Physiological mechanisms of slow canopy wilting in early maturity group soybeans were identified and the underlying QTLs were mapped and confirmed to protect soybean yield under drought in the field.
Keywords: Drought, QTL mapping, slow canopy wilting, soybean, transpiration, water use efficiency
Abstract
Slow canopy wilting (SW) is a water conservation trait controlled by quantitative trait loci (QTLs) in late maturity group soybeans [Glycine max (L.) Merr.]. Recently, two exotic (landraces) plant introductions (PI 567690 and PI 567731) were identified as new SW lines in early maturity groups. Here, we show that the two PIs share the same water conservation strategy of limited maximum transpiration rates as PI 416937. However, in contrast to PI 416937, the transpiration rates of these PIs were sensitive to an aquaporin inhibitor, indicating an independence between limited maximum transpiration and the lack of silver-sensitive aquaporins. Yield tests of selected recombinant inbred lines from two elite/exotic crosses provide direct evidence to support the benefit of SW in drought tolerance. Four SW QTLs mapped in a Pana×PI 567690 cross at multiple environments were found to be co-located with previous reports. Moreover, two new SW QTLs were mapped on chromosomes 6 and 10 from a Magellan×PI 567731 cross. These two QTLs explain the observed relatively large contributions of 20–30% and were confirmed in a near-isogenic background. These findings demonstrate the importance of SW in yield protection under drought and provide genetic resources for improving drought tolerance in early maturity group soybeans.
Introduction
Climate changes increase the occurrence of extreme weather patterns, including irregular rainfall patterns, higher temperature, and the consequent drought stress, which cause significant reductions in crop production (Lesk et al., 2016). These yield losses of major crops keep increasing, despite the progressive increase in yield through breeding and management practices since the 1960s (Boyer, 2013; Lobell et al., 2014; Rosenzweig et al., 2014). A decline of 30–82% in crop yields has been projected by the end of the century under the current warming scenarios (Hatfield et al., 2011).
Soybean has been estimated to have an ~40% reduction in yield as a result of drought (Specht et al., 1999). To overcome the negative impacts of drought stress in soybean, many strategies have been developed and adopted, mainly including agricultural practices and genetic improvement of soybean cultivars (Turner, 2000). Rainfall and irrigation water are being used more efficiently; however, adaptation of irrigation systems is region limited and would substantially increase the costs of soybean production (Kebede et al., 2014). Clearly, improving drought tolerance of soybean varieties is strategically crucial to protect yield gain (Devi et al., 2014; Ye et al., 2018a). Drought tolerance in soybean should be translated to traits enhancing yield stability rather than that increasing survivability under drought (Bartels et al., 2006; Blum, 2009; Passioura, 2010; Sinclair, 2011; Ye et al., 2018a). The essential factor to improve drought tolerance in crops, including soybean, is to use available water more effectively through the growing season so that the plant’s physiological activity is sustained through to maturity (Turner et al., 2001; Blum, 2009; Sinclair, 2018; Ye et al., 2018a).
Soybean genotypes differ in the time of onset and the severity of canopy wilting in response to drought (Sloane et al., 1990; Carter et al., 1999; Valliyodan et al., 2017). As soil dries, soybean genotypes with a slow canopy wilting (SW) phenotype have delayed canopy/leaf wilting when compared with fast canopy wilting (FW) lines (Charlson et al., 2009; Valliyodan et al., 2017). Field evaluations of plant introductions (PIs) from the USDA Soybean Germplasm Collection identified PI 416937 [maturity group (MG) VI] and PI 471938 (MG V)] as often expressing the SW phenotype relative to commercially available cultivars (Carter et al., 2006; Charlson et al., 2009). This trait was predicted using a simulation model to improve yield under drought by >80% of the growing seasons in most regions of the USA (Sinclair et al., 2010). Currently in the USA, the SW phenotype has been used as one of the indicators to screen drought tolerance in the field (Charlson et al., 2009) and several drought-tolerant soybean varieties for late MGs (V–VIII) have been developed by utilizing these two PIs, PI 416937 and PI 471938 (Devi et al., 2014).
One of the physiological bases for SW in PI 416937 was found to be associated with limited transpiration rate (TR) under atmospheric vapor pressure deficits (VPDs) of ~2.1 kPa or higher (Fletcher et al., 2007). This limitation on TR was shown to be associated with limited hydraulic conductance between the xylem and the guard cells in the leaves (Sinclair et al., 2008). This hydraulic restriction was suggested to be associated with lack of a specific silver-sensitive population of aquaporins, as the TR in PI 416937 was insensitive to AgNO3 treatment (Sadok and Sinclair, 2010). Interestingly, response in transpiration to increasing VPD of the other SW line, PI 471938, was found to be linear without a breakpoint. Furthermore, transpiration in PI 471938 was sensitive to the AgNO3 treatment, indicating the presence of an AgNO3-sensitive pathway in their leaves allowing higher hydraulic conductance (Sadok and Sinclair, 2010). These results indicate that there are at least two distinct water conservation mechanisms that are involved in the SW trait in soybean. In addition, other mechanisms, such as more water resource exploration by roots, could also result in the SW phenotype. PI 416937 was found to have a much larger root system in the field than other FW lines, which might be one factor to enhance the SW phenotype under drought (Hudak and Patterson, 1995).
Many quantitative trait locus (QTL) mapping efforts have been conducted to identify genomic regions associated with the SW trait (Valliyodan et al., 2017). Initial QTL mapping work was conducted in a recombinant inbred line (RIL) population derived from the Benning and PI 416937 cross. Seven QTLs were mapped in that population and the one on chromosome (Chr.) 12 was identified as a major QTL across all five independent environments with a phenotypic contribution up to 27% (Abdel-Haleem et al., 2012). In another QTL mapping study conducted using five populations under 15 environments (site or year), a total of 13 QTLs were mapped for canopy wilting with varied phenotypic contributions (R2=4–29%), and the majority of these QTLs (11/13) had donor alleles for SW from either PI 416937 or Jackson (Hwang et al., 2015). Among these 13 QTLs, eight were further confirmed and their confidence intervals were refined through Meta-QTL analysis (Hwang et al., 2016). A genome-wide association study (GWAS) was also conducted, and 23 putative loci were identified as being associated with canopy wilting from a diverse panel of 373 MG IV soybean genotypes (Kaler et al., 2017), indicating the complexity of the canopy wilting trait. Although statistically significant QTLs for SW have been reported in soybean, most were found to be unstable across independent environments and populations. For instance, the major QTL on Chr. 12 was not detectable in any populations or environments reported by Hwang et al. (2015, 2016); thus QTL confirmation needs to be performed to validate each individual QTL, especially in a near-isogenic background. In addition, effort was put into QTL mapping for response of transpiration to silver ions in the Benning×PI 416937 population. Four QTLs were identified (R2=17.7–24.7%), with the largest one located on Chr. 12 (Carpentieri-Pipolo et al., 2012). However, the donor allele at this major locus for insensitivity of transpiration to silver ions is from an FW parent, Benning (Carpentieri-Pipolo et al., 2012). This result is contradictory to the QTL mapping for SW in the same population, as the donor allele at this locus for SW is from the SW parent, PI 416937 (Abdel-Haleem et al., 2012). Thus, the correlation between the lack of silver-sensitive aquaporins and the SW phenotype involved in a limited maximum TR needs to be reconsidered.
