Abstract
Objective
Hypercholesterolaemia is a well-established risk factor for blood vessel damage, which can lead to cardiovascular diseases. An abundance of clinical data show that dipeptidyl peptidase-4 inhibitors protect against aortic damage in patients with diabetes. The goal of this study was to investigate the possible protective effects of teneligliptin against aortic damage in apolipoprotein E knockout (ApoE-/-) mice.
Methods
Eight-week-old male ApoE-/- mice were randomly divided into 3 groups: a control group fed a normal diet, a high-cholesterol diet (HD group), and an HD diet mixed with teneligliptin (HD + Tene group), and all the groups were fed with the different treatments for 6 weeks.
Results and Conclusion
The metabolic characteristics of total cholesterol, low-density lipoprotein-cholesterol, and high-sensitivity C-reactive protein were lower in ApoE-/- HD + Tene mice than in ApoE-/- HD mice. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) gene and protein expression were lower in the aortic tissue of ApoE-/- HD + Tene mice than in ApoE-/- HD mice. IL-6 and TNF-α gene expression were lower in ApoE-/- HD + Tene mice than in ApoE-/- HD mice. These results indicate that teneligliptin may provide a potential therapeutic target for the aortic damage from hypercholesterolaemia.
Keywords: Hypercholesterolaemia, Aortic damage, Dipeptidyl peptidase-4, ApoE−/– mice
What Is It about?
In this study, we evaluated whether the dipeptidyl peptidase-4 inhibitor teneligliptin reduced kidney damage in hypercholesterolaemia. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) induced aortic damage. Teneligliptin downregulates LOX-1 gene and protein expression via Pho-ERK signal transduction in aortic tissue. Teneligliptin reduces aortic damage according to decreased lipid deposition and inflammation. Usually, the dipeptidyl peptidase-4 inhibitor is used in the management of type 2 diabetes mellitus, and our study found that teneligliptin reduced aortic damage in hypercholesterolaemia.
Introduction
ApoE-/- mice are considered a well-accepted model of hypercholesterolaemia [1]. Hypercholesterolaemia leads to the development of cardiovascular disease, which involves disorders of the heart and blood vessels, and causes various fatal events [2, 3].
In ApoE-/- mice, hypercholesterolaemia accelerates lipid deposition, atherosclerosis, and chronic inflammation [4, 5]. However, the underlying pathophysiological mechanisms of the relationship between hypercholesterolaemia and aortic injury are not yet fully understood. Dipeptidyl peptidase-4 (DPP-4), also known as lymphocyte cell surface marker CD26, exists as a smaller soluble form in blood plasma. DPP-4 is widely expressed on T and B cells, subsets of macrophages, haematopoietic stem cells, and haematopoietic progenitor cells, as well as on epithelial, endothelial, and acinar cells of a variety of tissues [6, 7]. The complex biological roles of DPP-4 include cell membrane-associated activation of intracellular signal transduction pathways, cell-to-cell interaction, and enzymatic activity [8]. Inhibition of the DPP-4 system is a new approach to the management of type 2 diabetes by virtue of its effects on extending the half-life of glucose-dependent insulinotropic peptide (GLP-1) and glucagon-like peptide-1 [9].
DPP-4 inhibitors have been demonstrated to play a protective role in cardiovascular diseases, including hypertension, atherosclerosis, and peripheral vascular disease through both GLP-1-dependent and -independent effects [10, 11, 12]. Meta-analyses suggest a potentially beneficial effect of DPP-4 inhibitors on cholesterol, which could contribute to a reduction in cardiovascular risk [13, 14]. Much clinical data suggests that teneligliptin is associated with improvements in left ventricular function, particularly diastolic and endothelial functions [15]. However, the function of teneligliptin in hypercholesterolaemia-induced aortic injury is not clear.
