Abstract
Background
Many Alaska Native communities rely on a traditional marine diet that contains persistent organic pollutants (POPs). The indoor environment is also a source of POPs. Polybrominated diphenyl ethers (PBDEs) and perfluoroalkyl substances (PFASs) are present both in the traditional diet and the home indoor environment.
Objectives
We assessed exposure to PBDEs and PFASs among residents of two remote Alaska Native villages on St. Lawrence Island. Ninespine stickleback (Pungitious pungitious) and Alaska blackfish (Dallia pectoralis) were used to detect accumulation of these compounds in the local environment.
Methods
Concentrations of PBDEs and PFASs were measured in dust collected from 49 households on St. Lawrence Island, as well as in blood serum from 85 island residents. Resident ninespine stickleback and Alaska blackfish were used as sentinels to detect accumulation of PBDEs and PFASs in the food web.
Results
Serum concentrations of perfluorononanoic acid (PFNA) and perfluoroundecanoic acid (PFUnDA) were elevated, despite low concentrations of PFASs in dust samples. Concentrations of PBDEs in dust and serum were similar to those from the contiguous United States. Statistical associations between dust and serum concentrations are apparent for a small number of PBDEs, suggesting a possible route of exposure Predominant compounds were similar between human sera and stickleback; however, blackfish accumulated PFASs not found in either stickleback or human sera.
Conclusion
Household dust contributes to PBDE exposure, but not PFAS exposure. Elevated concentrations of long chain PFAS in serum are likely due to exposure from traditional foods. The presence of both PFASs and PBDEs in sentinel fish species suggests atmospheric deposition and bioaccumulation, as well as local environmental contamination.
CAPSULE
Alaska Natives on St. Lawrence Island are exposed to PBDEs and PFASs through global transport as well as local sources of pollution.
BACKGROUND
The Arctic is a hemispheric sink for persistent organic pollutants (POPs) due to global distillation and bioaccumulation of these compounds (AMAP 2009, 2015). However, relatively little is known about the indoor sources of POPs in remote northern regions. Perfluoroalkyl substances (PFASs) are used in many consumer applications to impart beneficial surface characteristics, such as water or stain proofing, or non-stick properties. Polybrominated diphenyl ethers (PBDEs) are a class of brominated flame retardants added to consumer products. Both classes of compounds are undergoing global phase out, but remain significant environmental contaminants (Rotander et al. 2012a; Rotander et al. 2012b). Importantly, both PFASs and PBDEs are present in arctic wildlife that serve as traditional food sources for indigenous peoples (Butt et al. 2010; Kelly et al. 2008).
PFASs and PBDEs have been the subject of numerous laboratory and epidemiological investigations. In humans, PFASs are associated with adverse reproductive outcomes, specifically reduced fetal growth (Lam et al. 2014), altered cholesterol levels (Steenland et al. 2010), and altered thyroid hormone concentrations in children (Ballesteros et al. 2016). The health effects associated with PBDE exposure include thyroid disruption, neurodevelopmental effects and reproductive changes (Linares et al. 2015; Noyes and Stapleton 2014). Both PFASs and PBDEs are associated with thyroid hormone perturbations among Inuit adults in Canada (Dallaire et al. 2009).
Results of studies within the contiguous United States and in other regions of the world clearly show that indoor air and dust can be significant sources of exposure to contaminants including POPs (Allen et al. 2007; Stapleton et al. 2012). Due to more energy efficient construction and increased use of synthetic building materials, modern buildings tend to have lower ventilation rates and higher indoor contaminant concentrations (Jones 1999; Sundell 2004). While research has been conducted on POP exposure from subsistence diets in the Arctic (Liberda et al. 2011; Ostertag et al. 2009), little is known about indoor sources of POPs for arctic populations. Overall body burdens of PBDEs are higher in North America than other regions of the world (Kim et al. 2016; Linares et al. 2015); however, little research exists on PBDEs or PFASs within the arctic region of the United States.
St. Lawrence Island, Alaska is the largest island in the Bering Sea, located 60 km from Siberia. Approximately 1,600 Yupik people distributed nearly evenly between the villages of Gambell and Savoonga reside on the island year round. The residents are largely reliant on traditional subsistence foods including reindeer (Rangifer tarandus), bowhead whale (Balaena mysticetus), Pacific walrus (Odobenus rosmarus), and ice seal species such as ringed (Pusa hispida), bearded (Erignathus barbatus), and spotted (Phoca largha) seals. In summer, ambient temperatures on St. Lawrence Island rarely exceed 10° C and so the home heating season extends virtually year round. Assuming relatively low ventilation rates, and a large percentage of the year spent indoors, the indoor environment may be an important exposure route of contaminants for residents of St. Lawrence Island. Previous research indicates that the residents of St. Lawrence Island are exposed to POPs from their traditional foods (Welfinger-Smith et al. 2011). The aim of this study was to assess human exposure to PBDEs and PFASs on St. Lawrence Island using an analysis of the indoor environment. Sentinel fish species were also examined to assess PBDE and PFAS contamination and bioaccumulation in local freshwater habitats.
METHODS
Participants were recruited through flyers posted in public spaces, or were directly recruited by bilingual (English-Yupik) research assistants. Inclusion criteria of recruited participants included being 18–45 years old, and having a willing adult participant of the opposite sex from the same home. Participants were excluded for a history of adverse outcomes during blood draws. A total of 85 individuals from 49 homes were recruited for the study. The median age of participants was 29 (range of 18–45). In some cases one or both participants opted not to provide a blood sample after the household dust sample was collected. In summary, the participant list included a total of 36 male-female pairs from a single home, 11 unpaired women and 2 unpaired men. From one home a dust sample was collected, but no blood samples. This research study was approved by the Alaska Area Institutional Review Board (Indian Health Service IRB) and informed consent was obtained by bilingual research assistants prior to participation.
Dust samples were collected during late winter to early spring (February-April) of 2013 and 2014 from homes using a single standard household vacuum cleaner equipped with a detachable stainless steel collection nozzle (Rudel et al. 2003). Samples were collected on cellulose extraction thimbles (Whatman Inc., Clifton NJ) by lightly drawing the suction nozzle over the surface of floors and furniture. Thimbles were stored in pre-cleaned glass jars with polypropylene lids. Participants were asked not to sweep or dust for one week prior to sampling. Analytes in dust were extracted directly from the filter without sieving.
Blood samples were collected from fasted participants ±1 day of the dust collection at their home. Approximately 20ml of venous blood was drawn into sterile vacutainers (Becton Dickinson, Franklin Lakes, NJ) and allowed to clot at room temperature for one hour before centrifugation at 3300rpm for 15 minutes. Serum was decanted and stored in pre-cleaned glass vials with PTFE lined caps for PBDE analysis, and stored in pre-cleaned polypropylene falcon tubes (Becton Dickinson, Franklin Lakes, NJ) for PFAS analysis. Serum samples and dust samples were immediately transferred to and stored at −20°C until analysis.
Quantification of analytes in dust and serum was conducted at Axys Analytical Services Ltd. (Sydney, British Columbia, Canada). Target analytes included perfluorobutanoic acid (PFBA), perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), perfluorododecanoic acid (PFDoA), perfluorobutane sulfonate (PFBS), perfluorohexane sulfonate (PFHxS), perfluorooctane sulfonate (PFOS), perfluorooctane sulfonamide (PFOSA), as well as BDE-7, 8+11, 10, 12+13, 15, 17+25, 28+33, 30, 32, 35, 37, 47, 49, 51, 66, 71, 75, 77, 79, 85, 99, 100, 105, 116, 119+120, 126, 128, 138+166, 140, 153, 154, 155, 181, 183, 190, 203, 206, 207, 208, and 209.
