In multiple Neurachne species, organelles and glycine decarboxylase show varying enhancement in the mestome sheath, suggesting that C2 photosynthesis gradually evolved from ancestral C3 plants.
Abstract
The Australian grass subtribe Neurachninae contains closely related species that use C3, C4, and C2 photosynthesis. To gain insight into the evolution of C4 photosynthesis in grasses, we examined leaf gas exchange, anatomy and ultrastructure, and tissue localization of Gly decarboxylase subunit P (GLDP) in nine Neurachninae species. We identified previously unrecognized variation in leaf structure and physiology within Neurachne that represents varying degrees of C3–C4 intermediacy in the Neurachninae. These include inverse correlations between the apparent photosynthetic carbon dioxide (CO2) compensation point in the absence of day respiration (C*) and chloroplast and mitochondrial investment in the mestome sheath (MS), where CO2 is concentrated in C2 and C4 Neurachne species; width of the MS cells; frequency of plasmodesmata in the MS cell walls adjoining the parenchymatous bundle sheath; and the proportion of leaf GLDP invested in the MS tissue. Less than 12% of the leaf GLDP was allocated to the MS of completely C3 Neurachninae species with C* values of 56–61 μmol mol−1, whereas two-thirds of leaf GLDP was in the MS of Neurachne lanigera, which exhibits a newly-identified, partial C2 phenotype with C* of 44 μmol mol−1. Increased investment of GLDP in MS tissue of the C2 species was attributed to more MS mitochondria and less GLDP in mesophyll mitochondria. These results are consistent with a model where C4 evolution in Neurachninae initially occurred via an increase in organelle and GLDP content in MS cells, which generated a sink for photorespired CO2 in MS tissues.
C4 photosynthesis is a complex trait resulting from the evolutionary reorganization of C3 leaf anatomy and biochemistry to form an efficient carbon dioxide (CO2) concentrating mechanism. How the C4 pathway evolved has been a central issue in plant biology since its discovery over 50 years ago (Downton, 1971). In the past 15 years, phylogenetic studies of C3 and C4 species enabled testing of hypotheses concerning when, where, and how the C4 pathway originated (for example, McKown et al., 2005; Christin et al., 2010, 2011a,b; Kadereit et al., 2012, 2014). Together with physiological and biochemical investigations, these studies support the hypothesis of Monson et al. (1984) that the C4 pathway arose via a series of innovations that initially enabled plants to refix a large fraction of photorespired CO2 via the shuttling of Gly from the mesophyll (M) to a sheath of cells surrounding the vascular tissue, where the Gly is decarboxylated (for review, see Sage et al., 2014). The sheath layer is most commonly the bundle sheath (BS) cells, although in some grasses the mestome sheath (MS) cells is where decarboxylation of Gly occurs (Khoshravesh et al., 2016). Associated with the shuttling of Gly is a shift in the expression of the mitochondrial enzyme Gly decarboxylase (GDC) from M cells to the vascular sheath tissue. This requires that the Gly produced in photorespiration diffuses from M to sheath cells for metabolism. Gly shuttling, also known as C2 photosynthesis, elevates CO2 levels in the BS two to three times that of M cells, thereby improving Rubisco efficiency (von Caemmerer, 2000; Keerberg et al., 2014). Photosynthetic enhancements in C2 species are greatest at CO2 levels below the current atmospheric value of 400 µmol mol−1, supporting a hypothesis that C2 photosynthesis is a low-CO2 adaptation that bridges the transition from C3 to C4 photosynthesis (Hattersley et al., 1986; Bauwe, 2011; Vogan and Sage, 2012). Consistent with this hypothesis, the C2 condition is present in more than 40 intermediate species that branch between C3 and C4 species on the phylogenetic trees of more than a dozen distinct C4 lineages (Sage et al., 2014; Lundgren and Christin, 2017; Schüssler et al., 2017).
An important early step proposed for C2 evolution is the physiological activation of the BS of C3 species (Gowik and Westhoff, 2011; Sage et al., 2014). Activation of the BS is facilitated by increases in the number and size of mitochondria and chloroplasts and repositioning of mitochondria plus a few chloroplasts to the centripetal pole of the BS, possibly to facilitate refixation of some photorespired CO2 produced in the leaf (Muhaidat et al., 2011; Sage et al., 2013; Voznesenskaya et al., 2013; Khoshravesh et al., 2016). Together, organelle enrichment and repositioning have been termed the “proto-Kranz” phase of early C4 evolution, and is recognized in close relatives of C3 and C2 species in the eudicot genera Flaveria, Heliotropium, and Salsola, and the monocot genus Steinchisma (Muhaidat et al., 2011; Sage et al., 2013; Khoshravesh et al., 2016; Schüssler et al., 2017). These observations support a hypothesis that proto-Kranz is a key early step in C2 evolution. Transition to the C2 condition occurs with further increases in mitochondrial size and numbers in the BS, increased chloroplast numbers in the BS, and a repositioning of most chloroplasts to the centripetal wall of BS cells (Brown and Hattersley, 1989; Muhaidat et al., 2011; Sage et al., 2013; Khoshravesh et al., 2016). Increases in vein density and BS cross-sectional area can occur in concert with organelle and GDC enrichment in sheath cells of proto-Kranz and C2 species, and the size and numbers of M cells often decline as well (McKown and Dengler 2007; Muhaidat et al., 2011; Sage et al., 2013; Khoshravesh et al., 2016). Because metabolite flux between M and sheath cells increases as the C2 and C4 pathways evolve, increased intercellular transport capacity between these compartments should also increase. C4 plants are known to have greater frequencies of plasmodesmata (PD) between M and BS cells than that in related C3 species (Danila et al., 2016, 2018), but it is not known where along the C3 to C4 transition the PD frequency increases.
Although research on C3–C4 intermediates provides many insights into C4 evolution, it is largely dominated by results from a handful of species. Over 80% of known intermediates are eudicot, with Flaveria species (Asteraceae) in particular dominating research on C4 origins (Monson and Rawsthorne, 2000; Schulze et al., 2013; Sage et al., 2014). By contrast, only a handful of C3–C4 intermediate monocots are known, which is surprising given the predominance of monocot taxa among the C4 flora. Despite accounting for 78% of all C4 species, only four of 30 C4 monocot lineages are known to have C3–C4 intermediate species (Hattersley and Stone, 1986; Khoshravesh et al., 2016; Lundgren et al., 2016; Sage, 2016). Of these, the grass genera Alloteropsis and Neurachne also contain C4 species, but have only one recognized intermediate species each; Steinchisma has five or six species exhibiting C2 photosynthesis, but it is not close to any C4 clade (Brown et al., 1983; Aliscioni et al., 2003; Grass Phylogeny Working Group II, 2012). This relatively low number of C3–C4 intermediates in the monocots limits investigations into C4 pathway evolution in grasses and sedges, and constrains development of theoretical models of C4 evolution. For example, the evolutionary model of Williams et al. (2013) predicts C4 evolved differently in grasses than eudicots; however, the low number of C3–C4 intermediates in the grasses limited parameterization of the monocot algorithms in their model.
The above considerations indicate that identification of additional C3–C4 intermediates in grasses and sedges would promote understanding of C4 evolution in the monocots. To this end, C3–C4 intermediates in the grass genera Alloteropsis and Homolepis have been recently identified (Khoshravesh et al., 2016; Lundgren et al., 2016). Another approach is to re-examine the grass genus Neurachne and its allies in the subtribe Neurachninae, where work in the 1980s identified one C2 species (Neurachne minor), two C4 species (Neurachne munroi, and Neurachne muelleri—formerly Paraneurachne muelleri), and four putative C3 species (Neurachne alopecuroidea, Neurachne lanigera, Neurachne queenslandica, Neurachne tenuifolia; Hattersley et al., 1982, 1986; Hattersley and Roksandic, 1983; Hattersley and Stone, 1986). Subsequent work discovered an additional C3 species, Neurachne annularis (Macfarlane, 2007), whereas Thyridolepis, a sister genus in the Neurachninae, contains three putative C3 species (Prendergast and Hattersley, 1985; Hattersley et al., 1986). This research indicates the potential of the Neurachninae to provide novel insights into C4 evolution, particularly in the initial transition from a C3 character state. Work with C3 relatives of C4 species describe certain anatomical shifts, such as increases in vein density, that may predispose lineages to evolve C4 (Muhaidat et al., 2011; Christin et al., 2013; Griffiths et al., 2013; Sage et al., 2013). Similar studies in putative C3 species of Neurachne could also identify anatomical traits that facilitated C4 evolution in this lineage (Hattersley et al., 1986; Brown and Hattersley, 1989). The Neurachninae also promises unique insights not available in other C4 lineages, in that the C4 Neurachne species exhibit a distinct form of Kranz anatomy termed “Neurachneoid,” where a suberized MS is the site of CO2 concentration (Dengler and Nelson, 1999; Edwards and Voznesenskaya, 2011). These cells are in turn surrounded by an outer sheath of parenchyma cells considered to have low photosynthetic activity (Hattersley et al., 1982, 1986). The combined resistance of the suberized MS wall and the parenchymatous sheath (PS) could have profound implications for how C4 evolved in Neurachne, and other grass lineages where CO2 is concentrated in the MS, such as Alloteropsis (Lundgren et al., 2019). In addition, it is now possible to evaluate evolutionary hypotheses in the Neurachninae with the publication of a species-level molecular phylogeny (Christin et al., 2012). The Neurachninae clade is young (<10 million years old), increasing the likelihood that unknown intermediate traits persist (Christin et al., 2012). Both C4 Neurachne species use the NADP-malic enzyme subtype of C4 photosynthesis (Hattersley and Stone, 1986; Moore and Edwards, 1989), which the molecular phylogeny indicates were independently acquired (Christin et al., 2012).
To develop a more comprehensive understanding of the tissue and cellular changes during C4 grass evolution, this study re-examined the physiological and structural diversity of nine species of the Neurachninae subtribe using leaf gas exchange, leaf anatomical and ultrastructural studies, and immunolocalization of the P-subunit of GDC (GLDP) and Rubisco large subunit (LSU). Structural assessments include quantitative analyses of MS versus M cell dimensions, organelle size and numbers, interveinal distance (IVD), and the relative contribution of MS, PS, and M tissue to the leaf volume. PD frequencies between MS and PS cells and between PS and M cells were also evaluated to assess potential changes in intercellular transport capacities along the evolutionary transition. Gas exchange analysis allowed us to relate modifications in leaf structure to physiological performance, with the parameter C* (the apparent CO2 compensation point in the absence of day respiration) being our main index of photorespiratory CO2 refixation after Gly shuttling (Sage et al., 2013). We used the recent phylogeny of Neurachninae (Christin et al., 2012) to interpret observed trait shifts in an evolutionary context.
RESULTS
Leaf Gas Exchange
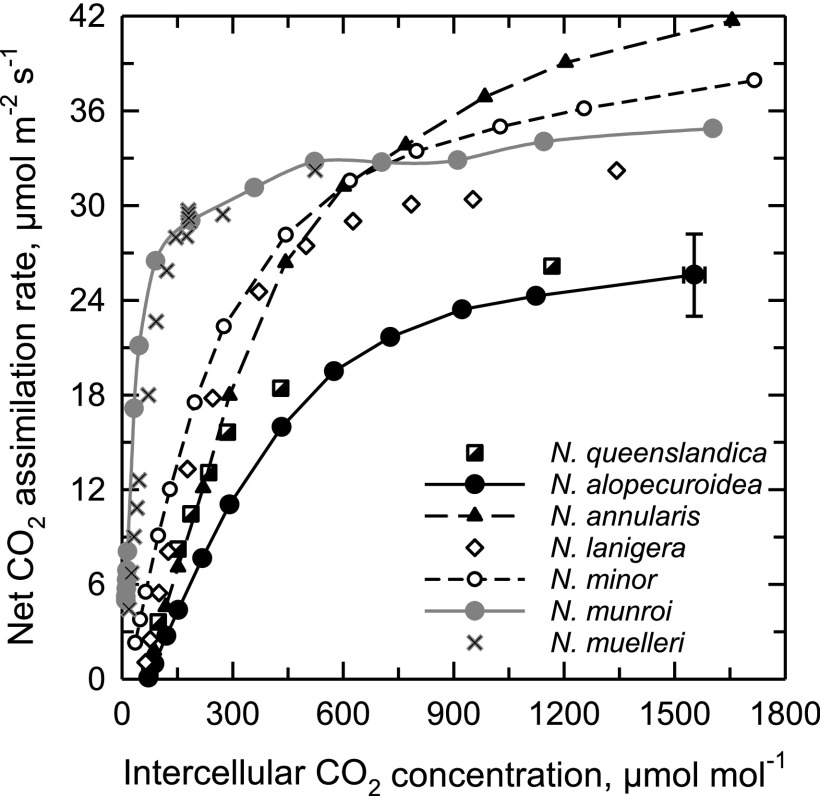
At 32°C, the response of net CO2 assimilation rate (A) to intercellular CO2 partial pressure (Ci) for N. muelleri and N. munroi was typical of C4 plants, showing a CO2 compensation point (Γ) near 0 µmol mol−1, a steep initial slope, and a CO2 saturation point below a Ci of 400 µmol mol−1 (Fig. 1; Table 1). N. alopecuroidea, N. queenslandica, and Thyridolepis mitchelliana exhibited A/Ci responses typical of C3 photosynthesis, with Γ > 60 µmol mol−1, a low initial slope relative to the C4 species, and a CO2 saturation point exceeding 1,000 μmol m−2 s−1 (Fig. 1; Table 1; A/Ci curves not shown for T. mitchelliana). We consider these three species “completely” C3 plants to distinguish them from N. annularis that is functionally C3 although it exhibits some intermediate structural characteristics. In N. annularis, Γ was C3-like at 65 μmol mol−1 at 32°C. The C2 species N. minor exhibited responses to Ci that were intermediate between the C3 and C4 responses, with a Γ of 16 μmol mol−1 at 32°C. In N. lanigera, Γ was 56 μmol mol−1 at 32°C, and 58 μmol mol−1 at 35°C; this latter Γ value was significantly less than in the Neurachne species that exhibi C3-like Γ values at 35°C. Carboxylation efficiency was statistically the same in all non-C4 species of Neurachninae that were examined at 32°C (Table 1).