Recently, two drought-tolerant exotic (unadapted) soybean germplasms, PI 567690 and PI 567731 (MG III), were identified to express the SW phenotype consistently in the field compared with two drought-sensitive cultivars, Pana and Magellan (Pathan et al., 2014). The early maturity group of these two PIs favors their use in breeding efforts for higher latitude regions. However, the mechanisms underlying the SW trait of these PIs have not yet been studied. Moreover, the impacts of SW trait on yield protection under drought obviously require further investigation. The objectives of this study were (i) to elucidate the physiological and genetic mechanisms involved in the SW trait in these two germplasm sources; (ii) to identify the effects of SW on yield protection under drought stress in similar genetic backgrounds; and (iii) to confirm selected major genetic factors (QTLs) regulating SW.
Materials and methods
Plant materials
Two mapping populations were developed from soybean Pana×PI 567690 and Magellan×PI 567731 crosses using a single-seed descent method to produce 258 and 160 F7:8-derived RILs, respectively (see Supplementary Fig. S1 at JXB online). The RILs were further advanced to increase seeds at the Delta Research Center (DC), University of Missouri, Portageville, MO, USA. Forty-six RILs from each of these populations were selected for yield test based on contrasting canopy wilting and other agronomic traits, including plant height, lodging, and maturity (Supplementary Table S1). Near-isogenic lines (NILs) of the major QTLs were identified from heterogeneous inbred families at the F7:8 generation (Supplementary Fig. S1). A single F7:8 plant, which has heterozygous genotypes at the QTL regions, was selected from the RIL families as described by Ye et al. (2015, 2018b). The NILs were selected in the progeny population of this F7:8 plant (Supplementary Fig. S1).
Plant cultivation and SW evaluation in the field
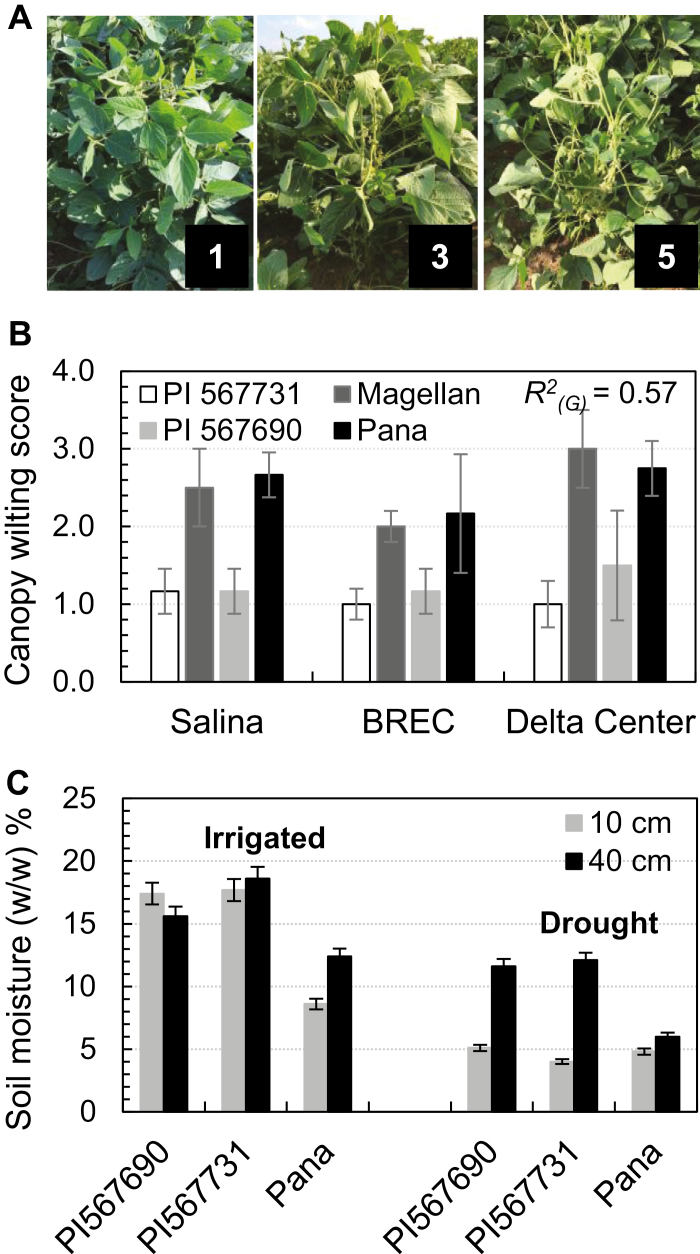
The parental lines and RIL populations were evaluated for the SW trait at the Bradford Research and Education Center (BREC), Columbia, MO, the DC, and Salina, KS, USA in 2013, 2014, and 2015. In Salina, KS, these RILs were planted in plots 3 m long and 0.75 m row space with 60 seeds per row. In Missouri, hill plots were planted with 0.75 m between rows and 0.75 m between hills. Ten seeds were sown per hill and, 3 weeks after germination, plants were thinned to four plants per hill. Hill plot was previously approved as an effective plot design to evaluate the SW phenotype, as the designed plant density (four plants per hill) can result in enough competition for water imposing stress on the plants under rain-fed conditions (Sloane et al., 1990). To develop NILs, seeds were sown in plots 3 m long and 0.75 m row space with 60 seeds per row at BREC. At the DC, water available to the plants was monitored using a Delta-T moisture PR2 probe (Delta-T instruments, Cambridge, UK) at depths of 10 cm and 40 cm. In all the fields, scoring for leaf wilting started when the plants were from R2 to R5 growth stages, when the canopy was fully developed (Fehr et al., 1971; Pathan et al., 2014). Canopy wilt scoring (WS) was rated when any of the FW check (Pana) showed obvious canopy wilting at late morning or mid-day. Data were recorded for leaf wilting using a 1–5 scale (1=no wilting, 2=few top leaves showed wilting, 3=half of the leaves showed wilting, 4=severe wilting, ~75% of the leaves showed wilting, and 5=severely wilted) (Fig. 1A).
Fig. 1.
Canopy wilting and water use of the parental lines in the field. (A) Field phenotyping for canopy wilting using the canopy wilting score (WS). WSs were used to represent canopy wilting levels from 1 to 5. (B) Canopy wilting of the parental lines under drought conditions. Data were recorded for the lines at three locations for 2–3 years. Significant genotypic differences were observed with P<0.0001, explaining 57% of phenotypic variations based on ANOVA across the three locations. (C) Soil moisture underneath the parental lines. Soil moisture was measured at 10 cm and 40 cm underneath the soil surface of the parental lines planted in the sandy soil at Delta Center, MO in 2014. Measurements were taken 3 weeks after the last rain. Columns and bars represent the mean and SDs of 10 replicates.