PFASs were quantified using reverse-phase high-performance liquid chromatography mass spectrometry on a Waters 2690 (Waters, Milford, MA coupled to a Micromass Quattro Ultima MS/MS (Waters, Milford, MA), using AXYS methods MLA-042 and MLA-041 (AXYS Analytical Services Ltd 2014a, b). PBDE concentrations were determined using isotope dilution high resolution gas chromatography high resolution mass spectrometry with a Hewlett Packard 5890 (Hewlett Packard, Palo Alto, CA) coupled to a Micromass Ultima (Waters, Milford, MA), according to EPA Method 1614A (AXYS Analytical Services Ltd 2015). The instruments were initially calibrated with a minimum of 6 calibration standards. Analytes were quantified using the internal standard method, using isotopically labeled surrogate standards for each of the 13 PFASs and BDE-15, 28, 47, 77, 99, 100, 126, 153, 154, 183, 197, and 209. Method detection limits for PFAS were 0.5 – 1 ng/g in serum, and 0.1 – 0.2 ng/g in dust. Method detection limits for PBDEs were 1 – 2 pg/g for the Di-BDE through Hepta-BDE and 10 – 20 pg/g for Nona-BDE and Deca-BDE. A blank and matrix spike were included in each analytical batch of approximately 13 samples. No PFASs were detectable in blank samples. PBDE concentrations in dust blanks were below 1% of sample concentrations. PBDE concentrations in serum blanks ranged from non-detect to over 100% of sample concentrations. If blanks contained quantifiable concentrations of analytes, this concentration was subtracted from the concentrations of samples in the analytical batch. If blank corrected concentrations were below zero, they were treated as <LOD. Matrix spike recoveries were typically between 90–110%, and never outside of 80–120% with one exception. The matrix spike recovery for PFUnDA was 73.5% for a single matrix spike dust sample. For descriptive statistics PBDE concentrations in serum are expressed here as ng/g lipid weight (lw). Total serum lipids were determined by enzymatic measurements of cholesterol, free cholesterol, triglycerides and phospholipids (Phillips et al. 1989).
Ninespine stickleback (Pungitius pungitius; hereafter ‘stickleback’) were collected from three locations on St. Lawrence Island. The largest sample was obtained in the Suqitughneq (Suqi) River watershed, including both upstream and downstream of a formerly used defense site. Stickleback were also collected from the Tapisaggak (Tapi) River located approximately 5 km east of the military site, and from Troutman Lake, a coastal lake situated adjacent to the village of Gambell. Alaska blackfish (Dallia pectoralis; hereafter ‘blackfish’) were collected from the Suqi River, but were absent from the other water bodies. None of the sites were known to be contaminated with PFASs or PBDEs at the initiation of the study.
All fish were collected with unbaited minnow traps, euthanized with an overdose of pH-neutral MS222 fish anesthetic, held on ice at the collection site, and then transferred to −80°C in the laboratory until analysis. PFAS and PBDE concentrations were determined using the same methods as for human serum. Percent recovery of PBDEs from matrix spikes was typically within 90–110%; recovery for BDE-154 was within 87–95%. Percent recovery for PFASs was within 80–120%. A single matrix spike for PFBS was 124%, while another single spike for PFPeA was 79.3%. The limit of quantification varied by compound, but was approximately 0.1–10 pg/g wet weight for PBDEs and 0.5–1 ng/g ww for PFASs. The concentrations of any analytes detected in blank samples were subtracted from the concentrations of samples in the analytical batch. Composite samples were used for stickleback only and were made up of approximately 10 individual whole stickleback totaling approximately 10g from the same study site.
Descriptive statistics for individual compounds are included only when the 95th percentile was greater than the limit of detection (LOD); full descriptive statistics are presented in the supplemental information. We assessed the relative contributions of individual compounds when comparing dust and serum samples. Percentage is given as the concentration of an individual compound divided by the total concentration of the sample (i.e. [ BDE-47]/[∑-PBDE]). The relative contribution was the mean percentage across all observations for a single compound.
Spearman’s correlations were used to assess the associations between concentration of compounds in dust and serum for chemicals present in greater than 50% of serum samples. Correlation analysis was not done on BDE-190 due to low detection rates in dust. Statistical analysis was performed in SAS 9.3 (SAS Institute Inc., Cary, NC), or Microsoft Excel (Microsoft Corporation, Redmond, WA).
RESULTS
Overall concentrations of PFASs in household dust were in the low ng/g range (Table 1). Approximately 95% of dust samples contained at least one detectable PFAS; however, detection rates of individual PFASs were low. PFOA was the most commonly detected compound, detected in 80% of homes at a median concentration of 0.76 ng/g. PFOS was detected in 71% of homes, with a median concentration of 1.40 ng/g. PFHpA was detected in 67% of homes with a median concentration of 0.39 ng/g. Only PFOA, PFOS, and PFHpA were detected in greater than 50% of homes.
Table 1:
Descriptive statistics for PFASs.
| Dust (ng/g) (n=49) | Serum (ng/ml) (n=85) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 95th | % detect | 25th | 50th | 75th | 95th | % detect | |
| PFOA | 0.34 | 0.76 | 1.74 | 3.37 | 80 | 0.75 | 1.01 | 1.44 | 2.14 | 92 |
| PFNA | <LOD | <LOD | 0.41 | 1.93 | 35 | 1.44 | 2.21 | 4.18 | 7.35 | 99 |
| PFDA | <LOD | <LOD | <LOD | 2.16 | 24 | <LOD | <LOD | 0.66 | 1.06 | 39 |
| PFUnDA | <LOD | <LOD | <LOD | 0.38 | 10 | <LOD | 0.72 | 1.04 | 1.72 | 72 |
| PFHxS | <LOD | <LOD | 0.41 | 3.13 | 27 | <LOD | <LOD | 1.07 | 2.74 | 32 |
| PFOS | <LOD | 1.40 | 3.62 | 23.56 | 71 | 3.03 | 4.55 | 6.50 | 12.32 | 99 |
LOD=level of detection; % detect=percent of samples above.
Both PFOS and PFNA were detected in >98% of serum samples, while PFOA was detected in 91.8% of serum samples (Table 1). PFOS contributed the greatest percentage of overall serum PFAS concentrations with median and maximum concentrations of 4.5 ng/ml and 16.0 ng/ml, respectively. PFNA was present at the second highest median and maximum concentrations of 2.2 ng/ml and 12.1 ng/ml, respectively. PFOA was present at a median concentration of 1 ng/ml and a maximum of 2.9 ng/ml.
All sampled homes had detectable concentrations of PBDEs in the dust (Table 2). Twenty-one individual congeners were present in 100% of dust samples. BDE-209 was present at the highest median concentration of 1,199.93 ng/g, followed by BDE-99 (362.98 ng/g) and BDE-47 (327.00 ng/g). BDE-47 and BDE-99 had the highest maximum concentrations of 98,799.98 ng/g and 136,999.98 ng/g, respectively. The maximum concentration of BDE-209 was 31,799.93 ng/g. Total PBDE concentrations ranged from 216.29 ng/g to 317,660.95 ng/g in dust samples. The highest concentrations of PBDEs in dust and serum were from the same home; serum and dust concentrations for these samples were approximately 40 and 50 times the interquartile range for ∑-PBDEs, respectively.