Figure 1.
The response of net CO2 assimilation rate to variation in intercellular CO2 concentration in seven species of Neurachne at 32°C. Each curve is a single representation of three to five measurements per species. The highest CO2 assimilation rate for N. alopecuroidea also presents ± se to show a typical se range.
Table 1. Summary of leaf gas exchange results for Neurachninae species.
Letters indicate common statistical groups at P < 0.05 (one-way ANOVA followed by a Tukey’s posthoc test). Mean ± se, n = 3–9 except for N. munroi where n = 2 at 35°C. ND, not determined.
| Species | C*, μmol µmol mol−1 | Γ at Saturating Light, µmol mol−1 | Carboxylation Efficiency μmol m−2 s−1 | ||
|---|---|---|---|---|---|
| 35°C | 32°C | 35°C | 32°C | 35°C | |
| T. mitchelliana | 61.1 ± 0.5 a | ND | 62.4 ± 0.5 c,d | ND | 0.22 ± 0.02 b |
| N. alopecuroidea | 60.9 ± 2.0 a | 71.1 ± 2.3 a | 78.5 ± 2.0 a | 0.05 ± 0.00 b | 0.05 ± 0.01 d |
| N. queenslandica | 55.9 ± 0.7 a | 62.8 ± 1.0 a,b | 69.2 ± 1.0 b | 0.10 ± 0.02 b | 0.08 ± 0.00 c,d |
| N. annularis | 54.4 ± 0.7 a | 65.0 ± 2.7 a,b | 66.9 ± 1.0 b,c | 0.09 ± 0.01 b | 0.13 ± 0.00 c |
| N. lanigera | 44.1 ± 0.9 b | 55.6 ± 0.9 b | 58.1 ± 1.7 d | 0.13 ± 0.01 b | 0.12 ± 0.03 c,d |
| N. minor | 16.7 ± 0.9 c | 15.6 ± 4.6 c | 14.3 ± 1.2 e | 0.11 ± 0.01 b | 0.09 ± 0.02 c,d |
| N. munroi | ND | 2.0 ± 0.4d | 1.7 ± 0.3 f | 0.50 ± 0.13 a | 0.51 ± 0.03 a |
| N. muelleri | ND | 0.3 ± 0.0d | ND | 0.27 ± 0.02 b | ND |
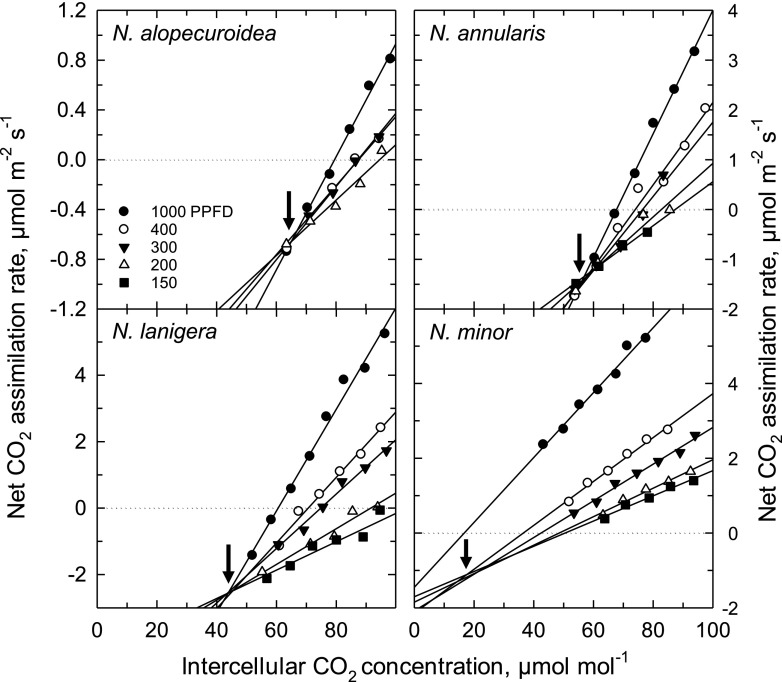
C* was measured at 35°C to enhance photorespiration and the ability to resolve differences between C3, proto-Kranz, and C2 species (Fig. 2; Table 1). In C4 plants, C* cannot be measured due to C4 metabolism, so Γ at high light was measured instead (for convenience in comparing species, C4 Γ is treated as equivalent to C* in the discussion of results). As shown in Figure 2, there is good convergence of the initial slopes of the A/Ci responses upon a common intercept at all light intensities (N. alopecuroidea, N. annularis, and N. lanigera), or light intensities at <500 µmol m−2 s−1 (N. minor). For N. alopecuroidea, N. queenslandica, N. annularis, and N. lanigera, all plots of the y-intercept versus the initial slope of the A/Ci response closely fit a linear response, with the slope of the response being similar for each species (Supplemental Fig. S1). For N. minor, by contrast, only the values <500 μmol m−2 s−1 fit a linear response; the value at high light did not correspond to the linear response due to a shift in the high-light A/Ci response to lower Ci (Supplemental Fig. S1). Mean C* values at 35°C for T. mitchelliana, N. alopecuroidea, and N. queenslandica were C3-like, being 56 μmol mol−1 to 61 μmol mol−1 (Table 1). For N. annularis, C* was 54 μmol mol−1, which is statistically equivalent to the C3 group; in N. lanigera, C* was 44 μmol mol−1, significantly less than the values of C3 Neurachninae species (Table 1). C* for N. minor was 17 µmol mol−1, a typical value for C2 species (Khoshravesh et al., 2016). Because C* reflects the magnitude of photorespiratory CO2 refixation (Busch et al., 2013; Sage et al., 2013), the observed C* values can be used to evaluate relationships between photosynthetic physiology and the underlying leaf structure.
Figure 2.
The response of net CO2 assimilation rate to variation in intercellular CO2 concentration (Ci) at <100 μmol CO2 mol−1 air in four species of Neurachne at 35°C and four to five light intensities. The Ci where the curves intercept is an estimate of C*, the apparent CO2 compensation point in the absence of day respiration (arrows). Curves are representative of three to eight A/Ci plots per species. Note the y axis range varies between the graphic representations. PPFD, photosynthetic photon flux density in μmol photons m−2 s−1.
Leaf and Cellular Architecture
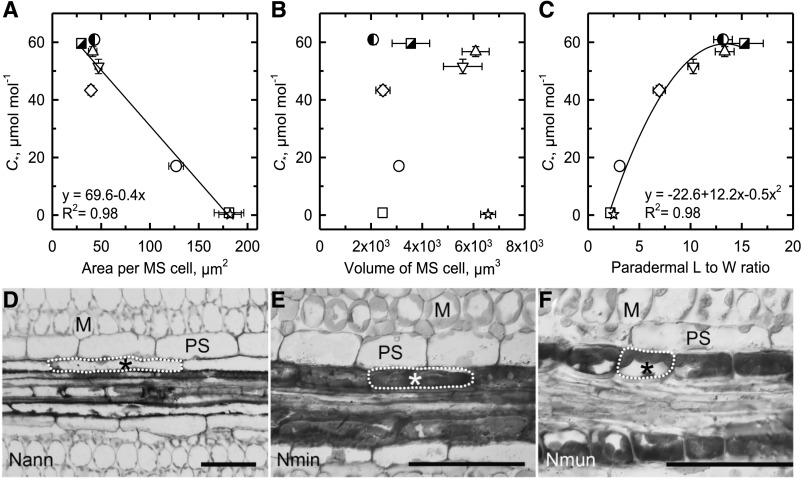
The leaf architecture of Neurachninae species varies dramatically between the C3 and C4 phenotypes, in a manner that is consistent with differences between these photosynthetic pathways in many monocot and eudicot clades where the C4 pathway evolved (Hattersley and Watson, 1975; Hattersley, 1984; Dengler and Nelson, 1999; Christin et al., 2013). Specifically, IVD, the ratio of M to MS tissue area in a cross section, and the M cell number between PSs of minor veins decreases from C3 to C4 species (Fig. 3, A–C; Supplemental Table S1). In species with C* values > 40 μmol mol−1, MS cells have a smaller cross-sectional area (<45 μm−2) in comparison to the larger (>100 μm−2) MS cells in species where C* < 20 μmol mol−1 (Fig. 3A; Supplemental Table S1). This increase in cross-sectional area of the MS cells in Neurachne as C* declines mirrors changes in BS cell area and width (W) in planar cross sections from many clades where C4 photosynthesis evolved, leading to the general view that C4 species have more voluminous BS or MS cells relative to their C3 relatives (Dengler and Nelson, 1999; Christin et al., 2013; Williams et al., 2013). To further evaluate changes in MS size and shape during C4 evolution, we estimated MS cell volume by multiplying the cross-sectional area times the cell length (L) in a paradermal section, which is possible because MS and BS cells in grasses are cylindrical in shape (Leegood, 2008). Our estimated volume of MS cells does not support the common view that MS cells become larger during C4 evolution. No increase in MS volume was observed in C4 relative to C3 or intermediate Neurachninae species and there is no relationship between MS cell volume and C* (Fig. 3B; Supplemental Table S1). This is because MS cells decrease in L (the dimension along the proximo-distal axis parallel to the veins in grass leaves) and increase in W (the dimension along the medio-lateral axis perpendicular to grass veins) as C* declines (Fig. 3, C–F). The MS cells in the C3 species such as N. alopecuroidea are long and relatively thin (high L:W), whereas in C4 species such as N. munroi, they are short and wide as shown by changes in L:W that decline from near 15 in C3 species to <5 in C4 species (Fig. 3, D–F; Supplemental Table S1). N. lanigera and N. minor have intermediate L:W, reflecting a transition from the C3- to C4-type MS cell. A second-order regression predicted 98% of the variation in this relationship (Fig. 3C). These morphological changes result in an increase in MS cell number along the length of the vascular strands (Fig. 3, D–F).
Figure 3.
The relationship between C* and dimensions of MS cells. A, MS cell area in planar cross section. B, MS cell volume. C, MS cell L:W in a paradermal section in eight species of Neurachnineae. Values were determined from planar cross sections of TEM images (A and B). Values were determined from light microscopic images of planar paradermal sections (C). Mean ± se; n = 3 plants per species; 10 images per plant. Significant regressions at P ≤ 0.05 are shown. N. alopecuroidea (◑), N. annularis (▽), N. lanigera (◇), N. minor (○), N. munroi (⬜), N. muelleri (☆), N. queenslandica (△), and T. mitchelliana (◪). See Supplemental Table S1 for values and statistical tests. D to F, Paradermal view of vascular sheath cells. Nann, N. annularis; Nmin, N. minor; Nmun, N. munroi. Single MS cells are outlined (dashed line). Scale bars = 50 μm; *, MS, M, and PS.
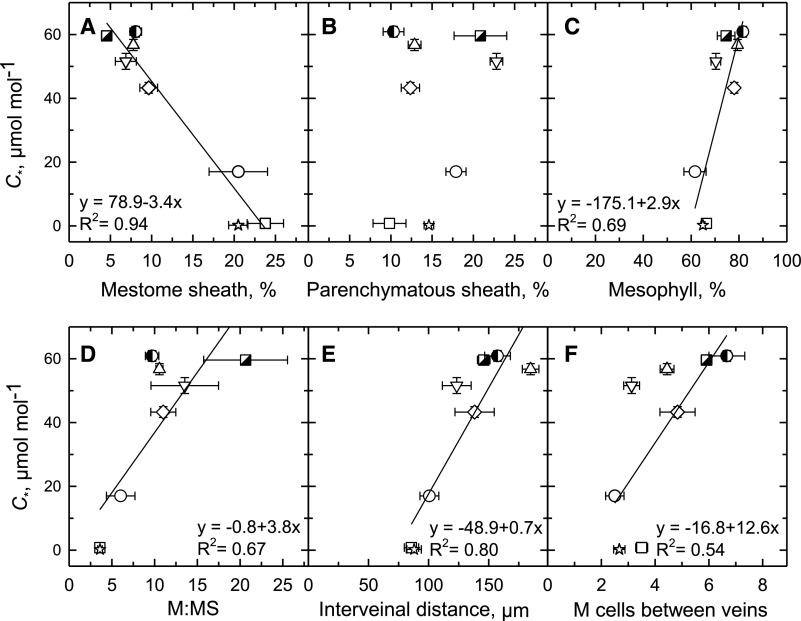
Increased MS cell width, as well as reductions in interveinal M cell number, contribute to modifications in the percent of leaf tissue occupied by MS and M tissue in C3 compared to C2 and in turn C4 species (Fig. 4, Supplemental Table S1; Supplemental Fig. S2). Whereas <10% of the cross-sectional area of photosynthetic tissue is occupied by MS in the C3 species with C* > 50 μmol mol−1, MS tissue accounts for >20% of the leaf cross-sectional area in species with C* < 20 μmol mol−1 (Fig. 4A). The reduction in C* from C3 to C2 to C4 species was also associated with a decline in the percent of M tissue in a cross section, from >80% of the cross-sectional image area at a C* >60 µmol mol−1 to ∼60% at a C* <20 µmol mol−1 (Fig. 4C). As M:MS, IVD, and interveinal M cell number decline, so does C* in a linear manner, with each regression having a significant slope (Fig. 4, D–F). No relationship was observed between percentage of PS tissue area and C* (Fig. 4B).