Materials and Methods
All animal studies were approved by the Animal Studies Committee of the First Affiliated Hospital of Dalian Medical University. ApoE-/- (B6.129P2-Apoetm1Unc/J) mice were purchased from Shanghai Slac Laboratory Animal Co., Ltd. (Shanghai, China). All mice were housed in a room with a 12:12-h light-dark cycle, with room temperature maintained at 24°C. At 8 weeks of age, the ApoE-/- male mice were randomly divided into 3 groups and fed with either a normal diet (n = 7), a high-cholesterol diet (n = 7), or teneligliptin (20 mg/kg/day; Mitsubishi Tanabe Pharma, Osaka, Japan) plus a high-cholesterol diet (n = 7). The high-cholesterol diet contained 1.5% cholesterol and 15% fat. The experimental diet was purchased from the Shanghai Slac Laboratory Animal Co., Ltd. Each group was fed their diet for 6 weeks. Blood samples were obtained from the inferior vena cava, collected in serum tubes, and stored at −80°C until used. Coronal sections of the aorta were fixed in 10% formalin and then embedded in paraffin for histological evaluation. The remainder of the aorta was snap-frozen in liquid nitrogen for mRNA or immunohistochemical analysis. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals. The study was approved by the ethical committee of the First Affiliated Hospital of Dalian Medical University.
Biochemical Measurements
Total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), and high-sensitivity C-reactive protein (hs-CRP) were measured using an automatic analyser (Dimension, Wilmington, DE, USA).
Morphologic Analysis and Immunohistochemistry
The aorta was dissected free from the surrounding connective tissue. Aorta samples were collected and fixed in 4% paraformaldehyde. Samples were embedded in paraffin and then were cut into slices using a microtome (Leica RM 2235 or Leica CM1850UV; Leica, Solms, Germany). Slices were then mounted onto glass slides and histological examinations were performed.
Immunohistochemistry was performed using the Histone Simple Stain Kit (Nichirei, Tokyo, Japan) according to the manufacturer's instructions. Briefly, paraffin-embedded sections were deparaffinized with xylene and then rehydrated in a descending series of ethanol washes. The sections were treated for 15 min with 3% H2O2 in methanol to inactivate endogenous peroxidases and then incubated at room temperature for 1 h with primary antibodies to collagen IV (rabbit anti-collagen IV antibody, 1:500; Abcam, England) and LOX-1 (rabbit anti-LOX-1 antibody, 1:250; Abcam). All sections were observed under an Olympus B ×40 upright light microscope (Olympus, Tokyo, Japan).
RNA Isolation and Real-Time RT-PCR
Total RNA was isolated from aorta using ISOGEN (Nippon Gene, Tokyo, Japan) according to the manufacturer's protocol. Complementary DNA (cDNA) was synthesized from total RNA using a first-strand cDNA synthesis kit (SuperScript VILO cDNA Synthesis Kit; Life Technologies, Carlsbad, CA, USA) according to the manufacturer's protocol. Gene expression was analysed quantitatively by real-time RT-PCR using fluorescent SYBR Green technology (Light Cycler; Roche Molecular Biochemicals). β-Actin cDNA was amplified and quantitated in each cDNA preparation in order to normalize the relative amounts of the target genes. Primer sequences are listed in Table 1.
Table 1.
Primer oligonucleotide sequences
| Gene | Primers |
|---|---|
| LOX-1 | F: 5′-CAAAGTCTCCCAACCAACCTGCAA-3′ R: 5′-ACATCCTGTCTTTCATGCGGCAAC-3′ |
| β-Actin | F: 5′-CGATGCCCTGAGGGTCTTT-3′ R: 5′-TGGATGCCACAGGATTCCAT-3′ |
| SR-A1 | F: 5′-GTTAAAGGTGATGGGGGACA-3′ R: 5′-TCCCCTTCTCTCCCTTTTGT-3′ |
| CD36 | F: 5′-CCTTAAAGGAATCCCCGTGT-3′ R: 5′-TGCATTTGCCAATGTCTAGC-3′ |
| ABCA1 | F: 5′-AGCCAGAAGGGAGTGTCAGA-3′ R: 5′-CATGCCATCTGGGTAAACCT-3′ |
| TNF-α | F: 5′-TCTCATGCACCACCATCAAGGACT-3′ R: 5′-ACCACTCTCCCTTTGCAGAACTCA-3′ |
| IL-6 | F: 5′-TACCAGTTGCCTTCTTGGGACTGA-3′ R: 5′-TAAGCCTCCGACTTGTGAAGTGGT-3′ |
LOX-1, lectin-like oxidized low-density lipoprotein receptor-1; SR-A, scavenger receptor-A; ABCA1, ATP-binding cassette transporter A1.