Table 2:
Descriptive statistics for PBDEs.
| Dust (ng/g) (n=49) |
Serum (ng/g lipid) (n=85) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 95th | % detect | 25th | 50th | 75th | 95th | % detect | |
| BDE-15 | 0.06 | 0.12 | 0.26 | 0.79 | 100 | 0.06 | 0.08 | 0.13 | 0.30 | 100 |
| BDE-17/25 | 0.89 | 1.58 | 4.41 | 15.86 | 100 | 0.02 | 0.03 | 0.05 | 0.09 | 95 |
| BDE-28/33 | 2.44 | 4.34 | 13.23 | 50.70 | 100 | 0.40 | 0.65 | 0.98 | 2.25 | 100 |
| BDE-37 | 0.03 | 0.06 | 0.14 | 0.41 | 94 | <LOD | <LOD | <LOD | 0.02 | 28 |
| BDE-47 | 170.97 | 327.00 | 1052.45 | 4599.99 | 100 | 5.95 | 9.25 | 16.94 | 34.14 | 99 |
| BDE-49 | 3.91 | 7.43 | 27.55 | 168.20 | 100 | 0.04 | 0.06 | 0.10 | 0.21 | 95 |
| BDE-51 | 0.44 | 0.93 | 3.49 | 20.78 | 100 | <LOD | <LOD | 0.01 | 0.02 | 45 |
| BDE-66 | 3.27 | 7.87 | 24.50 | 172.20 | 100 | 0.03 | 0.07 | 0.11 | 0.26 | 92 |
| BDE-71 | 0.45 | 0.90 | 3.28 | 15.06 | 98 | <LOD | <LOD | <LOD | 0.02 | 25 |
| BDE-75 | 0.17 | 0.45 | 1.69 | 7.03 | 96 | <LOD | 0.01 | 0.02 | 0.04 | 61 |
| BDE-79 | <LOD | 0.07 | 0.31 | 2.21 | 63 | <LOD | <LOD | <LOD | 0.02 | 15 |
| BDE-85 | 11.60 | 29.90 | 118.50 | 1058.00 | 100 | 0.09 | 0.18 | 0.35 | 0.87 | 91 |
| BDE-99 | 203.98 | 362.98 | 1684.97 | 6583.99 | 100 | 0.87 | 1.71 | 3.61 | 8.34 | 89 |
| BDE-100 | 42.40 | 93.10 | 323.25 | 1350.00 | 100 | 1.19 | 2.11 | 3.53 | 8.27 | 98 |
| BDE-119/120 | 0.44 | 1.03 | 3.90 | 14.60 | 94 | <LOD | <LOD | 0.03 | 0.06 | 44 |
| BDE-126 | <LOD | 0.14 | 0.43 | 2.54 | 65 | <LOD | <LOD | <LOD | 0.01 | 9 |
| BDE-128 | 0.12 | 0.26 | 1.08 | 6.88 | 88 | <LOD | <LOD | 0.04 | 0.08 | 39 |
| BDE-138/166 | 4.07 | 9.97 | 34.35 | 263.80 | 100 | 0.01 | 0.04 | 0.07 | 0.18 | 80 |
| BDE-140 | 0.92 | 2.37 | 7.89 | 61.56 | 100 | 0.03 | 0.05 | 0.07 | 0.18 | 96 |
| BDE-153 | 25.60 | 58.70 | 208.25 | 825.40 | 100 | 5.06 | 9.60 | 12.92 | 25.95 | 100 |
| BDE-154 | 17.50 | 41.70 | 147.25 | 603.40 | 100 | 0.10 | 0.16 | 0.36 | 0.79 | 95 |
| BDE-155 | 1.16 | 2.41 | 8.01 | 53.98 | 100 | 0.02 | 0.03 | 0.05 | 0.10 | 92 |
| BDE-183 | 2.52 | 5.42 | 9.72 | 50.68 | 100 | 0.11 | 0.18 | 0.32 | 1.08 | 100 |
| BDE-190 | <LOD | <LOD | <LOD | 1.83 | 18 | <LOD | 0.06 | 0.10 | 0.31 | 58 |
| BDE-203 | 2.43 | 4.63 | 8.65 | 27.01 | 100 | 0.17 | 0.30 | 0.49 | 1.29 | 100 |
| BDE-206 | 31.96 | 67.60 | 114.50 | 468.38 | 100 | 0.20 | 0.38 | 0.65 | 2.11 | 99 |
| BDE-207 | 44.50 | 98.09 | 191.67 | 738.50 | 100 | 1.15 | 2.00 | 4.09 | 9.60 | 100 |
| BDE-208 | 35.54 | 64.00 | 124.20 | 451.74 | 100 | 0.37 | 0.58 | 1.12 | 3.70 | 99 |
| BDE-209 | 693.00 | 1199.93 | 1885.00 | 8959.52 | 100 | 1.79 | 3.01 | 6.54 | 16.51 | 99 |
LOD=level of detection; % detect=percent of samples above LOD.
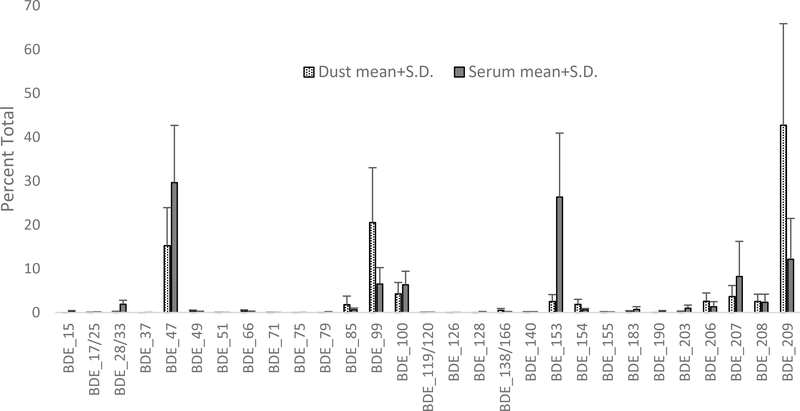
Six PBDEs were detected in 100% of serum samples, and another 10 were present in at least 95% of serum samples (Table 2). BDE-47 and BDE-153 contributed the greatest percentage of overall PBDE body burden (Figure 2). BDE-47 was present at the highest maximum concentration (551.9 ng/g lw) of any PBDE. BDE-153 had a maximum concentration of 54.8 ng/g lw. BDEs 99, 100, 207 and 209 also contributed relatively large percentages of overall PBDE body burden (Figure 2). BDE-99 was present at a median of 1.71 ng/g lw and a maximum of 180.7 ng/g lw. BDE-209 was present at median and maximum concentrations of 3.01 ng/g lw and 46.4 ng/g lw, respectively.
Figure 2:
Relative concentrations of PBDEs in dust and serum.
Associations between compounds in dust and serum samples
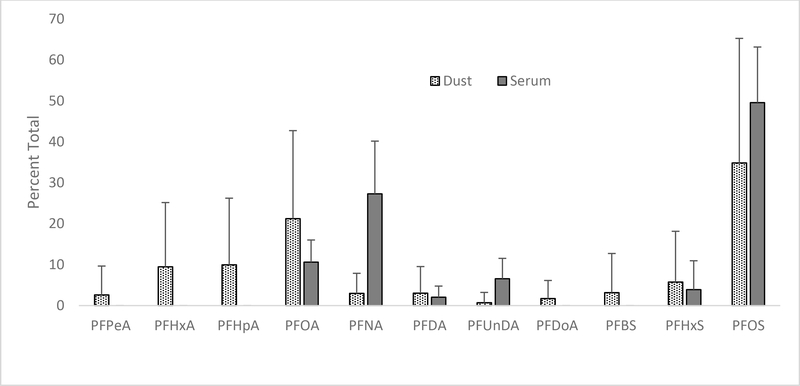
Overall there were relatively poor correlations between concentrations of individual PFASs in dust and in blood serum. Longer chain (≥8 carbon) carboxylates and sulfonates tended to make up the largest percentage of PFASs in serum samples (Fig. 1). PFOS was the predominant compound in both dust and serum samples, but there was no apparent relationship between dust and serum concentrations for either men or women (Table 3). PFNA was a major contributor to overall serum concentration, but a relatively minor contributor to overall dust concentration. Spearman’s correlations do not suggest associations between concentrations of dust borne PFASs and serum PFASs (Table 3).
