but their direct phosphorylation by these kinases still needs to be confirmed (Umezawa et al., 2013, Wang et al., 2013). SnRK2.8 phosphorylated ABF3 (redundantly to SnRK2 subclass 2 proteins) and additionally targeted EEL, another ABF transcription factor (Mizoguchi et al., 2010). Moreover, SnRK2.8 phosphorylated three 14-3-3 proteins, adenosine kinase, glyoxylase I, and ribose 5-phosphate isomerase, which links its function to the regulation of metabolic processes (Shin et al., 2007).
Recently subclass 1 SnRK2 protein kinases have been shown to regulate plant responses to osmotic stress at the posttranscriptional level. SnRK2.1, SnRK2.4, SnRK2.5, SnRK2.9, and SnRK2.10 phosphorylated VCS (VARICOSE), a member of the mRNA decapping complex and crucial component of 5′ mRNA decay pathways (Soma et al., 2017). Also, two dehydrins, ERD10 and ERD14, have been found as a direct phosphorylation targets of SnRK2.10 in responses to salt stress (Maszkowska et al., 2019). Moreover, SnRK2.4 and SnRK2.10 are involved in reactive oxygen species homeostasis upon salt stress, but the mechanism of this regulation remains unknown (Szymańska et al., 2019).
In Arabidopsis roots, SnRK2.4 and SnRK2.10 are activated within 30 s of exposure to salt and both were shown to function as positive regulators of root growth under saline conditions (McLoughlin et al., 2012). snrk2.4 knock-out mutants showed a decreased main (primary) root length in the presence of salt, whereas snrk2.10 knock-out mutants exhibited reduced lateral root density (McLoughlin et al., 2012). Consistent with this phenotype, SnRK2.10 was expressed in the vasculature at the sites of lateral root formation, whereas SnRK2.4 was localized to most of the tissues in the main root (McLoughlin et al., 2012).
Here, we set out to identify the molecular mechanism by which subclass 1 SnRK2 protein kinases control root growth and development under salt and osmotic stress. We confirmed that VCS is a phosphorylation target for ABA-independent subclass 1 SnRK2 protein kinases and possibly for ABA-dependent SnRK2.6 and identified phosphorylation sites targeted by these protein kinases. Root phenotyping showed the involvement of subclass 1 SnRK2s, VCS, and 5′ EXORIBONUCLEASE 4 (XRN4) in root growth in nonstress conditions as well as in reshaping of root system architecture under salt stress. Our study suggests that SnRK2.1, SnRK2.5, and SnRK2.9 play a role in main root growth under control conditions, whereas under salt stress all subclass 1 SnRK2s are likely to modulate lateral root growth. We propose that in response to salt stress, subclass 1 SnRK2s modulate root system architecture by regulation of the expression of aquaporins PIP2;3 and PIP2;5 as well as the auxin biosynthesis enzyme CYP79B2.
RESULTS
SnRK2.4 and SnRK2.10 Physically Interact with Proteins Involved in mRNA Metabolism
In order to identify putative up- and downstream SnRK2.4 and SnRK2.10 interactors, tandem affinity purification (TAP) using N- and C-terminal GS-rhino tag fusions of SnRK2.4 or SnRK2.10 expressed under control of the CaMV 35S promoter as baits was performed from Arabidopsis PSB-d (dark) cell suspension cultures (Van Leene et al., 2015). Commonly occurring proteins were treated as a background and were subtracted from the list of significant proteins (Van Leene et al., 2015). Eight proteins—VCS, VCR (VARICOSE RELATED), DCP2 (DECAPPING 2), RRP44B (RRP44 HOMOLOG B), XRN4, SnRK2.7, PAT1H1 (TOPOISOMERASE II-ASSOCIATED PROTEIN), and SnRK2.5—were copurified with both SnRK2.4 and SnRK2.10, whereas AREB3 was specific for SnRK2.4. ELP2 (ELONGATOR PROTEIN 2) and SnRK2.9 interacted only with SnRK2.10 (Table 1; Supplemental Table S1). A similar approach reported recently by Soma et al. (2017) for SnRK2.1 also identified VCS and VCR, and further confirmed VCS as a phosphorylation target of SnRK2 subclass 1 protein kinases. VCS and DCP2 are part of the decapping complex, involved in removal of the 5′mRNA cap, and other putative interactors identified here also function in mRNA metabolism processes. VCR, XRN4, and PAT1H1 have been previously shown to localize in cytoplasmic protein foci called processing bodies (P bodies), which are a site of mRNA degradation and sequestration (Xu et al., 2006; Weber et al., 2008; Roux et al., 2015). SnRK2.4 and SnRK2.1 relocalize to P bodies upon osmotic and salt stress, thus indicating involvement of mRNA decay or RNA sequestration from the translation machinery in response to salinity and osmotic stress (McLoughlin et al., 2012; Soma et al., 2017).
Table 1. List of putative SnRK2.4 and SnRK2.10 interactors.
Proteins were identified by LC-MS/MS after TAP from Arabidopsis suspension cultures. GSrhino N- and C-terminal fusions with SnRK2.4 and SnRK2.10 were used as baits with two technical replicates. Table presents proteins identified with at least two peptides for each sample.
| Purified Proteins | Bait | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GSrhino-SnRK2.4 1 | GSrhino-SnRK2.4 2 | SnRK2.4-GSrhino 1 | SnRK2.4-GSrhino 1 | SnRK2.4 Total | GSrhino-SnRK2.10 1 | GSrhino-SnRK2.10 2 | SnRK2.10-GSrhino 1 | SnRK2.10-GSrhino 1 | SnRK2.10 Total | ||
| AT1G10940 | ASK1, SNRK2-4, SNRK2.4, SRK2A Protein kinase superfamily protein | + | + | + | + | 4 | – | – | – | – | 0 |
| AT1G60940 | SNRK2-10, SNRK2.10, SRK2B SNF1-related protein kinase 2.10 | – | – | – | – | 0 | + | + | + | + | 4 |
| AT3G13300 | VCS Transducin/WD40 repeat-like superfamily protein | + | + | + | + | 4 | + | + | + | + | 4 |
| AT3G13290 | VCR varicose-related | + | + | + | + | 4 | + | + | + | + | 4 |
| AT5G13570 | DCP2, TDT, ATDCP2 decapping 2 | + | + | + | + | 4 | + | + | + | + | 4 |
| AT1G77680 | ATRRP44B, RRP44 HOMOLOG B, RRP44B, SOV, SUPPRESSOR OF VARICOSE | + | + | + | + | 4 | + | + | + | + | 4 |
| AT1G54490 | AIN1, EIN5, XRN4, ATXRN4 |exoribonuclease 4 | + | + | + | + | 4 | + | – | + | – | 2 |
| AT4G40010 | SNRK2-7, SNRK2.7, SRK2F SNF1-related protein kinase 2.7 | – | + | – | + | 2 | + | + | + | + | 4 |
| AT3G22270 | PAT1H1, Topoisomerase II-associated protein | + | – | – | – | 1 | + | + | + | + | 4 |
| AT5G63650 | SNRK2-5, SNRK2.5, SRK2H SNF1-related protein kinase 2.5 | + | – | – | – | 1 | + | + | + | – | 3 |
| AT3G56850 | AREB3, DPBF3 ABA-responsive element binding protein 3 | – | + | – | + | 2 | – | – | – | – | 0 |
| AT1G49540 | ELP2, AtELP2 elongator protein 2 | – | – | – | – | 0 | + | – | + | – | 2 |
| AT2G23030 | SNRK2-9, SNRK2.9 SNF1-related protein kinase 2.9 | – | – | – | – | 0 | + | – | + | – | 2 |
Peptides of identified interactors of SnRK2.4 and SnRK2.10 were searched for possible phosphorylation events in the purified complex. Eleven and six phosphopeptides were identified for VCS and VCR copurified with SnRK2.4, respectively. In a complex with SnRK2.10, we found seven VCS and two VCR phosphopeptides (Table 2; Supplemental Fig. S1). Among these, phosphorylation of six sites for VCS and two for VCR have been shown to be up-regulated by osmotic and/or salt stress (Table 2; Stecker et al., 2014; Maszkowska et al., 2019). No phosphopeptides were identified for the other proteins copurified with SnRK2.4 and SnRK2.10 (Table 1), suggesting that VCS and VCR are possible phosphorylation targets of SnRK2.4 and SnRK2.10, whereas other identified proteins are likely functioning in the same complex (Table 2).
Table 2. Phosphorylation sites Identified within the sequence of SnRK2.4, SnRK2.10, and their putative interactors.
Identified phosphorylated Ser and Tyr (pS, pT) are denoted in bold. For each peptide the localization in the protein is indicated with its start and end position. Peptide expectation value is the number of times an equal or higher score could be expected to be obtained purely by chance. Site analysis % is the probability that the phosphorylation was assigned correctly to particular residue. Sequences of peptides marked with an asterisk were used for design of the synthetic peptides used for in vitro kinase activity assays. Spectrum number corresponds to spectra presented in Supplemental Figure S1.