Western Blotting for Aortic Tissue
Proteins were extracted from renal cortical tissues using radio immunoprecipitation assay buffer (P0013B; Beyotime, Shanghai, China). Samples were electrophoresed on 10% SDS-PAGE gel, and proteins were transferred to polyvinylidene fluoride membrane (Immobilon, Millipore, Billerica, MA, USA). Membranes were blocked in Tris-buffered saline with 0.1% Tween-20 containing 5% skim milk, and then were incubated in primary antibody diluent (P0023A; Beyotime) and gently shaken overnight at 4°C. Primary antibodies against LOX-1 (rabbit anti-LOX-1 polyclonal antibody;1:250; Abcam), phosphor-Erk (rabbit anti-phosphor-Erk, polyclonal antibody 1:1,000; Cell Signaling Technology), and anti-β-actin antibody (1:1,000; Cell Signaling Technology) were used. Membranes were then incubated with secondary antibody (anti-rabbit Ig-G, 1:1,000; Cell Signaling Technology) for 1 h. This analysis was carried out independently 3 times. Protein levels are expressed as protein/β-actin ratios to minimize loading differences. The relative signal intensity was quantified using NIH ImageJ software.
Statistical Analysis
All data are presented as means ± SEM. Statistical analysis was performed using SPSS software version 23.0 (SPSS Inc., Chicago, IL, USA). Intergroup variation was measured by 1-way ANOVA and a subsequent Tukey test. The minimal level for significance was p < 0.05.
Results
Metabolic Characteristics
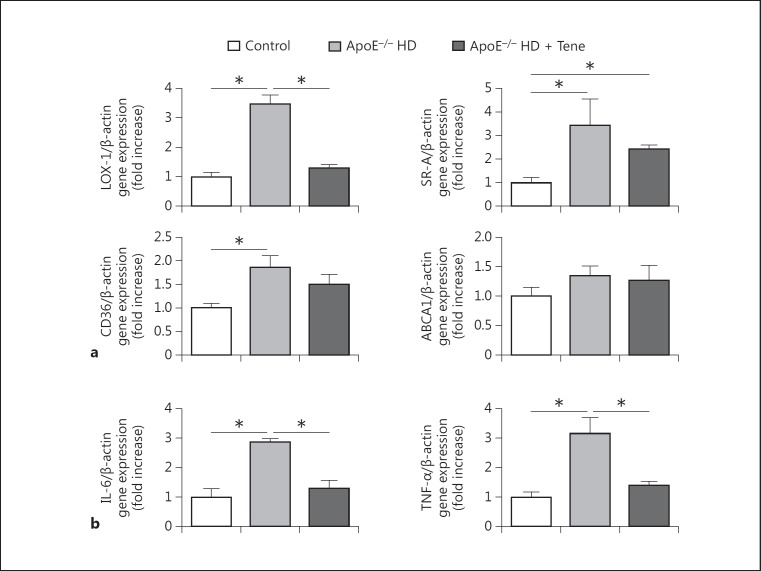
The metabolic characteristics of ApoE-/- mice after 6 weeks of dietary treatment are summarized in Table 2. In the ApoE-/- mice, TC and LDL were markedly increased in the HD group, but were significantly decreased in the HD + Tene group. There was no difference between the HD + Tene group and the normal diet group. Body weight did not differ among the 3 groups. Compared with the HD group, hs-CRP was significantly decreased in the HD + Tene group. Teneligliptin reduced LOX-1 gene expression in the aortic tissue of ApoE-/- mice with HD.
Table 2.
