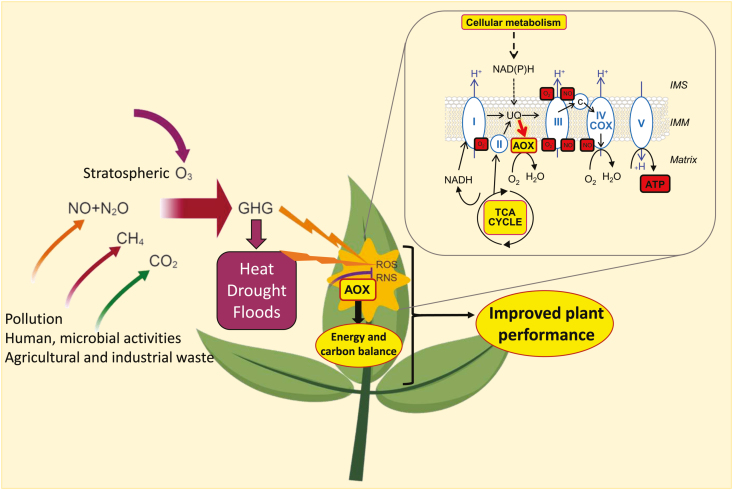
Elevated greenhouse gases (GHGs) induce adverse conditions directly and indirectly, causing decreases in plant productivity. To deal with climate change effects, plants have developed various mechanisms including the fine-tuning of metabolism. Plant respiratory metabolism is highly flexible due to the presence of various alternative pathways. The mitochondrial alternative oxidase (AOX) respiratory pathway is responsive to these changes, and several lines of evidence suggest it plays a role in reducing excesses of reactive oxygen species (ROS) and reactive nitrogen species (RNS) while providing metabolic flexibility under stress. Here we discuss the importance of the AOX pathway in dealing with elevated carbon dioxide (CO2), nitrogen oxides (NOx), ozone (O3), and the main abiotic stresses induced by climate change.
Recent advances in our understanding concerning the in vivo regulation of alternative oxidase (AOX) and its structural properties suggest that novel AOXs with altered regulatory properties could be used in future gene editing strategies. We suggest that fine-tuning modulation of the regulatory properties of AOX and targeting its expression in different plant tissues could improve plant growth and productivity under climate change conditions promoted by elevated greenhouse gasses (GHGs). Moreover, we also emphasize the need for extensive study on the interactive effects of major global change factors on AOX respiration and the importance of studies differentiating between the roles of AOX in sink versus source tissues under field conditions in order to improve plant productivity in response to elevated GHGs.
Climate change is associated with an elevation of the greenhouse gases such as (CO2), nitrogen oxides (NOx), ozone (O3), and methane (CH4), and with increased events of adverse conditions for plants including drought and high temperature stress as well as flooding (Min et al., 2011; Pall et al., 2011). Such abiotic stress conditions in combination with increasing biotic stresses are challenging plant and agricultural research to adopt new strategies for developing more climate-resilient crops with high yield and productivity in order to meet the enhanced global population food demand (Dhankher and Foyer, 2018). Considering that respiration and photosynthesis are the main components of plant carbon balance, alterations in respiration can potentially affect plant growth and productivity (Zhang et al., 2018; Amthor et al., 2019). In particular, the AOX pathway has been demonstrated to improve plant performance under different physiological conditions—mainly due to its roles both in providing metabolic flexibility and in lowering the level of mitochondrial reactive oxygen species (ROS) (Vanlerberghe, 2013; Selinski et al., 2018; Del-Saz et al., 2018a). As such, it probably functions to protect plants against the adverse effects of climate change (Fig. 1).
Fig. 1.
