Abstract
All currently available general anesthetic agents possess potentially lethal side effects requiring their administration by highly trained clinicians. Among these agents is etomidate, a highly potent imidazole-based intravenous sedative-hypnotic that deleteriously suppresses the synthesis of adrenocortical steroids in a manner that is both potent and persistent. We developed two distinct strategies to design etomidate analogs that retain etomidate’s potent hypnotic activity, but produce less adrenocortical suppression than etomidate. One strategy seeks to reduce binding to 11β-hydroxylase, a critical enzyme in the steroid biosynthetic pathway, which is potently inhibited by etomidate. The other strategy seeks to reduce the duration of adrenocortical suppression after etomidate administration by modifying the drug’s structure to render it susceptible to rapid metabolism by esterases. In this chapter, we describe the methods used to evaluate the hypnotic and adrenocortical inhibitory potencies of two lead compounds designed using the aforementioned strategies. Our purpose is to provide a case study for the development of novel analogs of existing drugs with reduced side effects.
1. INTRODUCTION
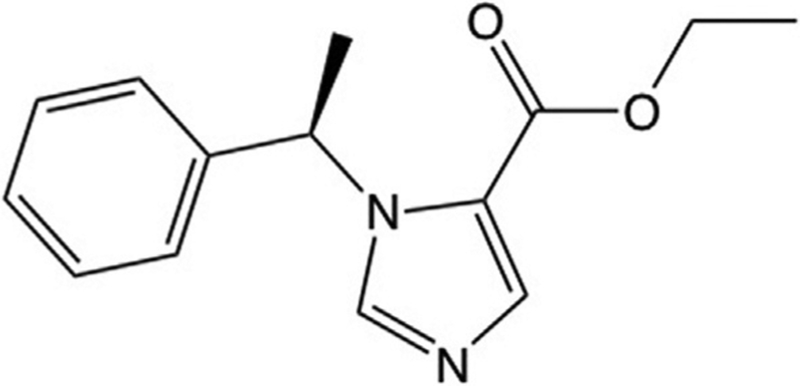
Etomidate is an intravenous sedative-hypnotic agent that was originally invented by Janssen Pharmaceuticals in the 1960s (Fig. 1) (Godefroi, Janssen, Vandereycken, Vanheertum, & Niemegeers, 1965). It emerged from their antifungal agent development program, which involved the synthesis of novel imidazole-containing compounds designed to suppress the biosynthesis of the fungal steroid ergosterol by inhibiting the cytochrome P450 enzyme 14α-demethylase. When tested in rats, etomidate exhibited both potent anesthetic activity and a very high therapeutic index (Godefroi et al., 1965; Janssen, Niemegeers, & Marsboom, 1975). Consequently, it was developed as an anesthetic induction and maintenance agent for use in the operating room, and as a sedative for critically ill patients in the intensive care unit. However, a decade after its introduction into clinical practice in 1972, etomidate was discovered to also produce a profound and persistent suppression of adrenocortical steroid synthesis that was associated with a significant increase in the mortality of critically ill patients who had received prolonged infusions (Ledingham & Watt, 1983; Watt & Ledingham, 1984). Such suppression is caused by etomidate binding to and inhibiting the function of 11β-hydroxylase, a cytochrome P450 enzyme that is found in the adrenal cortex and required for the biosynthesis of cortisol, corticosterone, and aldosterone (Allolio, Stuttmann, Leonhard, Fischer, & Winkelmann, 1984; de Jong, Mallios, Jansen, Scheck, & Lamberts, 1984; Duthie, Fraser, & Nimmo, 1985; Wagner & White, 1984; Wagner, White, Kan, Rosenthal, & Feldman, 1984). So ironically, a drug whose roots were as an antifungal agent intended to act by suppressing the production of ergosterol—a steroid that is absent in animals, but a critical component of the fungal cell membrane—instead potently inhibits a homologous enzyme found in humans and other animals that is required for the biosynthesis of adrenocortical steroids. Because of this undesirable side effect, etomidate is no longer used as a prolonged infusion to maintain sedation or anesthesia. However, it continues to be used as a single bolus to induce anesthesia because it maintains hemodynamic stability better than any other induction agent (Gooding & Corssen, 1977; Lamalle, 1976; Stockham et al., 1987). Nevertheless, significant concerns remain regarding even that brief use because although such a bolus produces anesthesia for several minutes, the associated suppression of steroid synthesis persists for hours or even days (den Brinker et al., 2008; Vinclair et al., 2007).
Fig. 1.
Molecular structure of etomidate.
Etomidate produces anesthesia by binding to and enhancing the function of γ-aminobutyric acid type A (GABAA) receptors (Forman, 2011). Thus, its anesthetic and steroidogenesis inhibitory activities are mediated by well-characterized but different protein targets with presumably distinct structure–activity relationships. This suggested to us that it might be possible to design etomidate analogs that retain etomidate-like anesthetic activity but are safer because they lack etomidate’s ability to produce clinically significant adrenocortical suppression.
