roGFP2 measurements demonstrate that ungerminated Arabidopsis pollen grains are highly oxidized. Glutathione-deficient flowers show increased cellular oxidation with poor pollen germination/tube growth, suggesting that the reduced state underpins growth functions.
Keywords: Flower development, glutathione, oxidative stress, pollen germination, redox regulation, redox-sensitive green fluorescent protein (roGFP)
Abstract
The importance of the glutathione pool in the development of reproductive tissues and in pollen tube growth was investigated in wild-type (WT) Arabidopsis thaliana, a reporter line expressing redox-sensitive green fluorescent protein (roGFP2), and a glutathione-deficient cad2-1 mutant (cad2-1/roGFP2). The cad2-1/roGFP2 flowers had significantly less reduced glutathione (GSH) and more glutathione disulfide (GSSG) than WT or roGFP2 flowers. The stigma, style, anther, germinated pollen grains, and pollen tubes of roGFP2 flowers had a low degree of oxidation. However, these tissues were more oxidized in cad2-1/roGFP2 flowers than the roGFP2 controls. The ungerminated pollen grains were significantly more oxidized than the germinated pollen grains, indicating that the pollen cells become reduced upon the transition from the quiescent to the metabolically active state during germination. The germination percentage was lower in cad2-1/roGFP2 pollen and pollen tube growth arrested earlier than in roGFP2 pollen, demonstrating that increased cellular reduction is essential for pollen tube growth. These findings establish that ungerminated pollen grains exist in a relatively oxidized state compared with germinating pollen grains. Moreover, failure to accumulate glutathione and maintain a high GSH/GSSG ratio has a strong negative effect on pollen germination.
Introduction
The tripeptide thiol γ-glutamylcysteine glycine (glutathione, GSH) is the most abundant low-molecular-weight thiol in plants and animals (Noctor et al., 2011). GSH synthesis in the cytosol and plastids of plant cells (Wachter et al., 2005; Galant et al., 2011) occurs via the sequential action of two ATP-dependent enzymes, γ-glutamylcysteine synthetase (γ-ECS), also called γ-glutamylcysteine ligase, and glutathione synthase (GS) (Cairns et al., 2006; Pasternak et al., 2008; Galant et al., 2011; Noctor et al., 2011). In the first step, γ-glutamylcysteine (γ-EC) is produced from cysteine and glutamate by the action of γ-ECS. In the second step, glycine is added to γ-EC to form GSH by the action of GS (Noctor et al., 2002; Sugiyama et al., 2004; Wachter et al., 2005; Galant et al., 2011). GSH synthesis is regulated by the availability of cysteine or glycine and by the post-translational redox activation of γ-ECS via the formation of an intramolecular disulfide bond (Cys186-Cys406 in Arabidopsis) (Noctor, et al., 2002; Gromes, et al., 2008). In this way GSH synthesis is regulated by the redox environment of the chloroplast, allowing rapid responses to biotic and abiotic stresses.
Knockout γ-ECS mutants are lethal (Cairns et al., 2006). However, there are a number of Arabidopsis mutants with defects in γ-ECS that allow a lower level of GSH synthesis (Cobbett et al., 1998; Ball et al., 2004). These mutants have significantly lower levels of GSH accumulation than the wild type (WT), and have no marked shoot phenotypes but variable effects on root system development and architecture (Schnaubelt et al., 2013). For example, very low levels of GSH are present in the rml1 mutant, leading to arrest of the root apical meristem (Vernoux et al., 2000; Schnaubelt et al., 2013). In contrast, the cad2-1 mutant contains ~30% of WT GSH levels and shows some effects on lateral root production (Schnaubelt et al., 2013).
GSH and γ-EC are exported from the chloroplasts to the cytosol by CLT transporters, but the mechanisms of import of GSH into mitochondria and other cellular compartments are largely unknown (Gigolashvili and Kopriva, 2014; Zechmann, 2014). GSH accumulates at high concentrations (5–10 mM) in plant cells and is directly involved in the maintenance of intracellular redox homeostasis, acting as a redox buffer (Meister et al., 1983; Schafer et al., 2001; López-Mirabal et al., 2008; Noctor et al., 2011, 2016). Like ascorbate, GSH serves to limit the accumulation of reactive oxygen species (ROS) within plant cells (Mittler et al., 2004; Foyer and Noctor, 2005, 2009, 2011; Noctor et al., 2012; Mittler, 2017). As a result of interactions with ROS, GSH is oxidized to glutathione disulfide (GSSG). GSSG is reduced back to GSH by the action of glutathione reductase (Schwarzländer et al., 2008; Marty et al., 2009; García-Quirós et al., 2017). GSH and GSSG are key players in cellular redox regulation, governing the redox potential and ensuring a stable redox environment within the cell. GSH and GSSG are also involved in the control of gene expression (Foyer et al., 2001; Maughan and Foyer, 2006). However, in vivo measurements of the glutathione pool have revealed that the cytosol, nuclei, and mitochondria contain very little GSSG, and that oxidative challenges are rapidly counterbalanced by the network of other antioxidants (Queval et al., 2009; Noctor et al., 2011, 2016; de Simone et al., 2017). Moreover, GSSG can be rapidly sequestered in the vacuole (Noctor et al., 2013).
