Abstract
Background
Metastatic breast cancer (MBC) represents a life-threatening disease with a median survival time of 18–24 months that often can only be treated palliatively. The majority of women suffering from MBC are those who had been previously diagnosed with locally advanced disease and subsequently experienced cancer recurrence in the form of metastasis. However, according to guidelines, no systemic follow-up for monitoring purposes is recommended for these women. The purpose of this article is to review current methods of recurrent risk assessment as well as non-invasive monitoring options for women at risk for distant disease relapse and metastasis formation.
Methods
We used PubMed and national guidelines, such as the National Comprehensive Cancer Network (NCCN), to find recently published studies on breast cancer recurrence risk assessment and systemic monitoring of breast cancer patients through non-invasive means.
Results
The options for recurrence risk assessment of locally invasive breast cancer has improved due to diverse genetic tests, such as Oncotype DX, MammaPrint, the PAM50 (now known as the “Prosigna Test”) assay, EndoPredict (EP), and the Breast Cancer Index (BCI), which evaluate a women's risk of relapse according to certain cancer-gene expression patterns. Different promising non-invasive urinary protein-based biomarkers with metastasis surveillance potential that have been identified are MMP-2, MMP-9, NGAL, and ADAM12. In particular, ααCTX, ββCTX, and NTX could help to monitor bone metastasis.
Conclusion
In times of improved recurrence risk assessment of women with breast cancer, non-invasive biomarkers are urgently needed as potential monitoring options for women who have an increased risk of recurrence. Urine as a bioliquid of choice provides several advantages – it is non-invasive, can be obtained easily and frequently, and is economical. Promising biomarkers that could help to follow up women with increased recurrence risk have been identified. In order for them to be implemented in clinical usage and national guideline recommendations, further validation in larger independent cohorts will be needed.
Keywords: Recurrent breast cancer, Metastatic breast cancer, Bone metastasis, Biomarker, Risk assessment
What Is It about?
Breast cancer represents the most prevalent neoplasia in women worldwide. Once metastasized, the average 5-year survival rate of breast cancer patients is only 22%. Despite the fact that ~30% of all women with primarily locally invasive breast cancer develop recurrent disease in the form of metastasis throughout their lives, there is only local – no systemic – follow-up examination of the breast recommended by national guidelines such as the National Comprehensive Cancer Network (NCCN). While genetic tests such as Oncotype DX, MammaPrint, PAM50, EndoPredict (EP), and the Breast Cancer Index (BCI) allow more precise evaluation of who will suffer from distant relapse, there is still the question of how to monitor women with locally invasive breast cancer as well as those with a high risk of recurrence in a feasible way. For women at risk for distant disease recurrence, urinary biomarkers may potentially fill the gap between genetic risk assessment and symptom-directed examinations. Ideally, directed use of an easily applicable biomarker recognizes relapsing disease in an early or oligometastatic state, before it becomes a clinically apparent and potentially a palliative situation. Among the most promising proteins that present as elevated in urines of women with metastatic breast cancer are MMP-2, MMP-9, NGAL, ADAM12, ααCTX, ββCTX, and NTX. Larger validation studies on these urinary protein biomarkers are still needed before implementation into clinical usage.
Introduction
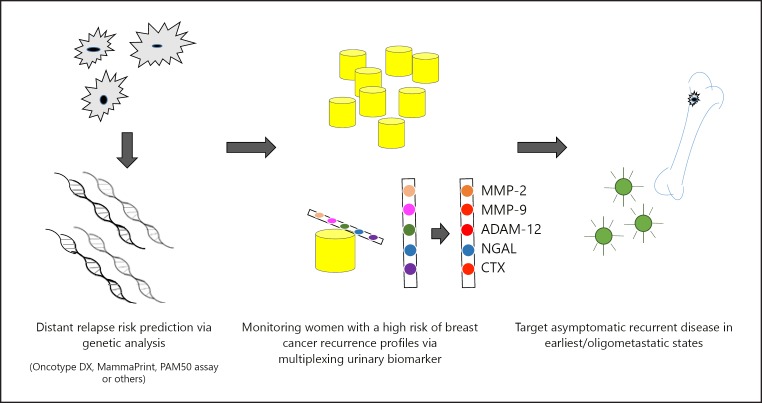
Breast cancer is the most prevalent and one of the deadliest neoplastic diseases in women worldwide [1]. In 2017, 252,710 newly diagnosed breast cancer cases and 40,610 deaths were predicted in the US [2]. The diagnosis of breast cancer as ductal carcinoma in situ or early stages I or II correlates with good outcomes and high survival rates (100%, 100%, and 93%, respectively, according to the American Cancer Society) [3]. Contributing to this prognosis is the possibility of complete surgical excision supported, if necessary, by radiotherapy [4]. However, when diagnosed in the more advanced stages III or IV, survival rates drop significantly to 72% and 22%, respectively [3]. To date, stage IV, defined as metastatic breast cancer (MBC), represents a life-threatening disease with a median survival time of 18–24 months that often can only be treated palliatively (Fig. 1) [5, 6].
Fig. 1.
Theoretic illustration a systemic monitoring sequence of breast cancer patients at high risk for disease recurrence through non-invasive urinary protein-based biomarkers.
While only 6–10% of the women are diagnosed with “de novo” MBC, the majority of women suffering from MBC are those who had been previously diagnosed with locally advanced disease and who are subsequently suffering from cancer recurrence in the form of metastasis [7]. Despite systemic chemo-, antibody, and hormonal therapy, ∼30% of these women will suffer from cancer recurrence at its common metastatic sites, i.e. the skeletal system, lungs, liver, and/or brain [6, 8, 9].
Recently, promising genetic tests focusing on an examination of the genetic expression patterns in tumor tissue, such as Oncotype DX, MammaPrint, PAM50 (now known as the “Prosigna test”) assay, EndoPredict (EP), and the Breast Cancer Index (BCI) have been established to evaluate a woman's precise risk of metastasis relapse. These help the treating physician choose the best possible therapy, resulting in a more radical treatment regimen in case of a high likelihood of recurrence [4, 10, 11]. Recently, genes have been validated that may precisely correlate with relapse manifestation to the bone, which is both the most common and earliest site of metastasis [12, 13, 14].
While national guidelines, such as the National Comprehensive Cancer Network (NCCN), have begun to support genetic risk stratification, they do not support imaging methods or tumor marker determination to monitor women at risk for systemic recurrence, downstream of their initial treatment [15, 16]. However, as the risk of recurrence threatens many women, more accurate methods to predict the probability of disease progression is strongly needed. In order to address this issue, diverse tissue-, blood-, urine-, or saliva-based proteins; DNA; miRNAs; exosomes; and circulating tumor cells (CTC) have been suggested as biomarkers for monitoring purposes [17, 18, 19, 20, 21, 22, 23]. Since closer monitoring for women at risk may translate into better outcomes, serial measurements of biomarkers have been proposed. Ideally, this approach should be as easy, cost-efficient, and as straightforward as possible to achieve maximum effectiveness. Biofluids such as urine and saliva are especially attractive since they can be obtained non-invasively and have become a focus of metastasis [23, 24, 25, 26, 27, 28, 29, 30].
This review offers an overview of current methods being used to identify women at risk for disease recurrence (particularly in bone), biomarkers that have been suggested to be utilized to monitor these women at risk (specifically in a non-invasive manner), and why the necessity of such biomarkers has become even more relevant today as breast cancer recurrence risk-stratification has become a possibility.
Characteristics of Metastatic and Recurrent Breast Cancer
Current data demonstrate that stage IV breast cancer, which is defined by the presence of distant metastasis, correlates to a 5-year-survival rate as low as 22% [3]. Women with stage 0 and I, suffering from carcinoma in situ and locally invasive cancer smaller than 20 mm in diameter without lymph node involvement or – if so – no greater than 2.0-mm lymph node metastasis, respectively, have a very high probability of surviving 5 years [3, 16].
Challenges begin as soon as cancerous cells enter the circulation. This may be already the case in cancer stages II and III, which are defined as the presence of metastasis in up to 9 ipsilateral axillary lymph nodes (pN2) and overall ipsilateral supraclavicular lymph node involvement, respectively. However, 5-year survival rates, being 93% and 72%, respectively, are still higher as compared to devastating low 5-year survival rates in case of metastasis to distant sites, as already mentioned above.