2. ETOMIDATE ANALOG DESIGN STRATEGIES
We developed two separate drug design strategies to produce etomidate analogs with reduced abilities to suppress adrenocortical function (Cotten et al., 2010, 2009; Raines, 2015). One strategy was pharmacodynamic in nature, whereas the other one was pharmacokinetic. The pharmacodynamic strategy was to alter etomidate’s molecular structure to reduce binding affinity to 11β-hydroxylase, while retaining potent GABAA receptor positive modulatory activity. The pharmacokinetic strategy was to design a rapidly metabolized “soft” analog of etomidate so that any adrenocortical suppression that is produced by the drug would last for only minutes (rather than hours or days) following drug administration.
2.1. Pharmacodynamic Strategy
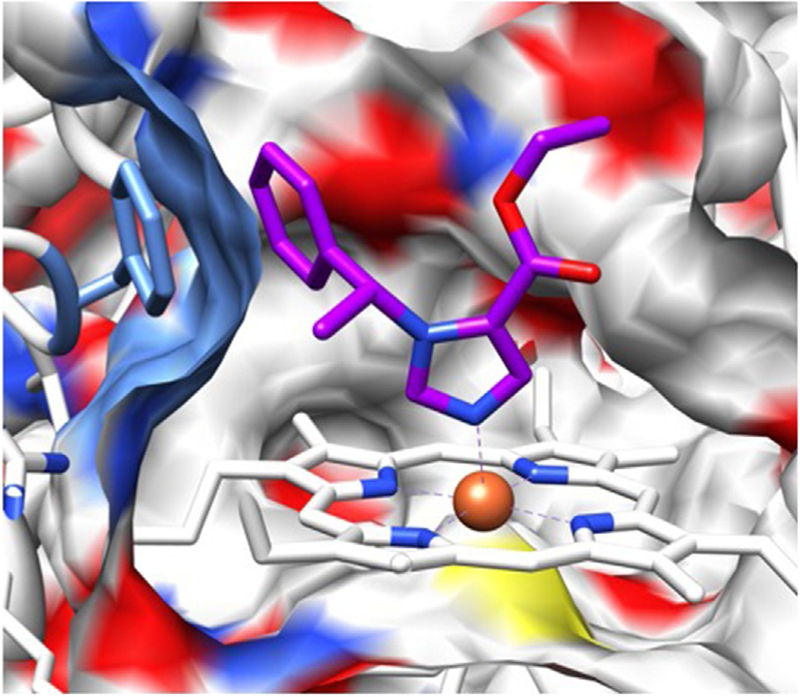
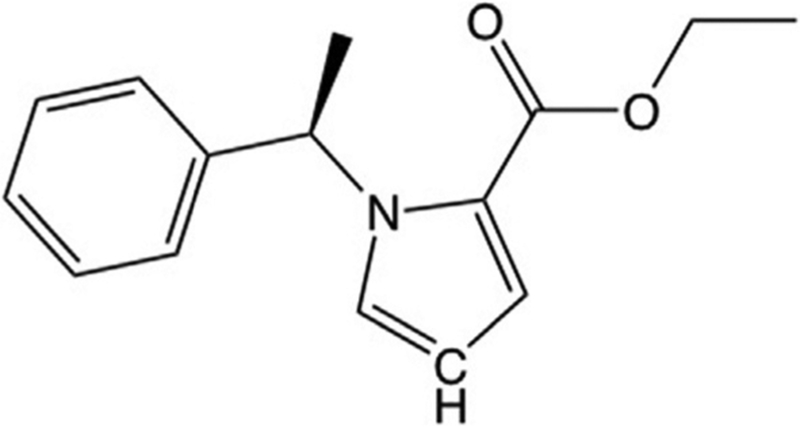
X-ray crystallographic structures of drug targets can suggest strategies for designing ligands (e.g., drugs) that bind—or in our case do not bind—with high affinity. Unfortunately, this was not available for 11β-hydroxylase at the time that we began to develop the pharmacodynamic strategy. However, there were published structures for several other P450 enzymes with imidazole-containing ligands bound. A common feature of these structures was the presence of a coordination bond between the aromatic nitrogen in the ligand’s imidazole ring and the heme iron at the enzyme’s active site (Ouellet, Podust, & de Montellano, 2008; Scott et al., 2004; Seward, Roujeinikova, McLean, Munro, & Leys, 2006). By analogy, we (and others) hypothesized that a similar coordination bond between the aromatic nitrogen in etomidate’s imidazole ring and 11β-hydroxylase’s active site heme iron was responsible for etomidate’s high binding affinity to this enzyme (Fig. 2) (Roumen et al., 2007). If correct, then etomidate’s binding affinity to 11β-hydroxylase could be greatly reduced by replacing this nitrogen with another atom(s) that was unable to form such a bond. This led us to synthesize carboetomidate, an etomidate analog in which the aromatic nitrogen was replaced by a methine (CH) group (Fig. 3).
Fig. 2.
