Abstract
Type 1 diabetes, a disorder characterized by immune-mediated loss of functional pancreatic beta cells, is a disease continuum with specific presymptomatic stages with defined risk of progression to symptomatic disease. Prognostic biomarkers have been developed for disease staging and for stratification of subjects that address the heterogeneity in rate of disease progression. Using biomarkers for stratification of subjects at different stages of type 1 diabetes will enable smaller and shorter intervention clinical trials with greater effect size. Addressing the heterogeneity of the disease will allow precision medicine-based approaches to prevention and interception of presymptomatic stages of disease and treatment and cure of symptomatic disease.
Keywords: Type 1 diabetes, Biomarkers, Autoimmune process, Autoantibodies, Beta cell, Dysglycemia, Staging, Stratification
Introduction
Type 1 diabetes (T1D) is a chronic, immune-mediated disease associated with destruction of the insulin-producing beta cells of the islets of the pancreas [1]. Approximately 40–50% of the risk of disease arises from genetics with the remaining risk arising from poorly defined environmental etiologies. The class I and II human leukocyte antigen (HLA) genes contribute about half of the genetic risk of disease with about 40–50 non-HLA genes accounting for the remainder of genetic risk [2, 3, 4]. The most prominent associated HLA genes are HLA class II haplotypes DRB1*0301-DQB1*0201 (DR3-DQ2) and DRB1*0401-DQB1*0302 (DR4-DQ8) with the highest risk occurring in the heterozygous DR3/4 genotype. Non-HLA genes include INS, CTLA4, PTPN22, and IL2RA in addition to multiple non-HLA SNPs that have been mapped to DNA regulatory sequences of immune cells [4, 5]. Several non-HLA susceptibility genes are expressed in human islets, and cytokines can alter their expression in the islet [6]. For example, the risk-associated GLIS3 gene product affects beta cell fragility through altering responses of beta cells to cytokines [7] or through the unfolded protein stress response [8] and thus enhances beta cell apoptotic and senescent fates. Environmental etiologies have not been well defined, but viral infections, the host microbiome, and food/diet have been invoked [9]. In contrast to genetics, environmental etiologies have not been validated and thus do not currently contribute to subject stratification in T1D prevention trials. However, some examples of gene-environment interactions include the interactions of the microbiome with the Fut-2 nonsecretor gene polymorphism, which increases risk of T1D and is associated with faster progression of presymptomatic T1D [10, 11, 12], and interactions of picornaviruses, which include enteroviruses, with polymorphisms of the innate immunity viral RNA receptor gene region IFIH1(MDA-5) [13, 14].
The highest prevalence of T1D is found in individuals of Northern European Caucasian backgrounds [4] and over the last 4–5 decades, the incidence of childhood-onset T1D has been increasing 2–4% in many countries in the developed world [15, 16], which demonstrates a major environmental contribution.
Why Stratification?
T1D has a high degree of disease heterogeneity based on its rate of progression from presymptomatic stages, progression from the time of clinical presentation, and the degree of glucose control and development of both short-term and long-term complications in established disease. Stratification of the disease at these various stages can take into account that heterogeneity, which has plagued multiple T1D clinical trials. Several T1D trials failed to reach their primary endpoint for the test population but demonstrated good responses in a subset of subjects [17, 18]. Effective subject stratification will permit the design of shorter and smaller trials and should result in greater effect size in trials [19]. As new tools and technologies to better characterize T1D are developed, T1D will be reclassified into subgroups of disease with unique, tailored preventive and therapeutic treatments.
Stages of T1D
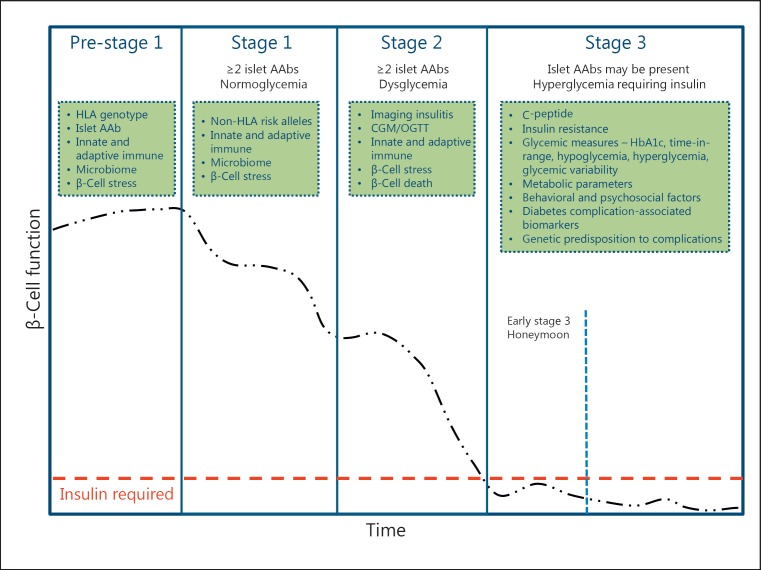
Based on insights from multiple natural history studies conducted over the last 2 decades [20, 21, 22, 23], distinct stages of T1D have been defined that reflect the underlying pathogenesis of the disease (Fig. 1) [24]. Early stages of T1D have been classified based on pathophysiology and prognostic outcomes that represent a continuum of the disease, including:
Fig. 1.
Progression and staging of type 1 diabetes. Type 1 diabetes is characterized by a progressive loss of beta cell function (black dashed-dotted line) over time. As the disease progresses, beta cell function falls below the threshold (red dashed line) required to maintain glucose control creating a requirement for insulin replacement therapy. Type 1 diabetes may be staged over the course of its progression starting with stage 1 at which point 2 or more of the 4 commonly measured islet autoantibodies are detected with normoglycemia. Stage 2 is marked by the appearance of dysglycemia associated with loss of beta cell function in addition to the presence of autoantibodies. Stage 3 is defined by hyperglycemia requiring insulin. In the green boxes are categories of biomarkers which could be leveraged to refine the staging paradigm, improve prognostic predictions, or subset individuals within a given stage of disease. The specifics of these biomarkers are discussed in the text related to the relevant stage.
Stage 1: pancreatic beta cell autoimmunity+/normoglycemia/presymptomatic T1D. Stage 1 is characterized by the development of 2 or more T1D-associated islet autoantibodies directed to insulin/proinsulin, GAD, IA-2, or ZnT8, which reflects the occurrence of beta cell-specific autoimmunity. The development of multiple islet autoantibodies in children with HLA risk genotypes followed from birth is associated with a 5-year and 10-year risk of progression to symptomatic, clinical disease of approximately 44 and 70%, respectively, and a lifetime risk approaching 100% [25, 26].
Stage 2: autoimmunity+/dysglycemia/presymptomatic T1D. With progressive loss of functional beta cell mass, the disease becomes associated with glucose intolerance, or dysglycemia, and this stage has a 5-year risk of symptomatic disease of approximately 75%, and lifetime risk approaching 100% [27].
Stage 3: symptomatic T1D. Stage 3 includes clinical symptoms and signs of diabetes (polyuria, polydipsia, weight loss, fatigue, diabetic ketoacidosis [DKA]) or is diagnosed in the presence of metabolic laboratory parameters established by the American Diabetes Association for a diagnosis of type 2 diabetes [28].
Established T1D. After diagnosis of stage 3 and with the initiation of insulin replacement therapy, a so-called “honeymoon” period may ensue associated with decreased insulin requirements until beta cell functional mass is further lost with increased insulin requirements. With established disease, subjects are at risk of short-term (hypoglycemia, DKA) and long-term (diabetic eye, kidney, neurologic disease) complications of the disease.
The order of this sequential progression of these stages is consistent, but the rate of progression from one stage to the next is variable. Biomarkers have been correlated with rate of progression (Fig. 1), and used in research studies [26, 29, 30, 31], but most have not been validated in longitudinal studies and none have reached a stage where they have become standard of care for informing treatment.
Several clinical trials have been designed that have taken advantage of the staging of T1D to preserve residual beta cell function in pre-stage 1, stage 1, stage 2, and stage 3 with a goal of delaying or preventing the onset of clinical, symptomatic disease and insulin dependence or improving glucose control in recent onset and established T1D. The TRIGR trial recruited subjects at pre-stage 1 with trial endpoints being progression to stage 1 in addition to progression to stage 3 [32]. An interception trial with CTLA-4-Ig (abatacept) has recruited stage 1 subjects with an endpoint of progression to stage 2 (NCT01773707), and a trial with anti-CD3 (tepluzimab) (NCT01030861) has recruited stage 2 subjects with an endpoint of progression to stage 3. Over the last decade, several stage 3 trials have been conducted to preserve residual functional beta cell mass at the time of onset of clinical disease [33], based on the evidence that preservation of residual beta cell mass is associated with decreased risk of the development of complications of T1D [34].