Metabolic data from the 4 groups after 6 weeks of dietary treatment
| ApoE−/– ND (n = 7) | ApoE−/– HD (n = 6) | ApoE−/– HD + Tene (n = 7) | |
|---|---|---|---|
| Body weight,g | 24.67±0.71 | 23.92±0.83 | 24.72±0.79 |
| TC, mg/dL | 572.65±101.35* | 2,302.73±326.62 | 650.52±62.28* |
| LDL-C, mg/dL | 146.27±42.75* | 663.52±92.13 | 174±23.89* |
| hs-CRP, ng/dL | 107.63±28.53** | 198.62±39.72 | 120.61±23.65** |
Data are expressed as means±SEM;n = 6–7 per group. TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein.
p < 0.01 vs. ApoE−/– HD;
p < 0.05 vs. ApoE−/– HD.
To investigate the mechanism of lipid accumulation in the aorta, aortic tissue gene expression of relevant receptors and the TP-binding cassette transporter A1 (ABCA1) were examined by RT-PCR. Compared with the normal diet group mice, LOX-1 gene expression was significantly increased in the aortic tissue of the ApoE-/- HD group mice. The increased expression of LOX-1 was suppressed in the ApoE-/- HD + Tene group. Compared with the normal diet group, expression of scavenger receptor-class A (SR-A) and CD36 were increased in the ApoE-/- HD mice; however, levels were similar to those of ApoE-/- HD + Tene mice. Expression of ABCA1 did not differ among the 3 groups (Fig. 1a). These results suggest that LOX-1, SR-A, and CD36 influence lipid accumulation in the aortic tissue of ApoE-/- HD mice. Compared to ApoE-/- HD mice, LOX-1, in particular, appears to be a critical factor for mitigation of lipid accumulation in the aortic tissue of ApoE-/- HD + Tene mice.
Fig. 1.
Scavenger receptors and proinflammatory gene expression in the aortic tissue of the 3 groups after 6 weeks of different treatments. a Relative mRNA expression of LOX-1, SRA, CD36, and ABCA1 in the aortic tissue of each group after 6 weeks of different treatment. b Relative mRNA expression of IL-6 and TNF-α in the aortic tissue of each groups after 6 weeks of dietary treatment. Data are expressed as the means ± SEM; n = 6–7 in each group. * p < 0.05.
Teneligliptin reduced TNF-α and IL-6 gene expression in the aortic tissue of ApoE-/- mice fed HD. To examine the involvement of proinflammatory cytokines in hypercholesterolaemic aortic damage, IL-6 and TNF-α gene expression were measured by real-time PCR. Both IL-6 and TNF-α were upregulated in ApoE-/- HD mice; however, this upregulation was attenuated in ApoE-/- HD + Tene mice (Fig. 1b).
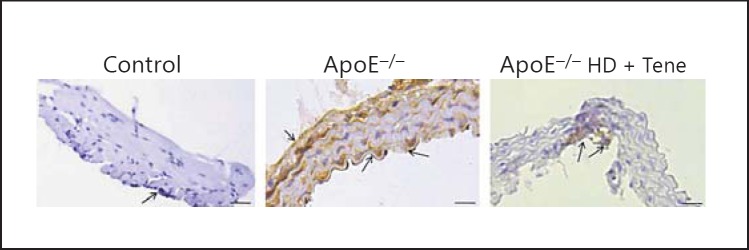
Teneligliptin reduced LOX-1 expression in aortic tissue assessed using immunohistochemistry. To evaluate LOX-1 expression in the aortic tissue, LOX-1 immunostaining was performed (Fig. 2). Compared with ApoE-/- HD mice, HD + Tene mice showed markedly reduced LOX-1 expression in aortic tissue. This result indicates that teneligliptin reduced LOX-1 expression in ApoE-/- HD mice.
Fig. 2.
LOX-1 expression in the aortic tissue of the 3 groups after 6 weeks of different treatments. Representative immunohistochemistry for LOX-1 in aortic tissue. Scale bar = 200 μm. Arrows indicate positive staining cells; n = 4 in each group.