In data analysis, the model for the WS trait was:
| (1) |
where µ is the grand mean, gi is the genetic effect of the ith genotype, lj is the effect of the jth location, tm is the effect of the mth year, (gl)ij is the interaction between the ith genotype and the jth location, (gt)im is the interaction between the ith genotype and the mth year, (glt)ijm is the interaction among the ith genotype, the jth location, and the mth year, bk(j) is the block (kth) effect within the jth location, and eijmk is a random error following N (0, σ2e). Estimation of variance components was performed by the GLM procedure in SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Evaluation of transpiration responses to VPD and silver nitrate
Transpiration responses of the four parental lines (Pana, Magellan, PI 567690, and PI 567731) were determined as previously described (Fletcher et al., 2007). Plants were grown for 4 weeks at 30/26 °C day/night temperature in a greenhouse at the North Carolina State University. Once four fully expanded leaves had been attained, plants were placed in individual chambers, in which VPD was varied. Three VPD exposures on two consecutive days were imposed in the following sequence: low (0.5–1.5 kPa), medium (1.5–2.5 kPa), and high (2.5 and higher kPa). The TR for the plant was expressed as water loss rate per unit of leaf area.
The TR for each genotype was regressed against the VPD for each genotype. For statistical analysis, two regression equations were applied using least-squares regression in Graphpad prism 2.01 (Graphpad Software Inc., San Diego, CA, USA). A key output of the regression analysis was the VPD value for the breakpoint (BP) where the two linear segments intersected.
| (2) |
where X0 is the BP between the two line-segments, S1 and S2 the slopes of the first and second line segments, respectively, and C1 and C2 are the constants of the first and second line segments, respectively. The slopes of the two linear regressions (S1 and S2) were statistically compared to determine if they differed significantly (P<0.05). If the slopes differed, the double-linear regression was retained. When the slopes were not significantly different, a simple linear regression was applied to all the data for that genotype.
Silver nitrate (AgNO3) treatment and subsequent data analysis were performed as previously outlined (Sadok and Sinclair, 2010). Plants were grown for 4 weeks in the greenhouse at a 30/26 °C day/night temperature scheme. The shoots were detached 4 weeks after emergence. The subsequent change in transpiration was measured gravimetrically. The VPD of the growth chamber was kept in the range of 3.3–4.0 kPa. The percentage reduction in transpiration rate (RTR) for each individual plant as a result of the inhibitor treatment was calculated using the following equation for 10 plants for each line.
| (3) |
where TR0=plant transpiration rate in water and TRX=plant transpiration rate in AgNO3 solution.
Yield tests under irrigated and rain-fed field conditions
Standard four row yield plots were planted to evaluate yield of the selected RILs in the sandy soil at two locations: Salina, KS, and the DC, MO. At each location, two sets of yield plots were planted with three replications, subjected to two treatments (irrigated and rain-fed). For each plot, 600 seeds were sown in 4 m rows with 0.75 m row space. Only the two center rows were harvested for yield evaluation. Drip irrigation was applied to plots once in 10 d without rainfall in July and August for irrigated treatments. Four and two irrigations were applied at Salina, KS, and the DC, MO, respectively. No irrigation was applied for rain-fed treatments at both locations. Rain-fed conditions were considered as relatively drought conditions compared with the irrigated conditions, especially the poor water-holding capacity of sandy soils.
Linkage map construction and QTL analysis
Genomic DNA of the parents and the RILs of the two RIL populations was extracted using a standard cetyltrimethylammonium bromide (CTAB) method (Doyle and Doyle, 1990) with minor modifications. Single nucleotide polymorphism (SNP) genotyping was performed using the BARCSoySNP6K Illumina Infinium BeadChips (Illumina Inc., San Diego, CA, USA) at the Washington University genotyping facility, St. Louis, MO, USA. The SNP alleles were called using the GenomeStudio Genotyping Module (Song et al., 2013). A genetic linkage map of each mapping population was constructed using the program JoinMap 3.0 (van Ooijen and Voorrips, 2001). A LOD (logarithm of odds) score of 3.0 was used for two-point analysis and a LOD score of 2.0 was used for all three-point and multipoint analysis. Putative QTLs for the traits studied were initially detected by the interval mapping (IM) method using the program MapQTL 5.0 (van Ooijen, 2004). Composite interval mapping (CIM) was then performed using the multi-QTL method and the appropriate cofactors (van Ooijen and Voorrips, 2001). A LOD score significance threshold value was estimated for each trait in each location by 1000 permutations to determine a QTL at the genome-wide significance level of P=0.05 (Doerge and Churchill, 1996). The name of the identified QTL was nominated following SoyBase nomenclature requirements as qSW_Gm standing for qtl_Slow Wilting_Glycine max_chromosome number.
Results
Canopy wilting and water usage of parental lines in the field
The four parental lines were planted at three locations over multiple years to evaluate canopy WSs (Fig. 1A). The two exotic parents, PI 567731 and PI 567690, showed almost no canopy wilting (WS <1.2) compared with the two elite parents, Magellan and Pana, across all of the three locations of both hill pots and row plots (Fig. 1B). Significant genotypic differences in WS were identified between the exotic and elite parents, explaining 57% of phenotypic variances across environments (locations and years) (Fig. 1B). Under the irrigation condition (non-stress), the FW line showed significantly less soil moisture at 10 cm and 40 cm depth soil layers, compared with the two SW lines (Fig. 1C). Under the rain-fed condition (drought), the surface soil moistures (10 cm depth) were all decreased to a level of ~5% (w/w); however, at deeper soil layers (40 cm depth), the two SW lines retained >12% moisture content compared with 7% by the FW line, Pana (Fig. 1C). These results suggest that the SW phenotype in these exotic lines is involved in water use efficiency and conservation.
Transpiration responses to VPD and an aquaporin inhibitor
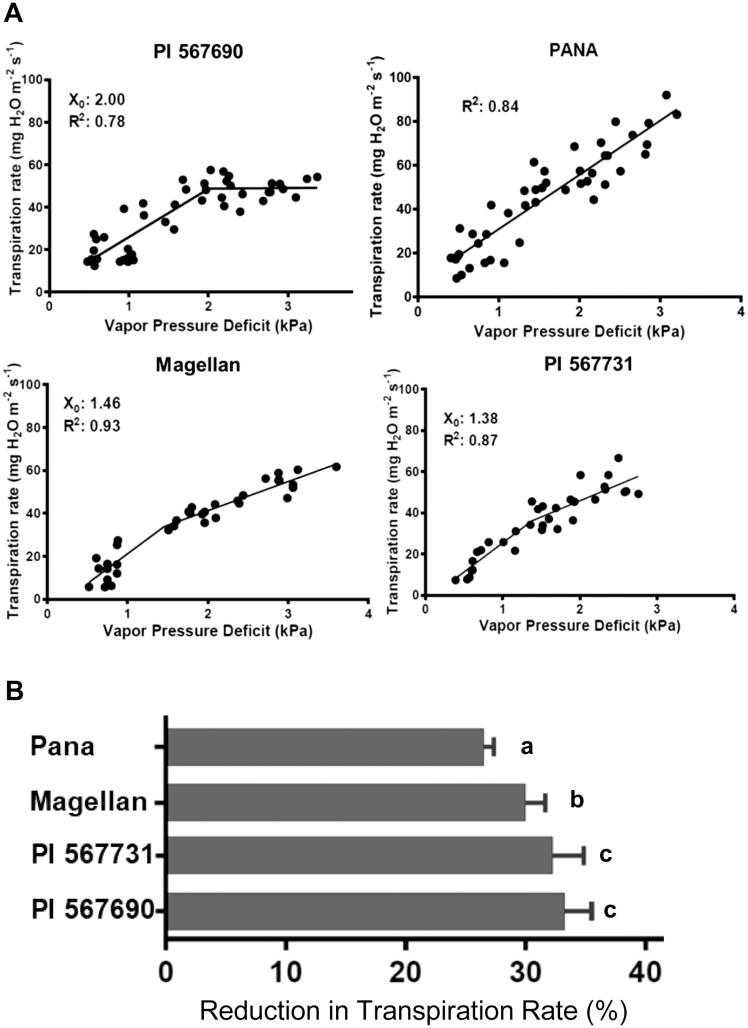
The responses of transpiration in the four parental lines to increasing VPD were further evaluated. Among these four lines, PI 567690, PI 567731, and Magellan were found to exhibit a BP in their TRs as VPD was increased (Fig. 2A), similar to PI 416937 (Fletcher et al. 2007). PI 567690 exhibited a BP in TR at 2.00 kPa, while PI 567731 and Magellan exhibited BPs at lower VPDs of 1.38 and 1.46 kPa, respectively (Fig. 2A). It was noted that PI 567690 showed the strongest suppression in transpiration when VPD was increased (Fig. 2A). The FS line, Pana, did not exhibit a BP, and a single linear regression was fit (non-BP line) (Fig. 2A). These results showed that the three lines with BPs in TRs (BP lines) had limited maximum TRs and could be candidate donors for SW (Fletcher et al., 2007).