Progression from Pre-Stage 1 T1D
Multiple parameters and biomarkers have been investigated to better predict risk of progression to stage 1 T1D. Only approximately 10–15% of individuals newly diagnosed with T1D have a family history, but family history increases risk of disease ∼10–100-fold higher than background population [24, 35]. Risk is higher for identical twins or with sharing of HLA genotype with the proband, is higher in offspring of fathers with T1D compared to offspring of mothers with T1D, and is ∼14-fold higher in DR3/DR4-positive siblings if the proband develops symptomatic diabetes before age 10 years [35, 36]. As noted above, HLA genotype accounts for half of the genetic risk, and specific HLA alleles are associated with higher risk of disease and have been used to stratify a high versus moderate risk population [35, 37], or protection or resistance from developing disease, such as occurs with HLA class II DRB1*1501 and DQA1*0102-DQB1*0602[38]. Both HLA genes [25] and non-HLA genotypes can be used to stratify subjects who have a faster rate of progression to stage 3 T1D [39, 40].
Several approaches beyond genetics are being investigated to predict early in life risk of developing T1D including: metabolomics and lipidomics [41, 42], type 1 interferon or inflammatory signature patterns with use of transcriptional profiling [30, 43, 44, 45, 46, 47], proteomics [48], and intestinal microbiome metagenomics and metabolites [49, 50, 51, 52, 53]. Epidemiological data can also be applied as illustrated by the risk of excessive weight gain in the first year of life, which is associated with increased risk of progression to stage 1 T1D [54, 55].
The environmental etiologies that trigger onset of islet autoimmunity are not well understood, but inflammation around the islets arising from various etiologies may lead to beta cell stress to precipitate immune-mediated beta cell dysfunction and destruction [56, 57]. Several candidate biomarkers (proinsulin/C-peptide ratio, hsp90, noncoding RNAs) for detecting beta cell stress in the periphery are being investigated, and although have not been studied at pre-stage 1 T1D, have been evaluated in later stages of presymptomatic or new onset T1D [29, 56, 58, 59, 60, 61].
Beta cell stress has been demonstrated to generate modifications (posttranslational modifications, generation of hybrid proteins arising from fusion of beta cell peptides, defective ribosomal proteins with altered reading frames) of beta cell proteins that elicit either antibody or T cell responses in established T1D [62, 63, 64, 65, 66]. Of interest to stratifying subjects at risk of progressing to stage 1 T1D is detection of antibodies to beta cell protein modifications prior to detection of antibodies to native beta cell epitopes. Antibodies to a modified form of insulin - oxidized insulin - have been demonstrated in at-risk children, occur in islet autoantibody-negative subjects, precede autoantibodies to native insulin, and can identify at an early stage risk of at-risk children progressing to stage 3 T1D [67]. It is possible that immune responses to beta cell protein modifications catalyze the breaking of immune tolerance to native beta cell epitopes and lead to autoimmune responses to native beta cell self-antigens [62, 68].
Alteration in islet-specific adaptive immunity in addition to innate immunity is observed in pre-stage 1 T1D. A specific gene signature is observed in altered naïve, beta cell antigen responsive CD4+ T cells that resembles a pre-T helper 1 (TH1)/TH17/T follicular helper cell response and occurs prior to the development of beta cell-specific antibodies or memory helper T cells [69].
Progression from Stage 1 T1D
The presence of multiple islet autoantibodies characterizes stage 1 T1D and the number of antibodies and their specificity, titer, and affinity as well as the age when first detected are associated with rate of progression to symptomatic disease. Faster rates of progression are observed with 3 or 4 versus 2 islet autoantibodies [25, 70, 71]. The presence of antibodies to IA-2 and ZnT8 as well as higher titers of antibody to Insulin and IA-2 are associated with a faster rate of progression [10, 70, 72, 73, 74]. Islet autoantibody seroconversion at a young age is associated with a faster rate of progression [25], and disease progression is also accelerated in children at stage 1 T1D with an increased BMI [75] and markedly accelerated in Hispanic children younger than 12 years of age who are overweight or obese [76]. A declining rate of progression is observed with increased age in stage 1 T1D relatives of individuals with T1D [77, 78].
HLA [25] and especially non-HLA genes [36, 79, 80, 81] are associated with a faster rate of progression from stage 1. There are differences in age and genetic predisposition for specificity of antibodies - insulin antibodies occur at an earlier peak age incidence (9–24 months) than GAD antibodies (∼36 months), with IA-2 and ZnT8 tending to occur later and rarely as the first autoantibody. Insulin antibodies have a higher association with HLA-DR4-DQ8 and GAD with HLA-DR3-DQ2 [35, 82, 83].
The presence of only a single islet autoantibody is associated with progression in approximately only 15% of subjects, with progression occurring more frequently at a younger age [84, 85], if associated with HLA-risk genotypes [85], when the autoantibody is directed to IA-2 [25], or if higher affinity single autoantibodies are generated [86, 87, 88, 89]. In young children with HLA risk of T1D, progression from a single to multiple autoantibodies occurs usually within 2 years [84]. Reversion of single islet autoantibodies over time is common, but complete reversion in the presence of multiple islet autoantibodies is relatively rare [90], though loss of insulin autoantibodies in the presence of multiple autoantibodies is associated with delayed progression [91].
Progression from Stage 2 T1D
Most, if not all, of the immune and beta cell biomarkers relevant in stage 1 will presumably be relevant in stage 2 as the underlying autoimmune process is not likely to differ in substantial ways. Instead, stage 2 of T1D is distinguished from stage 1 by the presence of dysglycemia signifying that the pancreatic islets are no longer capable of maintaining normal glucose control. The dysglycemia observed in stage 2 is likely the result of both the loss of beta cell mass and a decline in beta cell dysfunction [56]. Of note, emerging evidence suggests that beta cell mass may in fact be maintained until near the time of stage 2 to stage 3 transition [60]. There is also evidence that insulin resistance is present in at least a subset of stage 2 individuals [92, 93, 94, 95].
While the current staging concept relies on oral glucose tolerance test (OGTT)-based definitions of dysglycemia, the detection of elevated HbA1c, elevated fasting glucose, and impaired first-phase insulin response (FPIR) on an intravenous glucose tolerance test, which can be abnormal up to 5 years before stage 3 with an accelerated decline in the 1.5 years in progressors, have all been used to detect dysglycemia with impaired FPIR, the latter providing perhaps the earliest indicator of failing glucose control [96]. The OGTT can be analyzed for 2-h glucose levels, sum of glucose values every 30 min, peak C-peptide, C-peptide area under the curve, or 30-0 min C-peptide, which correlates with the FPIR on an IVGTT and is an earlier predictor of progression than the other OGTT parameters with changes 1–2 years before Stage 3 [31]. In addition, stimulated C-peptide, which changes later than the OGTT, may also be reduced in late stage 2 individuals, with an accelerated decrease beginning 6 months prior to stage 3 [97, 98]. Fasting C-peptide usually does not change during this period. As a given individual progresses toward the need for exogenous insulin, elevated HbA1c and/or abnormal fasting glucose measures may be observed [97]. In Stage 2, many individuals do not develop an increased HbA1c, but a HbA1c increasing by 10% or an HbA1c ≥5.9% on consecutive samples are associated with rapid progression to stage 3 [27, 99].
The possibility of relapsing-remitting disease (discussed below) and inherent variability in OGTT measures make this method of detecting dysglycemia somewhat unreliable, and repeated abnormal values may be required to be certain that someone has progressed to stage 2. It is possible that the use of continuous glucose monitoring could replace OGTT as a monitoring standard and would have several advantages over the use of OGTTs including convenience and lower overall costs [100, 101, 102, 103].
To improve stratification of stage 2 trials, composite predictive risk scores that integrate diverse data (genetics, immunologic, metabolic, age, etc.) have been used to predict rate of progression as well as onset of stage 3 T1D [31, 104].
Identification of Active Disease in Stage 1 and Stage 2 T1D
The ability to stratify subjects at stage 1 and stage 2 T1D who have ongoing active disease is important because of the likely possibility that presymptomatic T1D can be relapsing and remitting [105] and the requirement that certain types of interventions will only show efficacy in the face of active immune beta cell pathology. Several approaches should be considered. Changes in islet antibody titers do not directly correlate with active disease [26]. Subject-specific, expanded clones of islet antigen-reactive CD4+ memory T cells can be detected in the peripheral blood of individuals with T1D [106], and may prove to be an approach applicable to detecting active presymptomatic T1D in an individual. Circulating CXCR5+PD-1+ICOS+-activated circulating follicular T helper cells increase in the periphery in stage 2 T1D that has advanced close to Stage 3 T1D and may prove to be a useful biomarker of active disease [107]. Exhaustion of peripheral blood T cells associated with cellular unresponsiveness and loss of effector function with expression of a T-cell exhaustion signature correlates with clinical remission in several autoimmune diseases [108] and is being evaluated for correlation with both spontaneous remission in presymptomatic T1D and in therapy-induced remission in T1D [109]. Other immune assays are being developed to better predict active disease and rate of progression [29].