Figure 4.
The relationship between leaf structural parameters and C* in eight Neurachninae species. Values determined from light microscopic images of planar cross sections. Percent tissue values were calculated as the area of a given tissue in a leaf image divided by the sum of the areas of MS, PS, and M tissues in the same image, times 100% (Supplemental Fig. S2). A, Percent of image area composed of MS tissue. B, Percent of image area composed of PS tissue. C, Percent of image area comprised of M tissue. D, The ratio of M tissue area to MS tissue area in planar cross section. E, Mean distance between veins. F, The number of M cells between veins. Mean ± se; n = 3 plants per species; 10 images per plant. See Supplemental Table S1 for mean values and statistical tests. Significant linear regressions at P ≤ 0.05 are shown. N. alopecuroidea (◑), N. annularis (▽), N. lanigera (◇), N. minor (○), N. munroi (⬜), N. muelleri (☆), N. queenslandica (△), and T. mitchelliana (◪).
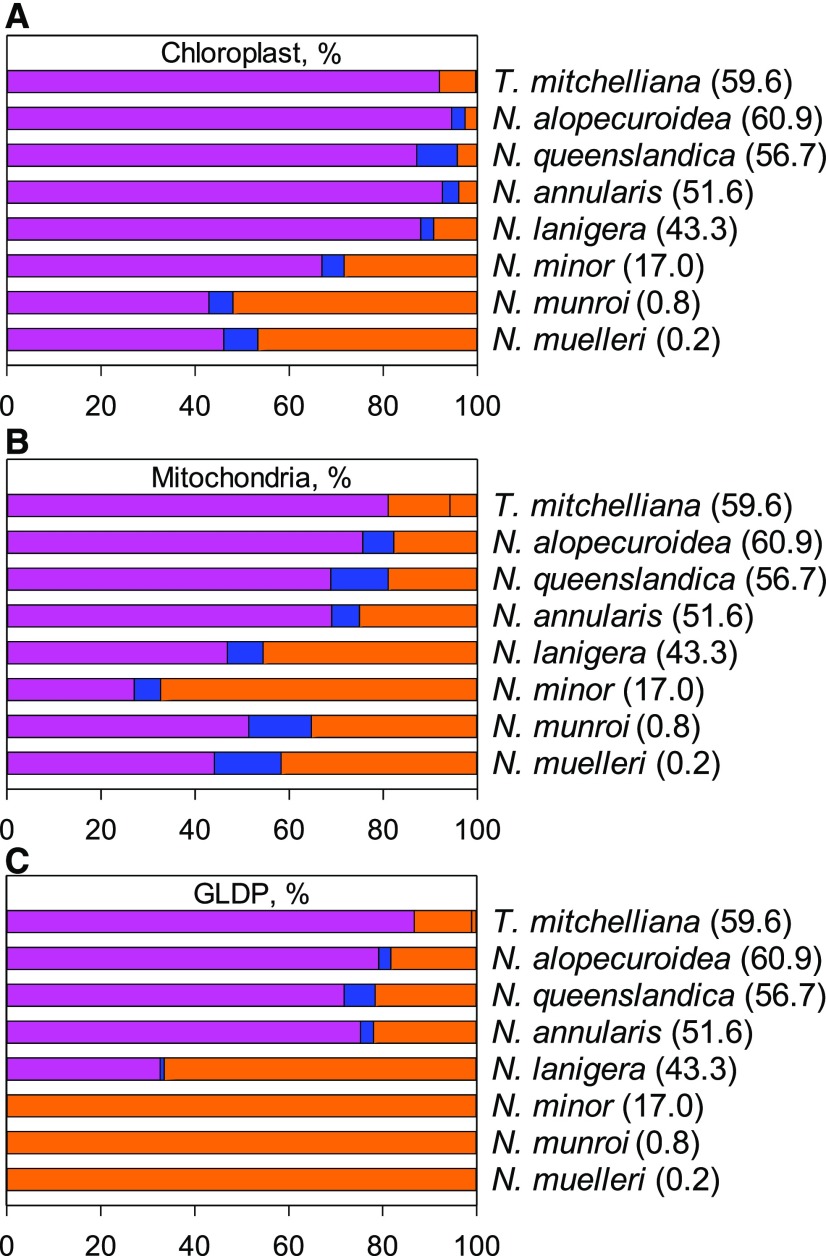
The allocation of chloroplasts to MS tissue also increases as C* declines from C3 to the C4 values in Neurachninae (Figs. 5 and 6; Supplemental Table S2; Supplemental Fig. S3). In the C3 species T. mitchelliana, N. queenslandica, N. alopecuroidea, and N. annularis, <5% of the chloroplast cross-sectional area within leaf photosynthetic tissue occurs in the MS (Figs. 5, A and B, and 6A). This fraction rises to >9% and 20% in N. lanigera and N. minor, respectively, and to near 50% in the C4 species (Figs. 5, C and D, and 6A; Supplemental Table S2). Increases in chloroplast size and number within the MS protoplast contribute to greater chloroplast investment in MS tissue of intermediate and C4 species (Figs. 5, E and F, and 6A; Table 2). Enhanced mitochondrial numbers and size contribute to greater mitochondrial investment in MS tissue as C* declines from C3 to C2 species (Figs. 5, A–D, and 6B; Table 2; Supplemental Table S2; Supplemental Fig. S3). In N. lanigera and N. minor, >40% and 60%, respectively, of the mitochondrial cross-sectional area within leaf photosynthetic cells occurs in the MS (Figs. 5, C and 5D, and 6B; Supplemental Table S2). In the MS of the C4 species, mitochondrial investment is lower than in C2 species, due to a reduction in mitochondrial numbers (Figs. 5, E and F, and 6B; Table 2; Supplemental Table S2).
Figure 5.
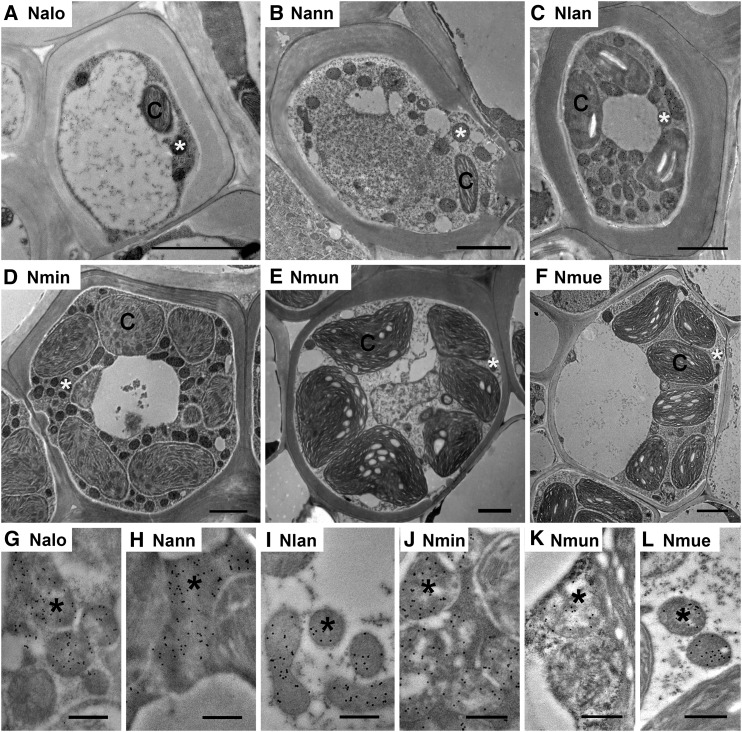
TEM images of MS cells of six Neurachne species. A to F, Low magnification images illustrating ultrastructure. G to L, Immunogold labeling of GLDP in MS mitochondria. Immunogold labeling of GLDP is indicated by black dots in mitochondria. Nalo, N. alopecuroidea; Nann, N. annularis; Nlan, N. lanigera; Nmin, N. minor; Nmun, N. munroi; Nmue,N. muelleri; C, chloroplast; *, mitochondrion. Scale bars = 2 μm (A–F); 500 nm (G–L).
Figure 6.
The allocation of organelles and GLDP among leaf tissue types. A, Chloroplast. B, Mitochondria. C, GLDP among M (magenta, left bar), PS (blue, middle bar), and MS (orange, right bar) tissues in eight species of Neurachnineae. Values in parentheses represent mean C* values for each species. n = 3 plants per species; 10 images per plant. See Supplemental Table S2 for mean values, statistical tests, and quantification procedure.
Table 2. Organelle parameters and gold density labeling of GLDP in tissues of Neurachninae species.
Values are from planar cross sections of TEM images. Mean ± se; n = 3 plants per species; 10 images per plant. Values followed by the same letter in each row are not significantly different (P ≥ 0.05) using a Tukey’s post hoc test for normally distributed data (*) and a Games–Howell test for all other data. See Supplemental Table S3 for peroxisome area per protoplast area. PK, Proto-Kranz phenotype.