Figure 1:
Relative concentrations of PFASs in dust and serum.
Table 3:
Spearman’s correlation coefficients between chemical concentrations in dust and serum.
| Women (n=47) | Men (n=38) | |||
|---|---|---|---|---|
| rs | p-value | rs | p-value | |
| PFOA | −0.008 | 0.95 | −0.10 | 0.56 |
| PFNA | −0.20 | 0.17 | −0.30 | 0.06 |
| PFUnDA | 0.09 | 0.53 | 0.01 | 0.94 |
| PFOS | 0.06 | 0.64 | 0.11 | 0.51 |
| BDE-15 | 0.13 | 0.37 | 0.28 | 0.09 |
| BDE-17/25 | 0.33 | 0.02 | 0.06 | 0.07 |
| BDE-28/33 | 0.34 | 0.02 | 0.29 | 0.08 |
| BDE-47 | 0.33 | 0.02 | 0.30 | 0.07 |
| BDE-49 | 0.42 | <0.005 | 0.39 | 0.02 |
| BDE-66 | 0.20 | 0.15 | 0.20 | 0.23 |
| BDE-75 | 0.25 | 0.07 | 0.18 | 0.29 |
| BDE-85 | 0.17 | 0.25 | 0.26 | 0.12 |
| BDE-99 | 0.18 | 0.24 | 0.18 | 0.28 |
| BDE-100 | 0.20 | 0.18 | 0.17 | 0.30 |
| BDE-138/166 | 0.1 | 0.47 | 0.02 | 0.87 |
| BDE-140 | −0.05 | 0.72 | 0.00 | 0.99 |
| BDE-153 | 0.70 | 0.65 | −0.09 | 0.59 |
| BDE-154 | 0.15 | 0.33 | 0.17 | 0.30 |
| BDE-155 | 0.03 | 0.82 | 0.11 | 0.51 |
| BDE-183 | −0.12 | 0.41 | −0.17 | 0.30 |
| BDE-203 | −0.09 | 0.53 | 0.06 | 0.71 |
| BDE-206 | 0.02 | 0.92 | 0.19 | 0.26 |
| BDE-207 | 0.12 | 0.39 | 0.17 | 0.30 |
| BDE-208 | 0.08 | 0.59 | 0.14 | 0.39 |
| BDE-209 | 0.14 | 0.33 | 0.26 | 0.11 |
In both dust and blood samples, the signatures of technical penta-BDE and deca-BDE mixtures were apparent. The most prevalent PBDE congeners in serum also tended to be prevalent in dust samples (Fig. 2); the exception is BDE-153, which was present at relatively low concentrations in dust samples. There were associations between select PBDE congeners in dust and blood serum (Table 3). Specifically, the dust borne concentration of BDE-49 was significantly associated with serum concentrations in both men and women. Dust and serum concentrations of BDE-17/25, BDE-28/33, and BDE-47 were significantly associated in women, but only marginally associated (p<0.10) in men.
Sentinel Fish
Blackfish and stickleback contained mutually exclusive subsets of PFASs. Three PFASs were detectable in stickleback samples: PFOS, PFNA, and PFOA (Table 4). PFNA was the most frequently detected PFAS in stickleback and PFNA concentrations were typically higher than PFOA concentrations in the same sample. Blackfish samples contained detectable concentrations of PFBS, PFHxS, and PFPeA. One blackfish contained PFBS at a concentration of 59.2 ng/g ww, the highest concentration of any PFAS found in fish during this study. Troutman Lake stickleback samples had substantially higher concentrations of PFASs than those in either the Suqi or the Tapi River. PFOS ranged from 10–16 ng/g ww in Troutman Lake samples, but was detectable in only one other stickleback sample at a concentration of 1.15 ng/g ww.
Table 4:
Descriptive statistics for PFASs in sentinel fish (ng/g ww)
| Stickleback | Blackfish | ||||
|---|---|---|---|---|---|
| Troutman Lake (nc =3) | Suqi River (n=29) | ||||
| PFOA | PFNA | PFOS | PFHxA | PFBS | PFPeA |
| 0.85 | 2.72 | 10.10 | 1.77 | <LOD | <LOD |
| 1.25 | 3.44 | 11.70 | 0.608 | 10.1 | <LOD |
| 1.51 | 4.13 | 16.10 | 0.6 | 9.99 | <LOD |
| 0.463 | <LOD | <LOD | |||
| 0.598 | 7.45 | <LOD | |||
| Suqi River (nc =9) | 0.638 | 10 | <LOD | ||
| PFOA | PFNA | PFOS | 0.617 | 1.59 | <LOD |
| <LOD | <LOD | <LOD | 0.476 | <LOD | <LOD |
| 0.55 | 0.85 | 1.15 | 0.7 | <LOD | <LOD |
| 0.65 | 0.51 | <LOD | 0.841 | <LOD | <LOD |
| <LOD | <LOD | <LOD | 0.669 | <LOD | <LOD |
| 0.58 | 0.83 | <LOD | 0.627 | <LOD | <LOD |
| <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| <LOD | 1.52 | <LOD | 0.75 | 16.3 | <LOD |
| <LOD | <LOD | <LOD | 0.661 | 17.4 | <LOD |
| <LOD | 0.59 | <LOD | <LOD | 2.46 | <LOD |
| <LOD | 2.3 | <LOD | |||
| <LOD | 4.98 | <LOD | |||
| Tapi River (nc =2) | <LOD | 16.9 | <LOD | ||
| PFOA | PFNA | PFOS | <LOD | 2.98 | <LOD |
| <LOD | <LOD | <LOD | <LOD | 59.2 | <LOD |
| 0.57 | 0.78 | <LOD | <LOD | 16.8 | 0.82 |
| <LOD | <LOD | <LOD | |||
| <LOD | <LOD | <LOD | |||
| <LOD | <LOD | <LOD | |||
| <LOD | <LOD | <LOD | |||
| <LOD | <LOD | <LOD | |||
| <LOD | <LOD | <LOD | |||
| <LOD | <LOD | <LOD | |||
LOD=level of detection; nc=number of composite samples, each composed of ~10 stickleback
The predominant PBDE congeners in fish samples were major penta-BDE congeners; BDE-47, 49, 99, and 100 were detected in all fish samples (Table 5). While BDE-209 was not frequently detected in fish, it was present at relatively high concentrations when detected. Excluding the Troutman Lake samples, PBDE concentrations were in the low ng/g lw with no sample having a ∑-PBDE concentration above 100 ng/g lw. Stickleback from Troutman Lake had substantially higher concentrations of PBDEs than those detected in either the Suqi or the Tapi River. The three composite samples from Troutman Lake, representing thirty individual fish, had ∑-PBDE of 1,176.44, 919.72 and 995.88 ng/g lw. BDE-209 was present in only one Troutman Lake stickleback sample at a concentration of 0.12 ng/g lw.