Active disease in stage 1 and stage 2 T1D could also be identified by detecting cellular infiltration in and around islets, so-called insulitis. Autopsy specimens from human new-onset T1D have shown that the degree of insulitis is quite variable, is not as prominent as in mouse models of the disease, and is often not detected in multiple islet autoantibody-positive cadaveric pancreata [110, 111]. Insulitis in younger, new-onset T1D subjects (less than 7 years of age) is associated with a more prominent CD20+ B cell infiltration response and with more marked loss of residual insulin-positive beta cells, suggesting a more aggressive loss of beta cells [112]. The infrequency of detection of insulitis in presymptomatic T1D may reflect a relapsing-remitting pattern of the disease and further reinforces the importance of detecting disease activity for stratifying for trials in the presymptomatic setting. Imaging of islet inflammation based on vascular leak, which allows leakage of the imaging agent into the pancreas, and accumulation of antigen-presenting cells, which retain the imaging agent, has been used to detect inflammation in the pancreas in new-onset T1D [113], and could be applied to presymptomatic stages of T1D to detect active insulitis. Imaging of insulitis based on detection of infiltrating T cells is also being investigated [114].
Alternative approaches to detect active disease include detection of pancreatic beta cell stress [56], using assays described above, or detection of beta cell death. Multiple beta cell death assays are under development that are based on detection in peripheral blood of circulating beta cell DNA (insulin, amylin, and others) that is differentially methylated/demethylated [115, 116, 117, 118]. Some of these assays have been shown to be able to detect beta cell death at stage 2 of T1D [115], but have not been well studied to date in longitudinal samples collected from subjects with presymptomatic T1D.
Early Stage 3 T1D
The distinction between stage 2 and early stage 3 is really a matter of degree. Currently, the proposal for stage 3 is to use the broad definition of diabetes that was developed for the use of insulin in the setting of type 2 diabetes and which may not be appropriate for T1D. T1D is rarely screened outside the research setting and because early symptoms are subtle, individuals can persist in a state of hyperglycemia for some time before stage 3 T1D is recognized. Unfortunately, this frequently means that the diagnosis of stage 3 is often made with the patient in a state of DKA. Emerging evidence suggests that the DKA that results from failure to recognize the disease in earlier stages may carry lasting consequences for the affected person's ability to achieve good glucose control [119], and may even predispose to additional DKA episodes.
Early stage 3 is characterized by a partial remission, or a so-called “honeymoon” phase, that is often observed after a newly diagnosed individual is placed on insulin for the first time. During this period of partial remission, insulin requirements decrease, sometimes so much that the use of basal insulin is all that is required. The typical honeymoon period, if observed, may last from a few months to more than a year. Predicting who will or will not enter a honeymoon period and the rate of decline of residual beta cell function in recent onset T1D is important for stratification for stage 3 T1D clinical trials. With insulin administration in new-onset T1D, beta cell dysfunction recovers with increase in stimulated C-peptide levels for ∼6 weeks [120], and then proceeds to decrease with a faster rate of decline in the first 12 months compared to the second year after onset of stage 3 [121]. The decline in C-peptide is more rapid in children than adults, with about 11% of subjects showing no decline from a baseline at 2–3 months to 2 years after onset of stage 3 [121]. Residual C-peptide can be detected in some individuals years to decades after diagnosis but more commonly with adult-onset versus childhood-onset T1D [122, 123]. Several biomarkers and genes have been identified and modeled that predict a lack or presence of a honeymoon/remission in stage 3 T1D [124, 125, 126, 127].
Established T1D: Progression from Stage 3
With the clinical presentation of frank insulin dependence, all forms of T1D are treated with combinations of rapid-acting (bolus) and long-acting (basal) insulins to compensate for the loss of endogenous insulin production. Better understanding of the etiopathogenesis of T1D is demystifying various assumptions, while several others remain unsolved. To list a few, it is now established while genetic predisposition is a critical factor, the majority of T1D occurs with no known family history; almost half of the newly diagnosed cases occur in adults; the epidemic of obesity has not spared T1D, thus invoking metabolic syndrome-like characteristics; insulin resistance - a less studied culprit - affects T1D even in the lean phenotypes; about a third of individuals retain up to a third of insulin production despite long-standing disease; and many other facts and findings [122, 128]. Additionally, psychosocial factors and family dynamics that often influence disease management and outcomes in significant ways, but are out of scope of this review. Some of these include, but are not limited to, socioeconomic status and awareness and access to treatments, ethnicity, and cultural considerations influencing disease perspective, key lifestyle factors including stress, diet, and exercise, family dynamics and partners, and several individual psychosocial and behavioral issues such as inherent fear of devices, lack of peer-to-peer networking, or under- or overcorrection for fear of severe hypoglycemia or long-term complications from hyperglycemia.
With the onset of stage 3 and throughout the duration of diabetes, all of the above affect glucose control to varying degrees among individuals and often in an individual from day to day - thus underscoring the often underappreciated heterogeneity in established T1D. Furthermore, the protracted glycemic insult and any genetic predisposition render individuals vulnerable to the development of long term vascular complications of the disease, again to varying degrees of severity in their manifestation. This is evidenced in many epidemiologic and observational study cohorts, most recently from the T1D Exchange [129].
With the advent of improved insulins and delivery systems such as insulin pumps, technology to measure glucose levels in real time such as continuous glucose monitors (CGMs), approval of the first automated hybrid closed loop systems (USA), emerging data from the use of adjunct therapies such as GLP1 analogs, SGLT inhibitors, and fixed dose and fixed ratio combinations of therapeutics with or without insulin, it behooves the research and clinical communities to exploit all options to understand the benefit/burden profiles of treatments and optimize care of patients [130, 131, 132]. This calls for deep phenotyping of individuals, including assessment of critical markers of disease onset and progression, such as age at onset, disease duration, body weight, insulin sensitivity, C-peptide level, adiposity, metabolic syndrome parameters, time in and out of desirable glucose range, glycemic variability, and plasma and urine markers suggestive of vascular diseases.
Clinical studies over the last decade have shown promising effects with use of devices such as insulin infusion pumps and CGMs; however, efficacy has been correlated with adherence to the therapies [133]. CGM is a powerful and the only technology that can measure exposures to various glucose levels and its variability at any point of time and for entire periods of use - which can be advantageous in monitoring effects of therapies and self-management, as well as avoidance of extremes of high and low blood glucose levels that often lead to devastating acute consequences [100, 134]. However, CGM use and data interpretation requires training and experience, has had slow adoption, and its potential remains to be fully realized. Likewise, use of therapies currently approved for the treatment of type 2 diabetes have improved outcomes in T1D such as body weight and insulin dose reductions, insulin sensitization, lowering HbA1c, increasing time in desired glucose range and even reducing glycemic variability; however, most studies have shown responses span the gamut from nonresponders to super-responders [135, 136, 137, 138, 139], thus emphasizing the need for stratified, precision medicine approaches. The novel class of SGLT inhibitors, both mono SGLT2 and dual SGLT1/2, are currently in pivotal T1D label expansion studies by manufacturers. Encouraging safety and efficacy results have been recently reported, and stratified analyses of all individuals will certainly enrich our understanding of T1D heterogeneity and facilitate the development of customized treatment approaches.
Tailoring the right therapy at the right dose to the right individual at the right stage is the Holy Grail for personalized medicine approaches. This has to be achieved in a standardized and simplified manner for easier adoption by health-care professionals and providers. There has been some success; however, current knowledge gaps for stratification of individuals for clinical trials and treatments will have to be addressed. Perhaps the need for prognostic and predictive markers to enable smart clinical trial design and develop stratified treatment is most urgent for diabetic long-term complications, which have a high degree of heterogeneity, protracted period with variable rates of progression toward end organ failures, and are often confounded with comorbidities. Not surprisingly, therapies to prevent or treat micro- and macrovascular complications have largely yielded mixed results in clinical studies with subsequent discontinuation of development by manufacturers, especially in diabetic nephropathy and neuropathy [140].
Recent success from various groups has been encouraging. For example, the use of a composite of levels of serum tumor necrosis factor receptor isotype 1 with or without the gold standard albumin-to-creatinine ratio significantly increased sensitivity and prognostic values, thus reducing the size of diabetic nephropathy clinical trials required to achieve statistical power in detecting treatment responses [141]. Furthermore, the rate of decline in estimated glomerular filtration rate (eGFR) has been suggested as a reliable marker for loss of renal function and for facilitating differentiation between rapid, moderate, and slow progression of diabetic nephropathy [142]. More studies such as these are required to enroll the appropriate individuals in trials as well as to predict response to therapies.
Another remaining gap in the field is the need for surrogate endpoints to accelerate drug development. Current clinical trials in diabetes complications require 2–4 years to observe primary treatment effect, which adds to the current barriers of entry. Fortunately, the JDRF and other funding organizations have invested significant resources for the discovery, development, and validation of prognostic and predictive biomarkers from longitudinal cohorts and interventional studies, as well as for understanding the natural history of disease progression, to ultimately de-convolute disease heterogeneity and lead to development of specific therapies and companion diagnostics for precision medicine approaches.