| Bait | Spectrum No. | Protein Name | Peptide Start | Peptide End | Peptide Ion Score | Peptide Expectation Value | Peptide Sequence | Phosphorylation Site Position in Protein Sequence | Site Analysis % | Phosphorylation Induced by Salt or Osmotic Stress Shown in |
|---|---|---|---|---|---|---|---|---|---|---|
| SnRK2.4 | 1 | VCS | 86 | 100 | 66.21 | 0.00002 | TLSYPTPPLNLQpSPR | S98 | 99.98 | – |
| 2 | VCS | 142 | 156 | 49.27 | 0.001 | SFPGGSGPIRVPpSCK | S154 | 98.83 | – | |
| 3 | VCS | 637 | 662 | 109.64 | 0.000000001 | TSGLPSQTSGAGSAYATLPQLPLpSPR * | S660 | 100.00 | Stecker et al., 2014 | |
| 4 | VCS | 637 | 662 | 58.26 | 0.0002 | pTSGLPSQTSGAGSAYATLPQLPLpSPR * | T637+S660 | 99.84 | Stecker et al., 2014 | |
| 5 | VCS | 637 | 662 | 44.00 | 0.004 | TSGLPSQTSGAGSApYATLPQLPLpSPR * | Y651+S660 | 98.77 | Stecker et al., 2014 | |
| 6 | VCS | 637 | 666 | 72.12 | 0.00001 | TSGLPSQTSGAGSAYATLPQLPLpSPRLpSSK * | S660+S664 | 86.13 | Stecker et al., 2014 | |
| 7 | VCS | 690 | 699 | 66.86 | 0.00001 | TPpSADYSVDR * | S692 | 99.76 | Stecker et al., 2014 | |
| 8 | VCS | 821 | 835 | 77.59 | 0.000001 | VFCSQVSNLpSTEMAR | S830 | 93.06 | – | |
| 9 | VCS | 821 | 835 | 80.73 | 0.000001 | VFCSQVpSNLSTEMAR | S827 | 99.86 | – | |
| 10 | VCS | 1149 | 1163 | 60.41 | 0.0001 | ESITSApSpSVAQALSR * | S1155 or S1156 | 49.95/49.95 | Stecker et al., 2014; Maszkowska et al., 2019 | |
| 11 | VCS | 1171 | 1203 | 41.43 | 0.003 | NLLALAAAGANSGGSNSLVpTQLpSGGPLGALLEK | S1193 or T1190 | 64.22/32.49 | – | |
| – | ||||||||||
| 12 | VCR | 678 | 687 | 60.03 | 0.00004 | TSpSADYFYVR * | S680 | 95.12 | Stecker et al., 2014; Maszkowska et al., 2019 | |
| 13 | VCR | 708 | 730 | 62.81 | 0.0001 | SKDTNVTPDDDVSGIRpSPSAFFK | S724 | 72.90 | – | |
| 14 | VCR | 708 | 730 | 76.19 | 0.000003 | SKDTNVTPDDDVSGIRSPpSAFFK | S726 | 73.91 | – | |
| 15 | VCR | 807 | 823 | 46.77 | 0.002 | ENIFCSQASNLpSTEMAR | S818 | 84.49 | – | |
| 16 | VCR | 862 | 877 | 64.88 | 0.00002 | LPESGpSSSGLVATNSK | S867 | 87.00 | – | |
| 17 | VCR | 1174 | 1199 | 42.17 | 0.002 | LALTAAGSNPLVTQLpSNGPLGALLEK | S1189 | 89.09 | Maszkowska et al., 2019 | |
| 18 | SnRK2.4 | 158 | 173 | 79.41 | 0.000001 | pSpTVGTPAYIAPEVLSR | S158 or T159 | 49.93/49.93 | Stecker et al., 2014 | |
| 19 | SnRK2.4 | 350 | 361 | 49.63 | 0.001 | TVKEVHApSGEVR | S357 | 99.88 | – | |
| SnRK2.10 | 20 | VCS | 86 | 100 | 84.61 | 0.0000003 | TLSYPTPPLNLQpSPR | S98 | 100.00 | – |
| – | ||||||||||
| 21 | VCS | 637 | 662 | 97.59 | 0.00000002 | TSGLPSQTSGAGSAYATLPQLPLpSPR * | S660 | 100.00 | Stecker et al., 2014 | |
| 22 | VCS | 637 | 662 | 42.10 | 0.0065 | TSGLPSQTSGAGSApYATLPQLPLpSPR * | Y651+S660 | 96.93 | Stecker et al., 2014 | |
| 23 | VCS | 690 | 699 | 54.82 | 0.0001 | TPpSADYSVDR * | S692 | 95.10 | Stecker et al., 2014; Maszkowska et al., 2019 | |
| 24 | VCS | 821 | 835 | 72.76 | 0.000004 | VFCSQVpSNLSTEMAR | S827 | 99.77 | – | |
| 25 | VCS | 821 | 835 | 75.56 | 0.000002 | VFCSQVSNLpSTEMAR | S830 | 93.09 | – | |
| 26 | VCS | 1217 | 1233 | 42.39 | 0.005 | LIpSERKYEESFTSALQR | S1219 | 99.90 | – | |
| 27 | VCR | 678 | 687 | 40.65 | 0.0034 | TSpSADYFYVR * | S680 | 86.03 | Stecker et al., 2014 | |
| 28 | VCR | 1213 | 1229 | 42.39 | 0.0049 | LIpSERKYEESFTSALQR | S1215 | 99.90 | – | |
| 29 | SnRK2.10 | 350 | 359 | 59.71 | 0.0001 | QVHApSMGEVR | S354 | 100.00 | – |
VCS Is a Direct Target of SnRK2.10, SnRK2.5, and SnRK2.6
In order to identify the phosphorylation sites of SnRK2 subclass 1 protein kinases targets, we performed in vitro kinase activity assays. We selected VCS, VCR, and DCP2 for verification, because they were previously found to by phosphorylated upon osmotic stress (Stecker et al., 2014). Purified recombinant protein kinases SnRK2.4 and SnRK2.10 were used for the in vitro phosphorylation reactions with synthetic peptides from VCS, VCR, and DCP2 proteins. Synthetic peptides were designed to contain the phosphopeptides identified in the TAP experiment and shown previously to be regulated by osmotic and/or salt stress (Table 2; Stecker et al., 2014; Maszkowska et al., 2019). Each protein kinase was incubated with a mixture consisting of three peptides representing the VCS sequence, and one peptide each from VCR and DCP2. In a separate reaction, MBP (myelin basic protein) was used as a positive control. SnRK2.4 did not phosphorylate itself nor any of the tested peptides; however, it was able to phosphorylate MBP, possibly because of its overall lower activity comparing with SnRK2.10 (Table 3; Supplemental Tables S2 and S3). Autophosphorylation of SnRK2.10 and phosphorylation of all three peptides from VCS and a peptide from VCR, but not from DCP2, were detected (Table 3; Supplemental Fig. S2; Supplemental Table S4).
Table 3. SnRK2.5, SnRK2.6, and SnRK2.10 phosphorylate VCS peptides.
Summary of the in vitro kinase activity assays performed with recombinant protein kinases and synthetic peptides. Position of start and end of the peptides used is relative to the first amino acid in the protein sequence. MBP is a known substrate for SnRK2 protein kinases and was used as a positive control. –, denotes no phosphorylation detected; NA, not applicable.
| Substrate | Protien Kinase | |||||
|---|---|---|---|---|---|---|
| Protein | Peptide Start | Peptide End | Peptide | SnRK2.5 | SnRK2.6 | SnRK2.10 |
| VCS | 636 | 668 | KTSGLPSQTSGAGSAYATLPQLPLSPRLSSK | T644/S645 | T644/S645 | S645 |
| 686 | 701 | LGGKTPSADYSVDRQM | – | S692 | S692 | |
| 1147 | 1170 | LKESITSASSVAQALSRELAETQR | S1156 | S1156 | S1155/S1156 | |
| VCR | 674 | 689 | LGGKTSSADYFYVRQT | – | S680 | S680 |
| DCP2 | 265 | 291 | CVWNARTSVGGNGTATVESQNRKSELR | – | – | – |
| MBP | NA | NA | NA | + | + | + |
| Protein Kinase Autophosphorylation | NA | NA | NA | T159 | S29 | T159 |
| – | S175/T176 | T269 | ||||
| – | – | S354 | ||||
To investigate whether other members of SnRK2 family can phosphorylate the same peptides as SnRK2.10, SnRK2.5 from the same subclass as SnRK2.4 and SnRK2.10, as well as SnRK2.6, from subclass 3 were tested. SnRK2.6 phosphorylated all the peptides, whereas SnRK2.5 phosphorylated VCR and two out of three VCS peptides. (Table 3; Supplemental Tables S5 and S6). Because the peptide corresponding to the DCP2 protein was hardly detectable, probably due to its low solubility, full-length DCP2 recombinant protein was purified and tested in an in vitro kinase assay. Also, full-length DCP2 was not phosphorylated in the presence of SnRK2.10, confirming it is not a direct substrate of this kinase in vitro (Supplemental Table S7). We conclude that VCS and VCR are direct targets of SnRK2.5, SnRK2.6, and SnRK2.10 and hypothesize that these protein kinases might be partially redundant.
Components of SnRK2 Subclass 1-Regulated 5′ mRNA Decay Pathway Contribute to Root Development and root System Architecture Responses to Salt Stress
SnRK2.4 and SnRK2.10 were shown previously to have a positive role in elongation of the main root and in lateral root emergence under salt stress, respectively (McLoughlin et al., 2012). Here, we tested the root system architecture of a quintuple knock-out mutant snrk2.1/2.4/2.5/2.9/2.10 impaired in all SnRK2 subclass I protein kinases (Fujii et al., 2011). Salt-induced changes in root growth were tested by transferring 4-d-old seedlings germinated on half-strength Murashige-Skoog medium to media supplemented with 0, 75, and 125 mm NaCl. At 6 d after transfer, we observed genotype-dependent changes in main root length (MRL). The quintuple mutant snrk2.1/2.4/2.5/2.9/2.10 displayed shorter MRL than ecotype Columbia-0 (Col-0) on all conditions tested. (Fig. 1A; Supplemental Fig. S3, A and D; Supplemental Dataset S1). Due to these differences in the MRL, lateral root growth was assessed by quantification of lateral root density (LRD) and aLRLperMRL (average lateral root length per main root length). Although no differences were observed regarding LRD at any condition tested, the response of aLRLperMRL to salinity differed between Col-0 and the snrk2.1/2.4/2.5/2.9/2.10 mutant, as indicated by two-way ANOVA testing the genotype by salt interaction (Supplemental Fig. S3D). The snrk2.1/2.4/2.5/2.9/2.10 mutant showed a higher aLRLperMRL on 125 mm NaCl (Fig. 1A; Supplemental Fig. S3, A and D; Supplemental Dataset S1). Altogether this suggests that SnRK2 subclass 1 protein kinases promote main root growth regardless of the conditions, whereas under high salinity they inhibit lateral root elongation.
Figure 1.
Components of SnRK2 subclass 1-regulated 5′ mRNA decay pathways contribute to root development and root system architecture responses to salt stress. Root system architecture of quintuple snrk2.1/2.4/2.5/2.7/2.9/2.10, amiRNA lines VCS#2 and VCS#4, xrn4-5, and xrn4-6. Seedlings (10 d old) were transferred to 0 and 125 mm NaCl at the 4-d-old stage. Main root length, average lateral root length per main root length, and lateral root density of Col-0, snrk2.1/2.4/2.5/2.7/2.9/2.10 (A), amiRNA lines VCS2 and VCS4 (B), xrn4-5 and xrn4-6 (C) on media supplemented with 0 or 125 mm NaCl are shown. Boxplots denotes span from 25th to the 75th percentile and are centered to the data median. Asterisk denotes p-value of pairwise comparison by least square method: ***<0.001, **<0.01, *<0.05, n > 30.