Table 5:
Descriptive statistics for PBDEs in sentinel fish (ng/g lipid)
| Troutman Lake Stickleback (nc=3) | Tapi River Stickleback (nc=2) | Suqi River Stickleback (nc=10) | Suqi River Blackfish (n=41) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C1 | C2 | median | min | max | % detect | median | min | max | % detect | |
| BDE-15 | 2.83 | 2.25 | 1.90 | 0.01 | 0.00 | 0.00 | <LOD | 0.03 | 50 | <LOD | <LOD | 0.03 | 15 |
| BDE-17/ 25 | 9.68 | 8.47 | 10.04 | <LOD | <LOD | <LOD | <LOD | 0.08 | 40 | <LOD | <LOD | 0.07 | 32 |
| BDE-28/33 | 39.16 | 33.60 | 30.43 | 0.05 | 0.03 | 0.02 | 0.01 | 0.07 | 100 | 0.03 | <LOD | 0.13 | 76 |
| BDE-37 | 0.74 | 0.66 | 0.62 | <LOD | <LOD | <LOD | <LOD | 0.02 | 20 | <LOD | <LOD | 0.07 | 34 |
| BDE-47 | 650.17 | 502.92 | 525.45 | 2.12 | 0.79 | 0.57 | 0.30 | 1.14 | 100 | 0.90 | 0.32 | 5.90 | 100 |
| BDE-49 | 111.33 | 91.25 | 97.45 | 0.42 | 0.34 | 0.25 | 0.10 | 0.55 | 100 | 0.25 | 0.05 | 1.14 | 100 |
| BDE-51 | 0.67 | 0.64 | 0.47 | 0.01 | <LOD | <LOD | <LOD | 0.04 | 10 | <LOD | <LOD | 0.01 | 2 |
| BDE-66 | 10.29 | 8.86 | 6.02 | 0.04 | <LOD | <LOD | <LOD | 0.19 | 30 | <LOD | <LOD | 0.13 | 32 |
| BDE-71 | 1.59 | 2.13 | 4.94 | <LOD | <LOD | <LOD | <LOD | 0.05 | 20 | <LOD | <LOD | 0.03 | 10 |
| BDE-75 | 0.80 | 0.70 | 0.52 | <LOD | <LOD | <LOD | <LOD | <LOD | 0 | <LOD | <LOD | 0.03 | 5 |
| BDE-79 | 0.39 | 0.43 | <LOD | 0.03 | <LOD | <LOD | <LOD | 0.01 | 10 | <LOD | <LOD | 0.05 | 22 |
| BDE-85 | 0.09 | 0.07 | 0.29 | 0.01 | <LOD | <LOD | <LOD | 0.01 | 40 | 0.02 | <LOD | 0.38 | 54 |
| BDE-99 | 170.36 | 133.13 | 168.01 | 0.41 | 0.30 | 0.31 | 0.20 | 0.98 | 100 | 0.49 | 0.03 | 6.85 | 100 |
| BDE-100 | 124.61 | 94.42 | 102.32 | 0.38 | 0.14 | 0.14 | 0.05 | 0.19 | 100 | 0.16 | 0.06 | 1.45 | 100 |
| BDE-119/120 | 1.85 | 1.38 | 3.17 | 0.03 | <LOD | <LOD | <LOD | 0.03 | 20 | <LOD | <LOD | 0.06 | 5 |
| BDE-126 | 0.20 | 0.15 | <LOD | 0.01 | <LOD | <LOD | <LOD | <LOD | 0 | <LOD | <LOD | <LOD | 0 |
| BDE-128 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0 | <LOD | <LOD | <LOD | 0 |
| BDE-138/166 | 0.02 | 0.02 | 0.09 | <LOD | <LOD | <LOD | <LOD | <LOD | 0 | <LOD | <LOD | 0.58 | 5 |
| BDE-140 | 0.08 | 0.06 | 0.10 | <LOD | <LOD | <LOD | <LOD | <LOD | 0 | <LOD | <LOD | <LOD | 0 |
| BDE-153 | 14.94 | 11.82 | 14.87 | 0.06 | 0.05 | 0.04 | <LOD | 0.08 | 90 | 0.06 | <LOD | 0.54 | 73 |
| BDE-154 | 33.00 | 24.25 | 27.02 | 0.14 | 0.08 | 0.07 | <LOD | 0.10 | 90 | 0.09 | <LOD | 0.50 | 90 |
| BDE-155 | 1.55 | 1.17 | 1.52 | 0.05 | 0.01 | 0.01 | <LOD | 0.03 | 60 | <LOD | <LOD | 0.04 | 12 |
| BDE-183 | 0.01 | 0.01 | <LOD | 0.01 | 0.04 | <LOD | <LOD | 0.04 | 20 | <LOD | <LOD | 0.32 | 37 |
| BDE-190 | <LOD | <LOD | <LOD | 0.01 | <LOD | <LOD | <LOD | <LOD | 0 | <LOD | <LOD | 0.02 | 5 |
| BDE-203 | <LOD | <LOD | <LOD | 0.03 | <LOD | <LOD | <LOD | 0.10 | 10 | <LOD | <LOD | 0.23 | 17 |
| BDE-206 | 0.02 | <LOD | <LOD | 0.01 | <LOD | <LOD | <LOD | 2.21 | 30 | <LOD | <LOD | 4.09 | 29 |
| BDE-207 | 0.01 | <LOD | <LOD | 0.02 | <LOD | <LOD | <LOD | 2.05 | 30 | <LOD | <LOD | 6.94 | 27 |
| BDE-208 | 0.01 | <LOD | <LOD | 0.02 | <LOD | <LOD | <LOD | 1.54 | 20 | <LOD | <LOD | 4.03 | 29 |
| BDE-209 | 0.12 | <LOD | <LOD | 0.41 | <LOD | <LOD | <LOD | 42.12 | 20 | <LOD | <LOD | 57.07 | 34 |
LOD=level of detection; % detect=percent of samples above LOD; nc=number of composite samples, each composed of ~10 stickleback
DISCUSSION
Concentrations of PFASs in household dust on St. Lawrence Island appear to be on the lower end of those reported worldwide in other studies. With a few exceptions, PFAS concentrations in the current study were in the low ng/g range. Several studies have discussed the geographic variability in indoor PFAS concentrations, and there appears to be great variation in both the prevalence and concentrations of these compounds in household dust (Goosey and Harrad 2011; Knobeloch et al. 2012). Studies from the contiguous United States have reported substantially higher concentrations PFASs (Fraser et al. 2013; Wu et al. 2015). A study of PFASs in household dust from Flanders, Belgium reported concentrations (0.2 – 336 ng/g) similar to those reported in the current study; median concentrations for all PFASs, including PFOS, PFOA and PFNA, were below 1 ng/g (D’Hollander et al. 2010).
When compared to age and sex adjusted data from the National Health and Nutrition Examination Survey (NHANES) (CDC 2007) serum concentrations of PFASs in St Lawrence Island residents were comparable to levels found in the general U.S. population. Notable exceptions are that PFNA and PFUnDA were elevated among St. Lawrence Island residents. Median serum PFNA concentrations of St. Lawrence Island residents were 2.74 and 2.13 ng/ml for men and women, respectively, compared to 1.4 and 1.0 ng/ml for men and women participating in NHANES. The median PFUnDA concentrations of St. Lawrence Island residents were also higher (men=0.74 ng/ml; women=0.72 ng/ml) than both men and women in NHANES (0.14 ng/ml). Serum PFOS concentrations were slightly lower in St. Lawrence Island residents than those reported in Yupik women from the Yukon-Kuskokwim River Delta region of Alaska, and those in Inuit from Nunavik Canada (AMAP 2009). Serum PFOS and PFOA concentrations on St. Lawrence Island were on the low end of the range reported for other arctic populations (AMAP 2015). Serum PFAS concentrations appeared dissimilar to those reported in residents of the Faroe Islands, who also consume marine mammals. While serum PFOS and PFOA concentrations were higher among the Faroese, PFNA was present at higher concentrations among residents of St. Lawrence Island (Grandjean et al. 2012). The median concentration of PFNA was higher among St. Lawrence Island residents than among Inuit men from Greenland, while PFUnDA concentrations were similar (Lindh et al. 2012).