Homology model of 11β-hydroxlase with etomidate computationally docked. A cross section through the surface of the active site cavity is shown. Etomidate is depicted in stick representation with blue nitrogens, red oxygens, and purple carbons. The heme iron is orange and coordinates with etomidate’s aromatic imidazole nitrogen. The authors thank Drs. Keith W. Miller and Sivananthaperumal Shanmugasundararaj for producing this figure.
Fig. 3.
Carboetomidate is an analog of etomidate in which the aromatic nitrogen in etomidate’s imidazole ring has been replaced with a methine group. This disrupts the ability of etomidate to form a coordination bond with the heme iron at the active site of 11β-hydroxylase, lowering its affinity for the enzyme.
2.2. Pharmacokinetic Strategy
As noted earlier, a remarkable feature of etomidate-induced adrenocortical suppression is that it persists long after etomidate’s anesthetic actions have worn off. This is attributable to etomidate’s vastly (~100-fold) higher potency for inhibiting 11β-hydroxylase vs positively modulating GABAA receptors. A typical anesthetic induction dose of etomidate represents an extreme overdose with respect to the drug’s ability to suppress adrenocortical function. Thus following such a dose, it takes much longer to recover adrenocortical function than to emerge from anesthesia.
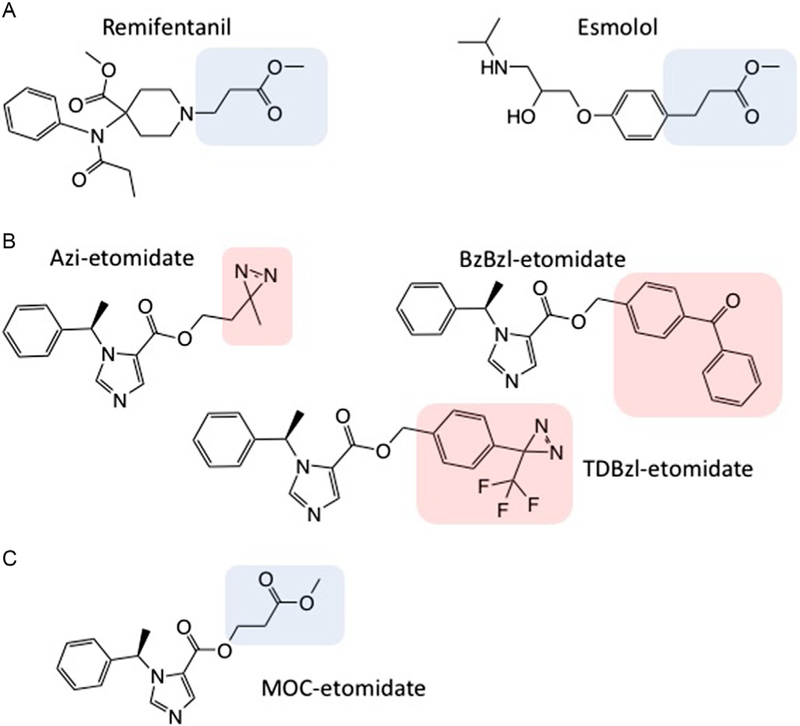
We hypothesized that we could significantly reduce the duration of adrenocortical suppression by modifying etomidate’s structure to accelerate the rate at which the drug is metabolized. This “soft analog” approach, which is exemplified by remifentanil and esmolol, often entails the addition of a metabolically labile ester moiety to render the drug highly susceptible to hydrolysis by nonspecific esterases (Fig. 4A) (Bodor & Buchwald, 2000; Buchwald & Bodor, 2002). The resulting carboxylic acid metabolites typically have pharmacological activities that are orders of magnitude lower than their corresponding parent drugs. A key consideration when designing a soft analog is where on the molecule to append the ester moiety. As previous studies had demonstrated that large photoactivatable moieties could be appended distal to etomidate’s existing (but metabolically stable) ester without disrupting either GABAA receptor modulatory or anesthetic activities, we elected to place the ester at this location (Fig. 4B) (Husain et al., 2006, 2010, 2003; Liao et al., 2005). Like remifentanil and esmolol, the ester was linked to the pharmacophore by a spacer composed of two methylene groups to reduce potential steric hindrance and facilitate esterase hydrolysis (Fig. 4C). We named this soft etomidate analog methoxycarbonyl-etomidate (MOC-etomidate).
Fig. 4.
(A) Molecular structures of remifentanil and esmolol. In each structure, the metabolically labile ester moiety along with the two-carbon linker are highlighted. (B) Molecular structures of photoactivatable etomidate analogs. These analogs retain etomidate’s potent GABAA receptor positive modulatory and anesthetic activities. In each structure, the photoactivatable moiety is highlighted. (C) Molecular structure of methoxycarbonyl-etomidate (MOC-etomidate).
3. TESTING NOVEL ETOMIDATE ANALOGS FOR HYPNOTIC ACTIVITY USING LOSS OF RIGHTING REFLEXES ASSAYS
When placed supine, animals will reorient themselves to return to their usual upright position. Because this righting reflex is lost when animals are anesthetized, it is commonly used as an animal correlate for hypnosis (unconsciousness). To confirm that carboetomidate and MOC-etomidate possessed hypnotic activities and to define their hypnotic potencies, we used tadpole and rat loss of righting reflexes (LoRR) assays.