Identification of VCS as a direct substrate of SnRK2 subclass 1 protein kinases, and the emerging role of mRNA metabolism factors in osmotic and salt stress responses, suggests that VCS, similarly to subclass 1 SnRK2s, might be involved in stress-regulated modulations of root system architecture (Kawa and Testerink, 2017; Soma et al., 2017). Therefore, we tested two artifical micro RNA (amiRNA) lines targeting VCS (VCS2 and VCS4; Soma et al., 2017) and two loss-of-function mutants in XRN4 (xrn4-5 and xrn4-6; Souret et al., 2004; Gy et al., 2007) in the same experimental set up as for snrk2.1/2.4/2.5/2.9/2.10. For both VCS amiRNA lines, their response to salt stress in main root and lateral root growth differed from Col-0 (Fig. 1B; Supplemental Fig. S3, B–D; Supplemental Dataset S2). Although changes in MRL varied in two amiRNA VCS lines tested, which could be attributed to the differences in the level of VCS expression in these lines, VCS had little effect on main root growth (Supplemental Fig. S4A). Both lines showed increased aLRLperMRL under control conditions, whereas under salt stress no differences were detected (Fig. 1C).
The observed decreased MRL, LRD, and aLRLperMRL of the xrn4-6 mutant under all conditions tested was dependent on genotype as well as on the interaction between genotype and the salt stress treatment. The xrn4-5 mutant had a shorter main root, whereas its decrease in LRD and aLRLpMRL in the presence of salt was dependent on the genotype-salinity interaction (Fig. 1C; Supplemental Fig. S3, C and D; Supplemental Dataset S3). Using reverse transcription quantitative PCR (RT-qPCR), we tested the expression of a possible XRN4 mRNA fragment, using primers located upstream of the transfer DNA insertion in both lines. No expression was detected for xrn4-6, confirming it is a true knock-out, whereas the same product was expressed two times higher in the xrn4-5 line than in Col-0 (Supplemental Fig. S4B). Both lines were previously shown to be loss-of-function mutants, producing truncated XRN4 proteins. Because the transfer DNA insertion in xrn4-5 is downstream of the one in xrn4-6, we cannot exclude the possibility that there is some remaining XRN4 activity in xrn4-5, which could explain the weaker phenotype of that line. We conclude that XRN4 has a positive role in lateral root formation and elongation under control and saline conditions.
Together, these results suggest that SnRK2 subclass 1 protein kinases, VCS, and XRN4 contribute to root growth under nonstress conditions as well as in remodeling root system architecture upon salt stress.
Impact of SnRK2 Subclass 1 Protein Kinases on the Salt-Induced Transcriptome in Arabidopsis Seedlings
To further investigate a functional link between SnRK2 subclass 1 protein kinases, mRNA decay pathways and salt stress, transcriptome profiling of Col-0, snrk2.4, double snrk2.4/2.10 (McLoughlin et al., 2012), and quintuple snrk2.1/2.4/2.5/2.9/2.10 (Fujii et al., 2011) knock-out mutants was performed. To select the most suitable duration of salt stress, a time-course experiment with Col-0 seedlings was performed. Seedlings (10 d old) grown in liquid half-strength MS media were treated with buffer (mock) or 150 mm NaCl. Kinase activity in the crude extract of proteins from whole seedlings was assessed by in-gel kinase assay using MBP as a substrate. Salt treatment resulted in rapid induction of SnRK2.4 and SnRK2.10 activity (37 kD band) within 30 s, which was reduced after 5 min and increased again after 24 h (Supplemental Fig. S5), similar to dynamics observed before for roots grown in hydroponics (McLoughlin et al., 2012). The changes in mRNA levels that we are interested in are likely the consequence of the action of the potential substrates of SnRK2 and may not be observed immediately after SnRK2 activation. Hence, a 1-h salt treatment was chosen for transcriptome profiling (measuring steady state transcript levels by RNA sequencing (RNA-seq) of Col-0, snrk2.4, double snrk2.4/2.10, and quintuple snrk2.1/2.4/2.5/2.9/2.10 mutants.
Because multiple members of the SnRK2 family in rice, wheat, and maize have been shown to be transcriptionally up-regulated by salt and osmotic stress (Kobayashi et al., 2004; Huai et al., 2008; Mao et al., 2010; Zhang et al., 2010, 2011), we checked whether the mRNA abundance of Arabidopsis SnRK2 is regulated by salt stress. The 1-h treatment with 150 mm NaCl resulted in a small up-regulation of only SnRK2.5, suggesting that under these conditions most of the Arabidopsis SnRK2 protein kinases are only regulated at the posttranscriptional level; yet we cannot exclude the possibility that they may be transcriptionally regulated at other time points (Supplemental Fig. S6).
We first investigated the effect of the mutations in genes encoding subclass 1 SnRK2 protein kinases under nonstress conditions (Supplemental Fig. S7A). We identified 44, 68, and 485 genes differentially expressed in snrk2.4, snrk2.4/2.10, and snrk2.1/2.4/2.5/2.9/2.10, respectively, as compared with Col-0 (Supplemental Table S8; Supplemental Fig. S8, A and C). Biological processes and molecular functions enriched among genes with altered expression in snrk2.1/2.4/2.5/2.9/2.10 indicate that subclass 1 SnRK2 protein kinases participate in responses to biotic and abiotic stress as well as secondary metabolite processes (Supplemental Fig. S8B). This suggests that subclass 1 SnRK2s are partially activated under control conditions used here, or have a function in their nonactive state.
Next, we investigated the effect of salt stress in Col-0. Differential gene expression analysis revealed that 1-h treatment of Col-0 seedlings with 150 mm NaCl resulted in a change in expression of more than two times in 1292 genes, among which 913 were up- and 379 were down-regulated (Supplemental Table S9). To assess the consequences of the mutations in SnRK2 subclass 1 protein kinases for the salt responses, for each snrk mutant tested, we checked which genes regulated by salt in Col-0 either (1) remain unaffected in mutant or (2) are affected to a different degree (the ratio of the fold change in response to salt stress is two times higher or lower in the mutant as compared with Col-0; genes up-regulated by salt in Col-0 are down-regulated in the mutant; genes down-regulated by salt in Col-0 are up-regulated in the mutant). To assess the impact of SnRK2 subclass 1 protein kinases specifically on the salt-induced transcriptome, genes for which expression was already altered in the mutant lines in control conditions were subtracted (Supplemental Fig. S7B).
Out of 1292 genes regulated by salt, abundance of 356, 426, and 434 was affected in snrk2.4, snrk2.4/2.10, and snrk2.1/2.4/2.5/2.9/2.10, respectively (Fig. 2, A and C; Supplemental Tables S10–S15). Salt response of 160 genes was altered in single, double, and quintuple mutants (Fig. 2C; Supplemental Table S16). Together, 684 genes were found in at least one mutant tested, suggesting that around 50% of the regulation observed in Col-0 depends on at least some of the subclass 1 SnRK2, whereas the other 50% implies existence of other regulatory pathways acting in parallel to them or a more complex mechanism (Fig. 2, A and C).
Figure 2.
SnRK2 subclass 1 protein kinases control various biological processes. A, Salt stress regulated expression profiles in Col-0 and snrk2.4, snrk2.4/2.10, and snrk2.1/2.4/2.5/2.7/2.9/2.10. Heatmap presents log2 fold changes (log2FC) in expression of the genes significantly affected by 1-h 150 mm NaCl treatment in Col-0 and corresponding log2FC value in mutants tested. Gray color indicates genes for which expression was not significantly changed (absolute value of log2FC > 1). B, GO categories enriched among the salt stress–regulated genes with altered expression in tested mutants. Heatmap presents corrected p-value (q-value) of the enrichment. Categories that were not enriched in individual genotypes are represented by gray squares (NA). C, Number of salt stress–regulated genes with expression altered in the tested mutants (left). Number of genes acting downstream of subclass 1 SnRK2 protein kinases that are substrates of VCS and have been reported to participate in root development (right) are shown. A detailed list of the genes is presented in Supplemental Table S16.
Among GO (gene ontology) categories enriched within salt stress–regulated genes with altered abundance in mutants tested, we found biotic and abiotic stress responses shared by all three mutants (Fig. 2B). Interestingly, kinase activity was enriched among genes affected by the snrk2.4 mutation, suggesting that SnRK2.4 alone can regulate, directly or indirectly, expression of other kinases; yet this was not the case for the higher order subclass 1 SnRK2s mutants (Fig. 2B). Several categories (responses to stress, secondary metabolic process, cell death) were enriched among both salt stress–regulated genes and genes with altered mRNA abundance in snrk2.1/2.4/2.5/2.9/2.10 under control conditions.
Expression of several osmotic stress-induced genes previously described to be dependent on SnRK2.2, SnRK2.3, and SnRK2.6 (RD29A, RD29B, RD26, NCED3, PKS5, KIN2, AREB1, HAI1, COR15A, DREB2A, ABI1; Yoshida et al., 2002; Fujita et al., 2009; Fujii et al., 2011) was not affected by any of the mutations tested here, indicating a separation of the effect of subclass 1 and 3 SnRK2 kinases in the regulation of the expression of, at least, these genes (Supplemental Tables S10–S15).
Given that SnRK2 subclass 1 protein kinases function upstream of the 5′ mRNA decay machinery, we analyzed the overlap of our transcriptome data with previously published data on mRNA decay rates and putative targets of VCS (Narsai et al., 2007; Perea-Resa et al., 2016; Sorenson et al., 2018). Out of 684 genes acting downstream of one or more subclass 1 SnRK2s in responses to salinity, 246 were covered in a microarray study by Narsai et al. (2007) and mRNA half-life of 127 of them was shorter than 3 h (Supplemental Table S17), suggesting that these transcripts have low stability. Moreover, 457 of the 684 identified here as SnRK2-dependent candidate genes were recently identified as potential targets of VCS (Sorenson et al., 2018; Supplemental Table S18;) and 29 genes had altered response to dehydration in VCS amiRNA lines (Soma et al., 2017; Supplemental Table S19). Additionally, salinity-dependent regulation of 92 of our candidates was found to be perturbed in the lsm1 (the Sm-like protein 1) mutant (Supplemental Table S20), a member of the decapping activator complex (Perea-Resa et al., 2016).