Fig. 2.
Differences in transpiration rates of the parental lines under increasing vapor pressure deficit (VPD) and silver nitrate treatments. (A) Transpiration rates of the parental lines under increasing VPD. The transpiration rates were calculated according to Equation 2. X0 is the breakpoint between the two-line segments and R2 represents the portion of variations explained by the model. (B) Responses of transpiration rates of the parental lines to an aquaporin inhibitor. AgNO3 was applied to the detached shoots (4 weeks after germination) and the percentage reduction in transpiration rate for each individual plant due to the inhibitor treatment was calculated using Equation 3. At 4 weeks after emergence, the transpiration response of detached shoots fed with silver ions was measured. Columns and bars represent the mean and SDs of 10 replicates. Duncan multiple comparison was performed to compare the means and categorize the data into ‘a,’ ‘b,’ and ‘c’ at P<0.001.
Detached shoots were treated with an aquaporin inhibitor (AgNO3) and significant RTRs were observed in the all four parental lines (Fig. 2B). The three BP lines (PI 567690, PI 567731, and Magellan) showed greater sensitivities in transpiration to the AgNO3 treatment than the non-BP line (Pana), unlike the nearly complete lack of sensitivity in transpiration to silver ions found previously in PI 416937 (Sadok and Sinclair, 2010). These results indicate that the limited transpiration imposed in the BP lines could be independent of the lack of a silver-sensitive aquaporin population hypothesized for PI 416937. In future, the focus should be on all aquaporin populations including both silver-sensitive and -insensitive aquaporin to understand the regulation of limited transpiration under high VPD.
Inheritance of SW and its effects on yield protection under drought
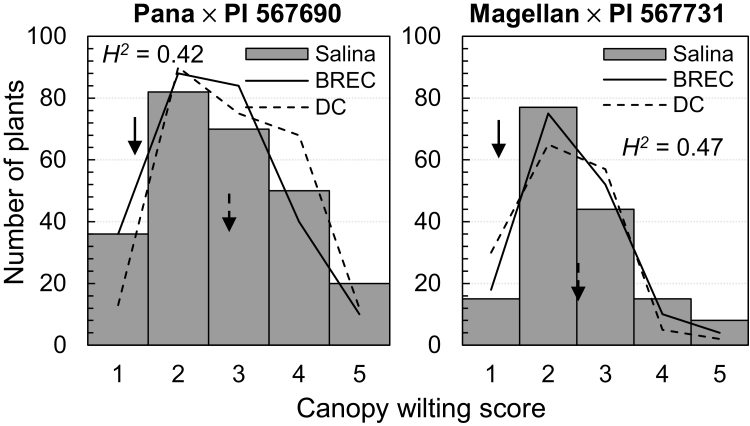
Two RIL populations were developed by crossing PI 567690 to Pana and PI 567731 to Magellan (Supplementary Fig. S1). WSs were rated for these RIL populations at three locations for 1–3 years (Fig. 1A). The WSs of the two RIL populations were quantitatively and normally distributed (Fig. 3), suggesting WS as a quantitative trait in these populations and no need for data normalization. Transgressive segregations in WSs were observed in both mapping populations for all the three locations (Fig. 3). These results indicated that both elite and exotic parents should have a donor locus or loci for SW. Broad-sense heritability was detected as 0.42 and 0.47 for the Pana×PI 567690 and Magellan×PI 567731 populations, respectively, across all environments (years and locations) (Fig. 3).
Fig. 3.
Phenotypic distributions of canopy wilting scores (WSs) of the two recombinant inbred line mapping populations. The means of WSs from multiple years for each location were used to calculate the phenotypic distributions. The arrows with solid lines or dashed lines point to the performance of the exotic or cultivated parents, respectively. The performance of canopy wilting was averaged for all environments (locations and years) for the parental lines. Broad-sense heritability (H2) was calculated for WSs across all environments (locations and years) using Equation 1.
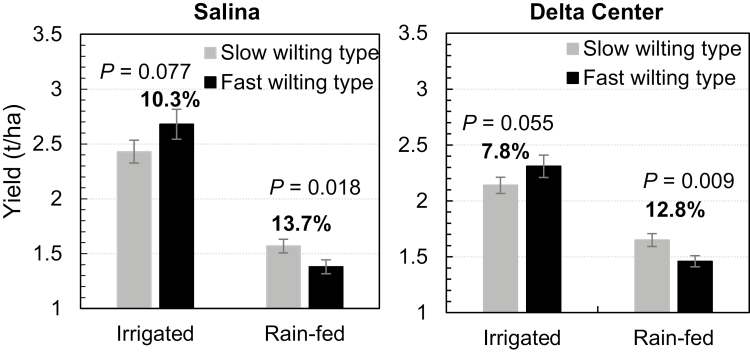
The evaluation of a possible relationship between canopy wilting and yield was carried out using 46 RILs from the two populations. These RILs were selected for extreme performance in WS with <1.5 (23 SW RILs) and >3 (23 FW RILs) (Supplementary Table S1). These 46 RILs have similar agronomic traits, including maturity, plant height, lodging (low), and determinant growth habit (Supplementary Table S1). Under non-stress (irrigated) conditions, the FW RILs showed a yield advantage over the SW RILs of 7.8–10.3% at both tested locations; however, these differences were not statistically significant (Fig. 4). Under drought stress (rain-fed) conditions, the FW RILs showed yield reductions of 48.5% and 36.8% at the two locations, while the SW RILs showed much lower yield reductions of 35.4% and 22.9%, respectively (Fig. 4). The SW RILs showed statistically significant yield advantages of 12.8% and 13.7% over the FW lines under water-limited conditions at both locations (Fig. 4). When yield of the selected RILs was evaluated separately for each population, a trend similar to the combination of the RILs from the two populations was observed (Supplementary Tables S2, S3); however, the difference was not significant between FW and SW RILs under either condition due to reduced sample size.