Disclosure Statement
The authors declare no conflict of interest.
Author Contributions
All authors contributed substantially to the work reported.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360:1646–1654. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- 3.Pociot F, Lernmark A. Genetic risk factors for type 1 diabetes. Lancet. 2016;387:2331–2339. doi: 10.1016/S0140-6736(16)30582-7. [DOI] [PubMed] [Google Scholar]
- 4.Rich SS. Genetics and its potential to improve type 1 diabetes care. Curr Opin Endocrinol Diabetes Obesity. 2017 doi: 10.1097/MED.0000000000000347. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onengut-Gumuscu S, Chen WM, Burren O, Cooper NJ, Quinlan AR, Mychaleckyj JC, Farber E, Bonnie JK, Szpak M, Schofield E, Achuthan P, Guo H, Fortune MD, Stevens H, Walker NM, Ward LD, Kundaje A, Kellis M, Daly MJ, Barrett JC, Cooper JD, Deloukas P, Todd JA, Wallace C, Concannon P, Rich SS. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47:381–386. doi: 10.1038/ng.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eizirik DL, Sammeth M, Bouckenooghe T, Bottu G, Sisino G, Igoillo-Esteve M, Ortis F, Santin I, Colli ML, Barthson J, Bouwens L, Hughes L, Gregory L, Lunter G, Marselli L, Marchetti P, McCarthy MI, Cnop M. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet. 2012;8:e1002552. doi: 10.1371/journal.pgen.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nogueira TC, Paula FM, Villate O, Colli ML, Moura RF, Cunha DA, Marselli L, Marchetti P, Cnop M, Julier C, Eizirik DL. GLIS3, a susceptibility gene for type 1 and type 2 diabetes, modulates pancreatic beta cell apoptosis via regulation of a splice variant of the BH3-only protein Bim. PLoS Genet. 2013;9:e1003532. doi: 10.1371/journal.pgen.1003532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dooley J, Tian L, Schonefeldt S, Delghingaro-Augusto V, Garcia-Perez JE, Pasciuto E, Di Marino D, Carr EJ, Oskolkov N, Lyssenko V, Franckaert D, Lagou V, Overbergh L, Vandenbussche J, Allemeersch J, Chabot-Roy G, Dahlstrom JE, Laybutt DR, Petrovsky N, Socha L, Gevaert K, Jetten AM, Lambrechts D, Linterman MA, Goodnow CC, Nolan CJ, Lesage S, Schlenner SM, Liston A. Genetic predisposition for beta cell fragility underlies type 1 and type 2 diabetes. Nat Genet. 2016;48:519–527. doi: 10.1038/ng.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340–2348. doi: 10.1016/S0140-6736(16)30507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollanen PM, Lempainen J, Laine AP, Toppari J, Veijola R, Vahasalo P, Ilonen J, Siljander H, Knip M. Characterisation of rapid progressors to type 1 diabetes among children with HLA-conferred disease susceptibility. Diabetologia. 2017;60:1284–1293. doi: 10.1007/s00125-017-4258-7. [DOI] [PubMed] [Google Scholar]
- 11.Smyth DJ, Cooper JD, Howson JM, Clarke P, Downes K, Mistry T, Stevens H, Walker NM, Todd JA. FUT2 nonsecretor status links type 1 diabetes susceptibility and resistance to infection. Diabetes. 2011;60:3081–3084. doi: 10.2337/db11-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ihara K, Fukano C, Ayabe T, Fukami M, Ogata T, Kawamura T, Urakami T, Kikuchi N, Yokota I, Takemoto K, Mukai T, Nishii A, Kikuchi T, Mori T, Shimura N, Sasaki G, Kizu R, Takubo N, Soneda S, Fujisawa T, Takaya R, Kizaki Z, Kanzaki S, Hanaki K, Matsuura N, Kasahara Y, Kosaka K, Takahashi T, Minamitani K, Matsuo S, Mochizuki H, Kobayashi K, Koike A, Horikawa R, Teno S, Tsubouchi K, Mochizuki T, Igarashi Y, Amemiya S, Sugihara S. FUT2 non-secretor status is associated with Type 1 diabetes susceptibility in Japanese children. Diabet Med. 2017;34:586–589. doi: 10.1111/dme.13288. [DOI] [PubMed] [Google Scholar]
- 13.Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, Guja C, Ionescu-Tirgoviste C, Widmer B, Dunger DB, Savage DA, Walker NM, Clayton DG, Todd JA. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38:617–619. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 14.Domsgen E, Lind K, Kong L, Huhn MH, Rasool O, van Kuppeveld F, Korsgren O, Lahesmaa R, Flodstrom-Tullberg M. An IFIH1 gene polymorphism associated with risk for autoimmunity regulates canonical antiviral defence pathways in Coxsackievirus infected human pancreatic islets. Sci Rep. 2016;6:39378. doi: 10.1038/srep39378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376:1419–1429. doi: 10.1056/NEJMoa1610187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 17.Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, Griffin KJ, Tsalikian E, Gottlieb PA, Greenbaum CJ, Sherry NA, Moore WV, Monzavi R, Willi SM, Raskin P, Keyes-Elstein L, Long SA, Kanaparthi S, Lim N, Phippard D, Soppe CL, Fitzgibbon ML, McNamara J, Nepom GT, Ehlers MR. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015;125:3285–3296. doi: 10.1172/JCI81722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ, Jr, Bode B, Aronoff S, Holland C, Carlin D, King KL, Wilder RL, Pillemer S, Bonvini E, Johnson S, Stein KE, Koenig S, Herold KC, Daifotis AG. Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–497. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staeva TP, Chatenoud L, Insel R, Atkinson MA. Recent lessons learned from prevention and recent-onset type 1 diabetes immunotherapy trials. Diabetes. 2013;62:9–17. doi: 10.2337/db12-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial - Type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 21.Mahon JL, Sosenko JM, Rafkin-Mervis L, Krause-Steinrauf H, Lachin JM, Thompson C, Bingley PJ, Bonifacio E, Palmer JP, Eisenbarth GS, Wolfsdorf J, Skyler JS. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10:97–104. doi: 10.1111/j.1399-5448.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 22.Hagopian WA, Erlich H, Lernmark A, Rewers M, Ziegler AG, Simell O, Akolkar B, Vogt R, Jr, Blair A, Ilonen J, Krischer J, She J. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421,000 infants. Pediatr Diabetes. 2011;12:733–743. doi: 10.1111/j.1399-5448.2011.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Insel RA, Dunne JL, Ziegler AG. General population screening for type 1 diabetes: has its time come? Curr Opin Endocrinol Diabetes Obes. 2015;22:270–276. doi: 10.1097/MED.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 24.Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark A, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964–1974. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, Bonifacio E, Eisenbarth GS. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonifacio E. Predicting type 1 diabetes using biomarkers. Diabetes Care. 2015;38:989–996. doi: 10.2337/dc15-0101. [DOI] [PubMed] [Google Scholar]
- 27.Krischer JP. The use of intermediate endpoints in the design of type 1 diabetes prevention trials. Diabetologia. 2013;56:1919–1924. doi: 10.1007/s00125-013-2960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Standards of Medical Care in Diabetes-2017 Abridged for Primary Care Providers. Clin Diabetes. 2017;35:5–26. doi: 10.2337/cd16-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watkins RA, Evans-Molina C, Blum JS, DiMeglio LA. Established and emerging biomarkers for the prediction of type 1 diabetes: a systematic review. Transl Res. 2014;164:110–121. doi: 10.1016/j.trsl.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabrera SM, Chen YG, Hagopian WA, Hessner MJ. Blood-based signatures in type 1 diabetes. Diabetologia. 2016;59:414–425. doi: 10.1007/s00125-015-3843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sosenko JM. Staging the progression to type 1 diabetes with prediagnostic markers. Curr Opin Endocrinol Diabetes Obes. 2016;23:297–305. doi: 10.1097/MED.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knip M, Akerblom HK, Becker D, Dosch HM, Dupre J, Fraser W, Howard N, Ilonen J, Krischer JP, Kordonouri O, Lawson ML, Palmer JP, Savilahti E, Vaarala O, Virtanen SM. Hydrolyzed infant formula and early beta-cell autoimmunity: a randomized clinical trial. JAMA. 2014;311:2279–2287. doi: 10.1001/jama.2014.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Battaglia M, Anderson MS, Buckner JH, Geyer SM, Gottlieb PA, Kay TWH, Lernmark A, Muller S, Pugliese A, Roep BO, Greenbaum CJ, Peakman M. Understanding and preventing type 1 diabetes through the unique working model of TrialNet. Diabetologia. 2017 doi: 10.1007/s00125-017-4384-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 35.Regnell SE, Lernmark A. Early prediction of autoimmune (type 1) diabetes. Diabetologia. 2017;60:1370–1381. doi: 10.1007/s00125-017-4308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillespie KM, Aitken RJ, Wilson I, Williams AJ, Bingley PJ. Early onset of diabetes in the proband is the major determinant of risk in HLA DR3-DQ2/DR4-DQ8 siblings. Diabetes. 2014;63:1041–1047. doi: 10.2337/db13-0994. [DOI] [PubMed] [Google Scholar]