Interestingly, not all women with MBC have the same low survival rates, and studies have shown that it is of profound significance whether a woman was diagnosed with metastatic disease “de novo” or “recurrent”. A large retrospective study by Dawood et al. [7] (n = 3,524) showed that the median overall survival (OS) of women with de novo MBC was 39.2 months compared to an average of only 27.2 months in women with relapsed disease. Similar outcomes have recently been confirmed by Lobbezoo et al. [8]. Unfortunately, the majority of MBC cases show recurrent disease. Approximately 1 out of 3 women with locally defined breast cancer stage II or III will suffer from secondary metastasis [8].
Once recurrence in the form of metastasis occurs, the quality of life and OS depend on different factors. One of these factors is the breast cancer metastasis molecular subtype, based on the estrogen/progesterone receptor and the HER2 receptor amplification status. Recently, Molnár et al. [31] showed that in case of recurrence, the best OS is associated with positive hormone receptor [HoR and negative human epidermal growth factor receptor 2 (HER2)] status of tumors (luminal A), followed by luminal B1 and B2 subtypes, defined as HoR+/HER2- with a Ki67 labeling index (LI) > 15% and HoR+/HER2+, respectively. Significant shorter OS was seen in patients with HoR-/HER2+ enhanced and HoR–/HER2– (triple negative cancer; TN) tumor subtypes [31]. Additional prognostic factors regarding the OS of distant relapse are: (a) the time-point of relapse [8], (b) the localization of recurrent disease, and (c) the metastasis load at the time of diagnosis.
Interestingly, the lapse of time until recurrence correlates with OS. While cases of distant recurrence appearing within the first 2 years after primary diagnoses are associated with significantly shorter OS, cases recurring later than 24 months do not seem to have a higher mortality risk as compared to women with de novo MBC [8]. Different underlying characteristics have been observed, which help to explain this phenomenon. In cases of early metastasis (< 24 months after initial treatment), Ki67 LI and tumor grade were significantly higher and the size of the tumor and nodal involvement were significantly more advanced in the primary tumor compared to cases with later metastasis formation [31, 32]. Moreover, a shorter metastasis-free interval was associated with HoR cancer subtypes, i.e. HER2+ or TN breast cancer, which has already been linked to a significant shorter OS regardless [31, 32]. Interestingly, the majority of patients with a short time to distant relapse and a more aggressive primary cancer phenotype were less than 50 years of age [32].
Bone metastases (BM) are the most common type of breast cancer metastases, regardless of de novo or recurrent breast cancer and regardless of the molecular cancer subtype. Further, the second most common manifestations are visceral metastases (liver and lungs), followed by metastases to the brain, which is the least common site of distant relapse [33, 34, 35]. Bone is the metastatic site associated with the highest morbidity due to skeletal related events leading to such complications as osseous pain, pathological fractures, spinal cord compression, and hypercalcemia [14, 36].
According to a recently published study focused on the metastasizing behavior of breast cancer subtypes based on the Surveillance, Epidemiology, and End Results (SEER)-registered database of 2017, luminal A breast cancer had the highest incidence of BM (58.5%), followed by luminal B (47.2%), TN (36.4%), and HER2-enriched subtypes (34.5%) [33]. In those two subtypes associated with the shortest OS, i.e. HER2-enriched and TN breast cancer, visceral metastases and brain metastases were more frequently present than in luminal A. Interestingly, when compared to luminal A breast cancers, HER2+ tumors were also observed to harbor a stronger tendency for multiple metastases overall, and logically the burden of distant metastasis seems to correlate with OS as well [31, 37]. Once recurrent breast cancer is diagnosed in a multi-localized state, OS decreases drastically. In the case of BM, Jacobson et al.[38] showed how OS is decreased as a function of the number of bone lesions present in bone scintigraphy. While the median survival was 53 months if only one focus was found, it dropped to a median survival of 22 months when > 3 osseous sites were involved. In contrast, follow-up studies of patients diagnosed early on with solitary MBC to the bone showed that disease remission – or complete cure in cases of oligometastatic BC – could be achieved by early therapeutic interventions such as radiotherapy, vertebroplasty and ky phoplasty, bisphosphonates, denosumab, and endocrine- and/or HER2-directed therapies [39, 40].
Therefore, it can be assumed that the time point in which metastasis progression is diagnosed is most crucial. With this is mind, a stringent systemic follow-up could be especially helpful within the first 2 years after treatment, specifying the potential target group for such monitoring.
Interestingly, regardless of the molecular subtype, the skeletal system seems to be both the most common site of breast cancer metastasis in general, but it also the earliest site of breast cancer distant relapse in up to 50% of all MBC cases [12].
Taken together, this suggests that a reliable surveillance method of the processes occurring within the skeletal system might contribute to diagnosing metastasis of recurrent breast cancer especially in women at risk.
Current Paradigms for Evaluating Women at Risk for Breast Cancer Recurrence
For women diagnosed with breast cancer today, two important procedures are conduct ed for the first recurrence risk evaluation: anatomical disease staging and analysis of the neoplasia on a molecular level [16, 41].
One important aspect of staging includes evaluating the tumor macroscopic anatomy, including tumor size (T), axillary lymph node involvement (N), and presence of distant metastasis (M). These data are summarized with the anatomical TNM stage of the disease: the further advanced this stage is, the higher the risk of recurrence [16, 41].
Breast cancer is further classified on a microscopic and even smaller level such as differentiating tumor type (e.g., ductal versus lobular carcinoma), its histological grade evaluated by means of the Nottingham grading system 1 through 3 (with 1 being defined as well differentiated), its molecular subtype, and even its underlying genetic profile [16, 41, 42, 43].
Since this information also harbors valuable prognostic information, the latest revision of the TNM classification conducted by the American Joint Commission of Cancer (AJCC) for breast cancer has proposed to recognize some of these factors as part of the TNM classification. Thus far, well-studied risk factors that correlate with a chance of distant recurrence are an advanced anatomic TNM stage (including large initial tumor size and extended lymph node involvement), a low tumor differentiation grade (Nottingham grade 3), a negative hormone receptor status, and HER2-amplification [41].
According to several studies, analysis of the five known molecular subtypes via PAM50 classification, HER2+ breast cancer, luminal B2, and TN cancer subtypes have the highest risk of distant relapse. Although subtypes luminal A and B1 are associated with the lowest risk for recurrence, they account for the most widespread subtype population and therefore may still compensate for a larger total number of women with recurrent metastasis despite the low er risk association [31, 44, 45]. Computer-based algorithms such as “Adjuvant!” and “PREDICT”have been established that incorporate these risk-factors as well as patient comorbidities and age, and offer a risk of recurrence estimation within a 10-year timeframe [46].
To evaluate a woman's risk for distant cancer recurrence even more precisely, powerful gene-based assays have been established over the past years that utilize gene-expression profiling in determining the cancer's molecular subtype. Oncotype DX® (Genomic Health Inc., Redwood City, CA, USA) analyzes 21 genes within breast cancer tissue via quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) and expression levels of these genes equal a “low”, “intermediate” or “high” recurrence score (RS), thereby evaluating the likelihood of distant metastasis within the subsequent 10 years [4, 10, 47]. In addition to providing information regarding recurrence prognoses, Oncotype DX has predictive ability, and can suggest who might benefit from additional chemotherapy. In node-positive cancers, for instance, a high RS can lead to a suggestion to add cardiotoxic anthracyclines to the chemotherapy regimen, while women with a low RS score would be unlikely to experience any further benefit by the addition of chemotherapy, regardless of the number of lymph nodes involved [47, 48].
A more extended panel of 70 genes is being quantified by MammaPrint® (Agendia, Irvine, CA, USA) with the goal of evaluating distant metastasis-free survival in both ER+ and ER– early breast cancer. Thus far, this assay's function is restricted to providing information about recurrence likelihood by classification into poor and good prognosis signature groups as well as in patients with early breast cancer [49, 50]. Both multigene assays, Oncotype DX and MammaPrint are FDA approved and included in NCCN guidelines. Additional genomic assays such as EndoPredict, Breast Cancer Index, and Genomic Grade are expanding the pool of possibilities to evaluate a woman's risk of recurrence [4, 16].