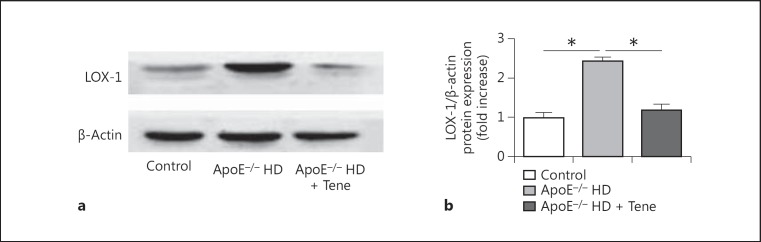
Teneligliptin reduced LOX-1 protein expression in the aortic tissue of the ApoE-/- HD mice. To evaluate LOX-1 protein expression in the aortic tissue, LOX-1 protein immunoblotting was performed (Fig. 3a). We found that compared with the ApoE-/- HD group, LOX-1 protein was significantly suppressed in the ApoE-/- HD + Tene group (Fig. 3b).
Fig. 3.
LOX-1 protein expression in the aortic tissue of the 3 groups after 6 weeks of different treatments. a Immunoblotting for LOX-1 protein expression in aortic tissue. b Bar graph shows quantification of LOX-1 protein expression. Data are expressed as means ± SEM; n = 3 in each group; * p < 0.05 vs. ApoE-/- HD.
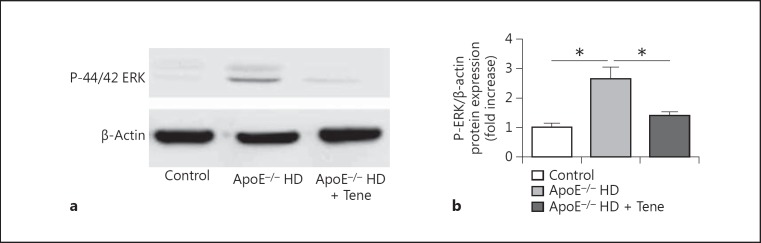
Teneligliptin reduced phosphor-ERK expression in the aortic tissue of ApoE-/- HD mice. Protein kinases play a role in foam cell formation and lipid deposition, thus phosphor-ERK protein immunoblotting was performed (Fig. 4a). We found that compared with the ApoE-/- HD group, phosphor-ERK was significantly suppressed in the ApoE-/- HD + Tene group (Fig. 4b).
Fig. 4.
Phosphor-ERK protein expression in the aortic tissue of the 3 groups after 6 weeks of different treatments. a Immunoblotting for phosphor-ERK protein expression in the aortic tissue. b Bar graph shows quantification of phosphor-ERK protein expression. Data are expressed as means ± SEM; n = 3 in each group; * p < 0.05 vs. ApoE-/- HD.
Discussion
This study demonstrates that teneligliptin has a protective effect against the progressive lipid deposition and atherosclerosis elicited by hypercholesterolaemia.
According to metabolic characteristics, we found that compared with ApoE-/- HD + Tene mice, TC and LDL-c were increased in ApoE-/- HD mice. The major risk factors for atherosclerosis are increased blood levels of TC and LDL [16]. Jialal and Devaraj [17] reported that suppression of blood TC and LDL contributed to the prevention of atherosclerosis. Several clinical studies have indicated that the hs-CRP level reflects the instability of atherosclerotic lesions and can be used as a biomarker for risk stratification of cardiovascular events [18, 19, 20, 21]. Our results indicate that teneligliptin influences cholesterol metabolism and hs-CRP; however, further studies are needed to clarify the mechanisms.