3.1. Tadpole Loss of Righting Reflex Assay
Although a variety of animals can and have been used as subjects for LoRR assays, small aquatic animals (e.g., tadpoles) offer unique advantages. First, they are inexpensive and easy to care for. Consequently, they allow one to study large numbers of animals easily, quickly, and at low cost. Second, anesthetic actions can be assessed at well-defined steady-state anesthetic concentrations. This may be contrasted with assays in which anesthetics are injected into animals, where anesthetic concentrations in the brain vary constantly over time after administration due to anesthetic distribution, redistribution, and metabolism. Finally, protein binding minimally impacts measurements of anesthetic potencies because the free in vivo anesthetic concentrations approximate the anesthetic concentrations in the aqueous environment. It should be noted though that for very hydrophobic anesthetics, absorption into tadpoles may reduce the aqueous concentration to a value that is significantly below the nominal concentration calculated from the mass of added anesthetic and the aqueous volume. For such drugs, depletion can be minimized by keeping the number of tadpoles per unit aqueous volume low (e.g., 1 tadpole per 100mL of water). For anesthetics possessing titratable chemical moieties, it is also preferable to buffer the pH at a constant near-physiological value (e.g., pH 7.4) to maintain a constant ratio of charged (i.e., hydrophilic) and uncharged (i.e., hydrophobic) molecular species regardless of the anesthetic concentration as only the uncharged species is likely to contribute significantly to hypnotic effects.
3.1.1. Equipment
100− to 1000−mL glass chamber(s)
Bent glass rod or other device to turn tadpoles supine
3.1.2. Animals and Buffers
Prelimb Rana pipens tadpoles (1–1.5cm in length, ~100 in number to complete a full concentration–response curve)
Tris–HCl buffer (2.5mM; pH 7.4) in dechlorinated water
3.1.3. Procedure
Add 50–500mL of the Tris–HCl buffer containing the desired concentration of test compound to the glass chamber.
Place five tadpoles in the buffer-containing chamber.
After a 10-min incubation time, test each tadpole for LoRR by turning it supine. A tadpole is defined as having LoRR if it does not turn back over to the prone position within 5s. This test is repeated every 10min until a stable behavioral response has been reached.
Remove tadpoles to fresh distilled water.
Tadpoles that had LoRR should be recovered separately to ensure reversibility and thus distinguish hypnosis from toxicity.
Notes
We have found that behavioral responses typically stabilize in ~30min.
By defining the effects of a range of test compound concentrations, a concentration–response curve can be generated and a median effective concentration (EC50) can be calculated.
3.2. Rat Loss of Righting Reflex Assay
After confirming that carboetomidate and MOC-etomidate possessed hypnotic activities in tadpoles (albeit at concentrations that were 5–10 times greater than that of etomidate), we evaluated their hypnotic activities in rats using an analogous LoRR. As with the tadpole LoRR assay, the outcome for each individual rat experiment is quantal: after receiving the anesthetic dose, the rat either has LoRR or its does not.
3.2.1. Equipment
Rat restraint tube
Volatile anesthetic delivery system (optional)
24-G intravenous catheters
Syringes
3.2.2. Animals and Reagents
Rats (e.g., Sprague–Dawley)
Heparin (10 units/mL)
3.2.3. Procedure
Gently restrain the rat in the restraint tube with the tail exiting through the back of the tube.
Place a 24-G intravenous catheter in one of the lateral tail veins and secure. This may be accomplished in either an awake rat or one that has been anesthetized with a volatile anesthetic (e.g., 3% isoflurane). If a volatile anesthetic has been used to immobilize the rat during catheter placement, then allow at least 1h for recovery before performing the LoRR test. After catheter placement, fill the catheter void volume with heparinized saline (10 units/mL) to prevent clotting.
Administer formulated test compound via syringe through the catheter followed by an 0.5mL saline flush.
Turn the restraint tube containing the rat over to place the rat in a supine position. Open the restraining tube and slide the rat out, keeping the rat in the supine position.
A rat is defined as having LoRR if it does not turn back over to the prone position within 5s.
The duration of LoRR is defined as the time from test compound injection until the time that the rat turns over onto all four paws.
Notes
Because anesthetics are typically hydrophobic, formulation can be challenging. We have commonly used dimethyl sulfoxide (DMSO) alone or cyclodextrin in water to solubilize test compounds for intravenous injection. Ideal intravenous injection volumes are 0.15–0.3mL. As DMSO causes hemolysis, it is not uncommon for the urine to become pink tinged following its injection.
We attach a saline-flushed 5-in. Microbore Extension Set (Hospira, Lake Forest, IL) to the catheter to facilitate test compound injection and flushing.
The onset of LoRR after injecting a test compound with hypnotic activity is typically less than 10s, although in some cases (e.g., carboetomidate) it may be longer.
By defining the effects of a range of test compound doses, a dose–response curve can be generated and a median effective dose (ED50) can be calculated.