Moreover, specific genetic alternations in breast cancer have been correlated with relapse to specific sites, with the bone being the most common and earliest site of metastasis [12, 13, 14]. Analyzing copy number imbalances in early breast cancers of diverse subtypes, Liu et al.[51] observed that a copy number loss at the site of chromosome 8p22 was associated with a 100% probability for BM in the cohort examined. In a two-step validation, Savci-Heijink et al. [52] unraveled a novel combination of 15 genes in primary breast tumor DNA which seem to be highly associated with breast cancer metastasis to the skeletal system. Primary breast cancers that developed BM showed overexpression of NAT1, BBS1, and PH-4, and downregulation of APOPEC3B, ATL2, C16orf61, C6orf167, KCNS1, MFAP3L, NIP7, NUP155, PALM2, PGD5, SFT2D2, and STEAP3 in 100% of the cases in which bone-only metastasis occurred. The code correctly identified 82.4% of the overall BM cases and, interestingly, was predictive for the bone being the first site of distant relapse in 85.2% of all MBC cases analyzed. Results were validated in an independent set of 376 women with breast cancers: of the 160 patients with manifest BM, 81.2% tested positive for this specific gene-signature [52].
Genetic sequencing of cancer cell DNA has now added more accurate information about breast cancer's behavior than long-established predictors like lymph nodal status, tumor size, and histological grade. One reason that the evaluation of metastasis risk and recurrence is so important is that it greatly impacts the therapeutic regime that is being chosen. Currently, this means the higher the risk, the more aggressive the therapy [16, 47, 50]. It will be the topic of future evaluations to determine the extent to which these predictions and subsequent therapies prevented distant recurrence and ultimately lead to improved OS.
Why There Is a Need for Monitoring Women with Breast Cancer
Despite follow-up care, almost 1 in 3 breast cancer patients will experience a relapse, with the highest incidence occurring during the first 2–3 years after therapy [6]. While follow-up therapy is confined to a local examination of the breast, 30% of all women initially diagnosed with early-stage breast cancer relapse at distant sites in the form of metastasis – in most cases primarily within the skeletal system [8, 9]. Nevertheless, national guidelines (NCCN) recommend follow-up strategies that focus on local recurrences exclusively, such as breast self-examination, physical examination by a physician, and bilateral mammography [16].
To date, no systemic follow-up has been implemented. According to the NCCN, “… in the absence of clinical signs and symptoms suggestive of recurrent disease, laboratory or imaging studies to screen for metastasis are not necessary” [16]. The panel further notes that there are no advantages in OS or disease palliation in cases where recurrence has been observed through biomarker-based monitoring in the past, concluding that “… the use of ‘tumor markers’ for breast cancer are not recommended” [16]. However, one of the reasons for this could be the fact that the main source considered by the NCCN guidelines is an article published by the American Society of Clinical Oncology, which included evidence-based clinical practice guidelines from the year 2000 (“2000 Update of Recommendations for the Use of Tumor Markers in Breast and Colorectal Cancer: Clinical Practice Guidelines of the American Society of Clinical Oncology”) and was written when the only three blood-based biomarkers discussed as surveillance biomarkers were CEA, CA 15–3 and CA 27.29, which, unfortunately, did not show an improvement in OS despite blood increases of all biomarker levels 2–18 months prior to clinical or radiological recurrence appearance [15, 16, 53, 54]. However, interventional and therapeutic strategies have improved since that time and tend to improve further each year so that diagnosis of a relapse in an early and oligometastatic state could now be beneficial, especially in patients with limited MBC [55, 56, 57, 58, 59].
Secondly, the data resulting from monitoring and follow-up biomarker studies considered in the NCCN guidelines are still before the era of a novel risk stratification methodology [15]. In the past years more specific risk factors for disease recurrence have been determined and validated, even within specific cancer sites such as the skeletal system, helping to identify the 30% of women who might develop distant metastasis in the future [8]. It has been shown in the past that without knowing who to focus on, follow-up markers might not be sensitive and specific enough [15]. Today, prognostic (genetic) risk markers have the ability to evaluate whether a relapse is likely to occur (at a specific site), while a well-directed and more selectively used follow-up marker could be informative with respect to the particular time point.
According to a national survey of oncologists in Italy, asymptomatic breast cancer patients are already undergoing extended and non-guideline-conform imaging and tumor marker testing, mainly due to significant patient-driven uncertainty and anxiety, inflicting immense costs on the health care systems [60]. One would imagine that women who are aware of their potential individual risk of recurrence would consider this information and the use of systematic follow-up monitoring when considering therapeutic options.
Recently, a large retrospective data analysis on more than 8,000 women with breast cancer by Blumen et al.[62] looked at the average per capita treatment costs based on an individual's cancer stage. Not surprisingly, stage IV breast cancer treatment costs were sig nificantly higher than those for early-stage disease and were the result of high differences in chemotherapy expenses and costs related to palliative care [61, 62]. This study, along side others, is suggestive of the fact that early diagnosis and treatment of breast cancer pa tients – also in case of early recurrence as metastatic disease where palliative settings can be prevented – may not only help prolong an individual's life, but also diminish the overall cost burden originating from cancer treatments and supportive care [62].
In summary, the development of accurate, non-invasive, and direct monitoring of high-risk inflammatory breast cancer (IBC) patients has significant clinical value for the following reasons:
1. There is a need to fill the gap between risk evaluation and therapy of MBC in case of recurrence. While established risk prediction indicates a recurrence event may happen, a surveillance marker may shed light on the time point when the recurrence may happen.
2. There is a need to ameliorate patient-driven uncertainty and anxiety as well as provide options for women who are aware of their risks.
3. There is a need to address cases that could still benefit from therapeutic interventions in an early oligometastatic state.
As noted by Puglisi et al., “The poor prognosis of patients with distant relapse justify a strong effort to identify a systemic surveillance strategy effective in improving outcome.” [56].
How Women with Breast Cancer Could Be Monitored (Non-Invasively)
A number of potential ways of monitoring patients with breast cancer have been explored, ranging from diverse imaging to body fluid analysis. A variety of targets in serum itself may be examined in order to measure metastatic cancer cell dissemination, including proteins, CTCs, cfDNA, miRNAs, and extracellular vesicles [63, 64, 65, 66, 67].
Multiple studies on serum biomarkers for MBC have been published. A systematic review by Berghuis et al.[66] listed 181 CTC- and 107 protein-based biomarkers studies, respectively, for MBC that have been published since 2006. With the increase of biomarker studies, validation and reproducibility have become critical criteria to identify useful potential biomarkers, and adhering to the “REporting recommendation for tumor MARKer prognostic studies” (REMARK) has been suggested for such studies [68]. However, few biomarkers have been explored, validated, and reproduced in multiple studies (9.8% biomarkers were represented in > 5 studies), showing how difficult it is to fulfill these guidelines and eventually change the current NCCN guidelines [16, 66]. Even the well-known breast cancer tumor markers CA 15–3, CA 27–29, and CEA do not fulfill the sensitivity and specificity requirements needed to meet the criteria of the American Society of Oncology for monitoring markers and are currently mostly used “off-label” in patient follow-ups [16, 69].
One strategy to improve the prognostic accuracy of follow-up biomarkers is multiplexing [70, 71]. Di Gioia et al. [72] conducted a study that both multiplexed different biomarkers and used two different methodologies for analysis, resulting in more reliable follow-up results: 813 patients with primary breast cancer underwent regular 6-week testing for the serum tumor biomarkers CEA, CA 15–3, and CA 125, respectively. In case of an increase of one or more markers, the women underwent MRI and/or an FDG-PET/CT scan [72]. Of the asymptomatic 44 patients who showed an increase in biomarker levels, metastatic recurrence was confirmed in 29 women via imaging (65.9%), and early intervention was possible [72].