The role of the alternative oxidase (AOX) pathway in mitigating the effects of climate change and improving plant growth. Environmental, human, and microbial activities lead to increased greenhouse gases (GHGs). These GHGs can elevate ROS and RNS directly or directly via inducing various stresses. AOX can reduce excess ROS and RNS while maintaining energy and carbon balance to improve plant growth. In the inset, a schematic representation of the plant mitochondrial electron transport chain (mETC) is shown. The mETC contains the classical components involved in oxidative phosphorylation [I, II, III, IV (or cytochrome oxidase, COX), and V], which yields ATP. Complexes I, III, III, and IV are also sources of superoxide (O2–) and nitric oxide (NO), which can be transformed into other ROS and RNS. The AOX is inserted at the inner mitochondrial membrane (IMM) and diverts electrons from the ubiquinone (UQ) pool by reducing O2 to H2O without proton (H+) translocation into the intermembrane space (IMS). In this way, the AOX can stabilize the reduction level of the UQ pool and other mETC components, thus preventing the formation of O2– and NO. At the same time, the AOX activity renders respiration independent of adenylate control, thus allowing the reoxidation of matrix and extramitochondrial NAD(P)H under high-energy charge or COX restriction. Several physiological situations can require the action of AOX to maintain or enhance the activities of the TCA cycle and other cellular metabolic processes under energy and carbon imbalance. Yellow and red boxes indicate induced and reduced molecule levels or processes by the action of AOX, respectively.
Susceptibility of plants to various abiotic and biotic stresses can be aggravated by climate change-induced ROS including the superoxide anion (O2–), hydrogen peroxide (H2O2), and the hydroxyl radical (·OH–) (Cassia et al., 2018). These ROS originate from various sources such as the mitochondria, chloroplast, peroxisome, and the plasma membrane NADPH oxidase (Mittler, 2017). Various stresses additionally induce nitric oxide (NO), which in turn reacts with ROS, leading to the production of reactive nitrogen species (RNS) such as peroxynitrite (ONOO–), nitric dioxide (NO2), nitrosyl anion (NO–), dinitrogen trioxide (N2O3), dinitrogen tetroxide (N2O4), and nitrous acid (HNO2). Low levels of these free radicals trigger important signals; however, if they are produced in higher levels, they can cause adverse effects such as damage to lipids, proteins, and DNA, and consequently impact on plant growth and development. Plants have accordingly evolved various machineries that can deal with elevated ROS, including ascorbate peroxidase (APX), catalase (CAT), superoxide dismutase (SOD), and other enzymes involved in the ascorbate–glutathione cycle (Choudhury et al., 2017). Among mitochondrial proteins, the AOX controls mitochondrial ROS production and plays a role in adaptive plasticity. Briefly, AOX protein is inserted in the inner membrane of plant mitochondria and branches the cytochrome c oxidase (COX) pathway at the level of the ubiquinone (UQ) pool bypassing two sites of proton translocation associated with ATP production (Fig. 1). In this manner, the AOX pathway can stabilize the UQ reduction level and prevent the production of excessive ROS. Furthermore, the activity of the AOX pathway renders respiration independent of adenylate control, thus allowing the continuation of respiratory metabolism, which is crucial for plants to cope with different stress conditions (Del-Saz et al., 2018a) such as those promoted by climate change.
The AOX pathway in modulating ROS and RNS induced by greenhouse gases
Different studies have reported that AOX expression is responsive to a variety of greenhouse gases such as O3, NO, and CO2. O3 is an important component of the stratosphere. It aids in filtering dangerous UV. In contrast, in the troposphere, O3 is deleterious to plant performance. O3 in the stratosphere is made by reaction between NOx, CO2, CH4, and volatile organic compounds (VOCs) in the presence of sunlight (Hickman, 2010). Increased O3 can cause adverse effects on plants. For instance, exposure of plants to O3 causes massive changes in transcription, translation, and metabolism, resulting in decreases in plant productivity of up to 15% (Wilkinson et al., 2012). In addition, increased formation of numerous free radicals has been reported in plants after O3 exposure (Fiscus et al., 2005). These radicals can disrupt various organelles, causing programmed cell death and reducing yield in various crops (Mills et al., 2011). AOX can protect plants against damage imposed by ozone.