Fig. 4.
Roles of the canopy wilting trait in yield protection under drought. The two sets of RILs were planted in the field under irrigated and rain-fed conditions at Salina (left) and Delta Center (right). Data shown are means ±SDs of yield plots of 23 RILs for either slow wilting or fast wilting types. Student’s t-test was performed to compare means of traits between the two sets of RILs. The percentages on the columns show the yield advantage of the corresponding wilting types over the others.
QTLs for SW under drought in the elite×exotic crosses
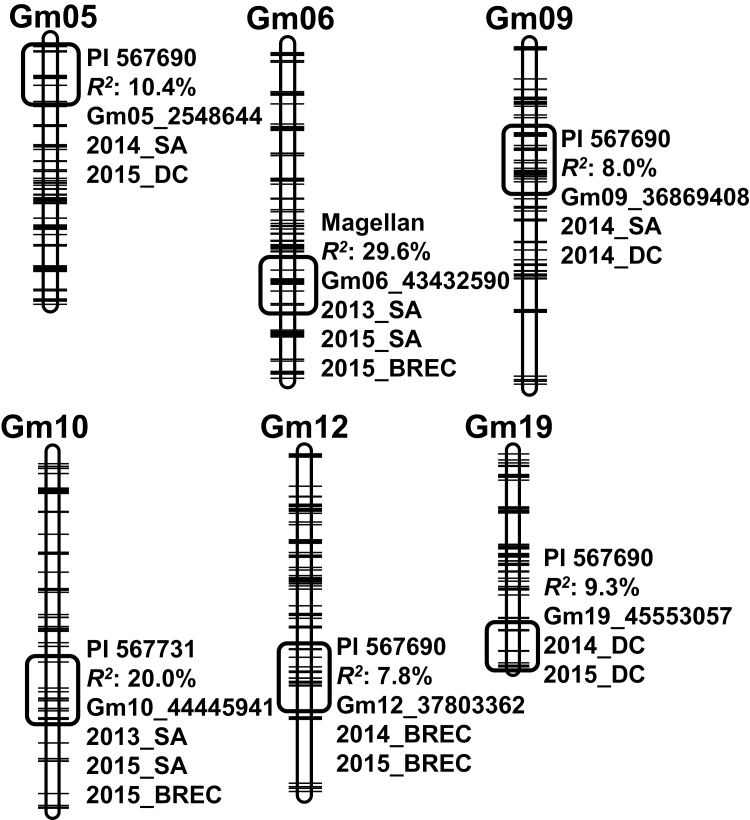
Linkage maps of the two RIL populations were constructed using 1601 and 2516 polymorphic SNP markers acquired from BARCSoySNP6K Illumina Infinium BeadChips genotyping with an average genetic interval of 1.5 cM (Table 1). Subsequent QTL analyses in the two RIL populations identified 10 QTLs associated with canopy WSs from each year and location (Table 2). From the Pana×PI 567690 population, eight QTLs were identified to locate at similar chromosomal positions to those previously reported by Abdel-Haleem et al. (2012) and Hwang et al. (2015) (Table 2). From the Magellan×PI 567731 population, two new QTLs, named as qSW_Gm06 and qSW_Gm10, were mapped as being distinct from those previously reported (Table 2).
Table 1.
Information of the constructed linkage maps for the two recombinant inbred line populations
| Pana×PI 567690 | Magellan×PI 567731 | |||
|---|---|---|---|---|
| Chr. | No. of markers | Average interval (cM) | No. of markers | Average interval (cM) |
| 1 | 82 | 1.1 | 144 | 1.6 |
| 2 | 74 | 1.9 | 147 | 1.0 |
| 3 | 89 | 1.6 | 114 | 1.9 |
| 4 | 95 | 1.2 | 130 | 1.5 |
| 5 | 54 | 2.2 | 102 | 1.7 |
| 6 | 87 | 1.3 | 126 | 1.6 |
| 7 | 56 | 2.1 | 144 | 1.5 |
| 8 | 134 | 2.1 | 221 | 1.1 |
| 9 | 80 | 1.3 | 130 | 1.5 |
| 10 | 81 | 1.2 | 91 | 2.0 |
| 11 | 63 | 1.4 | 73 | 2.6 |
| 12 | 62 | 1.3 | 110 | 1.6 |
| 13 | 96 | 1.4 | 179 | 1.1 |
| 14 | 66 | 1.4 | 95 | 1.8 |
| 15 | 69 | 1.6 | 113 | 1.8 |
| 16 | 68 | 1.4 | 107 | 1.7 |
| 17 | 83 | 1.6 | 106 | 1.7 |
| 18 | 85 | 1.3 | 151 | 1.3 |
| 19 | 56 | 1.6 | 93 | 1.4 |
| 20 | 121 | 1.8 | 140 | 1.3 |
| Total | 1601 | 1.5 | 2516 | 1.5 |
Table 2.
Summary of QTLs detected in previous and present genetic mapping
| QTL | Donor line | Abdel-Haleem et al. (2012) | Hwang et al. (2015) | This research |
|---|---|---|---|---|
| qSW_Gm02-1 | Benning, Jackson, PI 567690 | – | 2 populationsa | 1 environmenta |
| qSW_Gm02-2 | A5959, PI 416937, PI 567690 | 2 environmentsa | 1 population | 1 environment |
| qSW_Gm02-3 | PI 416937 | – | 2 populations | – |
| qSW_Gm04 | PI 416937, PI 567690 | 2 environments | – | 1 environment |
| qSW_Gm05 | PI 416937, Jackson, PI 567690 | 2 environments | 1 population | 2 environment |
| qSW_Gm06 | Magellan | – | – | 3 environments |
| qSW_Gm08 | Jackson, Nannong 1138-2 | – | 2 populations | – |
| qSW_Gm09 | Jackson, A5959, PI 567690 | – | 2 populations | 2 environments |
| qSW_Gm10 | PI 567731 | – | – | 3 environments |
| qSW_Gm11 | PI 416937, Jackson, PI 424140 | – | 3 populations | – |
| qSW_Gm12 | PI 416937, PI 567690 | 5 environments | – | 2 environments |
| qSW_Gm14 | Jackson, PI 416937 | – | 2 populations | – |
| qSW_Gm17-1 | KS4895, A5959, Benning, Pana | 2 environments | 4 populations | 1 environment |
| QSW_Gm17-2 | KS4895 | – | 2 populations | – |
| qSW_Gm19 | PI 416937, Jackson, PI 424140, PI 567690 | 2 environments | 3 populations | 2 environments |
a Numbers of environments or populations in which the QTL was detected in the corresponding study.
Among the 10 QTLs from the present mapping work, six were identified from two to three environments (either different year or different location) (Table 2; Fig. 5). Four of them were consistently mapped in two environments with favorable alleles from PI 567690 for SW, with each explaining 7.8% to 10.4% of total phenotypic variations (Fig. 5). The other two novel QTLs (qSW_Gm06 and qSW_Gm10) were mapped in the Magellan×PI 567731 cross in three environments (Fig. 5). Magellan and PI 567731 were found to contribute donor alleles for SW at qSW_Gm06 and qSW_Gm10, respectively (Fig. 5). Moreover, qSW_Gm06 and qSW_Gm10 were identified to have relatively large contributions to SW phenotypes, with each explaining 20–29.6% of total phenotypic variations (Fig. 5). It was noted that among the six QTLs from multiple environments, four QTLs (qSW_Gm05, qSW_Gm06, qSW_Gm09, and qSW_Gm10) were detected at both hill plots (BREC or DC) and row plots (SA), while the other two (qSW_Gm12 and qSW_Gm19) were detected only at hill plots (Fig. 5). Other confounding factors, such as plant density, could result in the difference in QTL detection between hill plots and row plots.