Together with nitric oxide (Airaki et al., 2011), ROS and GSH are key regulators of plant development (Considine and Foyer, 2014). These redox molecules are particularly important in the development of flowers and in pollen germination (Zechmann and Müller, 2010; Zechmann et al., 2011a, b; Traverso et al., 2013; Schippers et al., 2016). GSH functions together with NADPH-thioredoxin (TRX)-reductase NTR/TRX pathways to regulate auxin signalling during and after pollen germination (Zechmann et al., 2011a, b; Traverso et al., 2013). GSH depletion triggers alterations in auxin metabolism that lead to the inhibition of pollen germination. This observation is likely to be linked to changes in ROS accumulation that occur in the reproductive tissues during pollen and pistil development, pollen tube germination, and pollen–pistil interactions (Zafra et al., 2010). However, the relevance of glutathione-dependent pathways remains poorly understood, and too little is known about the compartment-specific changes in redox processes that occur during flower and pollen development. A combination of different techniques has been used to investigate the role of glutathione in fertilization and the germination of the pollen tube. Specific antibodies were used to characterize the abundance of GSH in flowers during development. In addition, the effects of low GSH in the cytosol and nuclei of Arabidopsis thaliana reproductive tissues were determined using in vivo redox-sensitive green fluorescent protein (roGFP) reporter lines (Meyer et al., 2007). roGFP-expressing WT (roGFP2) and cad2-1 mutant (cad2-1/roGFP2) reporter lines were used in the present study to estimate the redox state of the nuclei and cytosol of flower and pollen cells to provide some of the missing pieces of information required to decipher the role of glutathione in these key processes.
Materials and methods
Plant material and growth conditions
Seeds of WT Arabidopsis thaliana accession Columbia-0 (Col-0), Col-0 expressing roGFP2 (de Simone et al., 2017), and cad2-1 mutants expressing roGFP2 (cad2-1/roGFP2) were surface sterilized and washed twice with sterile water. After stratification for 2 days in the dark at 4 °C, seeds were sown on soil and grown in controlled environment chambers under 120–150 μmol m−2 s−1 irradiance with an 8 h/16 h day/night period, 22 °C/18 °C day/night temperatures, and a relative humidity of 60%. Samples were collected from 6-week-old plants bearing flowers at different stages (Alvarez-Buylla et al., 2010). Samples were collected from younger flower buds at flowering stage 6–7, with sepals that still enclose buds and anthers (at meiosis) and tetrads. Flowers were also collected from later stages of flowering, particularly flowering stage 11–12 (when petals, stamens and pollen at the bicellular–tricellular transition are present), flowering stage 13 (when stamens outstrip the gynoecium and anthers dehisce), and flowering stage 14 (when dehiscent anthers extend above the stigma). Each experiment was repeated three times and results are the average of three independent experiments.
Pollen germination
A total of 25–30 freshly opened flowers (at stage 14) of Arabidopsis were collected and allowed to dry at room temperature for 30 min in a 1.5 ml tube with the lid opened. A 1 ml volume of germination medium (Fan et al., 2001) was added and the pollen was concentrated according to Li (2011). The pollen grains were germinated at 28 °C and in the dark for 1, 2, and 3 h. Images of germinated pollen grains were taken in a Leica DMI600B or a Nikon Eclipse TE2000-U inverted epifluorescence microscope. The germination of the pollen was assessed by counting the germinated grains (those in which the pollen tube length was greater than the pollen diameter) at the different germination times, and expressing the number of germinated grains as a percentage of the total number of pollen grains. The length of the pollen tubes was calculated using the Straight tool of ImageJ software. Data presented are the means and SD of a minimum of nine different samples of each one of three experimental replicates.