These LoRR assays confirmed that both carboetomidate and MOC-etomidate produce hypnosis in tadpoles and rats (Cotten et al., 2010, 2009). Of note, the durations of LoRR produced in rats by even very high doses of MOC-etomidate (e.g., 40mg/kg, which is 8 × its ED50 for LoRR) were extremely short (~ 3min), consistent with very rapid in vivo metabolism (Cotten et al., 2009). For comparison, a half-equivalent hypnotic dose of etomidate (4mg/kg) produces nearly a half hour of LoRR in rats.
4. TESTING NOVEL ETOMIDATE ANALOGS FOR ADRENOCORTICAL INHIBITORY ACTIVITY
4.1. In Vivo Rat Adrenocortical Inhibition Assay
After having determined that carboetomidate and MOC-etomidate retain etomidate-like hypnotic action (and GABAA receptor positive modulatory activity, see chapter “Combining mutations and electrophysiology to map anesthetic sites on ligand-gated ion channels” by Forman in Vol. 602), we turned our attention to quantifying the extent to which these two etomidate analogs suppress adrenocortical function (Cotten et al., 2010, 2009). We used an adrenocorticotropic hormone (ACTH) stimulation test to evaluate their effects on corticosterone biosynthesis in rats. We pretreat each rat with dexamethasone prior to performing this assay to suppress endogenous ACTH production, thus reducing the baseline serum corticosterone concentration; if the baseline corticosterone concentration is already high due to (for example) the stress of handling, then interpreting the meaning of a similarly high corticosterone concentration after stimulating corticosterone biosynthesis with ACTH becomes difficult.
4.1.1. Equipment
Rat restraint tube
Volatile anesthetic delivery system (optional)
24-G intravenous catheters
Centrifuge
96-Well plate reader
4.1.2. Animals and Reagents
Rats (e.g., Sprague–Dawley)
Dexamethasone
ACTH1–24
Heparin (10 units/mL)
Enzyme-linked immunoabsorbant assay (ELISA) kit for the desired adrenocortical steroid
4.1.3. Procedure
Place a 24-G intravenous catheter in one of the lateral tail veins and secure as described above under rat LoRR assay.
Administer dexamethasone (0.2mg/kg intravenously) to the rat followed by an 0.5mL saline flush.
Two hours after administering the initial dose of dexamethasone, administer a second dexamethasone dose and the test compound (e.g., carboetomidate or MOC-etomidate) followed by an 0.5mL saline flush.
Administer ACTH1–24 (25μg/kg) intravenously either immediately after the test anesthetic (carboetomidate) or 15min later (MOC-etomidate) followed by an 0.5mL saline flush. For MOC-etomidate, the 15-min delay after administering ACTH1–24 provides the brief time required for metabolism to convert the drug to its carboxylic acid metabolite.
Fifteen minutes after administering ACTH1–24, draw a blood sample (~0.3mL) from the intravenous catheter and allow it to clot at room temperature.
Centrifuge the clotted blood sample at 16,000 × g for 5–15min to separate the serum from red blood cells and other cellular debris.
Determine the corticosterone concentrations in serum using an ELISA.
Notes
We use etomidate as a comparator for all etomidate analogs. An etomidate dose of 0.5–1mg/kg will reduce the adrenocortical response by approximately half (Pejo et al., 2014).
After collection, serum may be frozen and stored at − 20°C until the corticosterone concentration is measured. When we anticipate running multiple ELISAs on different days using a single blood sample, then we aliquot the blood into several vials that can be separately thawed to avoid multiple freeze–thaw cycles.
The concentrations of other adrenocortical steroids can also be measured from the same serum samples using the appropriate ELISA kit.
Many ELISAs can also be done with plasma. If using plasma, add the blood sample to a heparinized or EDTA-coated vacuum tube (e.g., BD Vacutainer). Gently invert the tube 5–10 times to ensure complete mixing with the anticoagulant. Immediately centrifuge at 16,000 × g for 5min and then aliquot the plasma into Eppendorf tubes for freezing at −20°C until ready to use.
After administering a single bolus using a dose sufficient to produce LoRR, neither carboetomidate nor MOC-etomidate significantly reduced ACTH-stimulated serum corticosterone concentrations in rats.
4.2. In Vitro 3H-Etomidate Competition Assay
We designed carboetomidate as an etomidate analog with low affinity for the etomidate binding site on 11β-hydroxylase, and results from both our rat adrenocortical inhibition assay and our computational docking studies suggested that we had achieved that goal (Cotten et al., 2010; Pejo et al., 2011; Shanmugasundararaj et al., 2013). As further confirmation, we developed a competitive binding assay between carboetomidate and 3H-etomidate (Pejo, Zhou, Husain, & Raines, 2016). In this assay, 3H-etomidate is used at a low nanomolar concentration to selectively bind to 11β-hydroxylase in adrenal tissue. The desired concentration of etomidate or carboetomidate (or any other test compound) is added, and after equilibration the mixture is filtered to separate free from enzyme-bound 3H-etomidate. Bound 3H-etomidate, which is retained on the filter, is then quantified by scintillation counting.