Another interesting new two-step validation approach to identify relapsing disease in breast cancer patients is by looking at cell-free DNA (cfDNA) amplification of the 1q21.3 sequence in blood. The amplification of chromosome 1q21.3 amplification (the chromosome involved in the S100 calcium-binding protein family A7, A8, and A9) as well as the IL-1 receptor-associated kinase 1 (IRAK1) encoding was seen in more than 70% of recurrent breast cancers, regardless of the subtype [67]. Unfortunately, one of the disadvantages of screening for cfDNA is the lack of standardization in detection [4]. Furthermore, a prior biopsy and analysis are needed to confirm that the mutation that is to be monitored is present, and high false-positive results in benign lesions have been observed [73].
In summary, the two disadvantages that remain with regular imaging and/or serum analysis (both protein- and cfDNA-based biomarkers) are that they are invasive and/or costly. Therefore, non-invasive follow-up biomarkers might be of particular long-term benefit, as long as the REMARK criteria remain fulfilled [68, 74]. Body fluids that theoretically meet the criteria as being non-invasively obtained are urine, saliva, tears, colostrum, and breast milk. Due to its easy and rapid accessibility, sufficient quantity, and economic analysis, most non-invasive biomarker studies focus on urine as compared to the other non-invasive methods listed above. Urine even harbors the potential for patients to regularly self-monitor their disease status, providing convenient check-ups from home [20]. Potential urinary breast cancer biomarkers described to date are mainly protein- or RNA-based [74]. RNA in the urine occurs mainly as exosomal microRNA (miRNA) since kidney-based nucleases degrade long-chain and/or free RNA types [75]. Erbes et al.[76] recently observed the urinary microRNA miR-155, miR-21, miR-125b, and miR451 to be significantly differentially expressed in women with breast cancer (n = 24) compared to healthy controls (n = 24). While dogma suggests that only proteins below the cutoff for glomerular filtration of ∼60 kDa may be present in urine, high-resolution techniques such as mass spectrometry have in fact detected high-molecular-weight proteins in urine from healthy individuals [77, 78]. One significant advantage of protein-based biomarker assessment in urine versus blood is that urine has fewer background proteins compared to serum, making the detection of low-abundance biomarkers relatively easier [74, 79, 80, 81]. The fact that elevated (protein) biomarkers in urine are not only limited to the presence of cancer within the urinary tract has been shown in a proof-of-concept study by Smith et al. [71]. Elevated brain tumor markers have been tracked in matched samples from patient brain tumors to cerebrospinal fluid and urine [71]. Upon surgical tumor removal, previously elevated urinary biomarker levels (MMP-9, MMP-9/NGAL, and MMP-2) dropped to undetectable levels, showing a strong correlation between the biomarkers and malignancy status [71].
As one of the pioneers in the field of urinary cancer biomarkers Moses et al.[82] published multiple studies on promising protein-based breast cancer biomarkers. Markers that were shown to correlate significantly with breast cancer progression and metastasis, are matrix-metalloproteinases (MMP) -2 and -9, neutrophil gelatinase-associated lipocalin (NGAL or lipocalin 2), the MMP-9/NGAL complex as well a disintegrin and metalloprotease 12 (ADAM12) [21, 82, 83, 84].
MMP-2 and MMP-9 were two of the first proteases to show enzymatic activity via zymography in urines of breast cancer patients and emerged as independent predictors for locally invasive and MBC [82].
Other groups have reported correlations between breast cancer progression and MMP-2 and -9 levels in sera [85, 86]. Primarily discovered as a high-molecular-weight MMP in the urine of advanced breast cancer patients, as shown by Yan et al.[83], the MMP-9/NGAL complex has been shown to prevent MMP-9 from autodegradation, suggesting its presence in (metastasizing) breast cancer. In an independent study of 49 patients via zymography including 22 patients with breast cancer and 27 healthy controls, the MMP-9/NGAL complex was present in 86.36% of urines of BC patients, but was absent in controls [87]. Yang et al.[88] reported that lipocalin 2 (Lcn2), a protein involved in breast cancer progression, stimulates breast tumor cell epithelial-to-mesenchymal transition and that free Lcn2 was significantly elevated in urines of MBC patients as compared to normal controls.
Roy et al.[84] showed that urinary ADAM12 levels increased significantly with disease progression, while Pories et al.[21] observed that – multiplexing – urinary MMP-9 and ADAM12 levels could serve as markers for breast cancer risk assessment in women with precursor lesions like LCIS and atypical hyperplasia. Further studies are needed to determine whether these particular markers could perform as tools to monitor IBC patients.
Hiramatsu et al. [90] studied N1N12-diacetylspermine as a potential urinary biomarker in breast cancer. This protein deriving from the polyamine metabolism in association with cell proliferation was significantly up-regulated in 80.3% of urines examined in 51 women with late-stage and metastasized breast cancer as compared to a healthy control cohort (n = 51). Interestingly, the same group of patients was simultaneously tested for their serum CA 15–3 and CEA levels. Even though these proteins have been suggested as potential monitoring biomarkers in the past, they accounted for a lower sensitivity of 60.8 and 58.8%, respectively [89, 90]. Beretov et al. [91] chose a liquid chromatography with tandem-mass spectrometry approach to discover novel urinary protein biomarker candidates for breast cancer metastasis. They found AGRIN, NEGR1, FIBA, and KIC10 to be exclusively elevated in MBC samples (n = 6), and missing in ductal carcinoma in situ (n = 6), locally invasive breast cancer (n = 8), benign breast disease (n = 6), and healthy urines. However, no validation of these protein candidates in larger cohorts has been conducted so far (Table 1).
Table 1.
Monitoring women with invasive breast cancer non-invasively: potential urinary biomarkers
| 1st author | Study year |
PMID | Study title | Urinary biomarkers | Details | Study, n |
|---|---|---|---|---|---|---|
| Moses et al. | 1998 | 9537238 | Increased incidences of matrix metalloproteinases in urine of cancer patients | MMP-2 MMP-9 MMP-9/NGAL |
The MMP-2 ' s, −9 ' s and the MMP-9/NGAL complex ' s urinary levels significantly correlated with breast cancer progression and metastasis | 9 |
| | ||||||
| Roy et al. | 2004 | 15381692 | ADAM 12 cleaves extracellular matrix proteins and correlates with cancer status and stage | ADAM12 | Significant increase of urinary ADAM12 levels were observed with progression of disease | 117 |
| | ||||||
| Fern á ndez et al. | 2005 | 16061852 | The matrix metallopro-teinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients | MMP-9/NGAL | MMP-9/NGAL-Complex was present in 86.36% urines of BC patients while being absent in controls | 49 |
| | ||||||
| Hiramatsu et al. | 2005 | PMC3389634 | N 1 N 12 -diacetylspermine (DiAcSpm) as a sensitive and specific novel marker for early- and late-stage colorectal and breast cancer | DiAcSpm | DiAcSpm was significantly upregulated in 80.3% of urines of women with late-stage and metastasized breast cancer as compared to a healthy control cohort and accounted for higher sensitivity than serum CEA and CA 15.3. | 134 |
| | ||||||
| Yang et al. | 2009 | 19237579 | Lipocalin 2 promotes breast cancer progression | Lipocalin 2 (NGAL) |
Lcn2 levels were significantly increased in urines of MBC patients (n = 20) as compared to normal controls (n = 46); (p = 0.03) | 46 |
| | ||||||
| Beretov et al. | 2015 | 26544852 | Proteomic analysis of urine to identify breast cancer biomarker candidates using a label-free LC-MS/MS approach | AGRIN NEGR1 FIBA, KIC10 |
In an MS analysis of women with MBC, AGRIN, NEGR1, FIBA, and KIC10 were detected to be exclusively elevated in MBC urine samples, but missing in DCIS, IBC and BBD urines | 26 |
| | ||||||
| Erbes et al. | 2015 | 25886191 | Feasibility of urinary microRNA detection in breast cancer patients and its potential as an innovative non-invasive biomarker | miR-155 miR-21 miR-125b miR451 | Four novel microRNAs were found to be significantly differentially expressed in urine concentration of women with breast cancer (n = 24) compared to healthy controls (n = 24) | 48 |
Recently, a combination of genes highly associated with breast cancer metastasis in the skeletal system has been identified that could help women with breast cancer predict their personal risk for recurrence [52]. Against this background, one logical consequence would be to implement surveillance mechanisms that represent occurrences on an osseous level – especially for those women at risk. This could ideally lead to an earlier time point for interfering, and noticing bone involvement early could help decrease the high morbidity associated with skeletal complications [23, 58].