Hypercholesterolaemia is a major independent risk factor for aortic damage and hyperlipidaemia promotes aortic lipid deposition and inflammation [22]. Cellular lipid homeostasis involves regulation of the influx, synthesis, catabolism, and efflux of lipids. An imbalance in these processes can result in conversion of macrophages and vascular smooth muscle cells into foam cells. This process is mediated by several independent pathways, including SR-A, class B (CD36), and LOX-1, and regulates expression of its target gene ABCA1 [23, 24, 25]. Proinflammatory genes (TNF-α and IL-6) were reported to be expressed at high levels and to contribute to cardiovascular disease in hyperlipidaemia [26, 27]. DPP-4 is a ubiquitous, type II cell surface glycoprotein and is widely expressed in all tissues [28]. Treatment with DPP-4 inhibitors, which increase GLP-1 levels, has been shown to exert numerous renoprotective effects. These effects include a reduction in blood glucose and lipid levels, inhibition of inflammation, and oxidative stress [29]. In the present study, we analysed gene expression of the LDL receptor and scavenger receptors which includes SR-A, CD36, and LOX-1. We found that LOX-1 gene expression was suppressed in the ApoE-/- HD + Tene group. LOX-1 was originally identified in endothelial cells and is a 50-kDa type II membrane glycoprotein that contains a short N-terminal cytoplasmic domain, a single transmembrane domain, a short neck or stalk region, and an ox-LDL-binding C-terminal extracellular C-type lectin-like domain. On the cell surface, LOX-1 is comprised of 3 homodimers bound to ox-LDL, and plays a leading role in ox-LDL uptake and foam cell formation [30, 31]. In contrast, deletion of LOX-1 reduced uptake of oxidized LDL and inhibited atherosclerosis in high-cholesterol diet-fed mice [32]. Thus, suppression of LOX-1 expression in ApoE-/- HD + Tene mice may reduce lipid deposition. Teneligliptin also reduced LOX-1 protein expression in the aortic tissue of ApoE-/- HD mice. Compared with ApoE-/- HD + Tene mice, proinflammatory gene (TNF-α and IL-6) expression was also reduced in ApoE-/- HD+ Tene mice. It was previously reported that DPP-4 inhibitors significantly suppressed atherosclerotic lesions in the aortic wall of ApoE-/- mice, a representative animal model of atherosclerosis [33, 34].
Our results showed that teneligliptin reduced LOX-1 protein expression in the aortic tissue of ApoE-/- mice. LOX-1 expression was attenuated by inhibitors of PKC and ERK, indicating that increased production of intracellular ROS and activation of the PKC/MAPK pathways are initial signaling events in the regulation of LOX-1 gene [35]. Hu et al. [36] reported that compared with normal vessel tissue or the aortic media of cholesterol-fed rabbits, there was a marked increase in the amount of ERK1/2 proteins from atherosclerotic lesions. In the present study, we found that Pho-ERK expression was attenuated in ApoE-/- HD + Tene mice, and it is possible that teneligliptin regulates LOX-1 by the phosphor-ERK pathway.
In conclusion, our data establish that teneligliptin contributes to the mitigation of hypercholesterolaemic aortic damage, as shown by downregulation of LOX-1, and suppression of lipid deposition and inflammation. These findings provide new insights into the role of teneligliptin in hypercholesterolaemic aortic injury and raise the possibility of a novel therapeutic intervention for the progression of cardiovascular disease.
Statement of Ethics
We obtained ethical review board approval for this study (KY2017-17).
Disclosure Statement
This study was supported by the Natural Science Foundation of Liaoning Province (No. 2013B020). The authors declare that they have no competing interests.
Acknowledgement
This work was supported by the Natural Science Foundation of Liaoning Province (No. 2013B020). Hongyang Liu designed this study, Ying Zhang helped in performing experiments, Ying Zhang, Lin Luo and Yingshu Liu analyzed data and interpreted the results of experiments, Nan Wang and Liyue Zhu prepared the figures, Hongyang Liu drafted the manuscript, and Guan Wang and Zuowei Pei helped in revising of manuscript. All authors read and approved the final manuscript.