In order to select genes for further validation, we focused on those with salt stress–regulated expression altered in single, double, and quintuple mutants tested. Although subclass 1 SnrK2 kinases are implicated in multiple biological processes, because of the previously found roles of SnRK2.4/2.10 in modulation of root architecture in salt (McLoughlin et al., 2012), we selected transcripts involved in root development. Out of 160 genes, we found 94 genes with a previously reported role in root development, out of which 91 were associated specifically with lateral root development (Fig. 2C; Supplemental Table S16; Péret et al., 2012; Voß et al., 2015) and 73 of these are potential substrates of VCS (Fig. 2C; Supplemental Table S16).
SnRK2 Subclass 1 Protein Kinases Regulate the Expression of Aquaporins, β-Glucosidase, and a Cytochrome P450
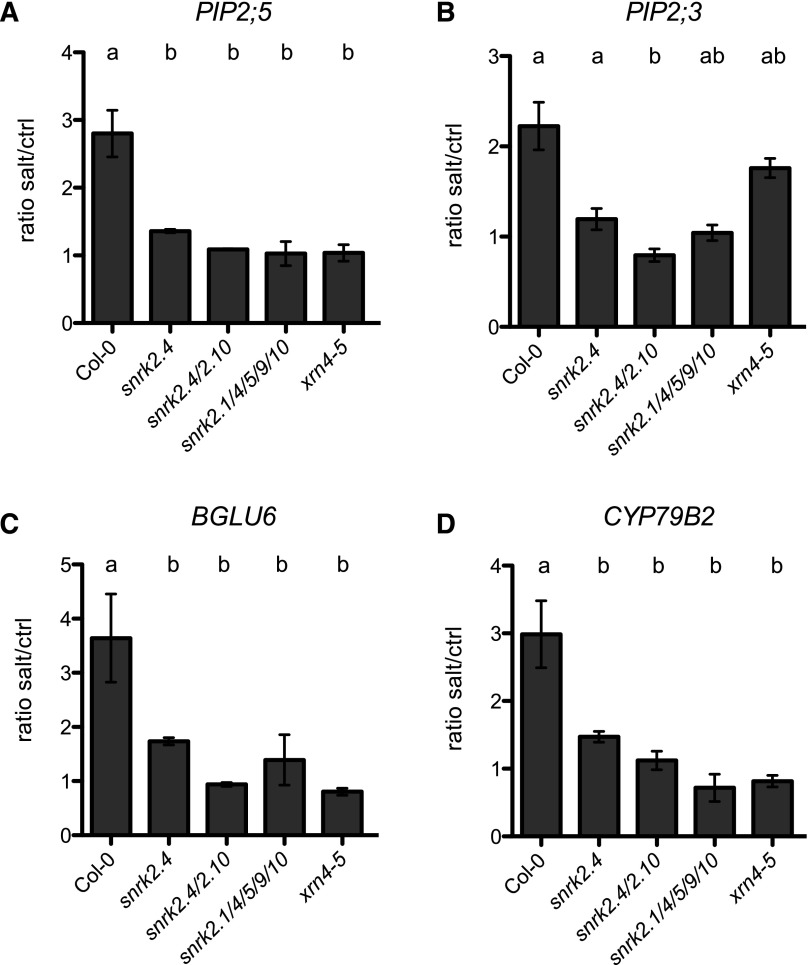
Out of 73 genes that act downstream of subclass 1 SnRK2 protein kinases, are probable targets of VCS, and have a potential role in the root development, we selected four for further characterization. Expression patterns of the four selected candidates were verified by RT-qPCR using RNA extracted from an independent biological experiment. Expression of selected genes was up-regulated by salt stress in Col-0, whereas this induction was not observed in at least one snrk2 mutant, suggesting different levels of redundancy between individual SnRK2 subclass 1 protein kinases (Fig. 3A; Supplemental Fig. S9). Salt stress–dependent induction of the expression of the aquaporin PLASMA MEMBRANE INTRINSIC PROTEIN 2;5 (PIP2;5) was dependent solely on SnRK2.4 (Fig. 3A; Supplemental Fig. S9A). Induction of another aquaporin, PIP2;3, and BETA GLUCOSIDASE 6 (BGLU6) required at least SnRK2.10 (Fig. 3, B, C, and E; Supplemental Fig. S9, B, C, and E). In response to salt, the induction of CYP79B2 (CYTOCHROME P450, FAMILY 79, SUBFAMILY B, POLYPEPTIDE 2) transcripts was reduced to 50% of the Col-0 response, despite only being present in a single snrk2.4 (Fig. 3D; Supplemental Fig. S9D). This response gradually decreased from single snrk2.4 to double snrk2.4/2.10, to being totally absent in the quintuple snrk2.1/2.4/2.5/2.9/2.10 mutant (Fig. 3D). This suggests that all subclass 1 SnRK2s govern the up-regulation of CYP79B2 by salt stress (Fig. 3D; Supplemental Fig. S9D). To assess whether the observed expression profiles might be a consequence of exoribonuclease activity of XRN4, we checked the steady state levels of selected transcripts in the xrn4-5 mutant, as it displayed altered root system architecture only under salt stress conditions (Fig. 1C). Differences in the xrn4-5 mutant were similar to those observed in snrk mutants for PIP2;5, CYP79B2, and BGLU6, but not for PIP2;3 (Fig. 3; Supplemental Fig. S9). We conclude that in response to salt stress, subclass 1 SnRK2 protein kinases regulate the expression of PIP2;3 PIP2;5, BGLU6, and CYP79B2. This might occur via VCS phosphorylation by subclass 1 SnRK2s, and subsequent effects on decapping activity, but could also be an effect of phosphorylation of proteins other than VCS.
Figure 3.
Salt-induced expression of PLASMA MEMBRANE INTRINSIC PROTEINS (PIP2;5, PIP2;3), BETA GLUCOSIDASE 6 (BGLU6), CYTOCHROME P450, FAMILY 79, SUBFAMILY B, POLYPEPTIDE 2 (CYP79B2) is dependent on SnRK2 subclass 1 protein kinases signaling. Expression of PIP2;5 (A), PIP2;3 (B), BGLU6 (C), CYP79B2 (D), as a ratio of the expression under salt stress salt and control (left), under control conditions (middle), and upon salt treatment (right) in Col-0, snrk2.4, snrk2.4/2.10, snrk2.1/2.4/2.5/2.9/2.10, and xrn4-5 lines is shown. Values present are averages of normalized expression levels of three biological replicates, and error bars denote se. Statistical comparison was done by one-way ANOVA followed by lsd post hoc test (P < 0.05). Different letters indicate significant differences.
DISCUSSION
SnRK2.4 and SnRK2.10 protein kinases are involved in early responses to osmotic stress and salinity. Their rapid activation within the first minutes after salt treatment is independent of ABA, and SnRK2.4 and SnRK2.10 have been shown to promote root growth in the presence of salt (McLoughlin et al., 2012). To understand the ABA-independent mechanism of early salt stress signaling leading to regulation of root growth, we aimed to identify components of SnRK2.4 and SnRK2.10 protein kinase pathways.
In planta copurification experiments showed that both kinases can physically interact with several proteins involved in 5′ mRNA decay, among which VCS and VCR were phosphorylated (Tables 1 and 2; Supplemental Table S1). Degradation of mRNA from its 5′ end requires removal of the 5′cap by the decapping complex followed by the digestion by 5′–>3′ exoribonuclease XRN4. Two of the proteins interacting with SnRK2.4 and SnRK2.10, VCS and DCP2, are members of the mRNA decapping complex (Xu et al., 2006). VCS physically interacts with DCP2 as well as DCP1, which is required for the activation of DCP2 decapping activity and assembly of a functional decapping complex (Xu et al., 2006; Goeres et al., 2007). Removal of 5′cap structure leaves the mRNA unprotected from the exoribonucleitic activity of XRN4 (Kastenmayer and Green, 2000). VCS and VCR were the most abundant interactors of SnRK2.4 and SnRK2.10, implying very stable interactions (Tables 1 and 2; Supplemental Table S1). This suggests that, besides being substrates of SnRK2.4 and SnRK2.10, VCS and VCR could be a part of a larger complex involving other proteins (Table 1). We hypothesize that other copurified proteins are not substrates for SnRK2.4 and SnRK2.10, but rather indirect interactors, that might stabilize the VCS-SnRK2.4/2.10 complex; however, this still requires confirmation. Similar copurification experiments reported by Soma et al. (2017) also identified VCS, VCR, and DCP2 as SnRK2.1 interactors and VCS as a phosphorylation target of all subclass 1 SnRK2 protein kinases. Moreover, it is unlikely that VCS is the only phosphorylation target of subclass 1 SnRK2 protein kinase, and other substrates are yet to be discovered.
Here we mapped the phosphorylation sites in VCS proteins that are targeted by subclass 1 SnRK2 protein kinases (Table 3). Direct phosphorylation of Ser-645, Ser-692, and Ser-1156 of VCS and Ser-680 of VCR by SnRK2.10 was confirmed in in vitro kinase activity assays (Table 3). Another kinase from the SnRK2 subclass 1 subfamily, SnRK2.5, was also able to phosphorylate Ser-645 and Ser-1156 of the VCS peptides, but not VCR, which could possibly be because its lower activity compared with SnRK2.10 (Table 3; Supplemental Table S5). A previous phosphoproteomic study identified VCS as a putative substrate also for ABA-dependent SnRK2 kinases, and VCS was weakly phosphorylated by SnRK2.2 protein kinase upon osmotic stress (Umezawa et al., 2013, Soma et al., 2017). In our in vitro assay SnRK2.6 was able to phosphorylate VCS and VCR at the same residues as SnRK2.10 (Table 3). Phosphorylation of Ser-645, Ser-692, and Ser-1156 of VCS and Ser-680 of VCR has been previously shown to be up-regulated by osmotic and/or salt stress in planta (Stecker et al., 2014; Maszkowska et al., 2019). Thus, our data now identify both ABA-dependent and independent SnRK2 protein kinases as protein kinases targeting the VCS and VCR phosphosites phosphorylated in vivo. Moreover, two out of three VCS phosphorylation sites, Ser 645 and Ser-690, are located in the intermediate VCS sequence (VCSm) region shown to be phosphorylated by SnRK2.1 SnRK2.4, SnRK2.5, and SnRK2.10 in vitro as well as in plants subjected to osmotic or salt stress (Soma et al., 2017). To date, the consequences of VCS phosphorylation remain unknown, but it is possible that this posttranslational modification can affect assembly of the decapping complex or activity of DECAPPING 2. Since the removal of the 5′ cap exposes an RNA molecule to the exoribonucleitic activity of XRN4, we hypothesize that SnRK2 protein kinases, via phosphorylation of VCS, control 5′ mRNA decay by either enhancing or inhibiting its action. The VCS phosphorylation sites targeted by subclass 1 SnRK2 protein kinases identified here can help to understand the mechanism of this regulation.