Fig. 5.
Chromosomal locations and genetic components of the two QTLs associated with canopy wilting score (WS) detected at multiple environments. The linkage maps were constructed based on genotyping results from Illumina 6K SNP arrays using JoinMap 4.0 (Table 1). The annotations of each QTL show the lines having the donor alleles for the slow wilting trait, the largest percentage contributions of the QTL to the total phenotypic variations at multiple environments, the nearest markers of the QTL, and environments detecting the QTL, respectively. Only QTLs detected in ≥2 environments are shown.
Isolation and confirmation of the major QTLs qSW_Gm06 and qSW_Gm10
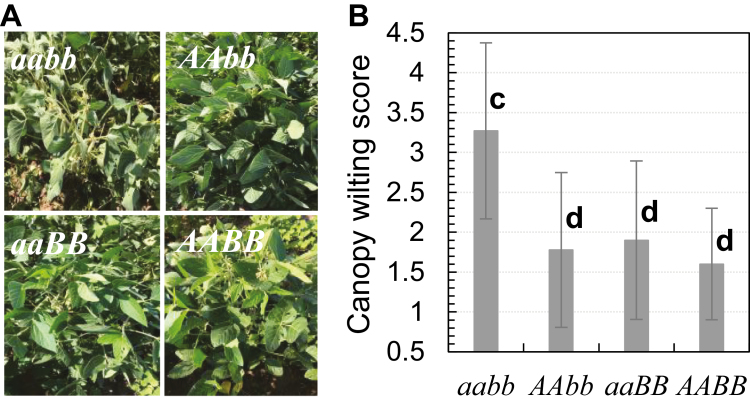
Previously, none of the SW QTLs was confirmed in the same genetic background, such as a near-isogenic background. As the two major SW QTLs (qSW_Gm06 and qSW_Gm10) were identified in the present study, efforts were made to isolate and confirm these two QTLs at a near-isogenic background. One RIL (RIL#049) in the F7:8 generation was identified to be heterozygous at the both qSW_Gm06 and qSW_Gm10 regions by genotyping of the QTL flanking markers: Gm06_43235059/Gm06_47338142 and Gm10_43894668/Gm10_44744804, respectively (Supplementary Fig. S1). The progenies of RIL#049 with homozygous genotypes at the two loci were selected as NILs for qSW_Gm06 and qSW_Gm10. These four selected NILs were named aabb, AAbb, aaBB, and AABB (Supplementary Fig. S1). ‘aa’ or ‘AA’ stands for the NILs with the donor allele for FW or SW at qSW_Gm06; while ‘bb’ or ‘BB’ stands for the NILs with the donor allele for FW or SW at qSW_Gm10. Line aabb showed an obvious wilting phenotype under drought (3 weeks without rainfall) (Fig. 6A). Adding SW alleles of either QTL significantly suppressed the canopy wilting from score 3.3 to <1.9 (Fig. 2B). The line AABB has the lightest appearance of canopy wilting; however, the differences among AAbb, aaBB, and AABB are not statistically significant, possibly due to small sample sizes and inadequate drought stress or the effects of undetected minor loci that are still segregating in the NILs (Fig. 6B). Yield test of these NILs is needed to confirm the yield benefit of these two QTLs under well-controlled drought in future studies. These efforts successfully isolated the two novel QTLs from a complex genetic background into a near-isogenic background using marker-assisted selection (MAS) and confirmed the effects of the SW QTLs for the first time.
Fig. 6.
Genotypic differences in canopy wilting under drought of qSW_Gm06 and 10. (A) Representative images of canopy wilting phenotypes of the near-isogenic lines (NILs) for qSW_Gm06 and 10. Images were taken at noon after 3 weeks without rainfall. ‘aa’ or ‘AA’ stand for the NILs with the donor allele for fast wilting or slow wilting at qSW_Gm06, while ‘bb’ or ‘BB’ stand for the NILs with the donor allele for fast wilting or slow wilting at qSW_Gm10. (B) Canopy wilting scores of the NILs. Bars represent means and SDs of 9–11 plants for each genotype. The letters ‘c’ or ‘d’ indicate results from Duncan’s multiple comparison test for not significant (same letter) or significant (different letter) at P<0.002.
Discussion
Physiological mechanisms of SW
The SW phenotype is a complex trait that could be involved in water conservation strategies in leaves and water resource exploration by roots (Valliyodan et al., 2017). Modification in transpiration was considered one important strategy for plants to adapt to water-limited conditions. Transpiration of plants increases with increasing atmospheric VPD, but, in some genotypes, the TR becomes limited when a certain VPD is reached (Sinclair and Bennett, 1998). This limited transpiration response may be important in conserving soil water for water deficit conditions. A visual consequence of the limited transpiration response can be delayed wilting (i.e. SW) under drought conditions (Fletcher et al., 2007). Previously, lack of a specific silver-sensitive population of aquaporins was hypothesized to explain the limited maximum TR of PI 416937 under increasing VPD (Sadok and Sinclair, 2010). In the present study, three lines (PI 567690, PI 567731, and Magellan) showed BPs in TR at a VPD of 1.38–2.00 kPa (Fig. 2A). PI 567690 was particularly interesting, because the slope above the BP was essentially horizontal, similar to that found in PI 416937 (Fig. 2A), suggesting that this PI should have stronger suppression in transpiration under drought. These four parental lines were subjected to AgNO3 treatment to determine if this test could confirm the previous hypothesis with PI 416937. However, unlike the findings in PI 416937, the transpiration of all of these lines was shown to be sensitive to the AgNO3 treatment and the three BP lines even showed greater sensitivity than the non-BP line (Fig. 2B). These results indicate that the mechanism for the limited maximum transpiration in SW genotypes could be independent of the previously hypothesized lack of silver-sensitive aquaporins.
Previously, PI 416937 was reported to have a much larger root system in the field than other FW lines, indicating the existence of other mechanisms enhancing the SW phenotype in addition to water conservation ability through limited transpiration responses (Hudak and Patterson, 1995). Therefore, other mechanisms, such as a larger and/or deeper root system, could also be present in the new SW lines in this research, which have not been identified yet. Dissecting the SW traits into individual genetic loci is needed to allow more in-depth mechanistic studies for a full understanding of this complex trait. The development of NILs for SW QTLs enabled zooming in on specific loci/genes and conducting more extensive mechanistic studies towards better understanding of physiological and molecular mechanisms for SW.