| Parameter by Tissue Type | Completely C3 Species | C3 PK | Modest C2a | C2 Speciesb | C4 Species | |||
|---|---|---|---|---|---|---|---|---|
| T. mitchelliana | N. queenslandica | N. alopecuroidea | N. annularis | N. lanigera | N. minor | N. munroi | N. muelleri | |
| Area per chloroplast, μm2 | ||||||||
| Mestome sheath | 0.6 ± 0.2 a | 4.1 ± 0.6 a | 3.4 ± 0.4 a | 3 ± 0.3 a | 3.5 ± 0.3 a | 8.3 ± 0.8 b | 19.2 ± 1 b | 8.6 ± 0.5 b |
| Mesophyll | 5.4 ± 0.4 a | 8.9 ± 0.7 b | 6.4 ± 0.4 a | 7.5 ± 0.4 a | 6.8 ± 0.4 a | 6.7 ± 0.3 a | 10.6 ± 0.9 b | 6.6 ± 0.4 a |
| Parenchymatous sheath | 3.5 ± 0.5 a,b | 4.8 ± 1 b | 2.8 ± 0.3 a | 2.9 ± 0.2 a | 2.3 ± 0.3 a | 3.7 ± 0.3 a,b | 7.7 ± 0.4 c | 3.4 ± 0.2 a,b |
| Area per mitochondrion, μm2 | ||||||||
| Mestome sheath | 0.08 ± 0.02 a | 0.21 ± 0.04 a | 0.22 ± 0.02 a | 0.3 ± 0.03 b | 0.3 ± 0.02 b | 0.36 ± 0.02 b | 0.42 ± 0.02 c | 0.32 ± 0.02 b |
| Mesophyll | 0.24 ± 0.06 a,b | 0.31 ± 0.03 b | 0.21 ± 0.03 a | 0.28 ± 0.02 a,b | 0.25 ± 0.02 a,b | 0.21 ± 0.02 a | 0.37 ± 0.03 c | 0.25 ± 0.02 a,b |
| Parenchymatous sheath | 0.15 ± 0.02 a | 0.21 ± 0.02 a | 0.15 ± 0.01 a | 0.16 ± 0.01 a | 0.18 ± 0.01 a | 0.21 ± 0.03 a | 0.37 ± 0.05 b | 0.21 ± 0.03 a |
| Chloroplast area/protoplast area, % | ||||||||
| Mestome sheath | 1.4 ± 0.4 a | 12.9 ± 2.7 b | 12.2 ± 2.9 b | 16.4 ± 1.9 b | 27.5 ± 2.8 c | 46.5 ± 3.3 d | 74.2 ± 2.4 e | 38.7 ± 1.7 d |
| Mesophyll | 15.7 ± 1.2 a,b | 21.6 ± 1.9 a,b | 38.2 ± 2.4 d | 33.4 ± 2.2 c,d | 24.4 ± 1.9 b,c | 21.4 ± 1.8 a,b | 18.8 ± 1.9 a,b | 11.4 ± 1.0 a |
| Parenchymatous sheath | 4.7 ± 0.8 a,b | 16.3 ± 3.8 c | 8.0 ± 1.0 c | 4.2 ± 0.5 a | 4.7 ± 0.6 a,b | 6.5 ± 0.7 a,b,c | 11.0 ± 1.0 c | 7.3 ± 0.8 b,c |
| Chloroplast #/protoplast area, μm−2 × 10−3 | ||||||||
| Mestome sheath | 20.0 ± 4.7 a | 32.4 ± 7.2 a,b | 34.8 ± 7.5 a,b | 56.3 ± 5.9 b,c | 84.0 ± 8.7 c | 62.6 ± 4.9 b,c | 41.9 ± 2.6 b | 47.8 ± 2.3 b |
| Mesophyll* | 29.9 ± 2.2 c | 26.8 ± 1.8 c,b | 58.4 ± 3.3 a | 45.3 ± 2.6 b | 36.7 ± 3.2 c | 30.7 ± 1.9 c | 19.0 ± 1.6 d,e | 17.5 ± 1.1 e |
| Parenchymatous sheath | 13.1 ± 1.6 a | 33.3 ± 5.5 c | 27.5 ± 1.7 c | 14.9 ± 1.4 a,b | 20.3 ± 2.0 a,b,c | 18.6 ± 1.6 a,b,c | 15.7 ± 1.8 a,b | 21.4 ± 2.0 b,c |
| Mitochondrion area/protoplast area, % | ||||||||
| Mestome sheath | 0.5 ± 0.1 a | 1.4 ± 0.4 a,b | 3.4 ± 0.7 b | 8.2 ± 0.8 c | 10.6 ± 1.1 c | 9.1 ± 0.8 c | 2.2 ± 0.2 b | 1.6 ± 0.1 b |
| Mesophyll | 0.4 ± 0. 1 a | 0.8 ± 0.1 b,c | 1.6 ± 0.2 d,e | 1.8 ± 0.2 e | 1.2 ± 0.2 c,d | 0.6 ± 0.1 a,b,c | 1.0 ± 0.1 c,d | 0.5 ± 0.1 a,b |
| Parenchymatous sheath | 0.2 ± 0.0 a | 1.0 ± 0.2 b,c,d | 0.9 ± 0.1 b,c | 0.5 ± 0.1 b | 1.0 ± 0.1 c,d | 0.7 ± 0.1 b | 1.4 ± 0.2 d | 0.8 ± 0.1 b,c |
| Mitochondrion #/protoplast area, μm−2 × 10−3 | ||||||||
| Mestome sheath | 47 ± 24 a | 100 ± 25 b,c | 164 ± 24 c,d | 313 ± 23 e,f | 389 ± 24 f | 246 ± 24 d,e | 53 ± 24 a,b | 53 ± 24 a,b |
| Mesophyll | 1.8 ± 0.2 a | 2.9 ± 0.3 a,b | 7.0 ± 0.7 c | 6.8 ± 0.6 c | 5.2 ± 0.8 b,c | 2.4 ± 0.3 a | 2.8 ± 0.3 a,b | 2.2 ± 0.2 a |
| Parenchymatous sheath | 1.2 ± 0.2 a | 4.9 ± 0.8 b,c | 5.6 ± 0.6 b,c | 3.4 ± 0.3 c | 6.0 ± 0.6 b | 3.5 ± 0.4 c | 4.3 ± 0.5 b,c | 3.5 ± 0.5 c |
| Gold particles/mitochondrion area, µm−2 | ||||||||
| Mestome sheath | 7.0 ± 3.5 a | 47.3 ± 3.7 b,c | 28.7 ± 3.5 b | 37.4 ± 3.4 b,c | 42.3 ± 3.5 bc | 49.8 ± 3.5 c | 23.8 ± 3.5 b | 28.1 ± 3.5 b |
| Mesophyll | 86.4 ± 5.8 a | 53.1 ± 3.5 a | 44.1 ± 2.1 a,b | 46.1 ± 2.4 a | 20.3 ± 1.7 b | 2.2 ± 0.3 c | 1.5 ± 0.3 c | 2.0 ± 0.3 c |
| Parenchymatous sheath | 53.9 ± 4.7 a | 25.4 ± 2.4 a | 16.2 ± 1.7 a | 18.5 ± 1.3 a | 4.0 ± 0.6 b | 2.5 ± 0.4 b | 1.3 ± 0.3 b | 3.3 ± 0.7 b |
| Gold particles/protoplast area, μm−2 | ||||||||
| Mestome sheath | 0.07 ± 0.28 a | 0.84 ± 0.29 b,c | 1.45 ± 0.28 c | 2.92 ± 0.27 d | 4.42 ± 0.28 d | 4.39 ± 0.27 d | 0.50 ± 0.27 b | 0.44 ± 0.28 b |
| Mesophyll | 0.32 ± 0.04 a,b | 0.42 ± 0.06 a,b | 0.76 ± 0.12 a,b | 0.83 ± 0.09 a | 0.29 ± 0.06 b | 0.01 ± 0.00c | 0.01 ± 0.00 c | 0.01 ± 0.00 c |
| Parenchymatous sheath | 0.13 ± 0.02a | 0.19 ± 0.03 a | 0.15 ± 0.02 a | 0.10 ± 0.01 a | 0.04 ± 0.01 b | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.02 ± 0.00 b |
Incomplete C2 phenotype.
Complete C2 phenotype, where all GDC is in the MS compartment.
Immunodetection of GLDP and Rubisco LSU
To quantify GLDP in tissues of the Neurachninae species, a polyclonal antiserum was generated against a highly conserved 16-amino acid-peptide present in eudicot and monocot GLDP isoforms. The specificity of the antiserum was determined by immunoblot analysis of Neurachne leaf extracts after denaturing PAGE (Supplemental Fig. S4A). A single polypeptide band of ∼98 kD corresponding to the molecular mass of GLDP was detected in all Neurachne leaf extracts (Supplemental Fig. S4B). The labeling shows lower content of GLDP protein, on a total protein basis, in leaves of the C4 species N. munroi and N. muelleri relative to that in leaves of N. annularis, N. queenslandica, N. lanigera, and N. minor (Supplemental Fig. S4B). Less intense GLDP labeling in N. alopecuroidea extracts is due to protein degradation, which is inherently high in this species compared with other Neurachninae even in the presence of protease inhibitors, and is evident in the Coomassie-stained gel (Supplemental Fig. S4A). The relative abundance of transcripts encoding GLDP was generally greater in the C3 and C2 species than in the C4 species (Supplemental Fig. S4D), reflecting the relative patterns of GLDP protein in the leaves of the various species (Supplemental Fig. S4B).
When the anti-GLDP serum was used to label sections of Neurachninae leaves, the density of GLDP labeling per protoplast planar area increased in MS cells as C* declined from near 60 μmol mol−1 in completely C3 species to the 44 μmol mol−1 observed in N. lanigera (Table 2). The density of GLDP labeling per protoplast area in the MS cells of N. annularis was double that of N. alopecuroidea, which had the highest density of GLDP gold labeling per protoplast area in the MS cells of the three completely C3 species (2.92 versus 1.45 gold particles μm−2; Table 2). The density of GLDP labeling per protoplast area in the MS cells was almost three times higher in N. lanigera and N. minor than the highest completely C3 mean (4.4 versus 1.45 particles μm−2; Table 2). These differences largely reflected the increase in mitochondrial area in the protoplast of MS cells of N. annularis, N. lanigera, and N. minor relative to the completely C3 species, because the amount of GLDP labeling per mitochondrion area did not consistently differ between the Neurachninae photosynthetic types (Fig. 5; Table 2). GLDP labeling per planar area of MS protoplasts was low in the C4 species relative to N. lanigera and N. minor, which reflects both low mitochondrial area in the C4 MS and reduced GLDP labeling per mitochondrion (Fig. 5, C–F and I–L; Table 2). Low mitochondrial area in the MS or BS cells is commonly observed in NADP-ME species, which include N. muelleri and N. munroi (Hattersley et al., 1986; Sage et al., 2014). T. mitchelliana exhibited very low amounts of GLDP labeling in the mitochondria of MS cells, which reflects low mitochondrial cross-sectional area and low GLDP content per mitochondrion (Table 2). Along with low values of chloroplast area in the MS cells, these results indicate the MS cells of C3 T. mitchelliana are photosynthetically inactive compared to those of the other completely C3 Neurachne species, which have higher organelle content and GLDP levels. In contrast to the patterns observed in the MS cells of the completely C3 species, the density of GLDP labeling per protoplast and mitochondrial planar area declined in M cells as C* declined, such that GLDP labeling of the M cells is negligible in the C4 species and N. minor (Table 2; Supplemental Fig. S5). The near-absence of GLDP labeling in M cells of N. minor corresponds to its robust C2 cycle as indicated by the low C* of 17 μmol mol−1 at 35°C. GLDP content per protoplast planar area also declined with C* in the PS cells (Fig. 6; Table 2). The increase in GLDP labeling in the MS cells coupled with its reduction in the M and PS cells results in an increase in the fraction of leaf GLDP labeling present in the MS tissue, and this correlates with a reduction in C* in the Neurachninae (Fig. 6C; Supplemental Table S2). The quantification of immunogold particles indicated 100% of the leaf GLDP was present in the MS tissue of N. minor, whereas 67% and 22% of leaf GLDP was present in the MS tissue in N. lanigera and N. annularis, respectively. In the MS tissue of the completely C3 species N. alopecuroidea, N. queenslandica, and T. mitchelliana, the fraction of GLDP labeling was <22%. In the two C4 species, all of the leaf GLDP labeling detected was in MS tissue (Fig. 6C; Supplemental Table S2).
Strong labeling of an ∼56-kD polypeptide, corresponding to the LSU of Rubisco, was detected in all Neurachninae species when blots of total leaf protein were labeled with an anti-Rubisco LSU antiserum (Supplemental Fig. S4C). Immunolabeling of the Rubisco LSU was detected in chloroplasts of MS and PS cells in all species, and in M cells of all species except the C4 N. munroi and N. muelleri (Supplemental Figs. S6–S8). Rubisco LSU immunolabeling in the PS cells was very low in N. annularis, N. lanigera, N. minor, and the two C4 species. The absence of Rubisco LSU immunolabeling in M cells of N. munroi and N. muelleri reflects their use of the C4 pathway.
PD Frequency
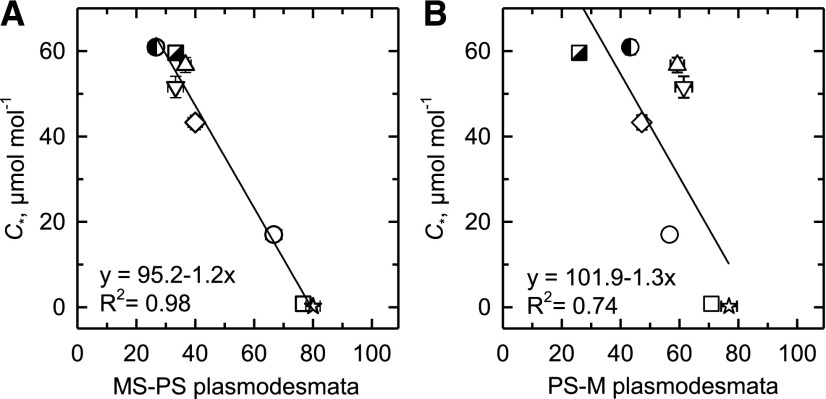
Because an increase in the frequency of PD between M and BS cells is characteristic of C4 relative to C3 species (Danila et al., 2016, 2018), we examined PD frequency in the MS, PS, and M walls of the different Neurachninae species to evaluate the hypothesis that PD frequency increases in C2 species to accommodate the increased flux of photorespiratory metabolites among M, PS, and MS cells. The PD frequency at the MS–PS cell wall boundary significantly increases in Neurachninae species when C* declines from >60 μmol mol−1 to <15 μmol mol−1 (Fig. 7A; Supplemental Table S3). The regression between PD frequency in the MS–PS wall and C* predicts 98% of the variation in the relationship. A significant inverse relationship was also present between PD frequency in the PS–M wall and C*, with an R2 of 0.74 (Fig. 7B; Supplemental Table S3). When species means were pooled by photosynthetic type, the C4 mean for PD frequency in the MS–MS cell wall was greater than the C3 mean (P ≤ 0.05).
Figure 7.
The relationship between C* and PD frequencies in walls between leaf tissue types. A, The frequency of PD between MS and PS cells. B, The frequency of PD between PS and M cells. Mean ± se; n = 3 plants per species; 10 images per plant. Significant linear regressions at P ≤ 0.05 are shown. N. alopecuroidea (◑), N. annularis (▽), N. lanigera (◇), N. minor (○), N. munroi (⬜), N. muelleri (☆), N. queenslandica (△), and T. mitchelliana (◪). See Supplemental Table S3 for mean values and statistical tests.