Subclass 1 SnRK2s and components of their signaling pathway, identified here as their phosphorylation substrates or proteins acting further downstream, play a role in root development and root responses to salt. At least one of the SnRK2.1, SnRK2.5, or SnRK2.9 isoforms promotes main root growth regardless of NaCl concentration (Fig. 1A; Supplemental Fig. S3, A–D). Activity of SnRK2.4 and SnRK2.10 under salt stress appeared higher than other members of subclass 1 (Supplemental Fig. S5), suggesting higher relevance of SnRK2.4 and SnRK2.10 signaling in response to salt stress over the other class 1 members. At the same time, expression of several salt stress–regulated transcripts was impaired in snrk2.1/2.4/2.5/2.9/2.10, but not in single and double mutants, implying that SnRK2.1, SnRK2.5, and SnRK2.9 signaling can also govern salt-induced changes in gene expression (Fig. 2, Supplemental Tables S10–S15). The decreased main root growth observed here in the quintuple snrk2.1/2.4/2.5/2.9/2.10 mutant regardless of NaCl concentration was also reported for the quadruple snrk2.1/2.4/2.5/2.10 mutant (Soma et al., 2017) and single snrk2.4 mutant (McLoughlin et al., 2012) exposed to salt stress. The quintuple snrk2.1/2.4/2.5/2.9/2.10 mutant had also longer lateral roots under high salinity conditions, suggesting that some of the SnRK2 subclass 1 protein kinases inhibit lateral root growth in response to salt stress (Fig. 1A), whereas interestingly, SnRK2.10 has been shown previously to be a positive regulator of lateral root formation under salt stress (McLoughlin et al., 2012). This suggests that redundancy among subclass 1 SnRK2 protein kinases depends on the environmental conditions and differs between main root and lateral roots.
Among downstream components of the subclass 1 SnRK2s-dependent pathway, VCS inhibited lateral root elongation under nonstress conditions, but this effect was absent under salt stress (Fig. 1B; Supplemental Fig. S3B), which may suggest that the functioning of the decapping machinery is modulated by the environment. The VCS amiRNA lines tested here were previously reported to be more affected in main root length than wild type after 15 d of exposure to salinity (Soma et al., 2017). Here, during a shorter duration of salt stress (6 d), we only observed mild alterations in the main root growth (Fig. 1B; Supplemental Fig. S3B). XRN4 promoted main root and lateral root growth under control and salt stress conditions (Fig. 1C; Supplemental Fig. S3C). Because the xrn4-5 mutant was previously shown to be hypersensitive to ABA at early vegetative stages, it is plausible that observed phenotypes are a consequence of altered ABA sensitivity (Wawer et al., 2018).
Alteration in responses to salt stress in the transcriptome of snrk2.4, snrk2.4/2.10, and snrk2.1/2.4/2.5/2.9/2.10 mutants included genes involved in many biological processes (Fig. 2B; Supplemental Tables S10–S15). Out of 160 genes affected in their responses to salinity in the single, double, and quintuple mutants tested, 110 are potential VCS substrates (Fig. 2C; Supplemental Tables S16 and S18). Expression of only 29 genes had an altered response to 5 h dehydration in VCS amiRNA lines (Supplemental Table S19; Soma et al., 2017), indicating that VCS might target different genes under osmotic and salt stress and this process depends on the duration of stress exposure. Although transcripts previously reported to be controlled by SnRK2-regulated mRNA decapping had enhanced mRNA decay rates (Soma et al., 2017), here we also identified transcripts up-regulated by salt and show that this pathway might also operate via stabilization of another set of mRNAs (Fig. 3). It is still not clear whether the regulation of the mRNA abundance of PIP2;3, PIP2;5; BGLU6, and CYP79B2 are consequences of the phosphorylation of VCS or other potential substrates on subclass 1 SnRK2s (Fig. 4). Some changes in transcript abundance in response to salt observed in snrk mutants may be also linked to the activity of XRN4, yet the mechanism of this control remains unclear (Fig. 3; Supplemental Fig. S9). If these transcripts would be direct targets of XRN4 5′ exoribonuclease activity, one would expect higher levels, rather than the observed lower levels in the xrn4-5 mutant compared with Col-0, suggesting that the observed changes are a consequence of the 5′ decay of transcripts acting upstream of PIP2;5, CYP79B2, and BGLU6. It is also plausible that in the absence of XRN4, the exosome becomes the dominant decay machinery (Zhang et al., 2015), but in that case, targeted transcript levels in xrn4-5 mutant would resemble ones in Col-0.
Figure 4.
Mode of action of salt stress–induced modulations of root system architecture (RSA) governed by SnRK2 protein kinases. SnRK2 protein kinases are autophosphorylated upon salt stress. Both ABA-independent (in yellow) and ABA-dependent (in pink) SnRK2 protein kinases can phosphorylate VCS. Additional phosphorylation targets were already identified for SnRK2.6, and for ABA-independent SnRK2s, other phosphorylation substrates remain unknown. Phosphorylation of VCS may affect proper functioning of the decapping complex and lead to either inhibition or enhancement of 5′ mRNA decay. The abundance of PIP2;3, PIP2;5, and CYP79B2 transcripts, among others, depends on the subclass 1 SnRK2s, yet it remains unknown whether it is a consequence of the phosphorylation any of the kinase targets and whether it is direct or indirect regulation. PIP2;3 and PIP2;5 regulate formation and elongation of lateral roots (LR) via control of water fluxes in lateral root primordia (LRP) and CYP79B2 via local auxin biosynthesis. XRN4 activity controls abundance of PIP2;3, PIP2;5, and CYP79B2 via an unknown mechanism. Transcripts for which abundance is affected by salt stress and that modulate main root (MR) elongation remain unknown. Drawings of proteins and processes were reproduced from the model published in Kawa and Testerink (2017). Dashed lines indicate proposed processes that have not been experimentally validated.
Out of 110 genes affected in their responses to salinity in the single, double, and quintuple mutants tested and previously described as potential VCS substrates, 73 have a role in root development (Fig. 2C; Supplemental Table S16 and S18). Aquaporins have already been shown to facilitate water transfer to the LR primordium from its overlying tissues and thereby controlling LR emergence, suggesting that the roles of PIP2;5, PIP2;3, and SnRK2.10 overlap (Péret et al., 2012). Overexpression of PIP2;5 in barley resulted in increased root growth under saline conditions (Alavilli et al., 2016). Interestingly, yet another aquaporin, PIP2;1, was phosphorylated by SnRK2.6 (Grondin et al., 2015), suggesting that function of aquaporins can be regulated by SnRK2 kinases via two mechanisms: ABA dependent via direct phosphorylation and indirectly at the transcript level by non-ABA dependent SnRK2s. Recently the cytochrome P450 protein CYP79B2 was shown to regulate lateral root formation and elongation under salt stress (Julkowska et al., 2017), and natural variation in salinity induced expression of CYP79B2 positively correlated with lateral root development under salt stress (Julkowska et al., 2017). Remarkably, CYP79B2 is expressed at the sites of lateral root formation, a similar localization of which was found for SnRK2.10 (Ljung et al., 2005; McLoughlin et al., 2012). CYP79B2, via the conversion of Trp to indole-3-acetaldoxime, is involved in local biosynthesis of auxin, the key hormone regulating root development (Mikkelsen et al., 2000; Ljung et al., 2005). This, together with the fact that the expression of PIP2;2 and PIP2;5 are regulated by auxin (Péret et al., 2012), suggests that subclass 1 SnRK2s may modulate root development in response to salinity via auxin-related processes. SnRK2.10 were also required for salinity-dependent induction of the flavonol glucosyltransferase BGLU6 (Fig. 3C), which by regulation of flavonol metabolism can contribute to scavenging reactive oxygen species accumulating upon salt stress (Agati et al., 2012; Ishihara et al., 2016).
To summarize, our results suggest that both subclass 1 SnRK2 protein kinases and the 5′ mRNA decay machinery can regulate root growth in the presence of salinity, but it is unclear yet how exactly these two pathways intersect. We propose that SnRK2.1, SnRK2.5, SnRK2.9, VCS, and XRN4 control root system architecture under control and salt stress conditions, whereas the role of SnRK2.4 and SnRK2.10 is limited to responses to salinity. mRNA abundance of genes encoding aquaporins PIP2;3 and PIP2;5 and cytochrome P450 protein CYP79B2 depends on subclass SnRK2s; yet whether they are under the control of mRNA decapping remains unknown. Finally, identification of phosphosites in VCS and VCR as SnRK2 targets, and the extensive set of subclass 1 SnRK2-dependent transcripts with roles in root system architecture modulations by salt stress and other biological processes, provide a foundation to further explore the role of SnRK2 phosphorylation in these processes.
MATERIAL AND METHODS
Identification of SnRK2.4 and SnRK2.10 Interactors
The coding regions of SnRK2.4 and SnRK2.10 were cloned under the CaMV 35S promoter for fusion with GSrhino tag in pH7m24GW2 vector for N- and pH7m34GW2 for C-teminal fusion with Multisite Gateway cloning as described by Van Leene et al. (2015). Arabidopsis (Arabidopsis thaliana) PSB-d cell suspension cultures were transformed and TAP of SnRK2.4 and SnRK2.10 protein complexes was performed according to the protocol described by Van Leene et al. (2015). Eluted proteins were identified on linear trap quadrupole (LTQ) OrbitrapVelos with two technical replicates per bait. Proteins identified with at least two peptides were considered as significant. The most abundant background proteins were subtracted, and final list of the putative interactors is presented in Table 1.
Protein Expression and Purification
Glutathione S-transferase fusions were obtained by cloning the full-length coding sequences of SnRK2.4, SnRK2.5, SnRK2.6, and SnRK2.10 into pGEX4T1 and DCP2 into pGEX-KG Gateway vector. All constructs were transformed to E. coli BL21 DE3 and their expression was induced for 3 h with 1 mm isopropylthio-β-galactoside at 18°C. Recombinant proteins were purified with glutathione S-transferase-Sepharose beads (GE Healthcare) as described in Julkowska et al. (2015).