References
- 1.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wijeysundera DN, Duncan D, Nkonde-Price C, Virani SS, Washam JB, Fleischmann KE, Fleisher LA. American College of Cardiology American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;22:2406–2425. doi: 10.1016/j.jacc.2014.07.939. [DOI] [PubMed] [Google Scholar]
- 3.Arsenault BJ, Kritikou EA, Tardif JC. Regression of atherosclerosis. Curr Cardiol Rep. 2012;4:443–449. doi: 10.1007/s11886-012-0285-7. [DOI] [PubMed] [Google Scholar]
- 4.Karshovska E, Zhao Z, Blanchet X, Schmitt MM, Bidzhekov K, Soehnlein O, von Hundelshausen P, Mattheij NJ, Cosemans JM, Megens RT, Koeppel TA, Schober A, Hackeng TM, Weber C, Koenen RR. Hyperreactivity of junctional adhesion molecule A-deficient platelets accelerates atherosclerosis in hyperlipidemic mice. Circ Res. 2015;116:587–599. doi: 10.1161/CIRCRESAHA.116.304035. [DOI] [PubMed] [Google Scholar]
- 5.Pei Z, Okura T, Nagao T, Enomoto D, Kukida M, Tanino A, Miyoshi K, Kurata M, Higaki J. Osteopontin deficiency reduces kidney damage from hypercholesterolemia in Apolipoprotein E-deficient mice. Sci Rep. 2016;6:28882. doi: 10.1038/srep28882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mentlein R. Dipeptidyl-peptidase IV (CD26) - role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 7.Gorrell MD, Gysbers V, McCaughan GW. CD26: A multifunctional integral membrane and secreted protein of activated lymphocytes. Scand J Immunol. 2001;54:249–264. doi: 10.1046/j.1365-3083.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- 8.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Mannucci E, Rotella CM. Future perspectives on glucagon-like peptide-1, diabetes and cardiovascular risk. Nutr Metab Cardiovasc Dis. 2008;18:639–645. doi: 10.1016/j.numecd.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira L, Teixeira-de-Lemos E, Pinto F, Parada B, Mega C, Vala H, Pinto R, Garrido P, Sereno J, Fernandes R, Santos P, Velada I, Melo A, Nunes S, Teixeira F, Reis F. Effects of sitagliptin treatment on dysmetabolism, inflammation, and oxidative stress in an animal model of type 2 diabetes (ZDF rat) Mediators Inflamm. 2010;2010:592760. doi: 10.1155/2010/592760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z, Xu X, Lu B, Moffatt-Bruce S, Durairaj R, Sun Q, Mihai G, Maiseyeu A, Rajagopalan S. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–2349. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang CY, Shih CM, Tsao NW, Lin YW, Huang PH, Wu SC, Lee AW, Kao YT, Chang NC, Nakagami H, Morishita R, Ou KL, Hou WC, Lin CY, Shyu KG, Lin FY. Dipeptidyl peptidase-4 inhibitor improves neovascularization by increasing circulating endothelial progenitor cells. Br J Pharmacol. 2012;167:1506–1519. doi: 10.1111/j.1476-5381.2012.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 14.Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther. 2012;29:14–25. doi: 10.1007/s12325-011-0088-z. [DOI] [PubMed] [Google Scholar]
- 15.Hashikata T, Yamaoka-Tojo M, Kakizaki R, Nemoto T, Fujiyoshi K, Namba S, Kitasato L, Hashimoto T, Kameda R, Maekawa E, Shimohama T, Tojo T, Ako J. Teneligliptin improves left ventricular diastolic function and endothelial function in patients with diabetes. Heart Vessels. 2016;31:1303–1310. doi: 10.1007/s00380-015-0724-7. [DOI] [PubMed] [Google Scholar]
- 16.Spady DK, Woollett LA, Dietschy JM. Dietschy, Regulation of plasma LDL-cholesterol levels by dietary cholesterol and fatty acids. Annu Rev Nutr. 1993;13:355–381. doi: 10.1146/annurev.nu.13.070193.002035. [DOI] [PubMed] [Google Scholar]
- 17.Jialal I, Devaraj S. The role of oxidized low density lipoprotein in atherogenesis. J Nutr. 1996;126:S1053–S1057. doi: 10.1093/jn/126.suppl_4.1053S. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;12:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 20.Matsushita K, Yatsuya H, Tamakoshi K, Yang PO, Otsuka R, Wada K, Mitsuhashi H, Hotta Y, Kondo T, Murohara T, Toyoshima H. High-sensitivity C-reactive protein is quite low in Japanese men at high coronary risk. Circ J. 2007;71:820–825. doi: 10.1253/circj.71.820. [DOI] [PubMed] [Google Scholar]