Phylogenetic Principal Component Analysis
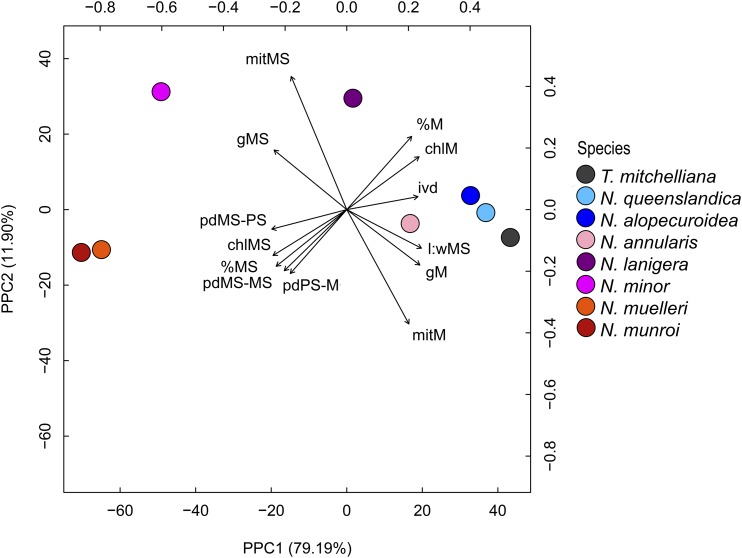
Of the many anatomical, cellular, and GLDP traits examined (Table 2; Supplemental Tables S1–S3), 13 are significantly correlated with C* (Supplemental Table S4). These 13 traits include IVD, MS L:W, the allocation of mitochondria, chloroplasts, and GLDP labeling in MS versus M tissue, as well as PD frequency between MS–PS, MS–MS, and PS–M cells. The pair comparison of phylogenetic independent contrasts between these 13 variables exhibited similar significance of correlation (Supplemental Fig. S9). To correct for phylogenetic signal, a phylogenetic principal component analysis (pPCA) was performed using the traits that exhibited high correlation in species values and the corresponding phylogenetic contrasts for C* values (Fig. 8; Revell, 2009, 2012). Five of seven principal components identified by pPCA had eigenvalues > 0.1 (Supplemental Table S5), and pPC1 and pPC2 explained 79% and 12% of variance, respectively (Fig. 8; Supplemental Table S5). In the pPCA plot, the values for N. annularis place it near the completely C3 species on the right-hand side of the pPC1 axis, but displaced toward the C4 species along pPC1. C4 species cluster on the left side of the pPC1 axis, whereas values for N. lanigera and N. minor are spaced between the C3 and C4 values along the pPC1 but shifted upwards along pPC2 axis relative to the C3 and C4 species (Fig. 8). pPC1 is negatively correlated with parameters associated with the MS tissue, and positively correlated with M tissue-related parameters. Species with C3, incomplete C2, complete C2, and C4 photosynthesis spread from right to left, respectively, along pPC1 in response to reductions in chloroplast, mitochondria, and GLDP investment in M tissue, and to increases in chloroplast, mitochondria, and GLDP in MS tissue (Fig. 8; Supplemental Table S6). pPC2 is negatively correlated with M tissue mitochondrial number and positively correlated with MS tissue mitochondrial number. The correlation of pPC2 with the remaining variables is negligible (<0.3). A linear model with C* as the response of the five first pPCs indicates that pPC1 significantly predicts the C* values (adjusted R2 = 0.99, P = 0.004). This is consistent with significant regressions between individual variables and C* in Figs. 3, 4, and 7.
Figure 8.
pPCA for eight species of Neurachnineae and correlation of 13 quantified variables with the first and second principal component. Data points indicate mean values for each species. Capital letters indicate cell or tissue type: M, PS, and MS. Small letters indicate measured variables as follows: chl, pecent chloroplast allocated to the specified tissue type; mit, percent mitochondria allocated to the specified tissue type; ivd, interveinal distance; g, GLDP density. See Supplemental Tables S1–S3 for mean values and allocation calculations.
Trait Reconstruction
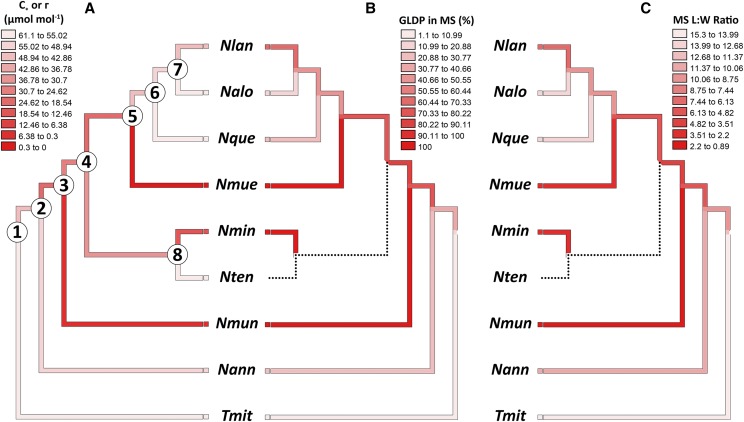
The Neurachninae is nested within a C3 clade in the Paniceae tribe (Grass Phylogeny Working Group II, 2012), and trait reconstructions support a hypothesis that the C3 state represents the ancestral condition in the Neurachninae, with two origins of C4 arising from an intermediate state (Fig. 9; Supplemental Table S7; Christin et al., 2012; Grass Phylogeny Working Group II, 2012). Ancestral state reconstruction of physiological (C* or Γ), cellular (percent GDC in MS), and anatomical (MS L:W) traits using the Neurachninae phylogeny of Christin et al. (2012) consistently show intermediate trait values along the internal branches of the phylogeny, as indicated in Figure 9 by darker (for C4) versus lighter (for C3) shading on the branches (see also Supplemental Table S7). The 95% confidence interval (CI) for C* and numerous other traits overlaps with the CI observed for C3 species at nodes 1, 7, or 8 only, supporting a hypothesis that the ancestral state of the internal nodes 2–6 was C2-like or proto-Kranz (Fig. 9; Supplemental Table S7). Although the trait reconstruction indicates the backbone of the Neurachne phylogeny corresponds to a C2-like condition. Alternatively, the backbone of the tree could be predominantly C3, with multiple origins of C2 and C4 species. Resolution of these alternative models may require discovery of additional populations and/or species of Neurachne, a possibility suggested by the relatively recent discovery of N. annularis by Macfarlane (2007).
Figure 9.
The molecular phylogeny of the Neurachninae (from Christin et al., 2012) with three traits mapped onto the tree. A, Values for C*. B, % of GDC in the MS tissue. C, L:W of MS cells in paradermal section. No data were available for N. tenuifolia, as indicated by the dotted branch. Nlan, N. lanigera; Nalo, N. alopecuroidea; Nque, N. queenslandica; Nmue, N. muelleri; Nmin, N. minor; Nten, N. tenuifolia; Nmun, N. munroi; Nnann, N. annularis; Tmit, T. mitchelliana. Ninety-five percent confidence intervals for C* and 12 other traits at ancestral nodes are presented in Supplemental Table S7.
DISCUSSION
In this work, we present a comprehensive analysis of changes in structure and enzyme distribution associated with the C3 to C4 transition in the grass subtribe Neurachninae. We demonstrate correlated shifts in multiple structural traits of leaves, and in the density and location of GLDP and Rubisco LSU in the observed C3, C2, and C4 species of the Neurachninae. These variations are correlated with C* and Γ, implying that simultaneous evolutionary modifications occurred to first assemble a C2 pathway and then upregulate the C4 pathway during the evolution of the two known C4 clades in the Neurachninae. A key question is whether the phenotypes in the extant species represent the evolutionary phases that occurred in the transition from C3 to C4 photosynthesis in Neurachne, as opposed to being distinct nonintermediate states, for example, as may arise by reversion or hybridization events (Kadereit et al., 2017). In other clades of C4 origin, phylogenetic data supports the hypothesis that intermediate phenotypes represent bona fide evolutionary states, as indicated by correspondence between phylogenetic and phenotypic gradients (McKown et al., 2005; Christin et al., 2011b; Yang and Berry, 2011; Sage et al., 2013; Fisher et al., 2015; Lundgren et al., 2016; Schüssler et al., 2017; Dunning et al., 2019). The Neurachninae phylogeny does not clearly follow these patterns, however. Hypotheses supported by the Neurachninae phylogeny of Christin et al. (2012; see also Fig. 9) include the following: (1) C3 is the ancestral condition in the Neurachninae as indicated by the phylogenetic sister position of C3 Thyridolepis and the nesting of the Neurachninae in a C3 clade by the Grass Phylogeny Working Group (GPWGII, 2012). (2) In Neurachne itself, N. annularis branches at the base of the genus, allowing us to infer the proto-Kranz state described here is the ancestral condition within Neurachne. (3) N. minor branches in a sister position to the two C4 species, supporting a hypothesis that it shares an intermediate common ancestor with the C4 species. (4) A phylogenetic reconstruction within Neurachne indicates the ancestral state along the backbone of the genus tree is an intermediate condition that could have given rise to C3 revertants, fully developed C2 species, and multiple origins of C4 species (Fig. 9).
The position of N. lanigera in the tree is intriguing because it is nested within a C3 clade that is sister to the C4 N. muelleri. This raises a number of possibilities. First, N. lanigera, N. alopecuroidea, and N. queenslandica may have been derived via reversion from an N. muelleri-like C4 ancestor. This is unlikely because unequivocal evidence of reversion from a C4 state has yet to be presented, and revertants may have poor fitness because they may not readily reacquire a functional C3 pathway in the M tissue (Christin et al., 2010; Oakley et al., 2014; Bräutigam and Gowik, 2016). In addition, Christin et al. (2012) present evidence indicating N. muelleri obtained a C4-adapted PEPC gene by lateral transfer from an ancestor of N. munroi. If N. lanigera, N. alopecuroidea, or N. queenslandica were revertants, they would be expected to share this introgressed gene, which they do not (Christin et al., 2012). It is also possible that the partial C2 physiology of N. lanigera is the result of C3 × C4 hybridization. Evidence of C3 × C4 hybridization in Neurachne was noted by Christin et al. (2012) through incongruence of chloroplast and nuclear genomes in polyploid N. queenslandica. The accession of N. lanigera used in this study is diploid (Christin et al., 2012), implying that it is not a hybrid. The available evidence supports a third interpretation, that N. lanigera ultimately arose de novo from an ancestral C3 state. Resolution of this issue will require additional populations or species of Neurachne and/or detailed analysis of genomic data, which are underway. For now, the most parsimonious interpretation is that N. lanigera is derived from the C3 condition by vertical selection, and thus may represent a genuine intermediate state in the evolution of full C2 photosynthesis. If it is assumed the N. annularis and N. minor are also derived vertically from C3 ancestors, and that C* reflects the degree of intermediacy, then a hypothetical model of C2 evolution can be generated for Neurachne.
The novel physiological and structural phenotypes shown here demonstrate the Neurachninae is more dynamic with respect to C3–C4 intermediacy than previously understood. Instead of containing just one C3–C4 intermediate species as described in Hattersley et al. (1986), the Neurachninae contains three intermediate species of differing character states. If the assumption that C* reflects degrees of evolutionary intermediacy is valid, then the results are consistent with a progressive model of C4 evolution as proposed for eudicot lineages such as Flaveria (Monson and Rawsthorne, 2000; Sage et al., 2013; Schulze et al., 2013; Bräutigam and Gowik, 2016). The structural and immunolocalization data provide some key details of how the C2 pathway may have been assembled in Neurachne, and in the process highlight similarities with Flaveria, but also differences associated with the co-option of the MS versus BS tissue as the compartment of CO2 concentration. To summarize, N. annularis has a higher organelle investment within the MS compared to the average of completely C3 species, whereas N. lanigera has greater organelle investment that is intermediate between N. annularis and N. minor. N. annularis shows higher labeling of GLDP per MS protoplast, nearly double that of completely C3 species, and approaching the high values observed in N. lanigera and N minor; however, N. annularis maintains a C3-like pattern of GLDP expression in the M tissue. GLDP labeling in the M tissue of N. lanigera is half that of N. annularis, and is negligible in N. minor, typical of other well-developed C2 species (Khoshravesh et al., 2016). N. annularis also exhibits initial characteristics of Kranz anatomy, as indicated by a 20% to 30% reduction in L:W of MS cells compared to the completely C3 species. The L:W of the MS cells is even lower in N. lanigera, with values that are 50% of the completely C3 species values, and in N. minor where L:W average 25% of the completely C3 values. These changes in physiological and cellular characteristics are associated with lower C* values: C* in N. annularis is at the low end of the C3 range, whereas C* in N. lanigera falls between the C3-like value of N. annularis and the completely C2 value of N. minor. From these results, we conclude N. annularis is largely C3 in function but shows evidence for an incipient C2 cycle in the MS tissue, similar to what has been described in BS tissue of proto-Kranz species of Flaveria, Heliotropium, Salsola, and Steinchisma (Muhaidat et al., 2011; Sage et al., 2013; Khoshravesh et al., 2016, Schlüssler et al., 2017). N. annularis differs from proto-Kranz eudicots by not exhibiting a centripetal arrangement of mitochondria, but the thick suberized wall, coupled with the PS layer, indicates the accumulation of photorespiratory CO2 released by MS mitochondria, thereby improving Rubisco efficiency. N. lanigera shows evidence for a stronger C2 cycle, but this is not fully optimized for C2 photosynthesis as it is in N. minor, largely due to the continued presence of GDC in the M cells. We conclude N. lanigera is an incomplete or partial C2 species (C2-like) that relies upon the MS mitochondria to metabolize some of the photorespiratory Gly from M tissue. In doing so, C* is lowered to 44 µmol mol−1 at 35°C. The near complete loss of GLDP expression in the M tissue of N. minor results in the vast majority of photorespiratory Gly being metabolized in the MS tissue. Because of an abundance of mitochondria in the MS tissue, this species has sufficient capacity to metabolize all the photorespiratory Gly coming from the M tissue, and consequently, to reduce C* to <20 μmol mol−1 as is often observed in completely C2 species (Monson and Rawsthorne, 2000). The pronounced anatomical shifts in N. minor relative to C3 species, including a lower MS cell L:W, greater MS volume, and higher PD frequency, demonstrate extensive cellular elaboration and developmental modification to support the C2 pathway. N. minor can thus be considered to express a form of Kranz anatomy that is highly specialized for efficient C2 function.