In Vitro Kinase Activity Assays
Peptides harboring putative phosphorylation sites were synthesized by GenScript (www.genscript.com). Sequences of used peptides can be found in Table 3. Each peptide (1 μm) was incubated with 0.1 μm of recombinant protein kinase in kinase reaction buffer (50 mm Tris-HCl, pH 7.5; 2 mm MgCl2; 1 mm dithiothreitol [DTT]; 1 mm ATP) in a final volume 60 μL for 6 h in 30°C. Then 20 μL of each reaction was used for direct trapping and collection of the synthetic peptides on 8 μg capacity OMIX RP tip (Agilent Technologies). The trapped peptides were eluted in 10 μL 50% (v/v) acetonitrile (ACN), 0.1% (v/v) trifluoroacetic acid (TFA), and a 3–5 μL fraction was dried in a speedvac and reconstituted in 6 μL 2% (v/v) ACN, 0.1% (v/v) TFA for liquid chromatography-mass spectrometry (LC-MS) analysis. The remaining 40 μL of each reaction was used for in-solution digestion. Samples were reduced with 10 mm DTT for 30 min at 60°C followed by alkylation with 20 mm iodoacetamide for 30 min at room temperature in darkness. An overnight digestion with 2 μg trypsin (Sigma) was performed at 37°C, stopped with TFA; peptides were collected with 50% (v/v) ACN, 0.1% (v/v) TFA on 8 μg capacity OMIX RP tip (Agilent Technologies), dried and reconstituted in 6 μL 2% (v/v) ACN, 0.1% (v/v) TFA for the analysis with LC-MS. For the kinase activity assays with full-length protein as a substrate, 0.4 μg of recombinant protein kinase and 2 μg of the substrate were used for the same reactions as with peptides in a total volume of 30 μL, digested with trypsin, collected, and analyzed as described above. MBP was used as a substrate for a positive control of protein kinase activity.
Mass Spectrometry Analysis
Mass spectrometry analyses were done with the amaZon Speed Iontrap with a CaptiveSpray ion source (Bruker) coupled to an EASY-nLC II (Proxeon, Thermo Fisher Scientific) chromatographic system. Peptide samples were injected and separated with an eluent flow of 300 nL × min−1 on an Acclaim PepMap100 (C18 75 µm 25 cm Dionex, Thermo Fisher Scientific) analytical column combined with an Acclaim PepMap100 precolumn (C18 100 µm 2 cm Dionex, Thermo Fisher Scientific) using a 30-min gradient of 0% to 50% (v/v) ACN and 0.1% (v/v) formic acid. Peptide precursor ions above a predefined threshold ion count were selected for low-energy collision-induced dissociation to obtain fragmentation spectra of the peptides. Technical replicates were performed with electron-transfer dissociation (ETD). Tandem mass spectrometry (MS/MS) data were processed with Data Analysis software (Bruker), and used for database searching with Mascot software (Version 2.5.1) in a custom-made database containing all SnRK protein kinases, MBP, and the synthetic peptides sequence information. Searches were simultaneously performed against a common contaminants database (compiled by Max Planck Institute of Biochemistry, Martinsried) to minimize false identifications. Mascot search parameters were as follows: a fixed modification of carbamidomethyl for Cys, variable modification of oxidized Met and Phospho(ST), trypsin with the allowance of one missed cleavage, peptide charge state +2, +3, and +4. Peptide and MS/MS mass error tolerances were 0.3 D for electrospray ionization-trap or electron-transfer dissociation-trap. For the sample with synthetic peptides no fixed modification was applied. The identified phosphopeptides were verified by manual inspection of MS/MS spectra in the raw data using the Data Analysis software.
Root System Architecture Assay
Seeds were surface sterilized with 20 ml dilute bleach and 600 μL 37.5% (v/v) HCl for 3 h followed by 1.5 h in laminar flow to evaporate chlorine gas. Seeds were stratified in 0.2% (w/v) agar at 4°C in the dark for 72 h. Seeds were germinated on half-strength Murashige-Skoog medium supplied with 0.5% (w/v) Suc, 0.1% (w/v) MES monohydrate, and 1% (w/v) agar with pH 5.8 (adjusted with KOH). Seeds were germinated on vertically positioned plates (70° angle) under long-day conditions (21°C, 70% humidity, 16/8h light/dark cycle). The 4-d-old seedlings of similar main root length were transferred to 0.5× MS media supplemented with 0, 75, or 125 mm NaCl. Root System Architecture of 10-d-old seedlings from control and salt stress conditions, respectively, was quantified with Smartroot Software (Lobet et al., 2011). Two independent biological experiments with 20 replicates per condition were performed. Individuals with main root length and lateral root number values outside the 3rd quartile were identified as outliers and removed from dataset. Statistical analysis was performed with ANOVA with experiment number as factor followed with pairwise genotype*treatment comparison by least square means test.
In-Gel Kinase Activity Assay
Arabidopsis seeds were surface sterilized with 20 mL dilute bleach and 600 μL 37.5% (v/v) HCl for 3 h and then placed for 1.5 h in laminar flow to evaporate chlorine gas. Seeds were stratified for 72 h at 4°C and grown under long-day conditions (21°C, 70% humidity, 16/8 light/dark cycle) in 100 mL liquid media containing 0.5× Murashige-Skoog basal salt, 0.5% (w/v) Suc, 1% (w/v) MES monohydrate, pH 5.8 (KOH) with shaking (120 rpm). The 10-d-old seedlings were treated with 150 mm NaCl in 0.1× Murashige-Skoog media (salt stress) or 0.1× Murashige-Skoog media (control) for 0, 0.5, 1, 2, 5, 10, and 30 min; 1, 6, and 24 h. Two independent biological experiments with four individual seedlings liquid cultures were performed. Seedlings were dried with paper towel and snap frozen in liquid nitrogen. Tissue was grounded and proteins were extracted with 1:3 (v/w) lysis buffer (50 mm Tris-HCl, pH 7.5; 5 mm EDTA; 5 mm EGTA; 2 mm DTT; 25 mm NaF; 1 mm Na3VO4; 50 mm β-glycerophosphate; 1× complete protease inhibitor cocktail; Promega) and spun down at 26,000 g for 30 min. Protein concentrations were determined by the Bradford protein assay (Bio-Rad). Crude protein extract (50 μg) was separated on 12% (w/v) polyacrylamide gel containing 0.2 mg/mL of MBP (Upstate). Gels were washed three times for 30 min at room temperature with washing buffer (25 mm Tris-HCl, pH 7.5; 0.5 mm DTT; 0.1 mm Na3VO4; 5 mm NaF; 0.5 mg/mL bovine serum albumin, 0.1% [v/v] Triton X-100) and then twice for 30 min and overnight at 4°C in renaturation buffer (25 mm Tris-HCl, pH 7.5; 0.5 mm DTT; 0.1 mm Na3VO4, 5 mm NaF). Gels were incubated in reaction buffer (25 mm Tris-HCl, pH 7.5; 2 mm EGTA; 12 mm MgCl2; 1 mm DTT; 0.1 mm Na3VO4) for 30 min at 37°C and then brought to reaction buffer containing 25 μm ATP and 50 μCi 32P γ-ATP for 1 h. Gels were washed 6 times in 1% (w/v) Na2H2P2O7, 5% (w/v) trichloroacetic acid (TCA), incubated for 30 min in 3% (v/v) glycerol, dried overnight, exposed to Storage Phospho Screen (Fuji) for 2 weeks, and scanned using a phosphoimager (Typhoon FLA 7000, GE Healthcare).
Transcriptome Profiling and RT-qPCR Validation
Arabidopsis seeds were surface sterilized with 20 ml dilue bleach and 600 μL 37.5% (v/v) HCl for 3 h and then placed for 1.5 h in laminar flow to evaporate chlorine gas. Seeds were stratified for 72 h at 4°C and grown under long-day conditions (21°C, 70% humidity, 16/8 light/dark cycle) in 100 mL liquid media containing 0.5× Murashige-Skoog basal salt, 0.5% (w/v) Suc, 1% (w/v) MES monohydrate, pH 5.8 (KOH) with shaking (120 rpm). The 10-d-old seedlings were treated with 150 mm NaCl in 0.1× Murashige-Skoog media (salt stress) or 0.1× Murashige-Skoog media (control) for 1 h. Three individual seedlings liquid cultures were used per each genotype and treatment combination. Seedlings were dried with paper towel and snap frozen in liquid nitrogen. Tissue (100 mg) was ground, and total RNA was extracted using Plant RNA extraction kit (Qiagen) according to the manufacturer’s instructions. RNA quality determination, library preparation, and sequencing with Illumina HiSeq 2500 was performed by Eurofins Genomics (Germany). The quality of the libraries was assessed before and after read processing with FastQC and Trimmomatic and then aligned to the Col-0 genome from The Arabidopsis Information Resource (TAIR) 10.30 database using TopHat algorithm (Kim et al., 2013). Transcripts were assembled, and their abundance was quantified using Cufflinks where significant changes in transcript abundance between samples were detected with Cuffdiff (Trapnell et al., 2012). Differentially expressed genes were selected based on a false discovery rate < 0.05 and absolute value of log2(fold change) > 1. GO categories enrichments were performed with agriGO analysis toolkit (Du et al., 2010) using TAIR10 annotation as a background with hypergeometric test and Hochberg multitest adjustment method (false discovery rate), significance level threshold 0.01, maximum number of mapping entries 5, and Plant GO Slim ontology. For the selected candidates, expression levels were confirmed by RT-qPCR on RNA extracted from an independent biological experiment performed under the same conditions as used for RNA-seq analysis. Complementary DNA was synthesized with ReverAid Kit (Fermentas) with oligo(dT) primer and 5 μL were used for each reaction with Eva-Green kit (Solis Biodyne). Three biological replicates were used per line and two technical replicates were made. The sequences of primers are indicated in Supplemental Table S21. The transcript level was normalized by expression of the reference gene MON1 (At2G28390) according to the following formula: ΔCt = 2(Ct candidate gen)/2(Ct reference gene).
Statistical Analysis
Statistical analysis of the root system architecture assays was performed with two-way ANOVA in R. A summary of the statistics can be found in Supplemental Datasets S1–S3.
Accession Numbers
The transcriptomic data from this article has been deposited in the Array Express (http://www.ebi.ac.uk/arrayexpress) under the accession E-MTAB-8073.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Spectra of the phosphorylated peptides identified in SnRK2.4 and SnRK2.10 TAP experiments.
Supplemental Figure S2. Spectra of the phosphorylated synthetic peptides identified in in vitro kinase activity assays.