Impact of SW on yield
The SW trait was considered to be especially desirable in environments of low humidity/high VPD for water conservation (Fletcher et al., 2007). In a simulation analysis, the imposition of the trait of limited maximum TRs was predicted to improve yield under drought by >75% (Sinclair et al., 2010). Previous analysis of soybean production in the USA indicated that those genotypes expressing the limited maximum TRs were likely to yield more due to significant water savings under non-irrigated and high VPD conditions (Sinclair et al., 2010). In the present study, we found that RILs with the SW trait showed a significantly greater yield under non-irrigated and high VPD conditions as compared with RILs with the FW phenotype (Fig. 4). These results offered experimental evidence for the first time to support the previous hypothesis that the SW trait is associated with drought tolerance in soybean.
However, when water supply is not limiting, a limiting maximum TR may result in yield penalty (Sinclair et al., 2005). PI 416937 was found to have a low yield potential (Cho et al., 2003). The low yield of PI 416937 was thought to be consistent with a limiting maximum TR (Fletcher et al., 2007). However, the low yield of this PI line could also be due to its exotic genetic background, as the exotic germplasms tend to have much lower yield potentials compared with cultivars. In the present study, we tested the yield potentials of both SW and FW RILs and found that the SW trait had yield penalties of 7.8–10.3% in irrigated environments, where water supply is sufficient (Fig. 4). In this experiment, the 46 tested RILs were selected from the same RIL populations to normalize the genetic backgrounds between SW and FW genotypes. Therefore, it can be concluded that a limiting maximum TR increases yield stability, but with associated yield penalty in optimal growth seasons or areas. These results can be interpreted as suggesting that the SW phenotype is particularly useful for breeding soybean varieties for environments with high VPD and low water supply.
Quantitative inheritance and complexity of the SW trait
Previously, >10 SW QTLs were mapped from several populations and/or environments. The majority of these QTLs (11/13) were contributed from PI 416937 and Jackson, while the FW parents still offered donor alleles at a few loci (Table 2). However, most of the mapped QTLs were found to be unstable across environments. For instance, the major QTL on Chr. 12, previously reported by Abdel-Haleem et al. (2012), was not detectable in the same population reported by Hwang et al. (2015, 2016). GWAS in the diverse panel also confirmed the complexity of SW, as 23 putative loci had a statistically significant association with this trait (Kaler et al., 2017). However, at this point, none of these QTLs was reported to be confirmed at a synchronized genetic background, such as a near-isogenic background.
In this study, six QTLs were mapped in two RIL populations under more than two environments (Table 2; Fig. 5). The majority of the QTLs (four out of six) were detected at both hill plots and row plots (Fig. 5), suggesting that hill plots can also be used to evaluate the canopy wilting trait. However, two QTLs were detected only in hill plots (Fig. 5), suggesting the existence of other confounding factors in hill plot planting, such as plant density. The difference in plant density can affect available water resources in the soil and may ultimately affect the development of canopy wilting phenotypes. In future studies, row plots are recommended to conduct canopy wilting and drought tolerance evaluation, if space and resources are available, as they can best represent actual agricultural production.
Development of NILs and confirmation of major QTLs
Among the six QTLs mapped under multiple environments, four were mapped from the Pana×PI 567690 population and these QTLs were found to overlap with the previously reported QTLs in different genetic backgrounds (Table 2). More interestingly, two new SW QTLs (qSW_Gm06 and qSW_Gm10) were mapped in the Magellan×PI 567731 population and each parent contributes to SW at one locus (Fig. 5). Although these two QTLs have relatively larger effects on SW and are more stable across different environments compared with the others (Fig. 5), further confirmation of these QTLs in a similar genetic background is still necessary. Therefore, these two QTLs were isolated into a near-isogenic background by MAS in the heterogeneous inbred families (see Supplementary Fig. S1). As expected, preliminary field evaluations of the NILs successfully confirmed these two QTLs for the SW phenotype (Fig. 6). The confirmation of the two SW QTLs in the NILs developed by MAS indicates that it is feasible and recommended to use MAS for this complex trait in soybean breeding, especially for the major QTLs, such as qSW_Gm06 and qSW_Gm10.
It was hypothesized that stacking of SW QTLs could improve drought tolerance significantly, especially under severe or extended drought periods (Valliyodan et al., 2017). In this research, stacking both QTLs showed a slight enhancement in SW performance compared with adding either QTL, although the difference is statistically insignificant (Fig. 6). This insignificance could be due to small sample sizes or inadequate drought stress (only 3 weeks without rainfall). Further evaluations of the benefit (canopy wilting and yield) of stacking SW QTLs are needed with a larger sample size under better controlled drought conditions.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. A scheme to develop recombinant inbred line (RIL) mapping populations and near-isogenic lines (NILs) for qSW_Gm06 and qSW_Gm10.
Table S1. Recombinant inbred lines selected for yield test.
Table S2. Yield of the selected contrasting recombinant inbred lines from the Pana×PI 567690 population under irrigated and rain-fed conditions.
Table S3. Yield of the selected contrasting recombinant inbred lines from the Magellan×PI 567731 population under irrigated and rain-fed conditions.
Acknowledgements
The authors gratefully acknowledge the financial support for this study provided by the United Soybean Board and USDA-NIFA (1006057). The authors acknowledge Mackensie Murphy and Dennis Yungbluth, Division of Plant Sciences, the University of Missouri, for their technical assistance.
References
- Abdel-Haleem H, Carter TE Jr, Purcell LC, King CA, Ries LL, Chen P, Schapaugh W Jr, Sinclair TR, Boerma HR. 2012. Mapping of quantitative trait loci for canopy-wilting trait in soybean (Glycine max L. Merr). Theoretical and Applied Genetics 125, 837–846. [DOI] [PubMed] [Google Scholar]
- Bartels D, Ditzer A, Furini A. 2006. What can we learn from resurrection plants? In: Ribaut J-M, ed. Drought adaptation in cereals. Binghamton, NY: The Haworth Press, Inc., 599–622. [Google Scholar]
- Blum A. 2009. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Research 112, 119–123. [Google Scholar]
- Boyer JS, Byrneb P, Cassmand KG et al. . 2013. The U.S. drought of 2012 in perspective: a call to action. Global Food Security 2, 139–143. [Google Scholar]
- Carpentieri-Pipolo V, Pipolo AE, Abdul-Haleem H, Boerma HR, Sinclair TR. 2012. Identification of QTLs associated with limited leaf hydraulic conductance in soybean. Euphytica 186, 679–686. [Google Scholar]
- Carter TE Jr, De Souza PI, Purcell LC. 1999. Recent advances in breeding for drought and aluminum resistance in soybean. In Kauffman H, ed. Proceedings of the World Soybean Conference VI Chicago, IL 4–7 August 1999 Champaign, IL: Superior Print., 106–125. [Google Scholar]