Overall concentrations of PBDEs in household dust from St. Lawrence Island were within the range of those found in the homes of the contiguous United States. Specifically, median concentration of BDE-209 in the current study (1,199 ng/g dust) is similar to the median concentration (1,200 ng/g dust) from a small study of dust collected from California homes (Dodson et al. 2012). However, the median values for other major congeners were higher in California. The median concentration of BDE-153 in dust in the current study was 58 ng/g compared to 150 ng/g in California; BDE-99 in the current study was 362 ng/g compared to 1,100 ng/g in California; and BDE-47 in the current study was 298 ng/g compared to 1,000 ng/g in California (Dodson et al. 2012). The major congeners of the commercial penta-BDE and deca-BDE mixtures were the major dust contaminants on St. Lawrence Island, which is similar to other studies in the U.S. (Meeker et al. 2009; Stapleton et al. 2005; Zota et al. 2008). Specifically, BDE-47, BDE-99 and BDE-209 were the predominant congeners in terms of both prevalence and percent of total concentration. Interestingly, BDE-209 made up a larger percentage of ∑-PBDEs than typically found in U.S. dust samples (Kim et al. 2016). This could be explained partially by the earlier phase out of penta-BDE and octa-BDE. The PBDE concentrations in dust are similar to levels that have been associated with endocrine disruption (Meeker et al. 2009).
Compared to age and sex adjusted data from NHANES, serum concentrations of major PBDE congeners were lower on St. Lawrence Island than in the general U.S. population. The only exception was BDE-153, which appeared comparable in terms of median and range concentration. BDE-153 is known to be more resistant to metabolism than other congeners (Lupton et al. 2009). Compared to a population of Inuit men from Canada, select congeners appeared elevated among St. Lawrence Island residents (O’Brien et al. 2012). Geometric mean concentrations of BDE-153 and BDE-47 on St. Lawrence Island were higher, at 8.65 and 10.19 ng/g lw, respectively, than corresponding data from the Canadian Inuit population, at 2.05 and 2.16 ng/g lw, respectively (Dallaire et al. 2009). St. Lawrence Island residents had serum PBDE concentrations similar to those of Yupik women from the Yukon-Kuskokwim River delta region of Alaska from the period 2004–2006, the highest reported in the circumpolar Arctic. However, BDE-47, 99 and 100 were slightly higher in women from Yukon-Kuskokwim delta, while BDE-153 and 209 concentrations were higher among St. Lawrence Island residents (AMAP 2009). This may be due in part to the increased use of deca-BDE in the intervening years. Research in the Canadian Arctic indicated that PBDE levels are higher in young Inuit children than in Inuit adults (O’Brien et al. 2012).
Sentinel Fish
Stickleback from Troutman Lake had exceptionally high ∑-PBDE and ∑-PFAS concentrations. ∑-PBDE concentrations are comparable to the range observed in pilot whales from the Faroe Islands (Rotander et al. 2012b), and several higher trophic level fish species from California (Brown et al. 2006). In all three composite stickleback samples, BDE-47 contributed over 50% of the ∑-PBDE concentration. PFOS and PFNA concentrations were also high in Troutman Lake stickleback samples, within the range reported for beluga whale liver samples from Alaska and other arctic regions (Reiner et al. 2011). However, substantially higher concentrations of PFASs have been reported in liver from polar bears from Alaska, and in whole body fish samples from US rivers (Kannan et al. 2005; Ye et al. 2008). Given the comparatively high concentrations of PBDEs and PFASs it is probable that stickleback from Troutman Lake are exposed to a local source of PFASs and PBDEs. While the source has not yet been investigated, contaminants may be leaching from local landfills. Village and military landfills and Troutman Lake are located on a cobble spit; this substrate may facilitate leaching of contaminants from the landfills.
Blackfish and stickleback samples contained mutually exclusive sub-sets of PFASs, even when collected from the same waters. The most frequently detected PFASs in stickleback were PFOA, PFOS, and PFNA. These compounds were also the predominant compounds in human sera samples in this study. PFOS and PFNA are commonly detected in marine biota from the circumpolar Arctic, and are likely present in traditional foods harvested on St. Lawrence Island (Butt et al. 2010). Stickleback appear to be an appropriate sentinel species for human exposure to PFASs in this region of the Arctic. In blackfish, PFBS was both the most frequently detected and present at the highest concentrations. Blackfish are resident freshwater species, while stickleback may be freshwater, anadromous, or marine. Blackfish from the upstream section of the Suqi River had higher concentrations of PFBS than those in the downstream section. Differences in metabolism and excretion of PFASs between species cannot be ruled out as the cause of the different body burdens. PFBS was not detected in any human sera samples.
A blackfish from the upstream section of the Suqi River contained PFBS at a concentration of 59.2 ng/g ww, the single highest concentration of any PFAS detected in either species of fish. PFBS is a degradation product of perfluorbutane sulfonyl fluoride compounds which are used as replacements for perfluorosulfonates, such as PFOS (D’Eon et al. 2006; Wang et al. 2013). The only other compound frequently detected in blackfish was PFHxS. Importantly, both PFBS and PFHxS appear to be increasing in concentration in humans (Glynn et al. 2012). Blackfish may be early indicators that these compounds are transported to the Arctic and bioaccumulate in animals.
Excluding Troutman Lake samples, overall ∑-PBDE concentrations in sentinel fish were similar to those reported in other arctic fish species (Letcher et al. 2010). Both stickleback and blackfish samples contained principally penta-BDE congeners, with lower concentrations of technical deca-BDE congeners. There were no apparent differences in accumulation of PBDEs between the fish species. Results from sentinel fish suggest that human dietary exposure from locally harvested aquatic animals is likely to be dominated by penta-BDE congeners.
Sources of exposure
Overall PFAS concentrations were low in dust samples. Longer chained PFASs were detected more frequently in serum and were present at higher concentrations than short chained PFASs. Short chained perfluorinated carboxylates are less bioaccumulative than those with long chains (Lasier et al. 2011). The low concentrations of PFASs in household dust, and overall weak correlations between PFASs in household dust and serum, suggest that people are exposed to other sources of these compounds. However, research into dietary exposure and other potential pathways would be necessary to test this hypothesis. The presence of PFAS in fish from remote sites is suggestive of atmospheric deposition.
Serum samples contained relatively high concentrations of odd numbered carbon chain PFASs, such as PFNA and PFUnDA. This pattern has been observed in arctic biota (Martin et al. 2004; Reiner et al. 2011) and is consistent with the hypothesis that global transport and transformation of fluorotelomer alcohols is the source of these compounds (Ellis et al. 2004). In the Faroe Islands the consumption of whale meat appears to be positively associated with serum PFOS and PFNA concentrations (Weihe et al. 2008). The presence of PFNA in stickleback samples provides evidence that this compound is accumulating in the local biota.
The dust and serum concentrations for a small number of PBDEs were weakly but significantly correlated. This suggests that dust may be a source of exposure, however this was not directly measured. The strength of the correlation is weak compared those reported from other adult populations (Johnson et al. 2010). It may be that dust is a less important route of exposure in this population than those in the contiguous United States. It is clear from sentinel fish data that major penta-BDE congeners bioaccumulate in local arctic biota and thus may be present in traditional foods. PBDEs were detected in fish from remote sites which suggests atmospheric deposition. Stickleback data suggest a point source of PBDEs and PFASs near the village of Gambell, possibly a local landfill. It is unclear to what extent residents could be exposed through this mechanism.