Sample preparation for immunohistochemistry
Samples were collected at the different developmental stages identified in the figure legends and immersed in a fixative solution (4% paraformaldehyde and 0.2% glutaraldehyde in 0.05 M sodium cacodylate buffer, pH 7.2). Further dehydration in an ethanol series and embedding in Unicryl resin followed by polymerization using UV light at –20 °C were performed. Thin sections (1 μm) were attached to 3-triethoxysilylpropylamine-treated slides.
Immunolabelling of glutathione
Thin sections were incubated in phosphate-buffered saline (pH 7.2) containing 2.5% bovine serum albumin (Sigma-Aldrich) for 1 h at room temperature and then treated with primary anti-GSH antibody (Agrisera prod. No. AS09 594) followed by secondary antibody (anti-rabbit IgG Alexa549-labelled secondary antibody; Molecular Probes). Negative controls were prepared by omitting the primary antibody. After washing three times, samples were mounted with citifluor (Ted Pella). Fluorescence microscopy observations were performed using a Nikon Eclipse TE2000-U inverted microscope fitted with a ProgRes MF Cool CCD microscope camera (Jenoptik). Fluorescence intensity was quantified using ImageJ software. Data presented are the average of a minimum of nine images obtained in three independent experiments.
Liquid chromatography–electrospray/mass spectrometry
Tissue glutathione contents were determined by liquid chromatography–electrospray/mass spectrometry (LC-ES/MS). Samples of open flowers and floral buds (green and white) of WT, roGFP2, and cad2-1/roGFP2 plants were frozen in liquid nitrogen, ground, and immediately homogenized in 1 ml 0.1 M HCl. After centrifugation, supernatants were extracted again. The supernatants were pooled and filtered through 0.45 mm polyvinylidene fluoride filters and analysed by LC-ES/MS on a Triple quadrupole mass spectrometer (Quattro micro) coupled to a liquid chromatograph (Waters Alliance 2695) with dual wavelength detector.
Confocal laser scanning microscopy and image analysis
Freshly opened flowers (day 0 according to Boavida et al., 2007) were incubated in 1 ml of germination medium (Fan et al., 2001) at 28 °C for 1 h in darkness. After the incubation, selected flowers were assembled on excavated slides containing a few drops of the germination medium. They were immediately observed with a C-1 confocal microscope (Nikon) fitted on a Nikon Eclipse TE2000-U inverted microscope, using an image capture schedule equivalent to that described by de Simone et al. (2017). Briefly, the image of reduced roGFP (green) was calculated by subtracting the image after excitation with 405 nm from the image obtained after excitation with 488 nm, by using ImageJ software. The final reduced roGFP/oxidized roGFP ratios were also determined with ImageJ, by using a threshold of 10. Finally, to better illustrate the redox potential differences in the images evaluated, the grayscale was converted to a false-colour scale image using the ImageJ ‘Fire’ query table. All measurements were taken with identical adjustments to be able to compare them in absolute terms (Meyer et al., 2007). The intensity per area was measured in each image by using ImageJ software, and these data were used to determine the degree of oxidation and the redox potential in each tissue examined according to the Nernst equation (de Simone et al., 2017). A minimum of 10 samples per treatment were analysed.
Statistical analysis
IBM SPSS Statistics software (version 21.0.0.0) was used to analyse the statistical significance of the results. The Shapiro–Wilk test combined with the Kolmogorov–Smirnov test was used to evaluate the normality of the data. A one-way ANOVA was performed in combination with a Duncan’s multiple range test and post hoc Bonferroni procedure to verify the statistical significance of the data.
Results
Immunolocalization of GSH during flower development
GSH was detected in the microsporocytes, tetrads, pollen grains, and anther tissues (including the tapetum) of the WT, roGFP2, and cad2-1/roGFP2 plants using anti-GSH antibody (Figs 1–3). GSH was also present in the gynoecium (including stigma), the embryo sacs, and other tissues of the ovary (Figs 4–6) of the WT and roGFP2 plants.