Intriguingly, Ederli et al. (2006) reported that exposure of tobacco plants to 300 ppb O3 strongly induced AOX expression. Moreover, Tosti et al. (2006) reported that O3 exposure resulted in the induction of AOX protein promoted by a crosstalk between ethylene and NO signalling. Furthermore, induction of AOX occurs via the inhibition of the cytochrome c pathway by O3, and at the same time the inhibition of the cytochrome pathway by O3 leads to production of H2O2. Consequently, the H2O2 produced causes further induction of AOX1a via retrograde signalling (Tosti et al., 2006). While the extent of the effect of O3 on in vivo AOX activity has not yet been determined, it follows that the overexpression of AOX in crop plants may offer plant resistance to O3 injury by reducing the levels of ROS.
Excess NOx (such as NO and NO2), which occur within the natural atmosphere, can be problematic since they are both components of GHGs as well as being inducers of other GHGs such as O3 (Hickman et al., 2010). NO, a free radical signal molecule, is a component of NOx. Excess NO can cause tyrosine nitration of proteins, thus inhibiting their activities. Several free radicals and metabolites such as pyruvate and citrate are inducers of AOX (Vanlerberghe, 2013). Among them, NO is an inducer of the AOX at the transcript and protein level (Huang et al., 2002; Kumari et al., 2019). Treatment of cell suspensions with NO leads to an increased capacity of the AOX pathway, and inhibition of AOX leads to increased NO sensitivity to cell death, suggesting that NO is induced to protect cells from cell death (Kumari et al., 2019). Fu et al. (2010) has shown that AOX is important in the prevention of cell death induced by Tobacco mosaic virus. Since O3 also causes cell death, the induced AOX can help in the protection from cell death (Overmeyer et al., 2005). The AOX pathway prevents excess ROS and NO production (Maxwell et al., 1999; Cvetkovska and Vanlerberghe, 2012; Alber et al., 2017; Vishwakarma et al., 2018). NO reacts with superoxide, leading to production of ONOO– which can cause tyrosine nitration and reduces function of various enzymes. In this context, the capacity of AOX to control both NO and ROS production makes it a very powerful machinery for the protection of plants against these molecules. Recently, it was demonstrated that AOX not only scavenges NO under normoxia induced by flg22 (flagellin) but also generates NO under hypoxia (Vishwakarma et al., 2018). In contrast to normoxia, hypoxia-induced NO does not react with superoxide, but rather is scavenged by phytoglobin1 via metaphytoglobin reductase activity. The scavenged NO has a role in recycling nitrate, maintenance of the redox status, and operation of the phytoglobin–NO cycle to generate ATP (Vishwakarma et al., 2018). NOx emissions also contribute to increased temperature, which indirectly can increase flash floods with the consequent hypoxic atmosphere in soil. Hence AOX can play a role under flooding conditions to improve energy efficiency and survival.
Although CO2 is important for photosynthesis, elevated CO2 can have some negative impacts on plants. The study of Loladze (2014) based on >130 species and crop species found that elevated CO2 can reduce mineral content on average by 8% and increases the ratio of soluble carbohydrates to proteins. Several important elements such as zinc and iron diminished in several food crops such as rice, wheat, and soybean in the presence of high CO2 (Myers et al., 2014). Elevated CO2 also induces ROS (Cheeseman, 2006) and, in order to detoxify ROS, plants also induce various antioxidants (AbdElgawad et al., 2016). Several reports suggest that AOX protein is highly responsive to elevated CO2 (Yoshida and Noguchi, 2009; Dahal and Vanlerberghe, 2018a). The relationships between yield, ROS production, and mineral nutrition in AOX-modified plants under elevated CO2 (eCO2) remain to be investigated and could provide more insights into the protective role of AOX.