4.2.1. Equipment
Centrifuge
Liquid scintillation counter
Electric homogenizer
Glass and teflon homogenizer
Vacuum filter
Disposable culture tubes (borosilicate glass; 13 × 100mm2)
4.2.2. Tissue, Buffers, and Reagents
Fresh or freshly frozen adrenal glands
Preparation buffer: 10mM HEPES, 1mM ethylenediaminetetracetic acid, 10 μg/mL leupeptin, 10 μg/mL chymostatin, 10 μg/mL pepstatin A, 2 μg/mL aprotinin, 1mM polymethanesulfonyl fluoride, and 10 μL/mL ethanol
Diisopropyl fluorophosphates (DFP)
10-mL syringe
23-G needle
Bicinchoninic acid (BCA) protein assay kit
3H-etomidate (specific activity ~10Ci/mM)
GF/B glass fiber filters
Polyethylenimine
Assay buffer: 11.9mM phosphates, 137mM NaCl, 200mM KCl, 1mM ethylenediaminetetracetic acid, and pH 7.4
4.2.3. Procedure
- Preparation of adrenal homogenate
- Cut the adrenal tissue into small pieces. Add the pieces to ice-cold preparation buffer (10mL buffer per 1g of adrenal tissue, rounded up). Homogenize the mixture on ice first using an electric homogenizer (6 × for 5s each) and then by hand using a glass and Teflon homogenizer (20 passes).
- Add DFP (1mM final concentration) and incubate for 60min at room temperature to inactivate esterases.
- Centrifuge the mixture (4°C, 43,100 × g) for 30min.
- Discard the supernatant and resuspend the pellet in fresh, cold preparation buffer (10mL buffer per 1g of adrenal tissue).
- Repeat the entire homogenization process with electric and manual homogenizers.
- Centrifuge the mixture (4°C, 43,100 × g) for 30min.
- Resuspend the pellet in 5–8mL of cold preparation buffer and briefly (5–10 s) mix with an electric homogenizer three times.
- Transfer the homogenate to a clean vial. Using a 10-mL syringe, draw and expel the homogenate through a 23-G needle three times to break up residual particulate matter.
- Aliquot 0.5–1mL into 1.5-mL eppendorf tubes and store at −80°C until used. Save an 0.1mL aliquot to quantify the protein concentration in the homogenate using the BCA kit.
- Competition assay
- Prepare 25mm GF/B glass fiber filters at least 2h in advance by soaking them in water containing 0.5% polyethylenimine.
- Prepare a 2-mL solution of 3H-etomidate (final concentration 2nM), adrenal homogenate (final concentration 0.07mg/mL), and the desired concentration of test compound in assay buffer.
- Gently mix the solution and allow it to equilibrate at room temperature for 15–30min.
- Vortex adrenal solution for 5s immediately before filtering. Pass 0.5mL of the mixture through a presoaked 25mm GF/B glass fiber filter under suction. Each solution can be run in triplicates by adding 0.5mL to three different filters.
- Wash the filter immediately with 5mL of buffer. Allow the buffer to pass through the filter and repeat wash.
- Dry the filters under a heat lamp for 2h and transfer to a scintillation vial containing Liquiscint scintillation cocktail.
- Quantify tritium radioactivity by liquid scintillation counting.
Notes
DFP is highly toxic. Use extreme care and adequate protection.
Displacement of 3H-etomidate by a high concentration of etomidate (e.g., 100μM) is >95%, indicative of minimal nonspecific 3H-etomidate binding.
Do not subject adrenal homogenates to repeated freeze–thaw cycles as this will reduce 3H-etomidate binding. We thaw homogenates in cold water and then keep them on ice until used (always within 6h of thawing).
Membrane proteins are susceptible to aggregation and degradation. Therefore, we mix solutions frequently but gently, and minimize vortexing.
By defining the extent of 3H-etomidate displacement produced by a range of test compound concentrations, a concentration–response curve can be generated and a half-inhibitory concentration (IC50) can be calculated.
We determined that the half-inhibitory concentrations of etomidate and carboetomidate were 26nM and 50μM, respectively. Thus, carboetomidate has an affinity for the etomidate binding site on 11β-hydroxylase that is 2000 × lower than etomidate.
5. SUMMARY AND CONCLUSION
In this chapter, we have presented two distinct strategies for producing etomidate analogs that retain etomidate’s ability to positively modulate GABAA receptors and produce hypnosis, but with substantially less ability to suppress adrenocortical steroid synthesis. We used a tadpole LoRR assay to detect hypnotic activity and to define the steady-state concentrations required to produce hypnosis. Such information is critical for defining the relevant concentration ranges for performing in vitro studies (e.g., GABAA receptor physiology). We also used a rat LoRR to define the dosing requirement for producing hypnosis in a small mammal and to assess each analog’s duration of action after single bolus administration. The abilities of etomidate analogs to bind to 11β-hydroxylase and suppress adrenocortical steroid synthesis were defined using an 3H-etomidate displacement assay and an ACTH stimulation test, respectively. Together, these studies showed that carboetomidate positively modulates GABAA receptors and produces hypnosis, but does not suppress adrenocortical steroid synthesis because it binds to 11β-hydroxylase with an affinity that is three orders of magnitude lower than etomidate. MOC-etomidate similarly positively modulates GABAA receptors and produces hypnosis, but does not produce measurable suppression of adrenocortical steroid synthesis because it is so rapidly metabolized. This rapid metabolism also confers upon MOC-etomidate a very brief duration of hypnotic action in rats even after the administration of a very high bolus dose. Additional soft etomidate analogs have been made since the development of MOC-etomidate with properties that are optimized for clinical use. One of these analogs (CPMM, also known as ABP-700) has reached human trials (Struys et al., 2017).