PD frequency through BS–M cell walls is known to be greater in C4 than C3 species (Hattersley et al., 1986; Danila et al., 2016, 2018). Data from this study likewise show more frequent PD through MS–PS cell walls, as well as through the PS–M and MS–MS cell walls of the C4 Neurachninae. Moreover, results demonstrated there is a significant relationship between C* and PD frequency through the MS–PS cell walls, and C* and PD frequency through PS–M walls. Assuming C* reflects the degree of C3–C4 intermediacy, these results are consistent with a model whereby the increase in PD is progressive across the C3–C4 transition in the Neurachninae. Because enhancement of PD frequency also occurs in proportion to greater organelle and GLDP labeling in the MS tissue, we conclude that a greater flux of photorespiratory metabolites is linked to formation of more PD. PD enhancement in the Neurachninae could be due to increased levels of GOLDEN2-LIKE (GLK) transcription factors, which have been hypothesized to promote activation of BS cells during C4 evolution (Waters et al., 2009). In rice (Oryza sativa), constitutive expression of GLK genes resulted in greater chloroplast and mitochondrial volume in BS and MS cells that corresponded with more Rubisco and GLDP content as well as higher PD frequency between M and BS cells (Wang et al., 2017), similar to what is observed in N. annularis and N. lanigera.
Declines in M cell number and size contribute to reductions in IVD in C4 relative to C3 leaves and are considered essential for optimal C4 function because fewer M cells reduce the mean diffusion distance for C4 cycle metabolites (Hattersley, 1984; Dengler and Nelson, 1999; Ogle, 2003; Griffiths et al., 2013). The reduction in IVD in Neurachninae species occurs in proportion to a decline in C*, and is due to progressively fewer M cells between veins. In the C4 N. munroi and N. muelleri, these changes converge upon approximately two M cells between the veins, a commonly observed number in most C4 lineages (Hattersley and Watson, 1975; Dengler and Nelson, 1999; Christin et al., 2013). Notably, reductions in IVD are apparent in N. annularis and N. lanigera compared to the completely C3 N. alopecuroidea and T. mitchelliana. In N. minor, two M cells are already apparent between veins and IVD approaches C4 values, indicating that the rapid flux of photorespiratory metabolites between M and MS tissues in this C2 species requires short diffusion distances that are similar to C4 values. If so, meeting the rapid transport imperative in C2 photosynthesis establishes the low transport distance required for efficient C4 function.
Enhanced BS cell size is commonly thought to be essential for C4 function by allowing for greater organelle volume, and creating a large vacuole in species lacking suberized lamellae (Dengler and Nelson, 1999; Christin et al., 2013). Large vacuoles can serve as a resistive barrier that facilitates CO2 trapping in sheath tissues (von Caemmerer and Furbank, 2003). Because it is uncertain where along the C3–C4 transition that the size of the Kranz sheath cell increases, we examined changes in MS cell morphology in three dimensions among the various photosynthetic types. In contrast to the common view, we observed no correlation between C* and MS volume. MS cells of C4 Neurachne species are not larger (that is, more voluminous) than their C3 counterparts, although they are wider and shorter. The MS cell dimensions changed such that the L:W was ∼80% lower in the C4 than in the C3 species. Danila et al. (2018) also noted C3 and C4 grasses have similar BS cell volume. Based on results from the Neurachninae and Danila et al. (2018), we conclude that Kranz anatomy in grasses does not consist of larger BS or MS cells, but only an increased width of BS or MS cells. As indicated by the proportional decline in C* and the L:W of MS cells, the evolutionary reorientation of the MS cells may have occurred progressively in Neurachne, with N. annularis representing an early stage in this process. The change in MS tissue shape arises from a reorientation in the polarity of cell expansion from a pattern emphasizing proximo-distal (lengthwise, or base to tip in a grass leaf) to medio-lateral (widthwise) expansion, with an increase in cell division along the proximo-distal axis. In doing so, the MS tissue volume (rather than cell volume) increases, creating additional space for organelles and possibly increasing the diffusive distance for CO2 efflux. The increase in MS cell division along the proximo-distal leaf axis is in contrast to declines in M cell division along the medio-lateral leaf axis, highlighting the contrasting shifts in tissue development from C3 to C2 to C4 phenotypes.
A Model of C2 Evolution in the Neurachninae
Understanding of the evolutionary origin of C4 photosynthesis is largely based on eudicot systems, notably Flaveria (Monson and Rawsthorne, 2000; Sage et al., 2013; Schulze et al., 2013; Covshoff et al., 2014). Evolution in monocots is less understood due to the low number of C3–C4 intermediates in grasses and sedges that have been identified and studied (Sage et al., 2011a; Lundgren and Christin, 2017). For monocots, this deficiency constrains development of evolutionary landscape models of C4 origin. Williams et al. (2013) used a Bayesian approach to fill in gaps in the evolutionary sequence of monocots and coupled this to a Markov model to compare evolutionary landscapes in eudicots and monocots. Only two C3–C4 intermediate monocots (N. minor and Steinchisma hians) were available to parameterize the monocot responses and both exhibit well-developed C2 photosynthesis (Williams et al., 2013; Khoshravesh et al., 2016). The model predicted significant divergence in the paths to C4 photosynthesis, with monocots delaying reallocation of GDC to the sheath cells relative to eudicots. Monocots were also predicted to delay chloroplast accumulation and enhancement of sheath size in comparison with eudicots, while accelerating reductions in vein spacing. Our results do not support these interpretations of Williams et al. (2013). The Neurachne data, along with recent results from Flaveria (Sage et al., 2013; Schulze et al., 2013), Heliotropium (Muhaidat et al., 2011), and Steinchisma (Brown et al., 1983; Khoshravesh et al., 2016) show similar patterns in organelle enhancement and GDC reallocation in grasses and eudicots. Instead of multiple distinct evolutionary pathways between monocots and eudicots, as concluded by Williams et al. (2013), the pattern we interpret is one of similarities between monocots and eudicots in terms of first acquiring the C2 pathway via organelle and GDC enrichment in the sheath cells, concurrent with reductions in IVD. This new information, along with recent data from Alloteropsis semialata intermediates (Lundgren et al., 2019), will be valuable in updating the Williams et al. (2013) model. Whereas significant differences in the general pattern of C2 evolution are not obvious, subtle differences are observed between Flaveria and Neurachne, which are two lineages with enough variation between intermediates to evaluate potential patterns of evolutionary change. The nature of the ancestral sheath tissue likely accounts for these variations.
From studies of both eudicots and Neurachne, the initial phase of C2 evolution appears to be the accumulation of organelles in the sheath tissue (BS in Flaveria, Heliotropium, and Euphorbia, MS in Neurachne), to strengthen, or “activate,” the photosynthetic potential of the sheath tissue (Muhaidat et al., 2011; Sage et al., 2011b, 2013; Schulze et al., 2013). In C3 Flaveria, Euphorbia, and Heliotropium, this activation occurs in tandem with increased BS width. In C3 Flaveria cronquistii, chloroplasts and mitochondria line the walls along the intercellular air spaces (IAS), allowing the BS cells to conduct diffusion-based C3 photosynthesis as occurs in the M cells (Sage et al., 2013). CO2 rapidly diffuses into the peripheral BS chloroplasts from the IAS while adjacent mitochondria quickly recycle photorespiratory metabolites (Bauwe et al., 2010). The shift to the proto-Kranz phase is observed in Flaveria pringlei and Flaveria robusta, which are branch sisters to F. cronquistii in the Flaveria phylogeny. In these two species, the mitochondria are repositioned along the centripetal wall of the BS cells, opposite most chloroplasts. Similar relocation of mitochondria occurs in the BS of proto-Kranz Heliotropium, Salsola, and Steinchisma species (Sage et al., 2014). This relocation leads Gly produced from glycolate made in the peripheral chloroplasts to be metabolized by centripetal mitochondria, with the result that photorespiratory CO2 accumulates in the inner region, enhancing Rubisco efficiency in nearby chloroplasts. This arrangement is proposed to establish a single-cell Gly shuttle that can scavenge some photorespiratory CO2 with slight benefits to the plant (Sage et al., 2013, 2014).
In Neurachne and Thyridolepis, MS cells have suberized outer walls and are surrounded by tightly packed PS cells, both of which can enhance resistance to diffusive efflux of CO2 generated by decarboxylation of Gly or C4 acids (Hattersley et al., 1986; von Caemmerer and Furbank, 2003; Alonso-Cantabrana et al., 2018). In the MS of C3 species, however, suberized walls and the PS sheath would reduce diffusive CO2 influx and thus starve chloroplasts of CO2. A boost in chloroplast numbers in the MS of C3 species would therefore be of little value unless CO2 were imported from another source, such as photorespiratory Gly. Because N. annularis grows in warm-temperate, semiarid landscapes of western Australia with hot summers (Macfarlane, 2007), high rates of photorespiration would produce an abundance of Gly that could potentially deliver CO2 to the MS cells. If elevated GDC activity reduces Gly concentration in the MS, a Gly diffusion gradient between M and MS cells would be established, thereby drawing in additional Gly. We hypothesize that the boost in organelles in the MS of N. annularis creates a sink for photorespiratory Gly and can thus increase photosynthetic potential in the MS cells in a manner that slightly enhances leaf photosynthetic efficiency. Because this enhancement results from the operation of a weak photorespiratory Gly shuttle, we propose N. annularis fits the criteria of the proto-Kranz species, even though there is no mitochondrial relocation within the MS cell as observed in eudicots. We hypothesize the suberized walls and PS in N. annularis slow CO2 efflux, thereby eliminating the need to reposition mitochondria, whereas in Flaveria and other eudicots, trapping of photorespired CO2 requires a wide vacuole between centripetal mitochondria and the outer periphery of the BS cells to form the barrier to CO2 efflux.
As indicated by the phenotypes of the C2-like species N. lanigera and Flaveria angustifolia, once numerous organelles are in place in the sheath tissue and a limited photorespiratory Gly shuttle is functioning, step-wise progression to an optimized C2 pathway is envisioned where parallel changes occur in (1) increased organelle number in the sheath tissue, (2) greater GDC content in the sheath tissue, (3) reduction of GDC in M cells, (4) increasing PD connections between sheath and M tissue, and (5) increasing Kranz-like anatomy via fewer M cells between veins and declining MS L:W, resulting in increased proportion of BS or MS cell area in cross section (Sage et al., 2013). Because the establishment of a Gly sink in the sheath tissue is beneficial during high photorespiratory episodes, it may stabilize the proto-Kranz character state in hot climates and by doing so become a foundation that enables subsequent evolution that strengthens the photorespiratory Gly shuttle (Sage et al., 2012; Mallmann et al., 2014; Bräutigam and Gowik 2016). As has been proposed for later stages in C4 evolution (from the C2 to C4 condition), this “facilitation” cascade could establish an evolutionary trajectory that resembles a Mount Fuji-type of fitness landscape (Heckmann et al., 2013).
CONCLUSION
The information presented here from the Neurachninae indicates how the structural requirements for C4 photosynthesis are largely met by the evolution of the C2 pathway, where MS tissue width, PD frequency, and IVD in N. minor equals or approaches C4 values. This illustrates how the evolution of C2 photosynthesis could establish the foundation of structure and metabolite transport upon which a C4 metabolic cycle may be assembled. We also provide evidence that is consistent with a model whereby the assembly of the C2 pathway occurs gradually, beginning with modifications in MS cell division and expansion, reduced M cell division, and greater organelle allocation to the MS cells. Assuming C* reflects the degree of evolutionary intermediacy, the results support a hypothesis that organelle enrichment initiates a progressive reallocation of GDC from M to MS tissue, with loss of GDC expression in M cells occurring after mitochondrial enhancement increases GDC capacity in the MS tissue. Pre-existing anatomy in Neurachne likely eliminates the need for the repositioning of organelles observed in eudicots, because the PS and thick MS wall enable CO2 trapping without a need for a large vacuole acting as a diffusive barrier. In light of these results, the Neurachninae, along with numerous eudicot groups such as Flaveria, indicates how evolutionary processes reconfigured the variable anatomy, biochemistry, and physiology of C3 ancestors into a common, complex trait that can address challenges imposed by global environmental change.
MATERIALS AND METHODS
Plant Material
Seeds or rhizomes of Thyridolepis mitchelliana, Neurachne alopecuroidea, Neurachne annularis, Neurachne lanigera, Neurachne minor, Neurachne queenslandica, Neurachne tenuifolia, Neurachne munroi, and Neurachne muelleri were collected in their natural habitat (Supplemental Table S8) and used to generate plants that were grown in naturally illuminated glasshouses at the Plant Growth Facility, University of Western Australia, Perth, Western Australia (latitude 33°59’ south). N. muelleri was classified by Blake (1972) as Paraneurachne muelleri but was previously known as N. muelleri (Hackel, 1895; Lazarides, 1970). As a recent molecular study showed the species branches within the Neurachne phylogeny (Christin et al., 2012), the original name is used here. T. mitchelliana, N. alopecuroidea, N. annularis, and N. tenuifolia were grown at mean temperatures of 22°C/18°C (day/night), whereas N. lanigera, N. minor, N. munroi, and N. muelleri were grown at mean temperatures of 30°C/25°C (day/night). N. queenslandica was grown at day/night temperatures of 28°C/18°C in a separate, sealed greenhouse, as it is not native to Western Australia and thus by regulatory statute required complete isolation from the outside environment. Plants were grown in a mixture of a commercial potting mix and soil collected from west-central Australia, and fertilized with slow release fertilizer for Australian native plants (Osmocote Plus Trace elements: Native Gardens, Scotts Australia). Growth photon flux density routinely reached 1,600 μmol m−2 s−1. Recently fully expanded leaves of mature plants were used for all measurements. All species were examined for gas exchange, microscopic imaging, and immunolocalization analysis except for N. tenuifolia, which died after preliminary sampling and was unavailable for immunolocalization and gas exchange analyses.