Limitations
This study sample is non-random. The data are cross-sectional and there is no assurance that a single dust sample is representative of historical indoor exposure. Small sample size limits statistical power to detect associations. While dust is known to be a significant source of exposure to PBDEs, it is not thought to be a significant source of PFASs. Indoor air samples would provide a more complete picture of indoor PFAS exposure. We cannot differentiate between exposure to PFASs and exposure to precursors such as fluorotelemer alcohols. As such, fluorotelemer alcohols may be an uncharacterized source of exposure. Deca-congeners are difficult to measure accurately because they can degrade during analysis (Stapleton 2006). Deca-BDE is also present at relatively high concentrations in indoor environments relative to serum, which poses a risk of sample contamination; these factors decrease confidence in concentration estimates. Dust samples were extracted directly from the filter without sieving; this could introduce fragments of commercial products that contain high concentrations of target analytes. Dietary exposure was not assessed. Composite samples of stickleback were necessary due to the small mass of individual fish; however, this method makes it impossible to determine the level of contamination of individual fish. A matrix spike for PFBS was outside the ideal recovery range (124%) in the analytical batch that contained the highest PFBS concentration. PFBS concentrations in these samples were probably overestimated; however, PFBS was detected in blackfish from other analytical batches with spike recoveries within 80–120%. No lab blanks contained detectable concentrations of PFBS.
CONCLUSION
Locally collected stickleback indicate a point source of PBDE and PFAS pollution near Gambell, while the lower concentration of these compounds in fish collected elsewhere on the island also suggest that both PFASs and PBDEs are present due to atmospheric deposition. Concentrations of PFASs in household dust from St. Lawrence Island are among the lowest reported in the literature. The proportion of PFNA is enriched in serum as compared to dust. PFNA and PFUnDA are elevated compared to the U.S. general population. Long-range transport of fluorotelomer alcohols and bioaccumulation in traditional foods may explain this pattern. PFNA is commonly detected in resident stickleback. PFBS is present in blackfish from St. Lawrence Island, but is not detectable in human serum. Concentrations of PBDEs in dust on St. Lawrence Island are within the range of previously published reports for the United States. Although within the range reported in the U.S., serum PBDE concentrations are above those of Canadian Inuit populations. Controlling exposure from household dust may reduce overall exposure to PBDEs in arctic homes. PFAS exposure is not likely to be significantly affected by dust mitigation strategies.
Supplementary Material
Acknowledgements
This study was supported by the National Institute of Environmental Health Sciences (NIH R01ES019620). The authors would like to thank the many individuals who contributed to accomplishing this research including Jane Kava, Jesse Gologergen, Erika Apatiki, Kristi Apangalook, Tiffany Immingan, Millie Kingeekuk, Susie Booshu, Sharon Campbell-Aningayou, Naomi Madsen, Shelley Klein-Apatiki, Carolyn Kava, Carol Gologergen, Bobby Ungwiluk, Kevin Zweifel, and Heidi Zimmer. The authors also thank Dr. Erin Bell, Dr. Michael Bloom, Dr. Kurunthachalam Kannan, and Dr. Lawrence Schell at the University at Albany, as well as anonymous reviewers, for their comments on the manuscript.
Contributor Information
Samuel Byrne, Department of Environmental Studies, 104 Memorial Hall, St. Lawrence University, Canton, NY. 13617 USA.
Samarys Seguinot-Medina, Alaska Community Action on Toxics, Anchorage, Alaska, USA.
Pamela Miller, Alaska Community Action on Toxics, Anchorage, Alaska, USA.
Vi Waghiyi, Alaska Community Action on Toxics, Anchorage, Alaska, USA.
Frank A. von Hippel, Department of Biological Sciences, Northern Arizona University, Flagstaff, AZ 86011
C. Loren Buck, Department of Biological Sciences and Center for Bioengineering Innovation, Northern Arizona University, Flagstaff, AZ, USA.
David O. Carpenter, Institute for health and the Environment, University at Albany, Albany, NY. USA
References
- Allen JG, McClean MD, Stapleton HM, Nelson JW, Webster TF. 2007. Personal Exposure to Polybrominated Diphenyl Ethers (PBDEs) in Residential Indoor Air. Environmental Science & Technology 41:4574–4579. [DOI] [PubMed] [Google Scholar]
- AMAP. 2009. Assessment 2009: Human health in the Arctic. Oslo, Norway:Arctic Monitoring and Asessment Programme (AMAP). [Google Scholar]
- AMAP. 2015. AMAP Assessment 2015: Human health in the Arctic. Oslo, Norway. [Google Scholar]
- AXYS Analytical Services Ltd. 2014a. Summary of AXYS Method MLA-042 Rev 09 Ver 06: Analytical Procedure for the Analysis of Perfluorinated Organic Compounds in Blood Serum by LC MS/MS
- AXYS Analytical Services Ltd. 2014b. Summary of AXYS Method MLA-041 Rev. 09 Ver. 03: Analytical Procedure for the Analysis of Perfluorinated Organic Compounds in Solid Samples by LC-MS/MS
- AXYS Analytical Services Ltd. 2015. Summary of AXYS method mla-033 rev. 06 ver. 03: Analytical method for the determination of brominated diphenyl ethers (BDE) and other brominated flame retardants (BFR).
- Ballesteros V, Costa O, Iñiguez C, Fletcher T, Ballester F, Lopez-Espinosa M-J. 2016. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies. Environment International. [DOI] [PubMed] [Google Scholar]
- Brown FR, Winkler J, Visita P, Dhaliwal J, Petreas M. 2006. Levels of PBDEs, PCDDs, PCDFs, and coplanar PCBs in edible fish from California coastal waters. Chemosphere 64:276–286. [DOI] [PubMed] [Google Scholar]
- Butt CM, Berger U, Bossi R, Tomy GT. 2010. Levels and trends of poly- and perfluorinated compounds in the arctic environment. Science of the Total Environment 408:2936–2965. [DOI] [PubMed] [Google Scholar]
- CDC. 2007. National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- D’Eon JC, Hurley MD, Wallington TJ, Mabury SA. 2006. Atmospheric chemistry of N-methyl perfluorobutane sulfonamidoethanol, C4F9SO2N(CH3)CH2CH2OH: kinetics and mechanism of reaction with OH. Environ Sci Technol 40:1862–1868. [DOI] [PubMed] [Google Scholar]
- D’Hollander W, Roosens L, Covaci A, Cornelis C, Reynders H, Campenhout KV, et al. 2010. Brominated flame retardants and perfluorinated compounds in indoor dust from homes and offices in Flanders, Belgium. Chemosphere 81:478–487. [DOI] [PubMed] [Google Scholar]
- Dallaire R, Dewailly E, Pereg D, Dery S, Ayotte P. 2009. Thyroid function and plasma concentrations of polyhalogenated compounds in Inuit adults. Environ Health Perspect 117:1380–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, et al. 2012. After the PBDE Phase-Out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environmental Science & Technology 46:13056–13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis DA, Martin JW, De Silva AO, Mabury SA, Hurley MD, Sulbaek Andersen MP, et al. 2004. Degradation of Fluorotelomer Alcohols: A Likely Atmospheric Source of Perfluorinated Carboxylic Acids. Environmental Science & Technology 38:3316–3321. [DOI] [PubMed] [Google Scholar]
- Fraser AJ, Webster TF, Watkins DJ, Strynar MJ, Kato K, Calafat AM, et al. 2013. Polyfluorinated compounds in dust from homes, offices, and vehicles as predictors of concentrations in office workers’ serum. Environ Int 60:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, et al. 2012. Perfluorinated Alkyl Acids in Blood Serum from Primiparous Women in Sweden: Serial Sampling during Pregnancy and Nursing, And Temporal Trends 1996–2010. Environmental Science & Technology 46:9071–9079. [DOI] [PubMed] [Google Scholar]