The AOX pathway in providing metabolic adaptations of plants to stresses aggravated by climate change
The AOX pathway provides flexibility in cellular energy and carbon metabolism under drought, elevated temperature, and CO2 (Del-Saz et al., 2018a; Dahal and Vanlerberghe, 2018a, b), which represent the major abiotic stresses challenging current agricultural productivity with regard to climate change. The beneficial role of such metabolic flexibility probably compensates the theoretical negative effects of AOX in reducing ATP and reductant availability required for growth (Vanlerberghe, 2013). In this context, Dahal and Vanlerberghe (2018b) importantly reported that plant growth was higher in tobacco plants overexpressing AOX as compared with wild-type plants after prolonged water deficit. The beneficial effect on growth has been linked to the ability of AOX to maintain a higher respiration in light, which improves chloroplast energy balance and photosynthesis (Dahal and Vanlerberghe, 2018b). The role of the AOX in improving photosynthesis has also been reported in other species under different conditions (reviewed by Del-Saz et al., 2018a) and is probably among the main reasons explaining the beneficial role of AOX in plant growth and productivity. Nevertheless, there is also evidence suggesting that the in vivo AOX activity favours the synthesis of tricarboxylic acid (TCA)-derived metabolites with specific roles in protecting against high light (Florez-Sarasa et al., 2016) and salinity stress (Del-Saz et al., 2016).
Adjustments to the partitioning of electrons between AOX and COX pathways were associated with changes in tissue energy demands of plants exposed to long-term elevated CO2 conditions (Gomez-Casanovas et al., 2007). In addition, changes in mitochondrial electron partitioning to AOX were related to the improvement of leaf carbon balance and respiratory efficiency under different CO2 growth conditions (Gonzàlez-Meler et al., 2009). Recently, AOX overexpression has been shown to prevent both carbohydrate and energy imbalances in leaves of tobacco plants grown at elevated CO2 (Dahal and Vanlerberghe, 2018a). All these studies suggest that increased AOX activity can be beneficial for plant growth under elevated CO2 conditions.
Future perspectives
As discussed above, the use of AOX-transgenic plants has provided important insights into the role of AOX in photosynthetic tissues and growth. However, the effects of AOX genetic modification on root growth and metabolism under stress have received much less attention (Smith et al., 2009, Keunen et al., 2016). Importantly, AOX has a role in the synthesis of carboxylates in white lupin (Florez-Sarasa et al., 2014), tobacco (Del-Saz et al., 2017), and tomato (Del-Saz et al., 2018b). The root exudation of carboxylates improves phosphate acquisition, which benefits photosynthesis and plant growth (Pang et al., 2018). On the other hand, information about the impact of the AOX pathway on the growth of other sink and reproductive tissues, such as tubers and fruits, is limited (Xu et al., 2012; Zidenga et al., 2012) and represents an important area for future research. Given the evidence for tissue-specific roles for AOX, the use of more sophisticated genetic approaches specifically targeting sink and/or source tissues (Sonnewald and Fernie, 2018) will be required for disentangling the roles of AOX and its impact on plant growth and productivity. Genetic engineering of respiration involving spatio-temporal changes of the target genes has been proposed as a crucial strategy to improve crop productivity (Amthor et al., 2019). Particularly, fine-tuning alterations of AOX have been predicted to be among the most efficient strategies to achieve high biomass gains (Amthor et al., 2019). In this respect, recent in vitro (Selinski et al., 2018) and in vivo (Florez-Sarasa et al., 2019) evidence on the predominant role of the TCA cycle intermediates on AOX regulation, together with new structural insights on its active site (May et al., 2017), is paving the way to design new AOXs with altered and desirable regulatory properties. Finally, the interactive effects of major global change factors on AOX respiration remain to be determined. Some studies have highlighted the importance of (photo) respiratory metabolism under stress combination (Obata et al., 2015; El Aou-Ouad et al., 2018), although AOX was not investigated in these studies. Thus, the specific role of AOX under stress combination remains to be explored by means of genetic approaches and in vivo activity measurements. Given the evidence reported about the AOX involvement in plant tolerance to several individual biotic and abiotic stresses (reviewed in Vanlerberghe, 2013; Saha et al., 2016; Del-Saz et al., 2018a), we envisage that AOX will provide a beneficial role for plants under combined stress conditions induced by climate change.
Acknowledgements
This work is supported by the DST-DAAD exchange program between KJG and ARF. This work in the KJG lab is partly supported by a Ramalingaswami Fellowship and IYBA from the Department of Biotechnology, Government of India. IFS has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 753301.