Supplemental Figure S3. Components of SnRK2 subclass 1-regulated 5′ mRNA decay pathways contribute to root development and root system architecture responses to salt stress.
Supplemental Figure S4. Expression of VCS (VARICOSE) and XRN4 (5′ EXORIBONUCLEASE 4) in amiRNA lines VCS #2 and VCS#4 and in T-DNA insertion lines xrn4-5 and xrn4-6.
Supplemental Figure S5. Rapid activation of SnRK2.4 and SnRK2.10 in Arabidopsis seedlings.
Supplemental Figure S6. Salt stress induced changes in mRNA abundance of genes encoding SnRK2 protein kinases.
Supplemental Figure S7. Overview of the process of the selection of salt stress regulated genes that are dependent on subclass 1 SnRK2 protein kinases.
Supplemental Figure S8. SnRK2 subclass 1 protein kinases regulate gene expression under nonstressed conditions.
Supplemental Figure S9. Salt-induced expression of PLASMA MEMBRANE INTRINSIC PROTEINS (PIP2;5, PIP2;3), BETA GLUCOSIDASE 6 (BGLU6), CYTOCHROME P450, FAMILY 79, SUBFAMILY B, POLYPEPTIDE 2 (CYP79B2) is dependent on SnRK2 subclass 1 protein kinases signaling.
Supplemental Table S1. Protein identification details obtained with the LTQ Orbitrap Velos (Thermo Fisher Scientific) and Mascot Distiller software (version 2.5.0, Matrix Science) combined with the Mascot search engine (version 2.5.0 for SnRK2.4, and version 2.5.1 for SnRK2.10, Matrix Science) using the Mascot Daemon interface and database TAIRplus (Van Leene et al., 2015).
Supplemental Table S2. Recombinant SnRK2.4 protein phosphorylates MBP, yet is activity is too low to detect its autophosporylation and phosphorylation of VCS, VCR and DCP2 peptides.
Supplemental Table S3. Peptides identified with MS/MS analysis of the in vitro kinase activity assay with SnRK2.4 and MBP as a substrate.
Supplemental Table S4. Peptides identified with MS/MS analysis of the in vitro kinase activity assay with SnRK2.10 and MBP as a substrate.
Supplemental Table S5. Peptides identified with MS/MS analysis of the in vitro kinase activity assay with SnRK2.5 and MBP as a substrate.
Supplemental Table S6. Peptides identified with MS/MS analysis of the in vitro kinase activity assay with SnRK2.6 and MBP as a substrate.
Supplemental Table S7. Peptides identified with MS/MS analysis of the in vitro kinase activity assay with SnRK2.10 and DCP2 as a substrate.
Supplemental Table S8. List of differentially expressed genes found in snrk2.4, snrk2.4/10 or snrk2.1/4/5/9/10 in ten days old seedlings.
Supplemental Table S9. List of genes with expression changed by salt. Ten days old seedlings grown in liquid cultures were treated with mock or 150 mm NaCl for 1 h.
Supplemental Table S10. List of genes with expression changed in Col-0 upon 1 h treatment with 150 mm NaCl, but not affected in snrk2.4 mutant.
Supplemental Table S11. List of genes with expression changed in Col-0 upon 1 h treatment with 150 mm NaCl, but not affected in snrk2.4/2.10 mutant.
Supplemental Table S12. List of genes with expression changed in Col-0 upon 1 h treatment with 150 mm NaCl, but not affected in snrk2.1/2.4/2.5/2.9/2.10 mutant.
Supplemental Table S13. List of genes which expression was regulated in snrk2.4 mutant, but to a different degree or in an opposite manner than in Col-0.
Supplemental Table S14. List of genes which expression was regulated in snrk2.4/10 mutant, but to a different degree or in an opposite manner than in Col-0.
Supplemental Table S15. List of genes which expression was regulated in snrk2.1/4/5/9/10 mutant, but to a different degree or in an opposite manner than in Col-0.
Supplemental Table S16. Overlap of the genes with expression altered in salt stress in snrk2.4, snrk2.4/10 and snrk2.1/4/5/9/10.
Supplemental Table S17. List of the genes overlapping between our candidate genes acting downstream of SnRK2 subclass 1 protein kinases and transcripts reported to have half-life shorter than 3 h as reported in Narsai et al. (2007).
Supplemental Table S18. List of the genes overlapping between our candidate genes acting downstream of SnRK2 subclass 1 protein kinases and transcripts reported to be targets of VCS (Sorenson et al., 2018).
Supplemental Table S19. List of the genes overlapping between our candidate genes acting downstream of SnRK2 subclass 1 protein kinases and transcripts with altered response to drought in amiRNA VCS lines (Soma et al., 2017).
Supplemental Table S20. List of the genes overlapping between our candidate genes acting downstream of SnRK2 subclass 1 protein kinases and transcripts reported to be dependent on LSM1 signaling (Perea-Resa et al., 2016).
Supplemental Table S21. List of the primers used for RT-qPCR.
Supplemental Dataset S1. ANOVA tables for RSA assays with snrk2.1/4/5/9/10.
Supplemental Dataset S2. ANOVA tables for RSA assays with VCS ami RNA lines.
Supplemental Dataset S3. ANOVA tables for RSA assays with xrn4-5 and xrn4-6.
ACKNOWLEDGMENTS
We thank Joanna Kufel for the xrn4-5 line (SAIL_681_E01) and helpful discussions, Jian-Kang Zhu for snrk2.1/2.4/2.5/2.9/2.10 seeds, and Kazuko Yamaguchi-Shinozaki for VCS amiRNA lines. The xrn4-6 line (SALK_014209) was obtained from NASC. We thank Ringo van Wijk and Jiorgos Kourelis for technical assistance.
Footnotes
This work was supported by the Netherlands Organization for Scientific Research-National Natural Science Foundation of China (ALW project 846.11.002 to C.T.); the European Research Council (Consolidator grant 724321 to C.T.); and the National Science Center (2016/23/B/NZ3/03182 to G.D.).
Articles can be viewed without a subscription.
References
- Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci 196: 67–76 [DOI] [PubMed] [Google Scholar]
- Alavilli H, Awasthi JP, Rout GR, Sahoo L, Lee BH, Panda SK (2016) Overexpression of a barley aquaporin gene, HvPIP2;5 confers salt and osmotic stress tolerance in yeast and plants. Front Plant Sci 7: 1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Barbier-Brygoo H, Laurière C (2004) Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J Biol Chem 279: 41758–41766 [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Droillard MJ, Barbier-Brygoo H, Laurière C (2007) Different phosphorylation mechanisms are involved in the activation of sucrose non-fermenting 1 related protein kinases 2 by osmotic stresses and abscisic acid. Plant Mol Biol 63: 491–503 [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Laurière C (2005) Osmotic signaling in plants: Multiple pathways mediated by emerging kinase families. Plant Physiol 138: 1185–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholc M, Ciesielski A, Goch G, Anielska-Mazur A, Kulik A, Krzywińska E, Dobrowolska G (2011) SNF1-related protein kinases 2 are negatively regulated by a plant-specific calcium sensor. J Biol Chem 286: 3429–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: Emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679 [DOI] [PubMed] [Google Scholar]
- Droillard M, Boudsocq M, Barbier-Brygoo H, Laurière C (2002) Different protein kinase families are activated by osmotic stresses in Arabidopsis thaliana cell suspensions. Involvement of the MAP kinases AtMPK3 and AtMPK6. FEBS Lett 527: 43–50 [DOI] [PubMed] [Google Scholar]
- Du Z, Zhou X, Ling Y, Zhang Z, Su Z (2010) agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res 38: W64–W70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fita A, Rodríguez-Burruezo A, Boscaiu M, Prohens J, Vicente O (2015) Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front Plant Sci 6: 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Verslues PE, Zhu JK (2007) Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell 19: 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Verslues PE, Zhu JK (2011) Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc Natl Acad Sci USA 108: 1717–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Zhu JK (2009) Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc Natl Acad Sci USA 106: 8380–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Nakashima K, Yoshida T, Katagiri T, Kidokoro S, Kanamori N, Umezawa T, Fujita M, Maruyama K, Ishiyama K, et al. (2009) Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol 50: 2123–2132 [DOI] [PubMed] [Google Scholar]
- Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2006) Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc Natl Acad Sci USA 103: 1988–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KA, et al. (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA 106: 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeres DC, Van Norman JM, Zhang W, Fauver NA, Spencer ML, Sieburth LE (2007) Components of the Arabidopsis mRNA decapping complex are required for early seedling development. Plant Cell 19: 1549–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin A, Rodrigues O, Verdoucq L, Merlot S, Leonhardt N, Maurel C (2015) Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 27: 1945–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gy I, Gasciolli V, Lauressergues D, Morel JB, Gombert J, Proux F, Proux C, Vaucheret H, Mallory AC (2007) Arabidopsis FIERY1, XRN2, and XRN3 are endogenous RNA silencing suppressors. Plant Cell 19: 3451–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huai J, Wang M, He J, Zheng J, Dong Z, Lv H, Zhao J, Wang G (2008) Cloning and characterization of the SnRK2 gene family from Zea mays. Plant Cell Rep 27: 1861–1868 [DOI] [PubMed] [Google Scholar]
- Ishihara H, Tohge T, Viehöver P, Fernie AR, Weisshaar B, Stracke R (2016) Natural variation in flavonol accumulation in Arabidopsis is determined by the flavonol glucosyltransferase BGLU6. J Exp Bot 67: 1505–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julkowska MM, McLoughlin F, Galvan-Ampudia CS, Rankenberg JM, Kawa D, Klimecka M, Haring MA, Munnik T, Kooijman EE, Testerink C (2015) Identification and functional characterization of the Arabidopsis Snf1-related protein kinase SnRK2.4 phosphatidic acid-binding domain. Plant Cell Environ 38: 614–624 [DOI] [PubMed] [Google Scholar]