One of the first non-invasive monitoring methods for BM was the determination of urinary calcium levels [92]. Multiplexed with serum CA 15–3 as “bone metastasis index”, patients with BM had significant elevations of their bone metastasis index as compared to locally confined breast cancer patients and provided a diagnostic efficacy of 90% [92]. By learning more about proteins and by-products involved in the osseous metabolism during the formation of BM due to breast cancer, more specific “bone turnover markers” were identified. As in cases of predominantly osteolytic BM in MBC, Houzé et al. [93] were one of the first to study the carboxy-terminally peptide of type I collagen (CTX) as a potential bone resorption marker in urine of women with breast cancer-positive BM and observed that CTX was significantly elevated compared to healthy pre- and postmenopausal controls (p < 0.01). Cloos et al. further specified studies on CTX by differentiating isomerized, racemised, and cross-linked versions of CTX, and found that newly synthesized, ∼12 kDa, non-isomerized alpha CTX peptides (αCTX) and the cross-linked chains of αCTX (ααCTX) are preferentially released into urine of patients with BM [28, 29, 94]. Comparing urine samples of 100 women with MBC to the bone to patients with IBC without bone involvement (n = 15) and normal controls (n = 31), this study reported that levels were even more elevated (p = 0.005 and p = < 0.0001, respectively) [29, 94]. The fact that αCTX and particularly ααCTX seem to be an interesting marker of monitor dysregulation in bone metabolism due to MBC was further supported by studies by Leeming et al., who observed that the number of distant BM (n = 40), i.e. 1, 2, and 3 metastases, correlated with a 38%, 57%, and 81% increase in ααCTX, respectively [25].
A less extensively studied bone turnover biomarker in urine includes another collagen I peptide-structure, the N-terminal telopeptide (NTx) [24, 27, 30]. NTx levels were significantly higher (p < 0.01) in a small cohort of 19 patients with recurrent BM compared to patients with either breast cancer patients without BM (n = 65) or patients with bone pathologies other than metastasis (n = 22) [27]. Serum-based carboxyterminal telopeptide of type I collagen (ICTP) has also been suggested as a useful biomarker to monitor women with BM [30].
Wu et al. [24] reported that both urinary NTx and serum ICTP levels were significantly higher in the urine of patients with BM compared to those with no bone involvement or healthy controls and that both markers decreased significantly with successful treatment (p < 0.05). Similar results have been observed by Leeming et al.[26], who compared urinary collagenous resorption marker levels of ααCTX, ββCTX (cross-linked isomerized carboxy-terminally telopeptide), and NTx individually in two cohorts: breast cancer patients with- and without BM. All three urine-based marker levels were significantly higher in the BM cohort (Table 2).
Table 2.
Monitoring women with invasive breast cancer non-invasively: potential urinary biomarkers for bone metastasis
| 1st Author |
Study year |
PMID | Study title | Urinary biomarkers | Details | Study, n |
|---|---|---|---|---|---|---|
| Yadav et al. | 1993 | 8138660 | CA 15.3 with urinary calcium excretion is useful in the diagnosis and monitoring of bone metastases from breast cancer | Urinary Ca 2 + | Multiplexed with serum CA 15-3, urine levels of Ca 2 + accounted for a 90% correct identification of MBC patients | 73 |
| | ||||||
| Houz é et al. | 1999 | 10217629 | Urinary carboxyterminal telopeptide of collagen I as a potential marker of bone metastases chemotherapy monitoring in breast cancer | CTX | CTX was significantly elevated in urines of breast cancer-positive bone metastases compared to healthy pre- and postmenopausal controls (p < 0.01) | 144 |
| | ||||||
| Ulrich et al. | 2001 | 11205705 | Cross-linked type I collagen C- and N-telopeptides in women with bone metastases from breast cancer | NTx | NTx had a sensitivity and specificity of 44% and 79% respectively in identifying BM from breast cancer (p < 0.01) | 106 |
| | ||||||
| Cloos et al. | 2003 | PMC165019 | Breast cancer patients with bone metastases are characterised by increased levels of nonisomerised type I collagen fragments | α CTX | Nonisomerized alpha CTX peptides showed to be increasingly released into urines of women with bone metastases | 178 |
| | ||||||
| Cloos et al. | 2004 | 15209436 | An immunoassay for measuring fragments of newly synthesized collagen type I produced during metastatic invasion of bone | αα CTX | αα CTX levels were higher concentrated in urines of MBC patients compared to IBC without bone involvement and normal controls with a significance of p = 0.005 and p < 0.0001, respectively | 156 |
| | ||||||
| Leeming et al. | 2006 | 16835341 | Alpha CTX as a biomarker of skeletal invasion of breast cancer: immunolocalization and the load dependency of urinary excretion | αα CTX | Bone metastases load correlates with the increase in urinary αα CTX levels | 40 |
| | ||||||
| Leeming et al. | 2006 | 16434583 | The Relative Use of Eight Collagenous and Noncollagenous Markers for Diagnosis of Skeletal Metastases in Breast, Prostate, or Lung Cancer Patients | αα CTX ββ CTX NTX |
Of the eight potential bone turnover markers tested, the three urinary-based αα CTX, ββ CTX and NTX were significantly higher concentrated in breast cancer patients with BM compared to those without | 90 |
| | ||||||
| Wu et al. | 2016 | 27647403 | Clinical significance of combined detection of urine NTX and serum ICTP for breast cancer patients with bone metastases | NTx | Urinary NTx was significantly higher in urines of BC patients with BM as compared to healthy controls (p < 0.05) and significantly decreased with successful treatment (p < 0.05) | 98 |
Conclusion
Today, women with invasive breast cancer can be stratified based on their risk of recurrence. This risk is not only reflected by the molecular cancer subtype, TNM stage, and cancer grade, but also by the tumor cell's genetics, and enables the identification of “high” versus “low” risk of recurrence as well as prognosis regarding the type of metastasis. For a long time, protein-based biomarkers have not fulfilled the reliability standards regarding sensitivity and specificity necessary for national guidelines. However, more precise patient information and the urgent need to provide surveillance strategies for women at risk might draw more attention to practical and non-invasive biomarkers that help to inform about the actual time point of a potential event. Ideally, follow-up should be easy, as non-invasive as possible, and economical.
Urine harbors a lot of these requirements. According to the literature, proteins up to 60–70 kDa can pass the blood-urine barrier under healthy conditions [61]. Recent studies using improved mass spectrometry methods have shown that even higher-molecular-weight proteins appear in the urine of healthy individuals [59]. In the case of an impaired kidney function, proteinuria becomes visible through urinalysis (> 150 mg protein) [62]. Nevertheless, even in the absence of kidney disease, proteins and especially polypeptides are known to pass the blood- urine barrier, making urine an interesting target for proteomics analysis. Using urine as body fluid for assessment of biomarker status has multiple advantages: it can be collected non-invasively, can easily be acquired, and is economical, making it especially attractive for regular and more frequent monitoring of cancer patients. Ideally, urine might even have self-monitoring potential for patients, enabling convenient check-ups from home. Furthermore, the urinary proteome is known to be less contaminated by high-abundant proteins as is the case with blood, where only 22 different proteins account for 99% of an analyzed aliquot, masking potential biomarkers and making additional cleaning steps essential [47, 57]. Additionally, urine is seen to be more stable as compared to blood, where more proteolytic activity can be measured [63].
While a variety of promising urinary biomarkers with potential to monitor breast cancer patients at overall risk for metastasis or, more specifically, at risk for bone metastasis, have been studied, none of these are currently used in clinical usage – mostly due to lack of validation in larger independent cohorts. So far, the most promising urinary biomarkers with surveillance potential for risk of (bone) metastases include MMP-2, MMP-9, NGAL, and ADAM12, as well as ααCTX, ββCTX, and NTX. Combining the information from individual studies conducted so far and multiplexing some of these potential biomarkers may lead to reliable monitoring options for women with invasive breast cancer that are at risk for recurrence and could help to detect and target recurrent disease at the earliest time point possible, subsequently promoting a longer OS with a better quality of life and a lower treatment-related cost burden.