References
- AbdElgawad H, Zinta G, Beemster GT, Janssens IA, Asard H. 2016. Future climate CO2 levels mitigate stress impact on plants: increased defense or decreased challenge? Frontiers in Plant Science 7, 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alber NA, Sivanesan H, Vanlerberghe GC. 2017. The occurrence and control of nitric oxide generation by the plant mitochondrial electron transport chain. Plant, Cell & Environment 40, 1074–1085. [DOI] [PubMed] [Google Scholar]
- Amthor JS, Bar-Even A, Hanson AD, Millar AH, Stitt M, Sweetlove LJ, Tyerman SD. 2019. Engineering strategies to boost crop productivity by cutting respiratory carbon loss. The Plant Cell 31, 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassia R, Nocioni M, Correa-Aragunde N, Lamattina L. 2018. Climate change and the impact of greenhouse gasses: CO2 and NO, friends and foes of plant oxidative stress. Frontiers in Plant Science 9, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman JM. 2006. Hydrogen peroxide concentrations in leaves under natural conditions. Journal of Experimental Botany 57, 2435–2444. [DOI] [PubMed] [Google Scholar]
- Choudhury FK, Rivero RM, Blumwald E, Mittler R. 2017. Reactive oxygen species, abiotic stress and stress combination. The Plant Journal 90, 856–867. [DOI] [PubMed] [Google Scholar]
- Cvetkovska M, Vanlerberghe GC. 2012. Alternative oxidase modulates leaf mitochondrial concentrations of superoxide and nitric oxide. New Phytologist 195, 32–39. [DOI] [PubMed] [Google Scholar]
- Dahal K, Vanlerberghe GC. 2018a Growth at elevated CO2 requires acclimation of the respiratory chain to support photosynthesis. Plant Physiology 178, 82–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal K, Vanlerberghe GC. 2018b Improved chloroplast energy balance during water deficit enhances plant growth: more crop per drop. Journal of Experimental Botany 69, 1183–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del-Saz NF, Florez-Sarasa I, Clemente-Moreno MJ, Mhadhbi H, Flexas J, Fernie AR, Ribas-Carbó M. 2016. Salinity tolerance is related to cyanide-resistant alternative respiration in Medicago truncatula under sudden severe stress. Plant, Cell & Environment 39, 2361–2369. [DOI] [PubMed] [Google Scholar]
- Del-Saz NF, Ribas-Carbo M, McDonald AE, Lambers H, Fernie AR, Florez-Sarasa I. 2018a An in vivo perspective of the role(s) of the alternative oxidase pathway. Trends in Plant Science 23, 206–219. [DOI] [PubMed] [Google Scholar]
- Del-Saz NF, Romero-Munar A, Cawthray GR, Aroca R, Baraza E, Flexas J, Lambers H, Ribas-Carbó M. 2017. Arbuscular mycorrhizal fungus colonization in Nicotiana tabacum decreases the rate of both carboxylate exudation and root respiration and increases plant growth under phosphorus limitation. Plant and Soil 416, 97–106. [Google Scholar]
- Del-Saz NF, Romero-Munar A, Cawthray GR, Palma F, Aroca R, Baraza E, Florez-Sarasa I, Lambers H, Ribas-Carbó M. 2018b Phosphorus concentration coordinates a respiratory bypass, synthesis and exudation of citrate, and the expression of high-affinity phosphorus transporters in Solanum lycopersicum. Plant, Cell & Environment 41, 865–875. [DOI] [PubMed] [Google Scholar]
- Dhankher OP, Foyer CH. 2018. Climate resilient crops for improving global food security and safety. Plant, Cell & Environment 41, 877–884. [DOI] [PubMed] [Google Scholar]
- Ederli L, Morettini R, Borgogni A, Wasternack C, Miersch O, Reale L, Ferranti F, Tosti N, Pasqualini S. 2006. Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants. Plant Physiology 142, 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Aou-Ouad H, Bota J, Obata T, Montero R, Fernie AR, Medrano H, Pou A, Florez-Sarasa I. 2018. Combined drought and virus infection trigger aspects of respiratory metabolism related to grapevine physiological responses. Journal of Plant Physiology 231, 19–30. [DOI] [PubMed] [Google Scholar]