- Julkowska MM, Koevoets IT, Mol S, Hoefsloot H, Feron R, Tester MA, Keurentjes JJB, Korte A, Haring MA, de Boer GJ, Testerink C (2017) Genetic components of root architecture remodeling in response to salt stress. Plant Cell 29: 3198–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmayer JP, Green PJ (2000) Novel features of the XRN-family in Arabidopsis: Evidence that AtXRN4, one of several orthologs of nuclear Xrn2p/Rat1p, functions in the cytoplasm. Proc Natl Acad Sci USA 97: 13985–13990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa D, Testerink C (2017) Regulation of mRNA decay in plant responses to salt and osmotic stress. Cell Mol Life Sci 74: 1165–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner A, Pekala I, Kaczanowski S, Muszynska G, Hardie DG, Dobrowolska G (2004) Biochemical characterization of the tobacco 42-kD protein kinase activated by osmotic stress. Plant Physiol 136: 3255–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL (2013) TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattori T (2004) Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell 16: 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywińska E, Bucholc M, Kulik A, Ciesielski A, Lichocka M, Dębski J, Ludwików A, Dadlez M, Rodriguez PL, Dobrowolska G (2016) Phosphatase ABI1 and okadaic acid-sensitive phosphoprotein phosphatases inhibit salt stress-activated SnRK2.4 kinase. BMC Plant Biol 16: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik A, Wawer I, Krzywińska E, Bucholc M, Dobrowolska G (2011) SnRK2 protein kinases--key regulators of plant response to abiotic stresses. OMICS 15: 859–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S (2009) A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA 106: 21419–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Assmann SM (1996) An abscisic acid-activated and calcium-independent protein kinase from guard cells of fava bean. Plant Cell 8: 2359–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang Y, Liu K, Ni Z, Fang Z, Sun Q, Gao J (2010) Identification and bioinformatics analysis of SnRK2 and CIPK family genes in sorghum. Agric Sci China 9: 19–30 [Google Scholar]
- Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg G (2005) Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 17: 1090–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobet G, Pagès L, Draye X (2011) A novel image-analysis toolbox enabling quantitative analysis of root system architecture. Plant Physiol 157: 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Mao X, Zhang H, Tian S, Chang X, Jing R (2010) TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. J Exp Bot 61: 683–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maszkowska J, Dębski J, Kulik A, Kistowski M, Bucholc M, Lichocka M, Klimecka M, Sztatelman O, Szymańska KP, Dadlez M, Dobrowolska G (2019) Phosphoproteomic analysis reveals that dehydrins ERD10 and ERD14 are phosphorylated by SNF1-related protein kinase 2.10 in response to osmotic stress. Plant Cell Environ 42: 931–946 [DOI] [PubMed] [Google Scholar]
- McLoughlin F, Galvan-Ampudia CS, Julkowska MM, Caarls L, van der Does D, Laurière C, Munnik T, Haring MA, Testerink C (2012) The Snf1-related protein kinases SnRK2.4 and SnRK2.10 are involved in maintenance of root system architecture during salt stress. Plant J 72: 436–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J (2001) The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J 25: 295–303 [DOI] [PubMed] [Google Scholar]
- Mikkelsen MD, Hansen CH, Wittstock U, Halkier BA (2000) Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J Biol Chem 275: 33712–33717 [DOI] [PubMed] [Google Scholar]
- Mizoguchi M, Umezawa T, Nakashima K, Kidokoro S, Takasaki H, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K (2010) Two closely related subclass II SnRK2 protein kinases cooperatively regulate drought-inducible gene expression. Plant Cell Physiol 51: 842–847 [DOI] [PubMed] [Google Scholar]
- Monks DE, Aghoram K, Courtney PD, DeWald DB, Dewey RE (2001) Hyperosmotic stress induces the rapid phosphorylation of a soybean phosphatidylinositol transfer protein homolog through activation of the protein kinases SPK1 and SPK2. Plant Cell 13: 1205–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsai R, Howell KA, Millar AH, O’Toole N, Small I, Whelan J (2007) Genome-wide analysis of mRNA decay rates and their determinants in Arabidopsis thaliana. Plant Cell 19: 3418–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Sarkeshik A, Nito K, Park SY, Wang A, Carvalho PC, Lee S, Caddell DF, Cutler SR, Chory J, Yates JR, Schroeder JI (2010) PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J 61: 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Resa C, Carrasco-López C, Catalá R, Turečková V, Novak O, Zhang W, Sieburth L, Jiménez-Gómez JM, Salinas J (2016) The LSM1-7 complex differentially regulates arabidopsis tolerance to abiotic stress conditions by promoting selective mRNA decapping. Plant Cell 28: 505–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, Li G, Zhao J, Band LR, Voß U, Postaire O, Luu DT, Da Ines O, Casimiro I, Lucas M, et al. (2012) Auxin regulates aquaporin function to facilitate lateral root emergence. Nat Cell Biol 14: 991–998 [DOI] [PubMed] [Google Scholar]
- Roux ME, Rasmussen MW, Palma K, Lolle S, Regué AM, Bethke G, Glazebrook J, Zhang W, Sieburth L, Larsen MR, Mundy J, Petersen M (2015) The mRNA decay factor PAT1 functions in a pathway including MAP kinase 4 and immune receptor SUMM2. EMBO J 34: 593–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Sato Y, Fukao Y, Fujiwara M, Umezawa T, Shinozaki K, Hibi T, Taniguchi M, Miyake H, Goto DB, Uozumi N (2009) Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem J 424: 439–448 [DOI] [PubMed] [Google Scholar]
- Shin R, Alvarez S, Burch AY, Jez JM, Schachtman DP (2007) Phosphoproteomic identification of targets of the Arabidopsis sucrose nonfermenting-like kinase SnRK2.8 reveals a connection to metabolic processes. Proc Natl Acad Sci USA 104: 6460–6465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichandra C, Gu D, Hu HC, Davanture M, Lee S, Djaoui M, Valot B, Zivy M, Leung J, Merlot S, Kwak JM (2009) Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett 583: 2982–2986 [DOI] [PubMed] [Google Scholar]
- Soma F, Mogami J, Yoshida T, Abekura M, Takahashi F, Kidokoro S, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2017) ABA-unresponsive SnRK2 protein kinases regulate mRNA decay under osmotic stress in plants. Nat Plants 3: 16204. [DOI] [PubMed] [Google Scholar]
- Sorenson RS, Deshotel MJ, Johnson K, Adler FR, Sieburth LE (2018) Arabidopsis mRNA decay landscape arises from specialized RNA decay substrates, decapping-mediated feedback, and redundancy. Proc Natl Acad Sci USA 115: E1485–E1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souret FF, Kastenmayer JP, Green PJ (2004) AtXRN4 degrades mRNA in Arabidopsis and its substrates include selected miRNA targets. Mol Cell 15: 173–183 [DOI] [PubMed] [Google Scholar]
- Stecker K, Minkoff BB, Sussman MR (2014) Phosphoproteomic analyses reveal early signaling events in the osmotic stress response. Plant Physiol 165: 1171–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymańska KP, Polkowska-Kowalczyk L, Lichocka M, Maszkowska J, Dobrowolska G (2019) SNF1-related protein kinases SnRK2.4 and SnRK2.10 modulate ROS homeostasis in plant response to salt stress. Int J Mol Sci 20: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testerink C, Dekker HL, Lim ZY, Johns MK, Holmes AB, Koster CG, Ktistakis NT, Munnik T (2004) Isolation and identification of phosphatidic acid targets from plants. Plant J 39: 527–536 [DOI] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7: 562–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Sugiyama N, Takahashi F, Anderson JC, Ishihama Y, Peck SC, Shinozaki K (2013) Genetics and phosphoproteomics reveal a protein phosphorylation network in the abscisic acid signaling pathway in Arabidopsis thaliana. Sci Signal 6: rs8. [DOI] [PubMed] [Google Scholar]
- Van Leene J, Eeckhout D, Cannoot B, De Winne N, Persiau G, Van De Slijke E, Vercruysse L, Dedecker M, Verkest A, Vandepoele K, et al. (2015) An improved toolbox to unravel the plant cellular machinery by tandem affinity purification of Arabidopsis protein complexes. Nat Protoc 10: 169–187 [DOI] [PubMed] [Google Scholar]
- Vlad F, Droillard MJ, Valot B, Khafif M, Rodrigues A, Brault M, Zivy M, Rodriguez PL, Merlot S, Laurière C (2010) Phospho-site mapping, genetic and in planta activation studies reveal key aspects of the different phosphorylation mechanisms involved in activation of SnRK2s. Plant J 63: 778–790 [DOI] [PubMed] [Google Scholar]
- Voß U, Wilson MH, Kenobi K, Gould PD, Robertson FC, Peer WA, Lucas M, Swarup K, Casimiro I, Holman TJ, et al. (2015) The circadian clock rephases during lateral root organ initiation in Arabidopsis thaliana. Nat Commun 6: 7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Xue L, Batelli G, Lee S, Hou YJ, Van Oosten MJ, Zhang H, Tao WA, Zhu JK (2013) Quantitative phosphoproteomics identifies SnRK2 protein kinase substrates and reveals the effectors of abscisic acid action. Proc Natl Acad Sci USA 110: 11205–11210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawer I, Golisz A, Sulkowska A, Kawa D, Kulik A, Kufel J (2018) mRNA decapping and 5′-3′ decay contribute to the regulation of ABA signaling in Arabidopsis thaliana. Front Plant Sci 9: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Nover L, Fauth M (2008) Plant stress granules and mRNA processing bodies are distinct from heat stress granules. Plant J 56: 517–530 [DOI] [PubMed] [Google Scholar]
- Xu J, Yang JY, Niu QW, Chua NH (2006) Arabidopsis DCP2, DCP1, and VARICOSE form a decapping complex required for postembryonic development. Plant Cell 18: 3386–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Tang N, Xian Z, Li Z (2015) Two SnRK2 protein kinases genes play a negative regulatory role in the osmotic stress response in tomato. Plant Cell Tissue Organ Cult 122: 421–434 (PCTOC) [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K (2002) ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol 43: 1473–1483 [DOI] [PubMed] [Google Scholar]
- Zhang H, Li W, Mao X, Jing R, Jia H (2016) Differential activation of the wheat SnRK2 family by abiotic stresses. Front Plant Sci 7: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Mao X, Jing R, Chang X, Xie H (2011) Characterization of a common wheat (Triticum aestivum L.) TaSnRK2.7 gene involved in abiotic stress responses. J Exp Bot 62: 975–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Mao X, Wang C, Jing R (2010) Overexpression of a common wheat gene TaSnRK2.8 enhances tolerance to drought, salt and low temperature in Arabidopsis. PLoS One 5: e16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhu Y, Liu X, Hong X, Xu Y, Zhu P, Shen Y, Wu H, Ji Y, Wen X, et al. (2015) Plant biology. Suppression of endogenous gene silencing by bidirectional cytoplasmic RNA decay in Arabidopsis. Science 348: 120–123 [DOI] [PubMed] [Google Scholar]