Whole-Leaf Gas Exchange
Leaf gas exchange measurements were performed on healthy, attached leaves using a model no. LI-6400XT gas exchange system (LICOR Biosciences). Responses of net CO2 assimilation rate (A) to intercellular CO2 concentration (Ci) were measured at a leaf temperature of 32°C and a light intensity of 1,500 μmol m−2 s−1. After a leaf was acclimated to these conditions inside the LI-6400XT leaf chamber, the A/Ci curves were measured with a sequence of reference CO2 concentrations of 400, 300, 200, 150, 100, 75, 50, 400, 400, 600, 800, 1,000, 1250, 1,500, and 2,000 μmol mol−1. Data were recorded in quick sequential order, with an equilibration time of between 90 and 180 s at each CO2 concentration. The CO2 compensation point in the absence of day respiration (C*) was measured using the common-intercept method at 35°C (Brooks and Farquhar, 1985). For the C* measurements, leaves were acclimated to a leaf temperature of 35°C, a light intensity of 1,000 μmol m−2 s−1, and a reference CO2 concentration of 400 μmol mol−1 until A reached steady state. CO2 responses were then measured at CO2 concentrations of 400, 120, 110, 100, 90, 80, 70, and 60 μmol mol−1 at five different light intensities of 1,000, 400, 300, 200, and 150 μmol m−2 s−1. At each light intensity, leaves were given an additional 10 min to acclimate before proceeding with the next CO2 step change. Typically, lower light intensity reduces the slope of the A versus Ci plot at low Ci, creating a distinct series of linear responses that can be used to estimate C* by plotting the regression slope of these responses versus their corresponding y-intercept (Walker and Ort, 2015). C* is then estimated as the slope of this relationship, assuming that M conductance is constant (Supplemental Fig. S1; Walker and Ort, 2015; Walker et al., 2016). Outlier points at high light were excluded from the regression analysis if they resulted from shifts in the A/Ci response to lower Ci, as can occur in C2 species at >800 μmol photons m−2 s−1 (Sage et al., 2013). In C4 plants, the common intercept method does not work for C* as Γ varies little with declining photosynthetic photon flux density (Table 1); hence, only Γ values were determined in C4 plants at saturating light.
Leaf Anatomy, Ultrastructure, and Immunolocalization
The internal anatomy of leaves was assessed on sections sampled from the middle of the most recent, fully expanded leaves (three plants per species; one leaf per plant; 10 images per leaf). Plants were sampled between 9 am and 12 am. The youngest cohort of fully expanded leaves was sampled in full sun for all procedures. Samples were fixed in 0.5% (v/v) glutaraldehyde, 4% (w/v) paraformaldehyde in sodium cacodylate buffer for light and transmission electron microscopy (TEM), and embedded in Spurr’s resin and London Resin White as described by Khoshravesh et al. (2017). Immunocytochemical detection of GLDP and Rubisco LSU followed Khoshravesh et al. (2016, 2017) with the same antibodies used for the immunoblots (Supplemental Fig. S4; see Supplemental Table S9 for more details). Light microscope images were obtained with an Axioplan (Zeiss) equipped with an image analysis system (model no. DP71, Olympus; Empix Imaging). A Philips 201 TEM equipped with an Advantage HR camera system (Advanced Microscopy Techniques) was used to capture leaf ultrastructure and immunolabeling results. To quantify all M, PS, and MS cellular features, and immunodetection events (gold particles) of GLDP and Rubisco LSU, TEM images were analyzed using a Cintiq graphics tablet (Wacom Technology) and ImageJ software (Schneider et al., 2012) as described in Sage et al. (2013) and Stata et al. (2014). To quantify protoplast area, we subtracted the cross-sectional area of the cell covered by cell walls from the cell area values. IVD was measured as the distance between PS tissue of minor veins, because the PS tissue is part of the vascular tissue complex. MS cell volume was estimated as the length along the proximo-distal axis parallel to the vein (in paradermal sections) times the area of the MS cell in planar cross section. Allocation coefficients representing the fraction of organelles and GLDP in specific tissues were calculated as: % organelle allocation = (planar area of an organelle within a tissue cross section divided by the sum of organelle area in all of the MS, M, and PS tissue within a sample image) × 100%; and percent GLDP allocation = (the number of immunogold particles within a tissue cross section divided by the total number of immunogold particles in the MS, M, and PS tissue within a sample image) × 100% (Wang et al., 2017). IAS were not included in tissue measurements. PD frequency was quantified as described by Wang et al. (2017); the presence and absence of PD in the walls between M, MS, and PS cells in planar cross sections of TEM images were recorded as absence = 0, presence = 1. Relative frequency of PD was then quantified as the number of cells that had PD connections with the adjacent cell, divided by the total number of observed cells for that cell-type, multiplied by 100%.
Data Analysis
For analysis of light microscopy and TEM images, samples were collected from one leaf of each of three distinct plants. Ten images from each leaf were analyzed with the values averaged to provide a mean for the plant. These individual plant values were the unit of replication for statistical analysis. For leaf gas exchange, three to eight measurements were conducted on two to seven plants per species, with each leaf serving as the unit of replication. Results were analyzed with the software SigmaPlot v12.5 (Systat Software) or SPSS v20.0. (IBM) using one-way ANOVA followed by a Tukey’s test of variance (for normally distributed data) or a Games–Howell test (when the variances were not equal) to evaluate statistical grouping of the species means, unless otherwise indicated.
To analyze whether the anatomical variables and GLDP labeling predict C* values in the Neurachninae, linear regression analysis was performed. Predictors were selected based on their correlation (Pearson correlation coefficient [r] ≥ |0.65|) with C*. To correct for phylogenetic signal, phylogenetic independent contrast on the mean value of selected traits was performed using the R package “ape” (Paradis et al., 2004), with evolutionary model = Brownian Motion. The list of predictors was then finalized by selecting traits exhibiting Pearson correlations coefficient (r) ≥ |0.65| with C* in the contrasting analysis. To eliminate collinearity between selected variables, a phylogenetic pPCA was performed using the phylo.pca function from the R package phytools v0.6-44 (Revell 2009, 2012). Then a linear regression (principal component regression) was performed using pPCs with eigenvalues > 0.1 as the predictors.
To evaluate possible ancestral character states, C*, percent GLDP in the MS, L:W of MS cell, plus other structural traits were mapped onto the Neurachninae phylogeny of Christin et al. (2012) using the program Mesquite (Maddison and Maddison, 2018) and maximum parsimony (Fig. 9). The 95% confidence intervals for physiological (C* or for C4 species, Γ), cellular, and anatomical traits were reconstructed using a maximum likelihood approach and Brownian Motion evolutionary models using R package “ape” (Paradis et al., 2004) and the Neurachninae phylogeny (Christin et al., 2012; Supplemental Table S7).
Supplemental Data
The following supplemental materials are available.
Supplemental Appendix 1. Supplemental Tables S1-S9.
Supplemental Table S1. Characteristics of cells and tissues from Neurachninae leaves.
Supplemental Table S2. The allocation of organelles and GLDP in photosynthetic tissues of eight Neurachninae species.
Supplemental Table S3. Peroxisome parameters and PD frequency in eight Neurachninae species.
Supplemental Table S4. Pearson correlations coefficients between measured parameters in this study.
Supplemental Table S5. Summary of pPCA.
Supplemental Table S6. Pearson correlations coefficients between traits and phylogenetic principal components.
Supplemental Table S7. Means and 95% confidence interval for reconstructed trait values at the phylogenetic nodes in Figure 9.
Supplemental Table S8. Species examined, with voucher information and source location.
Supplemental Table S9. Details of immunolocalization methods.
Supplemental Appendix 2. Supplemental Figures S1–S9.
Supplemental Figure S1. The relationship between the initial slope of the A/Ci response and its extrapolated y-intercept for five species of Neurachne.
Supplemental Figure S2. Light micrographs of leaf cross sections from nine Neurachninae species.
Supplemental Figure S3. TEM images of MS cells in N. queenslandica, N. tenuifolia, and T. mitchelliana.
Supplemental Figure S4. Immunoblot and transcript analyses of Neurachne GLDP and the Rubisco LSU.
Supplemental Figure S5. TEM images of GLDP immunolocalization in M cells of six Neurachne species.
Supplemental Figure S6. Immunolocalization of Rubisco LSU in C3 Neurachninae species.
Supplemental Figure S7. Immunolocalization of Rubisco LSU in N. annularis, N. lanigera, and N. minor.
Supplemental Figure S8. Immunolocalization of Rubisco LSU in C4 Neurachne species.
Supplemental Figure S9. Phylogenetic independent contrast plots of pair comparisons between 13 anatomical traits selected for pPCA and C*.
Footnotes
This work was supported by the Australian Research Council (Discovery Grant no. DP130102243 to M.L., R.F.S., and T.L.S.), the Canadian Natural Science and Engineering Research Council (grants no. RGPIN-04878-2015 to T.L.S. and no. RGPIN-2017-06476 to R.F.S.), the Australian Government through the Australian Research Council Centre of Excellence for Translational Photosynthesis (to F.A.B.), and a Queen Elizabeth II Graduate Scholarship in Science and Technology (to Ma.S.).
Articles can be viewed without a subscription.
References
- Aliscioni SS, Giussani LM, Zuloaga FO, Kellogg EA (2003) A molecular phylogeny of Panicum (Poaceae: Paniceae): Tests of monophyly and phylogenetic placement within the Panicoideae. Am J Bot 90: 796–821 [DOI] [PubMed] [Google Scholar]
- Alonso-Cantabrana H, Cousins AB, Danila F, Ryan T, Sharwood RE, von Caemmerer S, Furbank RT (2018) Diffusion of CO2 across the mesophyll-bundle sheath cell interface in a C4 plant with genetically reduced PEP carboxylase activity. Plant Physiol 178: 72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwe H. (2011) Photorespiration: The bridge to C4 photosynthesis In Raghavendra AS, and Sage RF, eds, C4 Photosynthesis and Related CO2 Concentrating Mechanisms. Springer, The Netherlands, pp 81–108 [Google Scholar]
- Bauwe H, Hagemann M, Fernie AR (2010) Photorespiration: Players, partners and origin. Trends Plant Sci 15: 330–336 [DOI] [PubMed] [Google Scholar]
- Blake ST. (1972) Neurachne and its allies (Gramineae). Contributions from the Queensland Herbarium 13: 1–53 [Google Scholar]
- Bräutigam A, Gowik U (2016) Photorespiration connects C3 and C4 photosynthesis. J Exp Bot 67: 2953–2962 [DOI] [PubMed] [Google Scholar]
- Brooks A, Farquhar GD (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Estimates from gas-exchange measurements on spinach. Planta 165: 397–406 [DOI] [PubMed] [Google Scholar]
- Brown RH, Bouton JH, Rigsby L, Rigler M; Ultrastructural Characteristics of Panicum Species in the Laxa Group (1983) Photosynthesis of grass species differing in carbon dioxide fixation pathways: VIII. Ultrastructural characteristics of Panicum species in the laxa group. Plant Physiol 71: 425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RH, Hattersley PW (1989) Leaf anatomy of C3-C4 species as related to evolution of c4 photosynthesis. Plant Physiol 91: 1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch FA, Sage TL, Cousins AB, Sage RF (2013) C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant Cell Environ 36: 200–212 [DOI] [PubMed] [Google Scholar]
- Christin P-A, Freckleton RP, Osborne CP (2010) Can phylogenetics identify C4 origins and reversals? Trends Ecol Evol 25: 403–409 [DOI] [PubMed] [Google Scholar]
- Christin P-A, Osborne CP, Chatelet DS, Columbus JT, Besnard G, Hodkinson TR, Garrison LM, Vorontsova MS, Edwards EJ (2013) Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proc Natl Acad Sci USA 110: 1381–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Osborne CP, Sage RF, Arakaki M, Edwards EJ (2011a) C4 eudicots are not younger than C4 monocots. J Exp Bot 62: 3171–3181 [DOI] [PubMed] [Google Scholar]
- Christin PA, Sage TL, Edwards EJ, Ogburn RM, Khoshravesh R, Sage RF (2011b) Complex evolutionary transitions and the significance of C3-C4 intermediate forms of photosynthesis in Molluginaceae. Evolution 65: 643–660 [DOI] [PubMed] [Google Scholar]
- Christin PA, Wallace MJ, Clayton H, Edwards EJ, Furbank RT, Hattersley PW, Sage RF, Macfarlane TD, Ludwig M (2012) Multiple photosynthetic transitions, polyploidy, and lateral gene transfer in the grass subtribe Neurachninae. J Exp Bot 63: 6297–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covshoff S, Burgess SJ, Kneřová J, Kümpers BMC (2014) Getting the most out of natural variation in C4 photosynthesis. Photosynth Res 119: 157–167 [DOI] [PubMed] [Google Scholar]