ACKNOWLEDGMENTS
Funded by Grants GM087316 and GM58448 from the National Institutes of Health, Bethesda, MD, and the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA.
REFERENCES
- Allolio B, Stuttmann R, Leonhard U, Fischer H, & Winkelmann W (1984). Adrenocortical suppression by a single induction dose of etomidate. Klinische Wochenschrift, 62(21), 1014–1017. [DOI] [PubMed] [Google Scholar]
- Bodor N, & Buchwald P (2000). Soft drug design: General principles and recent applications. Medicinal Research Reviews, 20(1), 58–101. [DOI] [PubMed] [Google Scholar]
- Buchwald P, & Bodor N (2002). Physicochemical aspects of the enzymatic hydrolysis of carboxylic esters. Pharmazie, 57(2), 87–93. [PubMed] [Google Scholar]
- Cotten JF, Forman SA, Laha JK, Cuny GD, Husain SS, Miller KW, et al. (2010). Carboetomidate: A pyrrole analog of etomidate designed not to suppress adrenocortical function. Anesthesiology, 112(3), 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten JF, Husain SS, Forman SA, Miller KW, Kelly EW, Nguyen HH, et al. (2009). Methoxycarbonyl-etomidate: A novel rapidly metabolized and ultra-shortacting etomidate analogue that does not produce prolonged adrenocortical suppression. Anesthesiology, 111(2), 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong FH, Mallios C, Jansen C, Scheck PA, & Lamberts SW (1984). Etomidate suppresses adrenocortical function by inhibition of 11 beta-hydroxylation. The Journal of Clinical Endocrinology and Metabolism, 59(6), 1143–1147. [DOI] [PubMed] [Google Scholar]
- den Brinker M, Hokken-Koelega AC, Hazelzet JA, de Jong FH, Hop WC, & Joosten KF (2008). One single dose of etomidate negatively influences adrenocortical performance for at least 24h in children with meningococcal sepsis. Intensive Care Medicine, 34(1), 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie DJ, Fraser R, & Nimmo WS (1985). Effect of induction of anaesthesia with etomidate on corticosteroid synthesis in man. British Journal of Anaesthesia, 57(2), 156–159. [DOI] [PubMed] [Google Scholar]
- Forman SA (2011). Clinical and molecular pharmacology of etomidate. Anesthesiology,114(3), 695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroi EF, Janssen PA, Vandereycken CA, Vanheertum AH, & Niemegeers CJ (1965). Dl-1-(1-Arylalkyl)Imidazole-5-carboxylate Esters. A novel type of hypnotic agents. Journal of Medicinal Chemistry, 8, 220–223. [DOI] [PubMed] [Google Scholar]
- Gooding JM, & Corssen G (1977). Effect of etomidate on the cardiovascular system. Anesthesia and Analgesia, 56(5), 717–719. [DOI] [PubMed] [Google Scholar]
- Husain SS, Nirthanan S, Ruesch D, Solt K, Cheng Q, Li GD, et al. (2006). Synthesis of trifluoromethylaryl diazirine and benzophenone derivatives of etomidate that are potent general anesthetics and effective photolabels for probing sites on ligand-gated ion channels. Journal of Medicinal Chemistry, 49(16), 4818–4825. [DOI] [PubMed] [Google Scholar]
- Husain SS, Stewart D, Desai R, Hamouda AK, Li SG, Kelly E, et al. (2010). p-Trifluoromethyldiazirinyl-etomidate: A potent photoreactive general anesthetic derivative of etomidate that is selective for ligand-gated cationic ion channels. Journal of Medicinal Chemistry, 53(17), 6432–6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain SS, Ziebell MR, Ruesch D, Hong F, Arevalo E, Kosterlitz JA, et al. (2003). 2-(3-Methyl-3H-diaziren-3-yl)ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate: A derivative of the stereoselective general anesthetic etomidate for photolabeling ligand-gated ion channels. Journal of Medicinal Chemistry, 46(7), 1257–1265. [DOI] [PubMed] [Google Scholar]
- Janssen PA, Niemegeers CJ, & Marsboom RP (1975). Etomidate, a potent nonbarbiturate hypnotic. Intravenous etomidate in mice, rats, guinea-pigs, rabbits and dogs. Archives Internationales de Pharmacodynamie et de Thérapie, 214(1), 92–132. [PubMed] [Google Scholar]
- Lamalle D (1976). Cardiovascular effects of various anesthetics in man. Four short-acting intravenous anesthetics: Althesin, etomidate, methohexital and propanidid. Acta Anaesthesiologica Belgica, 27(suppl), 208–224. [PubMed] [Google Scholar]
- Ledingham IM, & Watt I (1983). Influence of sedation on mortality in critically ill multiple trauma patients. Lancet, 1(8336), 1270. [DOI] [PubMed] [Google Scholar]