- 37.Ilonen J, Kiviniemi M, Lempainen J, Simell O, Toppari J, Veijola R, Knip M. Genetic susceptibility to type 1 diabetes in childhood - estimation of HLA class II associated disease risk and class II effect in various phases of islet autoimmunity. Pediatr Diabetes. 2016;17((suppl 22)):8–16. doi: 10.1111/pedi.12327. [DOI] [PubMed] [Google Scholar]
- 38.Pugliese A, Boulware D, Yu L, Babu S, Steck AK, Becker D, Rodriguez H, DiMeglio L, Evans-Molina C, Harrison LC, Schatz D, Palmer JP, Greenbaum C, Eisenbarth GS, Sosenko JM. HLA-DRB1*15:01-DQA1*01:02-DQB1*06:02 haplotype protects autoantibody-positive relatives from type 1 diabetes throughout the stages of disease progression. Diabetes. 2016;65:1109–1119. doi: 10.2337/db15-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steck AK, Xu P, Geyer S, Redondo MJ, Antinozzi P, Wentworth JM, Sosenko J, Onengut-Gumuscu S, Chen WM, Rich SS, Pugliese A. Can non-HLA single nucleotide polymorphisms help stratify risk in trialnet relatives at risk for type 1 diabetes? J Clin Endocrinol Metab. 2017;102:2873–2880. doi: 10.1210/jc.2016-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torn C, Hadley D, Lee HS, Hagopian W, Lernmark A, Simell O, Rewers M, Ziegler A, Schatz D, Akolkar B, Onengut-Gumuscu S, Chen WM, Toppari J, Mykkanen J, Ilonen J, Rich SS, She JX, Steck AK, Krischer J. Role of type 1 diabetes-associated SNPs on risk of autoantibody positivity in the TEDDY study. Diabetes. 2015;64:1818–1829. doi: 10.2337/db14-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oresic M, Gopalacharyulu P, Mykkanen J, Lietzen N, Makinen M, Nygren H, Simell S, Simell V, Hyoty H, Veijola R, Ilonen J, Sysi-Aho M, Knip M, Hyotylainen T, Simell O. Cord serum lipidome in prediction of islet autoimmunity and type 1 diabetes. Diabetes. 2013;62:3268–3274. doi: 10.2337/db13-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.La Torre D, Seppanen-Laakso T, Larsson HE, Hyotylainen T, Ivarsson SA, Lernmark A, Oresic M. Decreased cord-blood phospholipids in young age-at-onset type 1 diabetes. Diabetes. 2013;62:3951–3956. doi: 10.2337/db13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira RC, Guo H, Coulson RM, Smyth DJ, Pekalski ML, Burren OS, Cutler AJ, Doecke JD, Flint S, McKinney EF, Lyons PA, Smith KG, Achenbach P, Beyerlein A, Dunger DB, Clayton DG, Wicker LS, Todd JA, Bonifacio E, Wallace C, Ziegler AG. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes. 2014;63:2538–2550. doi: 10.2337/db13-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kallionpaa H, Elo LL, Laajala E, Mykkanen J, Ricano-Ponce I, Vaarma M, Laajala TD, Hyoty H, Ilonen J, Veijola R, Simell T, Wijmenga C, Knip M, Lahdesmaki H, Simell O, Lahesmaa R. Innate immune activity is detected prior to seroconversion in children with HLA-conferred type 1 diabetes susceptibility. Diabetes. 2014;63:2402–2414. doi: 10.2337/db13-1775. [DOI] [PubMed] [Google Scholar]
- 45.Levy H, Wang X, Kaldunski M, Jia S, Kramer J, Pavletich SJ, Reske M, Gessel T, Yassai M, Quasney MW, Dahmer MK, Gorski J, Hessner MJ. Transcriptional signatures as a disease-specific and predictive inflammatory biomarker for type 1 diabetes. Genes Immun. 2012;13:593–604. doi: 10.1038/gene.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen YG, Cabrera SM, Jia S, Kaldunski ML, Kramer J, Cheong S, Geoffrey R, Roethle MF, Woodliff JE, Greenbaum CJ, Wang X, Hessner MJ. Molecular signatures differentiate immune states in type 1 diabetic families. Diabetes. 2014;63:3960–3973. doi: 10.2337/db14-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao S, Wolanyk N, Chen Y, Jia S, Hessner MJ, Wang X. Investigation of coordination and order in transcription regulation of innate and adaptive immunity genes in type 1 diabetes. BMC Med Genomics. 2017;10:7. doi: 10.1186/s12920-017-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Toerne C, Laimighofer M, Achenbach P, Beyerlein A, de Las Heras Gala T, Krumsiek J, Theis FJ, Ziegler AG, Hauck SM. Peptide serum markers in islet autoantibody-positive children. Diabetologia. 2017;60:287–295. doi: 10.1007/s00125-016-4150-x. [DOI] [PubMed] [Google Scholar]
- 49.Dunne JL, Triplett EW, Gevers D, Xavier R, Insel R, Danska J, Atkinson MA. The intestinal microbiome in type 1 diabetes. Clin Exp Immunol. 2014;177:30–37. doi: 10.1111/cei.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Endesfelder D, zu Castell W, Ardissone A, Davis-Richardson AG, Achenbach P, Hagen M, Pflueger M, Gano KA, Fagen JR, Drew JC, Brown CT, Kolaczkowski B, Atkinson M, Schatz D, Bonifacio E, Triplett EW, Ziegler AG. Compromised gut microbiota networks in children with anti-islet cell autoimmunity. Diabetes. 2014;63:2006–2014. doi: 10.2337/db13-1676. [DOI] [PubMed] [Google Scholar]
- 51.Kostic AD, Gevers D, Siljander H, Vatanen T, Hyotylainen T, Hamalainen AM, Peet A, Tillmann V, Poho P, Mattila I, Lahdesmaki H, Franzosa EA, Vaarala O, de Goffau M, Harmsen H, Ilonen J, Virtanen SM, Clish CB, Oresic M, Huttenhower C, Knip M, Xavier RJ. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vatanen T, Kostic AD, d'Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hamalainen AM, Peet A, Tillmann V, Uibo R, Mokurov S, Dorshakova N, Ilonen J, Virtanen SM, Szabo SJ, Porter JA, Lahdesmaki H, Huttenhower C, Gevers D, Cullen TW, Knip M, Xavier RJ. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165:842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol. 2016;12:154–167. doi: 10.1038/nrendo.2015.218. [DOI] [PubMed] [Google Scholar]
- 54.Harder T, Roepke K, Diller N, Stechling Y, Dudenhausen JW, Plagemann A. Birth weight, early weight gain, and subsequent risk of type 1 diabetes: systematic review and meta-analysis. Am J Epidemiol. 2009;169:1428–1436. doi: 10.1093/aje/kwp065. [DOI] [PubMed] [Google Scholar]
- 55.Elding Larsson H, Vehik K, Haller MJ, Liu X, Akolkar B, Hagopian W, Krischer J, Lernmark A, She JX, Simell O, Toppari J, Ziegler AG, Rewers M. Growth and risk for islet autoimmunity and progression to type 1 diabetes in early childhood: the Environmental Determinants of Diabetes in the Young Study. Diabetes. 2016;65:1988–1995. doi: 10.2337/db15-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mirmira RG, Sims EK, Syed F, Evans-Molina C. Biomarkers of beta-cell stress and death in type 1 diabetes. Curr Diabetes Rep. 2016;16:95. doi: 10.1007/s11892-016-0783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark AL, Urano F. Endoplasmic reticulum stress in beta cells and autoimmune diabetes. Curr Opin Immunol. 2016;43:60–66. doi: 10.1016/j.coi.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watkins RA, Evans-Molina C, Terrell JK, Day KH, Guindon L, Restrepo IA, Mirmira RG, Blum JS, DiMeglio LA. Proinsulin and heat shock protein 90 as biomarkers of beta-cell stress in the early period after onset of type 1 diabetes. Transl Res. 2016;168:96–106. doi: 10.1016/j.trsl.2015.08.010. e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ocana GJ, Perez L, Guindon L, Deffit SN, Evans-Molina C, Thurmond DC, Blum JS. Inflammatory stress of pancreatic beta cells drives release of extracellular heat-shock protein 90alpha. Immunology. 2017;151:198–210. doi: 10.1111/imm.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez-Calvo T, Zapardiel-Gonzalo J, Amirian N, Castillo E, Lajevardi Y, Krogvold L, Dahl-Jorgensen K, von Herrath MG. Increase in pancreatic proinsulin and preservation of beta-cell mass in autoantibody-positive donors prior to type 1 diabetes onset. Diabetes. 