Disclosure Statement
The authors declare they have no conflicts of interest, including financial interests.
Author Contributions
All three authors had full access to all study data and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: E.M.S., K.Z., W.Z. Acquisition, analysis, and interpretation of data: E.M.S., K.Z., W.Z. Drafting of the manuscript: E.M.S. Critical revision of the manuscript for important intellectual content: E.M.S., K.Z., W.Z. Administrative, technical, or material support: E.M.S., K.Z., W.Z. Study supervision: K.Z., W.Z.
Acknowledgements
This paper was reviewed by Marsha Moses (PhD) and Roopali Roy (PhD), both from the Vascular Biology Program, Boston Children's Hospital, Harvard Medical School. We are indebted for their thorough thoughts, comments, and edits on this review article. Their expertise on urinary breast cancer biomarkers greatly improved the manuscript.
References
- 1.McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr. 2016 Mar;7((2)):418–9. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017 Jan;67((1)):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.ACS American Cancer Society: Breast Cancer Survival Rates by Stage [Google Scholar]
- 4.Le Du F, Ueno NT, Gonzalez-Angulo AM. Breast Cancer Biomarkers: Utility in Clinical Practice. Curr Breast Cancer Rep. 2013 Dec;5((4)):5. doi: 10.1007/s12609-013-0125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toss A, Venturelli M, Peterle C, Piacentini F, Cascinu S, Cortesi L. Molecular Biomarkers for Prediction of Targeted Therapy Response in Metastatic Breast Cancer: trick or Treat? Int J Mol Sci. 2017 Jan;18((1)):18. doi: 10.3390/ijms18010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerber B, Freund M, Reimer T. Recurrent breast cancer: treatment strategies for maintaining and prolonging good quality of life. Dtsch Arztebl Int. 2010 Feb;107((6)):85–91. doi: 10.3238/arztebl.2010.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010 Nov;21((11)):2169–74. doi: 10.1093/annonc/mdq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer. 2015 Apr;112((9)):1445–51. doi: 10.1038/bjc.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tinoco G, Warsch S, Glück S, Avancha K, Montero AJ. Treating breast cancer in the 21st century: emerging biological therapies. J Cancer. 2013;4((2)):117–32. doi: 10.7150/jca.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Breast Cancer Intergroup of North America Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010 Jan;11((1)):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Hoeven JJ. [70-Gene signature as an aid to treatment decisions in early-stage breast cancer] Ned Tijdschr Geneeskd. 2017;161:D1369. [PubMed] [Google Scholar]
- 12.Hayashi N, Iwamoto T, Qi Y, Niikura N, Santarpia L, Yamauchi H, et al. Bone metastasis-related signaling pathways in breast cancers stratified by estrogen receptor status. J Cancer. 2017 Apr;8((6)):1045–52. doi: 10.7150/jca.13690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003 Jun;3((6)):537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 14.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006 Oct;12((20 Pt 2)):6243s–9s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 15.Bast RC, Jr, Ravdin P, Hayes DF, Bates S, Fritsche H, Jr, Jessup JM, et al. American Society of Clinical Oncology Tumor Markers Expert Panel 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001 Mar;19((6)):1865–78. doi: 10.1200/JCO.2001.19.6.1865. [DOI] [PubMed] [Google Scholar]
- 16.NCCN NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Breast Cancer (Version 2.2017 - April 6, 2017) 2017:2017. [Google Scholar]
- 17.Olsson E, Winter C, George A, Chen Y, Howlin J, Tang MH, et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med. 2015 Aug;7((8)):1034–47. doi: 10.15252/emmm.201404913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanda DP, Sil H, Moulik S, Biswas J, Mandal SS, Chatterjee A. Matrix metalloproteinase-9 as a potential tumor marker in breast cancer. J Environ Pathol Toxicol Oncol. 2013;32((2)):115–29. doi: 10.1615/jenvironpatholtoxicoloncol.2013008166. [DOI] [PubMed] [Google Scholar]
- 19.Song J, Su H, Zhou YY, Guo LL. Prognostic value of matrix metalloproteinase 9 expression in breast cancer patients: a meta-analysis. Asian Pac J Cancer Prev. 2013;14((3)):1615–21. doi: 10.7314/apjcp.2013.14.3.1615. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Roy R, Jedinak A, Moses MA. Mining the Human Proteome: Biomarker Discovery for Human Cancer and Metastases. Cancer J. 2015 Jul-Aug;21((4)):327–36. doi: 10.1097/PPO.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 21.Pories SE, Zurakowski D, Roy R, Lamb CC, Raza S, Exarhopoulos A, et al. Urinary metalloproteinases: noninvasive biomarkers for breast cancer risk assessment. Cancer Epidemiol Biomarkers Prev. 2008 May;17((5)):1034–42. doi: 10.1158/1055-9965.EPI-07-0365. [DOI] [PubMed] [Google Scholar]
- 22.Jia D, Roy R, Moses MA. MMPs in Biology and Medicine, in Matrix Metalloproteinase Biology (eds I. Sagi and J. P. Gaffney); Matrix Metalloproteinase Biology. John Wiley & Sons, Inc, Hoboken, NJ, USA. 2015 [Google Scholar]
- 23.Jung K, Lein M. Bone turnover markers in serum and urine as diagnostic, prognostic and monitoring biomarkers of bone metastasis. Biochim Biophys Acta. 2014 Dec;1846((2)):425–38. doi: 10.1016/j.bbcan.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Wu CJ, Ma LX, Zhu J, Liu JJ, Cheng Y. [Clinical significance of combined detection of urine NTX and serum ICTP for breast cancer patients with bone metastases] Zhonghua Zhong Liu Za Zhi. 2016 Sep;38((9)):693–7. doi: 10.3760/cma.j.issn.0253-3766.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Leeming DJ, Delling G, Koizumi M, Henriksen K, Karsdal MA, Li B, et al. Alpha CTX as a biomarker of skeletal invasion of breast cancer: immunolocalization and the load dependency of urinary excretion. Cancer Epidemiol Biomarkers Prev. 2006 Jul;15((7)):1392–5. doi: 10.1158/1055-9965.EPI-05-0909. [DOI] [PubMed] [Google Scholar]
- 26.Leeming DJ, Koizumi M, Byrjalsen I, Li B, Qvist P, Tankó LB. The relative use of eight collagenous and noncollagenous markers for diagnosis of skeletal metastases in breast, prostate, or lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2006 Jan;15((1)):32–8. doi: 10.1158/1055-9965.EPI-05-0492. [DOI] [PubMed] [Google Scholar]
- 27.Ulrich U, Rhiem K, Schmolling J, Flaskamp C, Paffenholz I, Sälzer H, et al. Cross-linked type I collagen C- and N-telopeptides in women with bone metastases from breast cancer. Arch Gynecol Obstet. 2001 Jan;264((4)):186–90. doi: 10.1007/s004040000105. [DOI] [PubMed] [Google Scholar]
- 28.Cloos PA, Christgau S, Lyubimova N, Body JJ, Qvist P, Christiansen C. Breast cancer patients with bone metastases are characterised by increased levels of nonisomerised type I collagen fragments. Breast Cancer Res. 2003;5((4)):R103–9. doi: 10.1186/bcr607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cloos PA, Lyubimova N, Solberg H, Qvist P, Christiansen C, Byrjalsen I, et al. An immunoassay for measuring fragments of newly synthesized collagen type I produced during metastatic invasion of bone. Clin Lab. 2004;50((5-6)):279–89. [PubMed] [Google Scholar]
- 30.Wada N, Fujisaki M, Ishii S, Ikeda T, Kitajima M. Evaluation of bone metabolic markers in breast cancer with bone metastasis. Breast Cancer. 2001;8((2)):131–7. doi: 10.1007/BF02967492. [DOI] [PubMed] [Google Scholar]