- Goosey E, Harrad S. 2011. Perfluoroalkyl compounds in dust from Asian, Australian, European, and North American homes and UK cars, classrooms, and offices. Environment International 37:86–92. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Andersen E, Budtz-Jørgensen E, et al. 2012. SErum vaccine antibody concentrations in children exposed to perfluorinated compounds. Jama 307:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Stapleton HM, Sjodin A, Meeker JD. 2010. Relationships between Polybrominated Diphenyl Ether Concentrations in House Dust and Serum. Environmental Science & Technology 44:5627–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP. 1999. Indoor air quality and health. Atmospheric Environment 33:4535–4564. [Google Scholar]
- Kannan K, Yun SH, Evans TJ. 2005. Chlorinated, brominated, and perfluorinated contaminants in livers of polar bears from Alaska. Environmental Science & Technology 39:9057–9063. [DOI] [PubMed] [Google Scholar]
- Kelly BC, Ikonomou MG, Blair JD, Gobas FA. 2008. Bioaccumulation behaviour of polybrominated diphenyl ethers (PBDEs) in a Canadian Arctic marine food web. Science of the Total Environment 401:60–72. [DOI] [PubMed] [Google Scholar]
- Kim S-K, Kim K-S, Sang HH. 2016. Overview on relative importance of house dust ingestion in human exposure to polybrominated diphenyl ethers (PBDEs): International comparison and Korea as a case. Science of The Total Environment 571:82–91. [DOI] [PubMed] [Google Scholar]
- Knobeloch L, Imm P, Anderson H. 2012. Perfluoroalkyl chemicals in vacuum cleaner dust from 39 Wisconsin homes. Chemosphere 88:779–783. [DOI] [PubMed] [Google Scholar]
- Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S, et al. 2014. The Navigation Guide - evidence-based medicine meets environmental health: integration of animal and human evidence for PFOA effects on fetal growth. Environ Health Perspect 122:1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasier PJ, Washington JW, Hassan SM, Jenkins TM. 2011. Perfluorinated chemicals in surface waters and sediments from northwest Georgia, USA, and their bioaccumulation in Lumbriculus variegatus. Environ Toxicol Chem 30:2194–2201. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Bustnes JO, Dietz R, Jenssen BM, Jorgensen EH, Sonne C, et al. 2010. Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. Sci Total Environ 408:2995–3043. [DOI] [PubMed] [Google Scholar]
- Liberda EN, Wainman BC, Leblanc A, Dumas P, Martin I, Tsuji LJ. 2011. Dietary exposure of PBDEs resulting from a subsistence diet in three First Nation communities in the James Bay Region of Canada. Environ Int 37:631–636. [DOI] [PubMed] [Google Scholar]
- Linares V, Belles M, Domingo JL. 2015. Human exposure to PBDE and critical evaluation of health hazards. Archives of Toxicology 89:335–356. [DOI] [PubMed] [Google Scholar]
- Lindh CH, Rylander L, Toft G, Axmon A, Rignell-Hydbom A, Giwercman A, et al. 2012. Blood serum concentrations of perfluorinated compounds in men from Greenlandic Inuit and European populations. Chemosphere 88:1269–1275. [DOI] [PubMed] [Google Scholar]
- Lupton SJ, McGarrigle BP, Olson JR, Wood TD, Aga DS. 2009. Human liver microsome-mediated metabolism of brominated diphenyl ethers 47, 99, and 153 and identification of their major metabolites. Chemical Research in Toxicology 22:1802–1809. [DOI] [PubMed] [Google Scholar]
- Martin JW, Smithwick MM, Braune BM, Hoekstra PF, Muir DCG, Mabury SA. 2004. Identification of Long-Chain Perfluorinated Acids in Biota from the Canadian Arctic. Environmental Science & Technology 38:373–380. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Johnson PI, Camann D, Hauser R. 2009. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Science of the Total Environment 407:3425–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes PD, Stapleton HM. 2014. PBDE flame retardants. Endocrine Disruptors 2:e29430. [Google Scholar]
- O’Brien HT, Blanchet R, Gagné D, Lauzière J, Vézina C, Vaissière É, et al. 2012. Exposure to Toxic Metals and Persistent Organic Pollutants in Inuit Children Attending Childcare Centers in Nunavik, Canada. Environmental Science & Technology 46:4614–4623. [DOI] [PubMed] [Google Scholar]
- Ostertag SK, Tague BA, Humphries MM, Tittlemier SA, Chan HM. 2009. Estimated dietary exposure to fluorinated compounds from traditional foods among Inuit in Nunavut, Canada. Chemosphere 75:1165–1172. [DOI] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT Jr., Henderson LO, Needham LL. 1989. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Archives of environmental contamination and toxicology 18:495–500. [DOI] [PubMed] [Google Scholar]
- Reiner JL, O’Connell SG, Moors AJ, Kucklick JR, Becker PR, Keller JM. 2011. Spatial and temporal trends of perfluorinated compounds in beluga whales (Delphinapterus leucas) from Alaska. Environmental Science & Technology 45:8129–8136. [DOI] [PubMed] [Google Scholar]
- Rotander A, Kärrman A, Bavel Bv, Polder A, Rigét F, Auðunsson GA, et al. 2012a. Increasing levels of long-chain perfluorocarboxylic acids (PFCAs) in Arctic and North Atlantic marine mammals, 1984–2009. Chemosphere 86:278–285. [DOI] [PubMed] [Google Scholar]
- Rotander A, van Bavel B, Polder A, Rigét F, Auðunsson GA, Gabrielsen GW, et al. 2012b. Polybrominated diphenyl ethers (PBDEs) in marine mammals from Arctic and North Atlantic regions, 1986–2009. Environment International 40:102–109. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. 2003. Phthalates, Alkylphenols, Pesticides, Polybrominated Diphenyl Ethers, and Other Endocrine-Disrupting Compounds in Indoor Air and Dust. Environmental Science & Technology 37:4543–4553. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Dodder NG, Offenberg JH, Schantz MM, Wise SA. 2005. Polybrominated Diphenyl Ethers in House Dust and Clothes Dryer Lint. Environmental Science & Technology 39:925–931. [DOI] [PubMed] [Google Scholar]
- Stapleton HM. 2006. Instrumental methods and challenges in quantifying polybrominated diphenyl ethers in environmental extracts: a review. Analytical and Bioanalytical Chemistry 386:807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Sjödin A, Webster TF. 2012. Serum PBDEs in a North Carolina Toddler Cohort: Associations with Handwipes, House Dust, and Socioeconomic Variables. Environmental Health Perspectives 120:1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Fletcher T, Savitz DA. 2010. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ Health Perspect 118:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundell J 2004. On the history of indoor air quality and health. Indoor air 14:51–58. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbühler K. 2013. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environment International 60:242–248. [DOI] [PubMed] [Google Scholar]
- Weihe P, Kato K, Calafat AM, Nielsen F, Wanigatunga AA, Needham LL, et al. 2008. Serum Concentrations of Polyfluoroalkyl Compounds in Faroese Whale Meat Consumers. Environmental Science & Technology 42:6291–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welfinger-Smith G, Minholz JL, Byrne S, Waghiyi V, Gologergen J, Kava J, et al. 2011. Organochlorine and metal contaminants in traditional foods from St. Lawrence Island, Alaska. Journal of Toxicology and Environmental Health, Part A 74:1195–1214. [DOI] [PubMed] [Google Scholar]
- Wu X, Bennett DH, Calafat AM, Kato K, Strynar M, Andersen E, et al. 2015. Serum concentrations of perfluorinated compounds (PFC) among selected populations of children and Adults in California. Environmental research 136:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Strynar MJ, Nakayama SF, Varns J, Helfant L, Lazorchak J, et al. 2008. Perfluorinated compounds in whole fish homogenates from the Ohio, Missouri, and Upper Mississippi Rivers, USA. Environmental Pollution 156:1227–1232. [DOI] [PubMed] [Google Scholar]
- Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. 2008. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environmental Science & Technology 42:8158–8164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.