- Fiscus EL, Booker FL, Burkey KO. 2005. Crop responses to ozone: uptake, modes of action, carbon assimilation and partitioning. Plant, Cell & Environment 28, 997–1011. [Google Scholar]
- Florez-Sarasa I, Lambers H, Wang X, Finnegan PM, Ribas-Carbo M. 2014. The alternative respiratory pathway mediates carboxylate synthesis in white lupin cluster roots under phosphorus deprivation. Plant, Cell & Environment 37, 922–928. [DOI] [PubMed] [Google Scholar]
- Florez-Sarasa I, Obata T, Del-Saz NF, Reichheld JP, Meyer EH, Rodriguez-Concepcion M, Ribas-Carbo M, Fernie AR. 2019. The lack of mitochondrial thioredoxin TRXo1 affects in vivo alternative oxidase activity and carbon metabolism under different light conditions. Plant & Cell Physiology 60, doi:10.1093/pcp/pcz123. [DOI] [PubMed] [Google Scholar]
- Florez-Sarasa I, Ribas-Carbo M, Del-Saz NF, Schwahn K, Nikoloski Z, Fernie AR, Flexas J. 2016. Unravelling the in vivo regulation and metabolic role of the alternative oxidase pathway in C3 species under photoinhibitory conditions. New Phytologist 212, 66–79. [DOI] [PubMed] [Google Scholar]
- Fu LJ, Shi K, Gu M, Zhou YH, Dong DK, Liang WS, Song FM, Yu JQ. 2010. Systemic induction and role of mitochondrial alternative oxidase and nitric oxide in a compatible tomato–Tobacco mosaic virus interaction. Molecular Plant-Microbe Interactions 23, 39–48. [DOI] [PubMed] [Google Scholar]
- Gomez-Casanovas N, Blanc-Betes E, Gonzalez-Meler MA, Azcon-Bieto J. 2007. Changes in respiratory mitochondrial machinery and cytochrome and alternative pathway activities in response to energy demand underlie the acclimation of respiration to elevated CO2 in the invasive Opuntia ficus-indica. Plant Physiology 145, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzàlez-Meler MA, Blanc-Betes E, Flower CE, Ward JK, Gomez-Casanovas N. 2009. Plastic and adaptive responses of plant respiration to changes in atmospheric CO2 concentration. Physiologia Plantarum 137, 473–484. [DOI] [PubMed] [Google Scholar]
- Hickman JE, Wu S, Mickley LJ, Lerdau MT. 2010. Kudzu (Pueraria montana) invasion doubles emissions of nitric oxide and increases ozone pollution. Proceedings of the National Academy of Sciences, USA 107, 10115–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, von Rad U, Durner J. 2002. Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta 215, 914–923. [DOI] [PubMed] [Google Scholar]
- Keunen E, Florez-Sarasa I, Obata T, Jozefczak M, Remans T, Vangronsveld J, Fernie AR, Cuypers A. 2016. Metabolic responses of Arabidopsis thaliana roots and leaves to sublethal cadmium exposure are differentially influenced by ALTERNATIVE OXIDASE1a. Environmental and Experimental Botany 124, 64–78. [Google Scholar]
- Kumari A, Pathak PK, Bulle M, Igamberdiev AU, Gupta KJ. 2019. Alternative oxidase is an important player in the regulation of nitric oxide levels under normoxic and hypoxic conditions in plants. Journal of Experimental Botany 70, 4345–4354. [DOI] [PubMed] [Google Scholar]
- Loladze I. 2014. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 3, e02245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L. 1999. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proceedings of the National Academy of Sciences, USA 96, 8271–8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May B, Young L, Moore AL. 2017. Structural insights into the alternative oxidases: are all oxidases made equal? Biochemical Society Transactions 45, 731–740. [DOI] [PubMed] [Google Scholar]
- Mills G, Hayes F, Simpson D, Emberson L, Norris D, Harmens H, Büker P. 2011. Evidence of widespread effects of ozone on crops and (semi-) natural vegetation in Europe (1990–2006) in relation to AOT40- and flux-based risk maps. Global Change Biology 17, 592–613. [Google Scholar]