- Liao M, Sonner JM, Husain SS, Miller KW, Jurd R, Rudolph U, et al. (2005). R (+) etomidate and the photoactivable R (+) azietomidate have comparable anesthetic activity in wild-type mice and comparably decreased activity in mice with a N265M point mutation in the gamma-aminobutyric acid receptor beta3 subunit. Anesthesia and Analgesia, 101(1), 131–135. [DOI] [PubMed] [Google Scholar]
- Ouellet H, Podust LM, & de Montellano PR (2008). Mycobacterium tuberculosis CYP130: Crystal structure, biophysical characterization, and interactions with antifungal azole drugs. The Journal of Biological Chemistry, 283(8), 5069–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejo E, Feng Y, Chao W, Cotten JF, Le Ge R, & Raines DE (2011). Differential effects of etomidate and its pyrrole analogue carboetomidate on the adrenocortical and cytokine responses to endotoxemia. Critical Care Medicine, 40(1), 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejo E, Santer P, Jeffrey S, Gallin H, Husain SS, & Raines DE (2014). Analogues of etomidate: Modifications around etomidate’s chiral carbon and the impact on in vitro and in vivo pharmacology. Anesthesiology, 121(2), 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejo E, Zhou X, Husain SS, & Raines DE (2016). Sedative-hypnotic binding to 11beta-hydroxylase. Anesthesiology, 125(5), 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines DE (2015). The pharmacology of etomidate and etomidate derivatives. International Anesthesiology Clinics, 53(2), 63–75. [DOI] [PubMed] [Google Scholar]
- Roumen L, Sanders MP, Pieterse K, Hilbers PA, Plate R, Custers E, et al. (2007). Construction of 3D models of the CYP11B family as a tool to predict ligand binding characteristics. Journal of Computer-Aided Molecular Design, 21(8), 455–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EE, White MA, He YA, Johnson EF, Stout CD, & Halpert JR (2004). Structure of mammalian cytochrome P450 2B4 complexed with 4-(4-chlorophenyl) imidazole at 1.9-A resolution: Insight into the range of P450 conformations and the coordination of redox partner binding. The Journal of Biological Chemistry, 279(26), 27294–27301. [DOI] [PubMed] [Google Scholar]
- Seward HE, Roujeinikova A, McLean KJ, Munro AW, & Leys D (2006). Crystal structure of the Mycobacterium tuberculosis P450 CYP121-fluconazole complex reveals new azole drug-P450 binding mode. The Journal of Biological Chemistry, 281(51), 39437–39443. [DOI] [PubMed] [Google Scholar]
- Shanmugasundararaj S, Zhou X, Neunzig J, Bernhardt R, Cotten JF, Ge R, et al. (2013). Carboetomidate: An analog of etomidate that interacts weakly with 11beta-hydroxylase. Anesthesia and Analgesia, 116(6), 1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockham RJ, Stanley TH, Pace NL, King K, Groen F, & Gillmor ST (1987). Induction of anesthesia with fentanyl or fentanyl plus etomidate in high-risk patients. Journal of Cardiothoracic Anesthesia, 1(1), 19–23. [DOI] [PubMed] [Google Scholar]
- Struys M, Valk BI, Eleveld DJ, Absalom AR, Meyer P, Meier S, et al. (2017). A phase 1, single-center, double-blind, placebo-controlled study in healthy subjects to assess the safety, tolerability, clinical effects, and pharmacokinetics-pharmacodynamics of intravenous cyclopropyl-methoxycarbonylmetomidate (ABP-700) after a single ascending bolus dose. Anesthesiology, 127(1), 20–35. [DOI] [PubMed] [Google Scholar]
- Vinclair M, Broux C, Faure P, Brun J, Genty C, Jacquot C, et al. (2007). Duration of adrenal inhibition following a single dose of etomidate in critically ill patients. Intensive Care Medicine, 34(4), 714–719. [DOI] [PubMed] [Google Scholar]
- Wagner RL, & White PF (1984). Etomidate inhibits adrenocortical function in surgical patients. Anesthesiology, 61(6), 647–651. [DOI] [PubMed] [Google Scholar]
- Wagner RL, White PF, Kan PB, Rosenthal MH, & Feldman D (1984). Inhibition of adrenal steroidogenesis by the anesthetic etomidate. The New England Journal of Medicine, 310(22), 1415–1421. [DOI] [PubMed] [Google Scholar]
- Watt I, & Ledingham IM (1984). Mortality amongst multiple trauma patients admitted to an intensive therapy unit. Anaesthesia, 39(10), 973–981. [DOI] [PubMed] [Google Scholar]