2017;66:1334–1345. doi: 10.2337/db16-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sims EK, Chaudhry Z, Watkins R, Syed F, Blum J, Ouyang F, Perkins SM, Mirmira RG, Sosenko J, DiMeglio LA, Evans-Molina C. Elevations in the fasting serum proinsulin-to-C-peptide ratio precede the onset of type 1 diabetes. Diabetes Care. 2016;39:1519–1526. doi: 10.2337/dc15-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delong T, Wiles TA, Baker RL, Bradley B, Barbour G, Reisdorph R, Armstrong M, Powell RL, Reisdorph N, Kumar N, Elso CM, DeNicola M, Bottino R, Powers AC, Harlan DM, Kent SC, Mannering SI, Haskins K. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science. 2016;351:711–714. doi: 10.1126/science.aad2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGinty JW, Chow IT, Greenbaum C, Odegard J, Kwok WW, James EA. Recognition of posttranslationally modified GAD65 epitopes in subjects with type 1 diabetes. Diabetes. 2014;63:3033–3040. doi: 10.2337/db13-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGinty JW, Marre ML, Bajzik V, Piganelli JD, James EA. T cell epitopes and post-translationally modified epitopes in type 1 diabetes. Curr Diabetes Rep. 2015;15:90. doi: 10.1007/s11892-015-0657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Lummel M, Duinkerken G, van Veelen PA, de Ru A, Cordfunke R, Zaldumbide A, Gomez-Tourino I, Arif S, Peakman M, Drijfhout JW, Roep BO. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes. 2014;63:237–247. doi: 10.2337/db12-1214. [DOI] [PubMed] [Google Scholar]
- 66.Kracht MJ, van Lummel M, Nikolic T, Joosten AM, Laban S, van der Slik AR, van Veelen PA, Carlotti F, de Koning EJ, Hoeben RC, Zaldumbide A, Roep BO. Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat Med. 2017;23:501–507. doi: 10.1038/nm.4289. [DOI] [PubMed] [Google Scholar]
- 67.Strollo R, Vinci C, Napoli N, Pozzilli P, Ludvigsson J, Nissim A. Antibodies to post-translationally modified insulin as a novel biomarker for prediction of type 1 diabetes in children. Diabetologia. 2017;60:1467–1474. doi: 10.1007/s00125-017-4296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marrack P, Kappler JW. Do MHCII-presented neoantigens drive type 1 diabetes and other autoimmune diseases? Cold Spring Harbor Perspect Med. 2012;2:a007765. doi: 10.1101/cshperspect.a007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heninger AK, Eugster A, Kuehn D, Buettner F, Kuhn M, Lindner A, Dietz S, Jergens S, Wilhelm C, Beyerlein A, Ziegler AG, Bonifacio E. A divergent population of autoantigen-responsive CD4+ T cells in infants prior to beta cell autoimmunity. Sci Transl Med. 2017;9:eaaf8848. doi: 10.1126/scitranslmed.aaf8848. [DOI] [PubMed] [Google Scholar]
- 70.Steck AK, Vehik K, Bonifacio E, Lernmark A, Ziegler AG, Hagopian WA, She J, Simell O, Akolkar B, Krischer J, Schatz D, Rewers MJ. Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY) Diabetes Care. 2015;38:808–813. doi: 10.2337/dc14-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orban T, Sosenko JM, Cuthbertson D, Krischer JP, Skyler JS, Jackson R, Yu L, Palmer JP, Schatz D, Eisenbarth G. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009;32:2269–2274. doi: 10.2337/dc09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Achenbach P, Hummel M, Thumer L, Boerschmann H, Hofelmann D, Ziegler AG. Characteristics of rapid vs slow progression to type 1 diabetes in multiple islet autoantibody-positive children. Diabetologia. 2013;56:1615–1622. doi: 10.1007/s00125-013-2896-y. [DOI] [PubMed] [Google Scholar]
- 73.Gorus FK, Balti EV, Vermeulen I, Demeester S, Van Dalem A, Costa O, Dorchy H, Tenoutasse S, Mouraux T, De Block C, Gillard P, Decochez K, Wenzlau JM, Hutton JC, Pipeleers DG, Weets I. Screening for insulinoma antigen 2 and zinc transporter 8 autoantibodies: a cost-effective and age-independent strategy to identify rapid progressors to clinical onset among relatives of type 1 diabetic patients. Clinical Exp Immunol. 2013;171:82–90. doi: 10.1111/j.1365-2249.2012.04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gorus FK, Balti EV, Messaaoui A, Demeester S, Van Dalem A, Costa O, Dorchy H, Mathieu C, Van Gaal L, Keymeulen B, Pipeleers DG, Weets I. Twenty-year progression rate to clinical onset according to autoantibody profile, age, and HLA-DQ genotype in a registry-based group of children and adults with a first-degree relative with type 1 diabetes. Diabetes Care. 2017;40:1065–1072. doi: 10.2337/dc16-2228. [DOI] [PubMed] [Google Scholar]
- 75.Ferrara CT, Geyer SM, Liu YF, Evans-Molina C, Libman IM, Besser R, Becker DJ, Rodriguez H, Moran A, Gitelman SE, Redondo MJ. Excess BMI in childhood: a modifiable risk factor for type 1 diabetes development? Diabetes Care. 2017;40:698–701. doi: 10.2337/dc16-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tosur M, Geyer S, Rodriguez H, Libman De Gordon I, Baidal D, Redondo MJ. Ethnic differences in progression to type 1 diabetes in relatives at risk. Am Diabetes Assoc 77th Sci Sessions, San Diego. 2017 Jun; poster 285. [Google Scholar]
- 77.Bingley PJ, Gale EA. Progression to type 1 diabetes in islet cell antibody-positive relatives in the European Nicotinamide Diabetes Intervention Trial: the role of additional immune, genetic and metabolic markers of risk. Diabetologia. 2006;49:881–890. doi: 10.1007/s00125-006-0160-4. [DOI] [PubMed] [Google Scholar]
- 78.Jacobsen LM, Sosenko JM, Evans-Molina C, Dimeglio LA, Goland RS, Wilson DM, Atkinson MA, Aye T, Russell W, Wentworth JN, Geyer S, Boulware D, et al. The risk of progression to type 1 diabetes (T1D) in individuals of diverse ages with multiple autoantibodies. Am Diabetes Assoc 77th Sci Sessions, San Diego. 2017 Jun; poster 249. [Google Scholar]
- 79.Winkler C, Krumsiek J, Lempainen J, Achenbach P, Grallert H, Giannopoulou E, Bunk M, Theis FJ, Bonifacio E, Ziegler AG. A strategy for combining minor genetic susceptibility genes to improve prediction of disease in type 1 diabetes. Genes Immun. 2012;13:549–555. doi: 10.1038/gene.2012.36. [DOI] [PubMed] [Google Scholar]
- 80.Jin Y, Sharma A, Bai S, Davis C, Liu H, Hopkins D, Barriga K, Rewers M, She JX. Risk of type 1 diabetes progression in islet autoantibody-positive children can be further stratified using expression patterns of multiple genes implicated in peripheral blood lymphocyte activation and function. Diabetes. 2014;63:2506–2515. doi: 10.2337/db13-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lempainen J, Laine AP, Hammais A, Toppari J, Simell O, Veijola R, Knip M, Ilonen J. Non-HLA gene effects on the disease process of type 1 diabetes: from HLA susceptibility to overt disease. J Autoimmun. 2015;61:45–53. doi: 10.1016/j.jaut.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Krischer JP, Lynch KF, Lernmark A, Hagopian WA, Rewers MJ, She JX, Toppari J, Ziegler AG, Akolkar B. Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: the TEDDY Study. Diabetes Care. 2017;40:1194–1120. doi: 10.2337/dc17-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krischer JP, Lynch KF, Schatz DA, Ilonen J, Lernmark A, Hagopian WA, Rewers MJ, She JX, Simell OG, Toppari J, Ziegler AG, Akolkar B, Bonifacio E. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58:980–987. doi: 10.1007/s00125-015-3514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chmiel R, Giannopoulou EZ, Winkler C, Achenbach P, Ziegler AG, Bonifacio E. Progression from single to multiple islet autoantibodies often occurs soon after seroconversion: implications for early screening. Diabetologia. 2015;58:411–413. doi: 10.1007/s00125-014-3443-1. [DOI] [PubMed] [Google Scholar]
- 85.Bingley PJ, Boulware DC, Krischer JP. The implications of autoantibodies to a single islet antigen in relatives with normal glucose tolerance: development of other autoantibodies and progression to type 1 diabetes. Diabetologia. 2016;59:542–549. doi: 10.1007/s00125-015-3830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giannopoulou EZ, Winkler C, Chmiel R, Matzke C, Scholz M, Beyerlein A, Achenbach P, Bonifacio E, Ziegler AG. Islet autoantibody phenotypes and incidence in children at increased risk for type 1 diabetes. Diabetologia. 2015;58:2317–2323. doi: 10.1007/s00125-015-3672-y. [DOI] [PubMed] [Google Scholar]