- Danila FR, Quick WP, White RG, Furbank RT, von Caemmerer S (2016) The metabolite pathway between bundle sheath and mesophyll: Quantification of plasmodesmata in leaves of C3 and C4 monocots. Plant Cell 28: 1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danila FR, Quick WP, White RG, Kelly S, von Caemmerer S, Furbank RT (2018) Multiple mechanisms for enhanced plasmodesmata density in disparate subtypes of C4 grasses. J Exp Bot 69: 1135–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler NG, Nelson T (1999) Leaf structure and development in C4 plants In Sage RF, and Monson RK, eds, C4 Plant Biology. Academic Press, San Diego, pp 133–172 [Google Scholar]
- Downton WJ. (1971) Adaptive and evolutionary aspects of C4 photosynthesis In Hatch MD, Osmond CB, and Slatyer RO, eds, Photosynthesis and Photorespiration. Wiley Interscience, New York, pp 3–32 [Google Scholar]
- Dunning LT, Moreno-Villena JJ, Lundgren MR, Dionora J, Salazar P, Adams C, Nyirenda F, Olofsson JK, Mapaura A, Grundy IM, et al. (2019) Key changes in gene expression identified for different stages of C4 evolution in Alloteropsis semialata. J Exp Bot 70: 3255–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards GE, Voznesenskaya EV (2011) C4 photosynthesis: Kranz forms and single-cell C4 in terrestrial plants In Raghavendra AS, and Sage RF, eds, C4 Photosynthesis and Related CO2 Concentrating Mechanisms, Vol Vol 32 Springer, Dordrecht, pp 29–61 [Google Scholar]
- Fisher AE, McDade LA, Kiel CA, Khoshravesh R, Johnson MA, Stata M, Sage TL, Sage RF (2015) History of Blepharis (Acanthaceae) and the origin of C4 photosynthesis in section Acanthodium. Int J Plant Sci 176: 770–790 [Google Scholar]
- Gowik U, Westhoff P (2011) The path from C3 to C4 photosynthesis. Plant Physiol 155: 56–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass Phylogeny Working Group II (2012) New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol 193: 304–312 [DOI] [PubMed] [Google Scholar]
- Griffiths H, Weller G, Toy LFM, Dennis RJ (2013) You’re so vein: Bundle sheath physiology, phylogeny and evolution in C3 and C4 plants. Plant Cell Environ 36: 249–261 [DOI] [PubMed] [Google Scholar]
- Hackel E. (1895) Neurachninae muelleri n. sp. Österr Bot Zeitschr 45: 329 [Google Scholar]
- Hattersley PW. (1984) Characterization of C4 leaf anatomy in grasses (poaceae). Mesophyll: Bundle sheath area ratios. Ann Bot 53: 163–179 [Google Scholar]
- Hattersley PW, Roksandic Z (1983) Delta 13C values of C3 and C4 species of Australian Neurachne and its allies (Poaceae). Aust J Bot 31: 317–321 [Google Scholar]
- Hattersley PW, Stone NE (1986) Photosynthetic enzyme activities in the C3-C4 intermediate Neurachne minor Blake, S. T. (Poaceae). Aust J Plant Physiol 13: 399–408 [Google Scholar]
- Hattersley PW, Watson L (1975) Anatomical parameters for predicting photosynthetic pathways of grass leaves: The ‘maximum lateral cell count’ and the ‘maximum cells distant count’. Phytomorphology 25: 325–333 [Google Scholar]
- Hattersley PW, Watson L, Johnston CR (1982) Remarkable leaf anatomical variations in Neurachne and its allies (Poaceae) in relation to C3 and C4 photosynthesis. Bot J Linn Soc 84: 265–272 [Google Scholar]
- Hattersley PW, Wong S-C, Perry S, Roksandic Z (1986) Comparative ultrastructure and gas exchange characteristics of the C3–C4 intermediate Neurachne minor S. T. Blake (Poaceae). Plant Cell Environ 9: 217–233 [Google Scholar]
- Heckmann D, Schulze S, Denton A, Gowik U, Westhoff P, Weber AP, Lercher MJ (2013) Predicting C4 photosynthesis evolution: Modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153: 1579–1588 [DOI] [PubMed] [Google Scholar]
- Kadereit G, Ackerly D, Pirie MD (2012) A broader model for C4 photosynthesis evolution in plants inferred from the goosefoot family (Chenopodiaceae s.s.). Proc Biol Sci 279: 3304–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadereit G, Bohley K, Lauterbach M, Tefarikis DT, Kadereit JW (2017) C3-C4 intermediates may be of hybrid origin—a reminder. New Phytol 215: 70–76 [DOI] [PubMed] [Google Scholar]
- Kadereit G, Lauterbach M, Pirie MD, Arafeh R, Freitag H (2014) When do different C4 leaf anatomies indicate independent C4 origins? Parallel evolution of C4 leaf types in Camphorosmeae (Chenopodiaceae). J Exp Bot 65: 3499–3511 [DOI] [PubMed] [Google Scholar]
- Keerberg O, Pärnik T, Ivanova H, Bassüner B, Bauwe H (2014) C2 photosynthesis generates about 3-fold elevated leaf CO2 levels in the C3-C4 intermediate species Flaveria pubescens. J Exp Bot 65: 3649–3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshravesh R, Lundsgaard-Nielsen V, Sultmanis S, Sage TL (2017) Light microscopy, transmission electron microscopy, and immunohistochemistry protocols for studying photorespiration In Fernie AR, Bauwe H, and Weber APM, eds, Photorespiration: Methods and Protocols. Springer, New York, pp 243–270 [DOI] [PubMed] [Google Scholar]
- Khoshravesh R, Stinson CR, Stata M, Busch FA, Sage RF, Ludwig M, Sage TL (2016) C3-C4 intermediacy in grasses: Organelle enrichment and distribution, glycine decarboxylase expression, and the rise of C2 photosynthesis. J Exp Bot 67: 3065–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides M. (1970) The Grasses of Central Australia. Australian National University Press, Canberra, ACT, Australia [Google Scholar]
- Leegood RC. (2008) Roles of the bundle sheath cells in leaves of C3 plants. J Exp Bot 59: 1663–1673 [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Christin P-A (2017) Despite phylogenetic effects, C3-C4 lineages bridge the ecological gap to C4 photosynthesis. J Exp Bot 68: 241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren MR, Christin P-A, Escobar EG, Ripley BS, Besnard G, Long CM, Hattersley PW, Ellis RP, Leegood RC, Osborne CP (2016) Evolutionary implications of C3-C4 intermediates in the grass Alloteropsis semialata. Plant Cell Environ 39: 1874–1885 [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Dunning LT, Olofsson JK, Moreno-Villena JJ, Bouvier JW, Sage TL, Khoshravesh R, Sultmanis S, Stata M, Ripley BS, et al. (2019) C4 anatomy can evolve via a single developmental change. Ecol Lett 22: 302–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane TD. (2007) A new species of Neurachne (Poaceae) from Western Australia. Nuytsia 17: 215–222 [Google Scholar]
- Maddison WP, Maddison DR (2018) Mesquite: A modular system for evolutionary analysis. Version 3.51 http://www.mesquiteproject.org
- Mallmann J, Heckmann D, Bräutigam A, Lercher MJ, Weber AP, Westhoff P, Gowik U (2014) The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria. eLife 3: e02478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown AD, Dengler NG (2007) Key innovations in the evolution of Kranz anatomy and C4 vein pattern in Flaveria (Asteraceae). Am J Bot 94: 382–399 [DOI] [PubMed] [Google Scholar]
- McKown AD, Moncalvo JM, Dengler NG (2005) Phylogeny of Flaveria (Asteraceae) and inference of C4 photosynthesis evolution. Am J Bot 92: 1911–1928 [DOI] [PubMed] [Google Scholar]
- Monson RK, Edwards GE, Ku MSB (1984) C3-C4 intermediate photosynthesis in plants. Bioscience 34: 563–574 [Google Scholar]
- Monson R, Rawsthorne S (2000) CO2 assimilation in C3-C4 intermediate plants In Leegood R, Sharkey T, and von Caemmerer S, eds, Photosynthesis: Physiology and Metabolism, Vol Vol 9 Springer, Dordrecht, pp 533–550 [Google Scholar]
- Moore BD, Edwards GE (1989) Metabolism of (CO2) 14C by leaves of different photosynthetic types of Neurachne species. Plant Sci 60: 155–161 [Google Scholar]
- Muhaidat R, Sage TL, Frohlich MW, Dengler NG, Sage RF (2011) Characterization of C3-C4 intermediate species in the genus Heliotropium L. (Boraginaceae): Anatomy, ultrastructure and enzyme activity. Plant Cell Environ 34: 1723–1736 [DOI] [PubMed] [Google Scholar]
- Oakley JC, Sultmanis S, Stinson CR, Sage TL, Sage RF (2014) Comparative studies of C3 and C4 Atriplex hybrids in the genomics era: Physiological assessments. J Exp Bot 65: 3637–3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle K. (2003) Implications of interveinal distance for quantum yield in C4 grasses: A modeling and meta-analysis. Oecologia 136: 532–542 [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K (2004) APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290 [DOI] [PubMed] [Google Scholar]
- Prendergast H, Hattersley P (1985) Distribution and cytology of Australian Neurachne and its allies (Poaceae), a group containing C3, C4 and C3-C4 Intermediate species. Aust J Bot 33: 317–336 [Google Scholar]
- Revell LJ. (2012) Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3: 217–223 [Google Scholar]
- Revell LJ. (2009) Size-correction and principal components for interspecific comparative studies. Evolution 63: 3258–3268 [DOI] [PubMed] [Google Scholar]
- Sage RF. (2016) A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: Species number, evolutionary lineages, and Hall of Fame. J Exp Bot 67: 4039–4056 [DOI] [PubMed] [Google Scholar]
- Sage RF, Christin PA, Edwards EJ (2011a) The C4 plant lineages of planet Earth. J Exp Bot 62: 3155–3169 [DOI] [PubMed] [Google Scholar]
- Sage RF, Khoshravesh R, Sage TL (2014) From proto-Kranz to C4 Kranz: Building the bridge to C4 photosynthesis. J Exp Bot 65: 3341–3356 [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F (2012) Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol 63: 19–47 [DOI] [PubMed] [Google Scholar]
- Sage TL, Busch FA, Johnson DC, Friesen PC, Stinson CR, Stata M, Sultmanis S, Rahman BA, Rawsthorne S, Sage RF (2013) Initial events during the evolution of C4 photosynthesis in C3 species of Flaveria. Plant Physiol 163: 1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage TL, Sage RF, Vogan PJ, Rahman B, Johnson DC, Oakley JC, Heckel MA (2011b) The occurrence of C2 photosynthesis in Euphorbia subgenus Chamaesyce (Euphorbiaceae). J Exp Bot 62: 3183–3195 [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze S, Mallmann J, Burscheidt J, Koczor M, Streubel M, Bauwe H, Gowik U, Westhoff P (2013) Evolution of C4 photosynthesis in the genus Flaveria: Establishment of a photorespiratory CO2 pump. Plant Cell 25: 2522–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüssler C, Freitag H, Koteyeva N, Schmidt D, Edwards G, Voznesenskaya E, Kadereit G (2017) Molecular phylogeny and forms of photosynthesis in tribe Salsoleae (Chenopodiaceae). J Exp Bot 68: 207–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stata M, Sage TL, Rennie TD, Khoshravesh R, Sultmanis S, Khaikin Y, Ludwig M, Sage RF (2014) Mesophyll cells of C4 plants have fewer chloroplasts than those of closely related C3 plants. Plant Cell Environ 37: 2587–2600 [DOI] [PubMed] [Google Scholar]
- Vogan PJ, Sage RF (2012) Effects of low atmospheric CO2 and elevated temperature during growth on the gas exchange responses of C3, C3-C4 intermediate, and C4 species from three evolutionary lineages of C4 photosynthesis. Oecologia 169: 341–352 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. (2000) Biochemical Models of Leaf Photosynthesis. CSIRO Publishing, Collingwood, Australia [Google Scholar]
- von Caemmerer S, Furbank RT (2003) The C4 pathway: An efficient CO2 pump. Photosynth Res 77: 191–207 [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Akhani H, Roalson EH, Edwards GE (2013) Structural and physiological analyses in Salsoleae (Chenopodiaceae) indicate multiple transitions among C3, intermediate, and C4 photosynthesis. J Exp Bot 64: 3583–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BJ, Ort DR (2015) Improved method for measuring the apparent CO2 photocompensation point resolves the impact of multiple internal conductances to CO2 to net gas exchange. Plant Cell Environ 38: 2462–2474 [DOI] [PubMed] [Google Scholar]
- Walker BJ, Skabelund DC, Busch FA, Ort DR (2016) An improved approach for measuring the impact of multiple CO2 conductances on the apparent photorespiratory CO2 compensation point through slope-intercept regression. Plant Cell Environ 39: 1198–1203 [DOI] [PubMed] [Google Scholar]
- Wang P, Khoshravesh R, Karki S, Tapia R, Balahadia CP, Bandyopadhyay A, Quick WP, Furbank R, Sage TL, Langdale JA (2017) Re-creation of a key step in the evolutionary switch from a C3 to C4 leaf anatomy. Curr Biol 27: 3278–3287.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Wang P, Korkaric M, Capper RG, Saunders NJ, Langdale JA (2009) GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 21: 1109–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BP, Johnston IG, Covshoff S, Hibberd JM (2013) Phenotypic landscape inference reveals multiple evolutionary paths to C4 photosynthesis. eLife 2: e00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Berry PE (2011) Phylogenetics of the Chamaesyce clade (Euphorbia, Euphorbiaceae): Reticulate evolution and long-distance dispersal in a prominent C4 lineage. Am J Bot 98: 1486–1503 [DOI] [PubMed] [Google Scholar]