- Mittler R. 2017. ROS are good. Trends in Plant Science 22, 11–19. [DOI] [PubMed] [Google Scholar]
- Min SK, Zhang X, Zwiers FW, Hegerl GC. 2011. Human contribution to more-intense precipitation extremes. Nature 470, 378–381. [DOI] [PubMed] [Google Scholar]
- Myers SS, Zanobetti A, Kloog I, et al. 2014. Increasing CO2 threatens human nutrition. Nature 510, 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T, Witt S, Lisec J, Palacios-Rojas N, Florez-Sarasa I, Yousfi S, Araus JL, Cairns JE, Fernie AR. 2015. Metabolite profiles of maize leaves in drought, heat, and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiology 169, 2665–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmeyer K, Brosché M, Pellinen R, Kuittinen T, Tuominen H, Ahlfors R, Keinänen M, Saarma M, Scheel D, Kangasjärvi J. 2005. Ozone-induced programmed cell death in the Arabidopsis radical-induced cell death1 mutant. Plant Physiology 137, 1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall P, Aina T, Stone DA, Stott PA, Nozawa T, Hilberts AG, Lohmann D, Allen MR. 2011. Anthropogenic greenhouse gas contribution to flood risk in England and Wales in autumn 2000. Nature 470, 382–385. [DOI] [PubMed] [Google Scholar]
- Pang J, Ryan MH, Lambers H, Siddique KH. 2018. Phosphorus acquisition and utilisation in crop legumes under global change. Current Opinion in Plant Biology 45, 248–254. [DOI] [PubMed] [Google Scholar]
- Saha B, Borovskii G, Panda SK. 2016. Alternative oxidase and plant stress tolerance. Plant Signaling & Behavior 11, e1256530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinski J, Scheibe R, Day DA, Whelan J. 2018. Alternative oxidase is positive for plant performance. Trends in Plant Science 23, 588–597. [DOI] [PubMed] [Google Scholar]
- Smith CA, Melino VJ, Sweetman C, Soole KL. 2009. Manipulation of alternative oxidase can influence salt tolerance in Arabidopsis thaliana. Physiologia Plantarum 137, 459–472. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Fernie AR. 2018. Next-generation strategies for understanding and influencing source–sink relations in crop plants. Current Opinion in Plant Biology 43, 63–70. [DOI] [PubMed] [Google Scholar]
- Tosti N, Pasqualini S, Borgogni A, Ederli L, Falistocco E, Crispi S, Paolocci F. 2006. Gene expression profiles of O3-treated Arabidopsis plants. Plant, Cell & Environment 29, 1686–1702. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC. 2013. Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. International Journal of Molecular Sciences 14, 6805–6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwakarma A, Kumari A, Mur LAJ, Gupta KJ. 2018. A discrete role for alternative oxidase under hypoxia to increase nitric oxide and drive energy production. Free Radical Biology & Medicine 122, 40–51. [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Mills G, Illidge R, Davies WJ. 2012. How is ozone pollution reducing our food supply? Journal of Experimental Botany 63, 527–536. [DOI] [PubMed] [Google Scholar]
- Xu F, Yuan S, Zhang DW, Lv X, Lin HH. 2012. The role of alternative oxidase in tomato fruit ripening and its regulatory interaction with ethylene. Journal of Experimental Botany 63, 5705–5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Noguchi K. 2009. Differential gene expression profiles of the mitochondrial respiratory components in illuminated Arabidopsis leaves. Plant & Cell Physiology 50, 1449–1462. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Fernie AR. 2018. On the role of the tricarboxylic acid cycle in plant productivity. Journal of Integrative Plant Biology 60, 1199–1216. [DOI] [PubMed] [Google Scholar]
- Zidenga T, Leyva-Guerrero E, Moon H, Siritunga D, Sayre R. 2012. Extending cassava root shelf life via reduction of reactive oxygen species production. Plant Physiology 159, 1396–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]