- 87.Miao D, Steck AK, Zhang L, Guyer KM, Jiang L, Armstrong T, Muller SM, Krischer J, Rewers M, Yu L. Electrochemiluminescence assays for insulin and glutamic acid decarboxylase autoantibodies improve prediction of type 1 diabetes risk. Diabetes Technol Ther. 2015;17:119–127. doi: 10.1089/dia.2014.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fouts A, Pyle L, Yu L, Miao D, Michels A, Krischer J, Sosenko J, Gottlieb P, Steck AK. Do electrochemiluminescence assays improve prediction of time to type 1 diabetes in autoantibody-positive TrialNet subjects? Diabetes Care. 2016;39:1738–1744. doi: 10.2337/dc16-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sosenko JM, Yu L, Skyler JS, Krischer JP, Gottlieb PA, Boulware D, Miao D, Palmer JP, Steck AK. The use of electrochemiluminescence assays to predict autoantibody and glycemic progression toward type 1 diabetes in individuals with single autoantibodies. Diabetes Technol Ther. 2017;19:183–187. doi: 10.1089/dia.2016.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vehik K, Lynch KF, Schatz DA, Akolkar B, Hagopian W, Rewers M, She JX, Simell O, Toppari J, Ziegler AG, Lernmark A, Bonifacio E, Krischer JP. Reversion of beta-cell autoimmunity changes risk of type 1 diabetes: TEDDY Study. Diabetes Care. 2016;39:1535–1542. doi: 10.2337/dc16-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Endesfelder D, Hagen M, Winkler C, Haupt F, Zillmer S, Knopff A, Bonifacio E, Ziegler AG, Zu Castell W, Achenbach P. A novel approach for the analysis of longitudinal profiles reveals delayed progression to type 1 diabetes in a subgroup of multiple-islet-autoantibody-positive children. Diabetologia. 2016;59:2172–2180. doi: 10.1007/s00125-016-4050-0. [DOI] [PubMed] [Google Scholar]
- 92.Xu P, Cuthbertson D, Greenbaum C, Palmer JP, Krischer JP. Role of insulin resistance in predicting progression to type 1 diabetes. Diabetes Care. 2007;30:2314–2320. doi: 10.2337/dc06-2389. [DOI] [PubMed] [Google Scholar]
- 93.Bingley PJ, Mahon JL, Gale EA. Insulin resistance and progression to type 1 diabetes in the European Nicotinamide Diabetes Intervention Trial (ENDIT) Diabetes Care. 2008;31:146–150. doi: 10.2337/dc07-0103. [DOI] [PubMed] [Google Scholar]
- 94.Fourlanos S, Narendran P, Byrnes GB, Colman PG, Harrison LC. Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia. 2004;47:1661–1667. doi: 10.1007/s00125-004-1507-3. [DOI] [PubMed] [Google Scholar]
- 95.Akerman L, Ludvigsson J, Swartling U, Casas R. Characteristics of the pre-diabetic period in children with high risk of type 1 diabetes recruited from the general Swedish population - the ABIS study. Diabetes Metab Res Rev. doi: 10.1002/dmrr.2900. DOI: 10.1002/dmrr.2900. [DOI] [PubMed] [Google Scholar]
- 96.Veijola R, Koskinen M, Helminen O, Hekkala A. Dysregulation of glucose metabolism in preclinical type 1 diabetes. Pediatr Diabetes. 2016;17((suppl 22)):25–30. doi: 10.1111/pedi.12392. [DOI] [PubMed] [Google Scholar]
- 97.Sosenko JM, Palmer JP, Rafkin-Mervis L, Krischer JP, Cuthbertson D, Matheson D, Skyler JS. Glucose and C-peptide changes in the perionset period of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2008;31:2188–2192. doi: 10.2337/dc08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS. Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2006;29:643–649. doi: 10.2337/diacare.29.03.06.dc05-1006. [DOI] [PubMed] [Google Scholar]
- 99.Helminen O, Aspholm S, Pokka T, Hautakangas MR, Haatanen N, Lempainen J, Ilonen J, Simell O, Knip M, Veijola R. HbA1c predicts time to diagnosis of type 1 diabetes in children at risk. Diabetes. 2015;64:1719–1727. doi: 10.2337/db14-0497. [DOI] [PubMed] [Google Scholar]
- 100.Kovatchev BP. Metrics for glycaemic control - from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol. 2017;13:425–436. doi: 10.1038/nrendo.2017.3. [DOI] [PubMed] [Google Scholar]
- 101.Steck AK, Dong F, Taki I, Hoffman M, Klingensmith GJ, Rewers MJ. Early hyperglycemia detected by continuous glucose monitoring in children at risk for type 1 diabetes. Diabetes Care. 2014;37:2031–2033. doi: 10.2337/dc13-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van Dalem A, Demeester S, Balti EV, Decochez K, Weets I, Vandemeulebroucke E, Van de Velde U, Walgraeve A, Seret N, De Block C, Ruige J, Gillard P, Keymeulen B, Pipeleers DG, Gorus FK. Relationship between glycaemic variability and hyperglycaemic clamp-derived functional variables in (impending) type 1 diabetes. Diabetologia. 2015;58:2753–2764. doi: 10.1007/s00125-015-3761-y. [DOI] [PubMed] [Google Scholar]
- 103.Helminen O, Pokka T, Tossavainen P, Ilonen J, Knip M, Veijola R. Continuous glucose monitoring and HbA1c in the evaluation of glucose metabolism in children at high risk for type 1 diabetes mellitus. Diabetes Res Clin Pract. 2016;120:89–96. doi: 10.1016/j.diabres.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 104.Xu P, Krischer JP. Prognostic classification factors associated with development of multiple autoantibodies, dysglycemia, and type 1 diabetes - a recursive partitioning analysis. Diabetes Care. 2016;39:1036–1044. doi: 10.2337/dc15-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol. 2007;7:988–994. doi: 10.1038/nri2192. [DOI] [PubMed] [Google Scholar]
- 106.Cerosaletti K, Barahmand-Pour-Whitman F, Yang J, DeBerg HA, Dufort MJ, Murray SA, Israelsson E, Speake C, Gersuk VH, Eddy JA, Reijonen H, Greenbaum CJ, Kwok WW, Wambre E, Prlic M, Gottardo R, Nepom GT, Linsley PS. Single-cell RNA sequencing reveals expanded clones of islet antigen-reactive CD4+ T cells in peripheral blood of subjects with type 1 diabetes. J Immunol. 2017;199:323–335. doi: 10.4049/jimmunol.1700172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Viisanen T, Ihantola EL, Nanto-Salonen K, Hyoty H, Nurminen N, Selvenius J, Juutilainen A, Moilanen L, Pihlajamaki J, Veijola R, Toppari J, Knip M, Ilonen J, Kinnunen T. Circulating CXCR5+PD-1+ICOS+ follicular T helper cells are increased close to the diagnosis of type 1 diabetes in children with multiple autoantibodies. Diabetes. 2017;66:437–447. doi: 10.2337/db16-0714. [DOI] [PubMed] [Google Scholar]
- 108.McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523:612–616. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Long SA, Thorpe J, DeBerg HA, Gersuk V, Eddy J, Harris KM, Ehlers M, Herold KC, Nepom GT, Linsley PS. Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes. Sci Immunol. 2016;1:eaai7793. doi: 10.1126/sciimmunol.aai7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pugliese A. Insulitis in the pathogenesis of type 1 diabetes. Pediatr Diabetes. 2016;17((suppl 22)):31–36. doi: 10.1111/pedi.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A, Atkinson MA. Insulitis and beta-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65:719–731. doi: 10.2337/db15-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leete P, Willcox A, Krogvold L, Dahl-Jorgensen K, Foulis AK, Richardson SJ, Morgan NG. Differential insulitic profiles determine the extent of beta-cell destruction and the age at onset of type 1 diabetes. Diabetes. 2016;65:1362–1369. doi: 10.2337/db15-1615. [DOI] [PubMed] [Google Scholar]
- 113.Gaglia JL, Harisinghani M, Aganj I, Wojtkiewicz GR, Hedgire S, Benoist C, Mathis D, Weissleder R. Noninvasive mapping of pancreatic inflammation in recent-onset type-1 diabetes patients. Proc Natl Acad Sci USA. 2015;112:2139–2144. doi: 10.1073/pnas.1424993112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Signore A, Capriotti G, Chianelli M, Bonanno E, Galli F, Catalano C, Quintero AM, De Toma G, Manfrini S, Pozzilli P. Detection of insulitis by pancreatic scintigraphy with 99mTc-labeled IL-2 and MRI in patients with LADA (Action LADA 10) Diabetes Care. 2015;38:652–658. doi: 10.2337/dc14-0580. [DOI] [PubMed] [Google Scholar]