- 21.Shimada K, Fujita M, Tanaka A, Yoshida K, Jisso S, Tanaka H, Yoshikawa J, Kohro T, Hayashi D, Okada Y, Yamazaki T, Nagai R, JCAD Investigators Elevated serum C-reactive protein levels predict cardiovascular events in the Japanese Coronary Artery Disease (JCAD) Study. Circ J. 2009;73:78–85. doi: 10.1253/circj.cj-08-0295. [DOI] [PubMed] [Google Scholar]
- 22.Guo X, Yamada S, Tanimoto A, Ding Y, Wang KY, Shimajiri S, Murata Y, Kimura S, Tasaki T, Nabeshima A, Watanabe T, Kohno K, Sasaguri Y. Overexpression of peroxiredoxin 4 attenuates atherosclerosis in apolipoprotein E knockout mice. Antioxid Redox Signal. 2012;10:1362–1375. doi: 10.1089/ars.2012.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrass CK. Cellular lipid metabolism and the role of lipids in progressive renal disease. Am J Nephrol. 2004;24:46–53. doi: 10.1159/000075925. [DOI] [PubMed] [Google Scholar]
- 24.Glass CK, Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 25.Vaughan AM, Tang C, Oram JF. ABCA1 mutants reveal an interdependency between lipid export function, apoA-I binding activity, and Janus kinase 2 activation. J Lipid Res. 2009;50:285–292. doi: 10.1194/jlr.M800366-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao Y, Xiong Y, Wang H, Chu S, Zhong R, Wang J, Wang G, Ren X, Yu J. APOC3 induces endothelial dysfunction through TNF-α and JAM-1. Lipids Health Dis. 2016;15:153. doi: 10.1186/s12944-016-0326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Fang Q, Zhong P, Chen L, Wang L, Zhang Y, Wang J, Li X, Wang Y, Wang J, Liang G. EGFR inhibition blocks palmitic acid-induced inflammation in cardiomyocytes and prevents hyperlipidemia-induced cardiac injury in mice. Sci Rep. 2016;6:24580. doi: 10.1038/srep24580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deacon CF, Holst JJ. Dipeptidyl peptidase IV inhibition as an approach to the treatment and prevention of type 2 diabetes: a historical perspective. Biochem Biophys Res Commun. 2002;294:1–4. doi: 10.1016/S0006-291X(02)00359-5. [DOI] [PubMed] [Google Scholar]
- 29.Walker PD, Kaushal GP, Shah SV. The major matrix degrading enzyme in renal tubules, produces a novel nidogen fragment in vitro and in vivo. Kidney Int. 1998;53:1673–1680. doi: 10.1046/j.1523-1755.1998.00949.x. [DOI] [PubMed] [Google Scholar]
- 30.Sawamura T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 31.Gao S, Geng YJ. LOX-1: a male hormone-regulated scavenger receptor for atherosclerosis. Vascul Pharmacol. 2013;59:138–143. doi: 10.1016/j.vph.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Hu C. LOX-1 deletion decreases collagen accumulation in atherosclerotic plaque in low-density lipoprotein receptor knockout mice fed a high-cholesterol diet. Cardiovasc Res. 2008;79:287–293. doi: 10.1093/cvr/cvn110. [DOI] [PubMed] [Google Scholar]
- 33.Terasaki M, Nagashima M, Nohtomi K, Kohashi K, Tomoyasu M, Sinmura K, Nogi Y, Katayama Y, Sato K, Itoh F, Watanabe T, Hirano T. Preventive effect of dipeptidyl peptidase-4 inhibitor on atherosclerosis is mainly attributable to incretin's actions in nondiabetic and diabetic apolipoprotein E-null mice. PLoS One. 2013;8:e70933. doi: 10.1371/journal.pone.0070933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terasaki M, Nagashima M, Watanabe T, Nohtomi K, Mori Y, Miyazaki A, Hirano T. Effects of PKF275-055, a dipeptidyl peptidase-4 inhibitor, on the development of atherosclerotic lesions in apolipoprotein E-null mice. Metabolism. 2012;6:974–977. doi: 10.1016/j.metabol.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Sawamura T, Renier G. Glucose enhances human macrophage LOX-1 expression: role for LOX-1 in glucose-induced macrophage foam cell formation. Circ Res. 2004;94:892–901. doi: 10.1161/01.RES.0000124920.09738.26. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, Dietrich H, Metzler B, Wick G, Xu Q. Hyperexpression and activation of extracellular signal-regulated kinases (ERK1/2) in atherosclerotic lesions of cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 2000;20:18–26. doi: 10.1161/01.atv.20.1.18. [DOI] [PubMed] [Google Scholar]