- 31.Molnár IA, Molnár BA, Vízkeleti L, Fekete K, Tamás J, Deák P, et al. Breast carcinoma subtypes show different patterns of metastatic behavior. Virchows Arch. 2017 Mar;470((3)):275–83. doi: 10.1007/s00428-017-2065-7. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Ahn SG, Lee HM, Park JT, Han K, Lee SA, et al. Metastasis-Free Interval Is Closely Related to Tumor Characteristics and Has Prognostic Value in Breast Cancer Patients with Distant Relapse. J Breast Cancer. 2015 Dec;18((4)):371–7. doi: 10.4048/jbc.2015.18.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Q, Li J, Zhu S, Wu J, Chen C, Liu Q, et al. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget. 2017 Apr;8((17)):27990–6. doi: 10.18632/oncotarget.15856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong Y, Liu YR, Ji P, Hu X, Shao ZM. Impact of molecular subtypes on metastatic breast cancer patients: a SEER population-based study. Sci Rep. 2017 Mar;7((1)):45411. doi: 10.1038/srep45411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Zhang C, Zhang J, Kong L, Zhu H, Yu J. The prognosis analysis of different metastasis pattern in patients with different breast cancer subtypes: a SEER based study. Oncotarget. 2017 Apr;8((16)):26368–79. doi: 10.18632/oncotarget.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahim T, Mercatali L, Amadori D. A new emergency in oncology: bone metastases in breast cancer patients (Review) Oncol Lett. 2013 Aug;6((2)):306–10. doi: 10.3892/ol.2013.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savci-Heijink CD, Halfwerk H, Hooijer GK, Horlings HM, Wesseling J, van de Vijver MJ. Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res Treat. 2015 Apr;150((3)):547–57. doi: 10.1007/s10549-015-3352-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobson AF, Shapiro CL, Van den Abbeele AD, Kaplan WD. Prognostic significance of the number of bone scan abnormalities at the time of initial bone metastatic recurrence in breast carcinoma. Cancer. 2001 Jan;91((1)):17–24. doi: 10.1002/1097-0142(20010101)91:1<17::aid-cncr3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 39.Di Lascio S, Pagani O. Oligometastatic breast cancer: a shift from palliative to potentially curative treatment? Breast Care (Basel) 2014 Feb;9((1)):7–14. doi: 10.1159/000358750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marino N, Woditschka S, Reed LT, Nakayama J, Mayer M, Wetzel M, et al. Breast cancer metastasis: issues for the personalization of its prevention and treatment. Am J Pathol. 2013 Oct;183((4)):1084–95. doi: 10.1016/j.ajpath.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 42.Rakha EA, Reis-Filho JS, Baehner F, Dabbs DJ, Decker T, Eusebi V, et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res. 2010;12((4)):207. doi: 10.1186/bcr2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeffrey SS, Lønning PE, Hillner BE. Genomics-based prognosis and therapeutic prediction in breast cancer. J Natl Compr Canc Netw. 2005 May;3((3)):291–300. doi: 10.6004/jnccn.2005.0016. [DOI] [PubMed] [Google Scholar]
- 44.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010 Jul;28((20)):3271–7. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 45.Shim HJ, Kim SH, Kang BJ, Choi BG, Kim HS, Cha ES, et al. Breast cancer recurrence according to molecular subtype. Asian Pac J Cancer Prev. 2014;15((14)):5539–44. doi: 10.7314/apjcp.2014.15.14.5539. [DOI] [PubMed] [Google Scholar]
- 46.Shachar SS, Muss HB. Internet tools to enhance breast cancer care. NPJ Breast Cancer. 2016 Apr;2((1)):16011. doi: 10.1038/npjbcancer.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martei YM, Matro JM. Identifying patients at high risk of breast cancer recurrence: strategies to improve patient outcomes. Breast Cancer (Dove Med Press) 2015 Oct;7:337–43. doi: 10.2147/BCTT.S91981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004 Dec;351((27)):2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 49.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002 Jan;415((6871)):530–6. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 50.Buyse M, Loi S, van't Veer L, Viale G, Delorenzi M, Glas AM, et al. TRANSBIG Consortium Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006 Sep;98((17)):1183–92. doi: 10.1093/jnci/djj329. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Zhou R, Baumbusch LO, Tsavachidis S, Brewster AM, Do KA, et al. Genomic copy number imbalances associated with bone and non-bone metastasis of early-stage breast cancer. Breast Cancer Res Treat. 2014 Jan;143((1)):189–201. doi: 10.1007/s10549-013-2796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Savci-Heijink CD, Halfwerk H, Koster J, van de Vijver MJ. A novel gene expression signature for bone metastasis in breast carcinomas. Breast Cancer Res Treat. 2016 Apr;156((2)):249–59. doi: 10.1007/s10549-016-3741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molina R, Jo J, Filella X, Zanón G, Farrus B, Muñoz M, et al. C-erbB-2, CEA and CA 15.3 serum levels in the early diagnosis of recurrence of breast cancer patients. Anticancer Res. 1999 Jul-Aug;19(4A):2551–5. [PubMed] [Google Scholar]
- 54.Nicolini A, Colombini C, Luciani L, Carpi A, Giuliani L. Evaluation of serum CA15-3 determination with CEA and TPA in the post-operative follow-up of breast cancer patients. Br J Cancer. 1991 Jul;64((1)):154–8. doi: 10.1038/bjc.1991.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi T, Ichiba T, Sakuyama T, Arakawa Y, Nagasaki E, Aiba K, et al. Possible clinical cure of metastatic breast cancer: lessons from our 30-year experience with oligometastatic breast cancer patients and literature review. Breast Cancer. 2012 Jul;19((3)):218–37. doi: 10.1007/s12282-012-0347-0. [DOI] [PubMed] [Google Scholar]
- 56.Puglisi F, Fontanella C, Numico G, Sini V, Evangelista L, Monetti F, et al. Follow-up of patients with early breast cancer: is it time to rewrite the story? Crit Rev Oncol Hematol. 2014 Aug;91((2)):130–41. doi: 10.1016/j.critrevonc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S, et al. ESO-MBC Task Force International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010 Apr;102((7)):456–63. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng YC, Ueno NT. Improvement of survival and prospect of cure in patients with metastatic breast cancer. Breast Cancer. 2012 Jul;19((3)):191–9. doi: 10.1007/s12282-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chia SK, Speers CH, D'yachkova Y, Kang A, Malfair-Taylor S, Barnett J, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007 Sep;110((5)):973–9. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 60.Gianmauro Numico CP. Giovanni Ucci, Stefania Gori, Massimo Di Maio, Maurizio Cancian, Francesco De Lorenzo, Marco Venturini, and Associazione Italiana di Oncologia Medica (AIOM): how are cared surviving cancer patients? Results of an Italian national survey to medical oncologists (MO) about clinical and organizational features of follow-up (FU) J Clin Oncol. 2012;•••:30. [Google Scholar]
- 61.Jay N, Nuemi G, Gadreau M, Quantin C. A data mining approach for grouping and analyzing trajectories of care using claim data: the example of breast cancer. BMC Med Inform Decis Mak. 2013 Nov;13((1)):130. doi: 10.1186/1472-6947-13-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blumen H, Fitch K, Polkus V. Comparison of Treatment Costs for Breast Cancer, by Tumor Stage and Type of Service. Am Health Drug Benefits. 2016 Feb;9((1)):23–32. [PMC free article] [PubMed] [Google Scholar]
- 63.Woo HK, Sunkara V, Park J, Kim TH, Han JR, Kim CJ, et al. Exodisc for Rapid, Size-Selective, and Efficient Isolation and Analysis of Nanoscale Extracellular Vesicles from Biological Samples. ACS Nano. 2017 Feb;11((2)):1360–70. doi: 10.1021/acsnano.6b06131. [DOI] [PubMed] [Google Scholar]