- Carter TE Jr, Orf J, Purcell L, Specht J, Boerma HR, Chen P, Sinclair T, Rufty T. 2006. Tough times, tough plants—new soybean genes defend against drought and other stresses. In: Proceedings of the Soybean Seed Research Conference, 33rd, Chicago, IL 5–8 December 2006 CD-ROM. Alexandria, VA: American Seed Trade Association. [Google Scholar]
- Charlson DV, Bhatnagar S, King CA, Ray JD, Sneller CH, Carter TE Jr, Purcell LC. 2009. Polygenic inheritance of canopy wilting in soybean [Glycine max (L.) Merr.]. Theoretical and Applied Genetics 119, 587–594. [DOI] [PubMed] [Google Scholar]
- Cho Y, Njiti VN, Chen Y, Lightfoot DA, Wood AJ. 2003. Trigonelline concentration in field-grown soybean in response to irrigation. Biologia Plantarum 46, 405–410. [Google Scholar]
- Devi MJ, Sinclair TR, Chen P, Carter TE. 2014. Evaluation of elite southern maturity soybean breeding lines for drought tolerant traits. Agronomy Journal 106, 1947–1954. [Google Scholar]
- Doerge RW, Churchill GA. 1996. Permutation tests for multiple loci affecting a quantitative character. Genetics 142, 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. 1990. Isolation of plant DNA from fresh tissue. Focus 12, 13–15. [Google Scholar]
- Fehr WR, Caviness CE, Burmood DT, Pennington JS. 1971. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Science 6, 929–931. [Google Scholar]
- Fletcher AL, Sinclair TR, Allen LH. 2007. Transpiration responses to vapour pressure deficit in well-watered ‘slow-wilting’ and commercial soybean. Environmental and Experimental Botany 61, 145–151. [Google Scholar]
- Hatfield JL, Boote KJ, Kimball BA, Ziska LH, Izaurralde RC, Ort D, Thomson AM, Wolfe D. 2011. Climate impacts on agriculture: implications for crop production. Agronomy Journal 103, 351–370. [Google Scholar]
- Hudak CM, Patterson RP. 1995. Vegetative growth analysis of a drought resistant soybean plant introduction. Crop Science 35, 464–471. [Google Scholar]
- Hwang S, King CA, Chen P, et al. . 2016. Meta-analysis to refine map position and reduce confidence intervals for delayed-canopy-wilting QTLs in soybean. Molecular Breeding 36, 91. [Google Scholar]
- Hwang S, King CA, Ray JD, et al. . 2015. Confirmation of delayed canopy wilting QTLs from multiple soybean mapping populations. Theoretical and Applied Genetics 128, 2047–2065. [DOI] [PubMed] [Google Scholar]
- Kaler AS, Ray JD, Schapaugh WT, King CA, Purcell LC. 2017. Genome-wide association mapping of canopy wilting in diverse soybean genotypes. Theoretical and Applied Genetics 130, 2203–2217. [DOI] [PubMed] [Google Scholar]
- Kebede H, Fisher DK, Sui R, Reddy KN. 2014. Irrigation methods and scheduling in the delta region of Mississippi: current status and strategies to improve irrigation efficiency. American Journal of Plant Sciences 5, 2917–2928. [Google Scholar]
- Lesk C, Rowhani P, Ramankutty N. 2016. Influence of extreme weather disasters on global crop production. Nature 529, 84–87. [DOI] [PubMed] [Google Scholar]
- Lobell DB, Roberts MJ, Schlenker W, Braun N, Little BB, Rejesus RM, Hammer GL. 2014. Greater sensitivity to drought accompanies maize yield increase in the U.S. Midwest. Science 344, 516–519. [DOI] [PubMed] [Google Scholar]
- Passioura JB. 2010. Scaling up: the essence of effective agricultural research. Functional Plant Biology 37, 585–591. [Google Scholar]
- Pathan SM, Lee JD, Sleper DA, et al. . 2014. Two soybean plant introductions display slow leaf wilting and reduced yield loss under drought. Journal of Agronomy and Crop Science 200, 231–236. [Google Scholar]
- Rosenzweig C, Elliott J, Deryng D. et al. . 2014. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proceedings of the National Academy of Sciences, USA 111, 3268–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadok W, Sinclair TR. 2010. Transpiration response of ‘slow-wilting’ and commercial soybean [Glycine max (L.) Merr.] genotypes to three aquaporin inhibitors under high evaporative demand. Journal of Experimental Botany 61, 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR. 2011. Challenges in breeding for yield increase for drought. Trends in Plant Science 16, 289–293. [DOI] [PubMed] [Google Scholar]
- Sinclair TR. 2018. Effective water use required for improving crop growth rather than transpiration efficiency. Frontiers in Plant Science 9, 1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, Bennett JM. 1998. Water. In: Sinclair TR, Gardner FP, eds. Principles of ecology in plant production. Wallingford, Oxon: CAB International, 103–120. [Google Scholar]
- Sinclair TR, Hammer GL, van Oosterom EJ. 2005. Potential yield and water-use efficiency benefits in sorghum from limited maximum transpiration rate. Functional Plant Biology 32, 945–952. [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Messina CD, Beatty A, Samples M. 2010. Assessment across the United States of the benefits of altered soybean drought traits. Agronomy Journal 102, 475–482. [Google Scholar]
- Sinclair TR, Zwieniecki MA, Holbrook NM. 2008. Low leaf hydraulic conductance associated with drought tolerance in soybean. Physiologia Plantarum 132, 446–451. [DOI] [PubMed] [Google Scholar]
- Sloane RJ, Patterson RP, Carter TE. 1990. Field drought tolerance of a soybean plant introduction. Crop Science 30, 118–123. [Google Scholar]
- Song Q, Hyten DL, Jia G, Quigley CV, Fickus EW, Nelson RL, Cregan PB. 2013. Development and evaluation of SoySNP50K, a high-density genotyping array for soybean. PLoS One 8, e54985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht JE, Hume DJ, Kumudini SV. 1999. Soybean yield potential—a genetic and physiological perspective. Crop Science 39, 1560–1570. [Google Scholar]
- Turner NC. 2000. Drought resistance: a comparison of two frameworks. In: Saxena NP, Johansen C, Chauhan YS, Rao RCN, eds. Management of agriculture and drought: agronomic and genetic options. Enfield, NH: Scientific Publishers Inc., 89–102. [Google Scholar]
- Turner NC, Wright GC, Siddique KHM. 2001. Adaptation of grain legumes (pulses) to water-limited environments. Advances in Agronomy 71, 193–231. [Google Scholar]
- Valliyodan B, Ye H, Song L, Murphy M, Shannon JG, Nguyen HT. 2017. Genetic diversity and genomic strategies for improving drought and waterlogging tolerance in soybeans. Journal of Experimental Botany 68, 1835–1849. [DOI] [PubMed] [Google Scholar]
- van Ooijen JW. 2004. MapQTL 5, software for the mapping of quantitative trait loci in experimental populations.. Wageningen, the Netherlands; Kyazma BV. [Google Scholar]
- van Ooijen JW, Voorrips RE. 2001. JoinMap 3.0: software for the calculation of genetic linkage maps. Wageningen, the Netherlands: Plant Research International. [Google Scholar]
- Ye H, Feng J, Zhang L, Zhang J, Mispan MS, Cao Z, Beighley DH, Yang J, Gu XY. 2015. Map-based cloning of seed dormancy1-2 identified a gibberellin synthesis gene regulating the development of endosperm-imposed dormancy in rice. Plant Physiology 169, 2152–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Roorkiwal M, Valliyodan B, Zhou L, Chen P, Varshney RK, Nguyen HT. 2018a Genetic diversity of root system architecture in response to drought stress in grain legumes. Journal of Experimental Botany 69, 3267–3277. [DOI] [PubMed] [Google Scholar]
- Ye H, Song L, Chen H, et al. . 2018. b A major natural genetic variation associated with root system architecture and plasticity improves waterlogging tolerance and yield in soybean. Plant, Cell & Environment 41, 2169–2182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.