- 115.Herold KC, Usmani-Brown S, Ghazi T, Lebastchi J, Beam CA, Bellin MD, Ledizet M, Sosenko JM, Krischer JP, Palmer JP. Beta cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest. 2015;125:1163–1173. doi: 10.1172/JCI78142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Akirav EM, Lebastchi J, Galvan EM, Henegariu O, Akirav M, Ablamunits V, Lizardi PM, Herold KC. Detection of beta cell death in diabetes using differentially methylated circulating DNA. Proc Natl Acad Sci USA. 2011;108:19018–19023. doi: 10.1073/pnas.1111008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Olsen JA, Kenna LA, Spelios MG, Hessner MJ, Akirav EM. Circulating differentially methylated amylin DNA as a biomarker of beta-cell loss in type 1 diabetes. PLoS One. 2016;11:e0152662. doi: 10.1371/journal.pone.0152662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lehmann-Werman R, Neiman D, Zemmour H, Moss J, Magenheim J, Vaknin-Dembinsky A, Rubertsson S, Nellgard B, Blennow K, Zetterberg H, Spalding K, Haller MJ, Wasserfall CH, Schatz DA, Greenbaum CJ, Dorrell C, Grompe M, Zick A, Hubert A, Maoz M, Fendrich V, Bartsch DK, Golan T, Ben Sasson SA, Zamir G, Razin A, Cedar H, Shapiro AM, Glaser B, Shemer R, Dor Y. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc Natl Acad Sci USA. 2016;113:E1826–E1834. doi: 10.1073/pnas.1519286113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long-term glycemic control. Diabetes Care. 2017;40:1249–1255. doi: 10.2337/dc17-0558. [DOI] [PubMed] [Google Scholar]
- 120.DiMeglio LA, Cheng P, Beck RW, Kollman C, Ruedy KJ, Slover R, Aye T, Weinzimer SA, Bremer AA, Buckingham B. Changes in beta cell function during the proximate post-diagnosis period in persons with type 1 diabetes. Pediatr Diabetes. 2016;17:237–243. doi: 10.1111/pedi.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Greenbaum CJ, Beam CA, Boulware D, Gitelman SE, Gottlieb PA, Herold KC, Lachin JM, McGee P, Palmer JP, Pescovitz MD, Krause-Steinrauf H, Skyler JS, Sosenko JM. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes. 2012;61:2066–2073. doi: 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Davis AK, DuBose SN, Haller MJ, Miller KM, DiMeglio LA, Bethin KE, Goland RS, Greenberg EM, Liljenquist DR, Ahmann AJ, Marcovina SM, Peters AL, Beck RW, Greenbaum CJ. Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care. 2015;38:476–481. doi: 10.2337/dc14-1952. [DOI] [PubMed] [Google Scholar]
- 123.Williams GM, Long AE, Wilson IV, Aitken RJ, Wyatt RC, McDonald TJ, Wong FS, Hattersley AT, Williams AJ, Bingley PJ, Gillespie KM. Beta cell function and ongoing autoimmunity in long-standing, childhood onset type 1 diabetes. Diabetologia. 2016;59:2722–2726. doi: 10.1007/s00125-016-4087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nagl K, Hermann JM, Plamper M, Schroder C, Dost A, Kordonouri O, Rami-Merhar B, Holl RW. Factors contributing to partial remission in type 1 diabetes: analysis based on the insulin dose-adjusted HbA1c in 3,657 children and adolescents from Germany and Austria. Pediatr Diabetes. 2017;18:428–434. doi: 10.1111/pedi.12413. [DOI] [PubMed] [Google Scholar]
- 125.Marino KR, Lundberg RL, Jasrotia A, Maranda LS, Thompson MJ, Barton BA, Alonso LC, Nwosu BU. A predictive model for lack of partial clinical remission in new-onset pediatric type 1 diabetes. PLoS One. 2017;12:e0176860. doi: 10.1371/journal.pone.0176860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brorsson CA, Nielsen LB, Andersen ML, Kaur S, Bergholdt R, Hansen L, Mortensen HB, Pociot F, Storling J. Genetic risk score modelling for disease progression in new-onset type 1 diabetes patients: increased genetic load of islet-expressed and cytokine-regulated candidate genes predicts poorer glycemic control. J Diabetes Res. 2016;2016:9570424. doi: 10.1155/2016/9570424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Floyel T, Brorsson C, Nielsen LB, Miani M, Bang-Berthelsen CH, Friedrichsen M, Overgaard AJ, Berchtold LA, Wiberg A, Poulsen P, Hansen L, Rosinger S, Boehm BO, Ram R, Nguyen Q, Mehta M, Morahan G, Concannon P, Bergholdt R, Nielsen JH, Reinheckel T, von Herrath M, Vaag A, Eizirik DL, Mortensen HB, Storling J, Pociot F. CTSH regulates beta-cell function and disease progression in newly diagnosed type 1 diabetes patients. Proc Natl Acad Sci USA. 2014;111:10305–10310. doi: 10.1073/pnas.1402571111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, Maahs DM, Tamborlane WV. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38:971–978. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 129.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA. The T1D Exchange clinic registry. J Clin Endocrinol Metab. 2012;97:4383–4389. doi: 10.1210/jc.2012-1561. [DOI] [PubMed] [Google Scholar]
- 130.Bode BW, Garg SK. The emerging role of adjunctive noninsulin antihyperglycemic therapy in the management of type 1 diabetes. Endocr Pract. 2016;22:220–230. doi: 10.4158/EP15869.RA. [DOI] [PubMed] [Google Scholar]
- 131.Comee M, Peters A. The changing therapeutic armamentarium for patients with type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2016;23:106–110. doi: 10.1097/MED.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 132.Bacha F, Klinepeter Bartz S. Insulin resistance, role of metformin and other non-insulin therapies in pediatric type 1 diabetes. Pediatr Diabetes. 2016;17:545–558. doi: 10.1111/pedi.12337. [DOI] [PubMed] [Google Scholar]
- 133.Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 134.Schnell O, Barnard K, Bergenstal R, Bosi E, Garg S, Guerci B, Haak T, Hirsch IB, Ji L, Joshi SR, Kamp M, Laffel L, Chantal M, Polonsky WH, Snoek F, Home P. Role of continuous glucose monitoring in clinical trials: recommendations on reporting. Diabetes Technol Ther. 2017;19:391–399. doi: 10.1089/dia.2017.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Libman IM, Miller KM, DiMeglio LA, Bethin KE, Katz ML, Shah A, Simmons JH, Haller MJ, Raman S, Tamborlane WV, Coffey JK, Saenz AM, Beck RW, Nadeau KJ. Effect of metformin added to insulin on glycemic control among overweight/obese adolescents with type 1 diabetes: a randomized clinical trial. JAMA. 2015;314:2241–2250. doi: 10.1001/jama.2015.16174. [DOI] [PubMed] [Google Scholar]
- 136.Mathieu C, Zinman B, Hemmingsson JU, Woo V, Colman P, Christiansen E, Linder M, Bode B. Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: The ADJUNCT ONE Treat-To-Target Randomized Trial. Diabetes Care. 2016;39:1702–1710. doi: 10.2337/dc16-0691. [DOI] [PubMed] [Google Scholar]
- 137.Ahren B, Hirsch IB, Pieber TR, Mathieu C, Gomez-Peralta F, Hansen TK, Philotheou A, Birch S, Christiansen E, Jensen TJ, Buse JB. Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO Randomized Trial. Diabetes Care. 2016;39:1693–1701. doi: 10.2337/dc16-0690. [DOI] [PubMed] [Google Scholar]
- 138.Sands AT, Zambrowicz BP, Rosenstock J, Lapuerta P, Bode BW, Garg SK, Buse JB, Banks P, Heptulla R, Rendell M, Cefalu WT, Strumph P. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care. 2015;38:1181–1188. doi: 10.2337/dc14-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ahmed-Sarwar N, Nagel AK, Leistman S, Heacock K. SGLT-2 inhibitors: is there a role in type 1 diabetes mellitus management? Ann Pharmacother. 2017;51:791–796. doi: 10.1177/1060028017710481. [DOI] [PubMed] [Google Scholar]
- 140.Fineberg D, Jandeleit-Dahm KA, Cooper ME. Diabetic nephropathy: diagnosis and treatment. Nat Rev Endocrinol. 2013;9:713–723. doi: 10.1038/nrendo.2013.184. [DOI] [PubMed] [Google Scholar]
- 141.Yamanouchi M, Skupien J, Niewczas MA, Smiles AM, Doria A, Stanton RC, Galecki AT, Duffin KL, Pullen N, Breyer MD, Bonventre JV, Warram JH, Krolewski AS. Improved clinical trial enrollment criterion to identify patients with diabetes at risk of end-stage renal disease. Kidney Int. 2017;92:258–266. doi: 10.1016/j.kint.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Krolewski AS, Skupien J, Rossing P, Warram JH. Fast renal decline to end-stage renal disease: an unrecognized feature of nephropathy in diabetes. Kidney Int. 2017;91:1300–1311. doi: 10.1016/j.kint.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]