- 64.Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016 Sep;18((1)):90. doi: 10.1186/s13058-016-0753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banys-Paluchowski M, Witzel I, Riethdorf S, Rack B, Janni W, Fasching PA, et al. Clinical Relevance of Serum HER2 and Circulating Tumor Cell Detection in Metastatic Breast Cancer Patients. Anticancer Res. 2017 Jun;37((6)):3117–28. doi: 10.21873/anticanres.11669. [DOI] [PubMed] [Google Scholar]
- 66.Berghuis AM, Koffijberg H, Prakash J, Terstappen LW, IJzerman MJ. Detecting Blood-Based Biomarkers in Metastatic Breast Cancer: A Systematic Review of Their Current Status and Clinical Utility. Int J Mol Sci. 2017 Feb;18((2)):18. doi: 10.3390/ijms18020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chromosome 1q21.3 Amplification Is Linked to Breast Cancer Recurrence. Cancer Discov. 2017 doi: 10.1038/nm.4405. [DOI] [PubMed] [Google Scholar]
- 68.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of NCI-EORTC Working Group on Cancer Diagnostics REporting recommendations for tumor MARKer prognostic studies (REMARK) Breast Cancer Res Treat. 2006 Nov;100((2)):229–35. doi: 10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 69.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007 Nov;25((33)):5287–312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 70.Borrebaeck CA. Precision diagnostics: moving towards protein biomarker signatures of clinical utility in cancer. Nat Rev Cancer. 2017 Mar;17((3)):199–204. doi: 10.1038/nrc.2016.153. [DOI] [PubMed] [Google Scholar]
- 71.Smith ER, Zurakowski D, Saad A, Scott RM, Moses MA. Urinary biomarkers predict brain tumor presence and response to therapy. Clin Cancer Res. 2008 Apr;14((8)):2378–86. doi: 10.1158/1078-0432.CCR-07-1253. [DOI] [PubMed] [Google Scholar]
- 72.Di Gioia D, Stieber P, Schmidt GP, Nagel D, Heinemann V, Baur-Melnyk A. Early detection of metastatic disease in asymptomatic breast cancer patients with whole-body imaging and defined tumour marker increase. Br J Cancer. 2015 Mar;112((5)):809–18. doi: 10.1038/bjc.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roth C, Pantel K, Müller V, Rack B, Kasimir-Bauer S, Janni W, et al. Apoptosis-related deregulation of proteolytic activities and high serum levels of circulating nucleosomes and DNA in blood correlate with breast cancer progression. BMC Cancer. 2011 Jan;11((1)):4. doi: 10.1186/1471-2407-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pedroza-Díaz J, Röthlisberger S. Advances in urinary protein biomarkers for urogenital and non-urogenital pathologies. Biochem Med (Zagreb) 2015;25((1)):22–35. doi: 10.11613/BM.2015.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gasparri ML, Casorelli A, Bardhi E, Besharat AR, Savone D, Ruscito I, et al. Beyond circulating microRNA biomarkers: urinary microRNAs in ovarian and breast cancer. Tumour Biol. 2017 May;39((5)):1010428317695525. doi: 10.1177/1010428317695525. [DOI] [PubMed] [Google Scholar]
- 76.Erbes T, Hirschfeld M, Rücker G, Jaeger M, Boas J, Iborra S, et al. Feasibility of urinary microRNA detection in breast cancer patients and its potential as an innovative non-invasive biomarker. BMC Cancer. 2015 Mar;15((1)):193. doi: 10.1186/s12885-015-1190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marimuthu A, O'Meally RN, Chaerkady R, Subbannayya Y, Nanjappa V, Kumar P, et al. A comprehensive map of the human urinary proteome. J Proteome Res. 2011 Jun;10((6)):2734–43. doi: 10.1021/pr2003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Julian BA, Suzuki H, Suzuki Y, Tomino Y, Spasovski G, Novak J. Sources of Urinary Proteins and their Analysis by Urinary Proteomics for the Detection of Biomarkers of Disease. Proteomics Clin Appl. 2009 Aug;3((9)):1029–43. doi: 10.1002/prca.200800243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramsey SD, Henry NL, Gralow JR, Mirick DK, Barlow W, Etzioni R, et al. Tumor marker usage and medical care costs among older early-stage breast cancer survivors. J Clin Oncol. 2015 Jan;33((2)):149–55. doi: 10.1200/JCO.2014.55.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou M, Conrads TP, Veenstra TD. Proteomics approaches to biomarker detection. Brief Funct Genomics Proteomics. 2005 May;4((1)):69–75. doi: 10.1093/bfgp/4.1.69. [DOI] [PubMed] [Google Scholar]
- 81.Good DM, Zürbig P, Argilés A, Bauer HW, Behrens G, Coon JJ, et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics. 2010 Nov;9((11)):2424–37. doi: 10.1074/mcp.M110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moses MA, Wiederschain D, Loughlin KR, Zurakowski D, Lamb CC, Freeman MR. Increased incidence of matrix metalloproteinases in urine of cancer patients. Cancer Res. 1998 Apr;58((7)):1395–9. [PubMed] [Google Scholar]
- 83.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001 Oct;276((40)):37258–65. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 84.Roy R, Wewer UM, Zurakowski D, Pories SE, Moses MA. ADAM 12 cleaves extracellular matrix proteins and correlates with cancer status and stage. J Biol Chem. 2004 Dec;279((49)):51323–30. doi: 10.1074/jbc.M409565200. [DOI] [PubMed] [Google Scholar]
- 85.Wu ZS, Wu Q, Yang JH, Wang HQ, Ding XD, Yang F, et al. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer. 2008 May;122((9)):2050–6. doi: 10.1002/ijc.23337. [DOI] [PubMed] [Google Scholar]
- 86.La Rocca G, Pucci-Minafra I, Marrazzo A, Taormina P, Minafra S. Zymographic detection and clinical correlations of MMP-2 and MMP-9 in breast cancer sera. Br J Cancer. 2004 Apr;90((7)):1414–21. doi: 10.1038/sj.bjc.6601725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fernández CA, Yan L, Louis G, Yang J, Kutok JL, Moses MA. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin Cancer Res. 2005 Aug;11((15)):5390–5. doi: 10.1158/1078-0432.CCR-04-2391. [DOI] [PubMed] [Google Scholar]
- 88.Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, et al. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci USA. 2009 Mar;106((10)):3913–8. doi: 10.1073/pnas.0810617106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hou MF, Chen YL, Tseng TF, Lin CM, Chen MS, Huang CJ, et al. Evaluation of serum CA27.29, CA15-3 and CEA in patients with breast cancer. Kaohsiung J Med Sci. 1999 Sep;15((9)):520–8. [PubMed] [Google Scholar]
- 90.Hiramatsu K, Takahashi K, Yamaguchi T, Matsumoto H, Miyamoto H, Tanaka S, et al. N(1),N(12)-Diacetylspermine as a sensitive and specific novel marker for early- and late-stage colorectal and breast cancers. Clin Cancer Res. 2005 Apr;11((8)):2986–90. doi: 10.1158/1078-0432.CCR-04-2275. [DOI] [PubMed] [Google Scholar]
- 91.Beretov J, Wasinger VC, Millar EK, Schwartz P, Graham PH, Li Y. Proteomic Analysis of Urine to Identify Breast Cancer Biomarker Candidates Using a Label-Free LC-MS/MS Approach. PLoS One. 2015 Nov;10((11)):e0141876. doi: 10.1371/journal.pone.0141876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yadav GC, Rao A, Motawy MM, Safadi N, Ahmed MJ. CA 15.3 with urinary calcium excretion is useful in the diagnosis and monitoring of bone metastases from breast cancer. Int J Biol Markers. 1993 Oct-Dec;8((4)):208–14. doi: 10.1177/172460089300800402. [DOI] [PubMed] [Google Scholar]
- 93.Houzé P, Bellik B, Extra JM, Bouro F, Bousquet B. Urinary carboxyterminal telopeptide of collagen I as a potential marker of bone metastases chemotherapy monitoring in breast cancer. Clin Chim Acta. 1999 Mar;281((1-2)):77–88. doi: 10.1016/s0009-8981(98)00209-5. [DOI] [PubMed] [Google Scholar]
- 94.Hashim AA, Ali SA, Emara IA, El-Hefnawy MH. CTX Correlation to Disease Duration and Adiponectin in Egyptian Children with T1DM. J Med Biochem. 2016 Jan;35((1)):34–42. doi: 10.1515/jomb-2015-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]