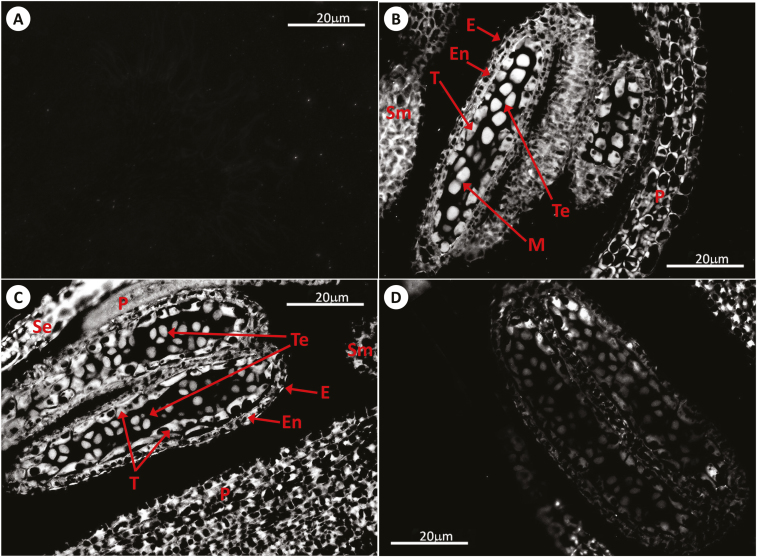
Fig. 1.
Immunolocalization of GSH by antibody-associated fluorescence microscopy in Arabidopsis anthers at stages 6–7 of development. (A) Negative control (meiocytes); (B) WT (meiocytes); (C) roGFP2 (tetrads); (D) cad2-1/roGFP2 (meiocytes). E, epidermis; En, endothecium; M, meiosis; P, petal; Se, sepal; Sm, stamen; T, tapetal layer; Te, tetrad.
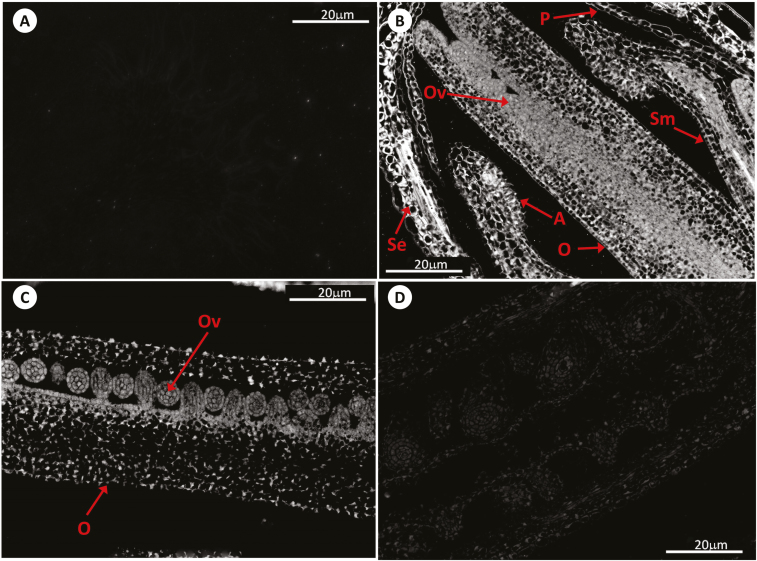
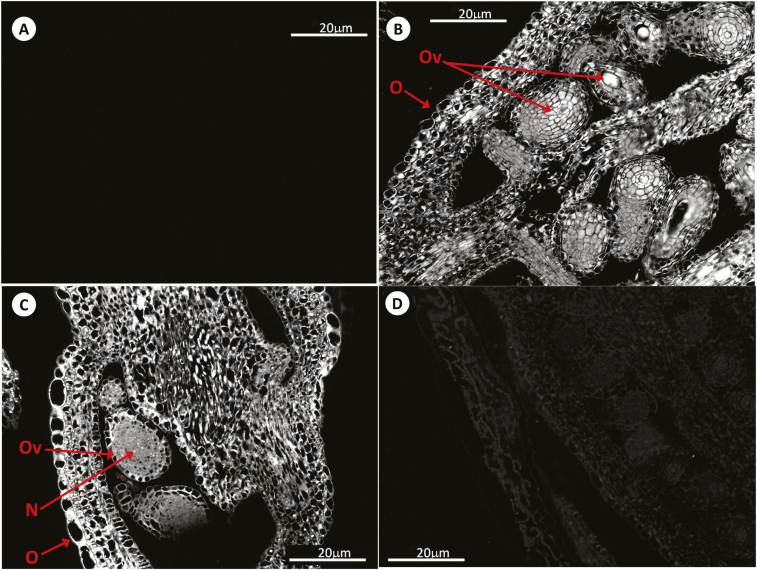
Fig. 4.
Immunolocalization of GSH by antibody-associated fluorescence microscopy in Arabidopsis flowers (pistils) at stage 11–12. (A) Negative control; (B) WT; (C) roGFP2; (D) cad2-1/roGFP2. A, anther; O, ovary; Ov, ovum; P, petal; Se, sepal; Sm, stamen.
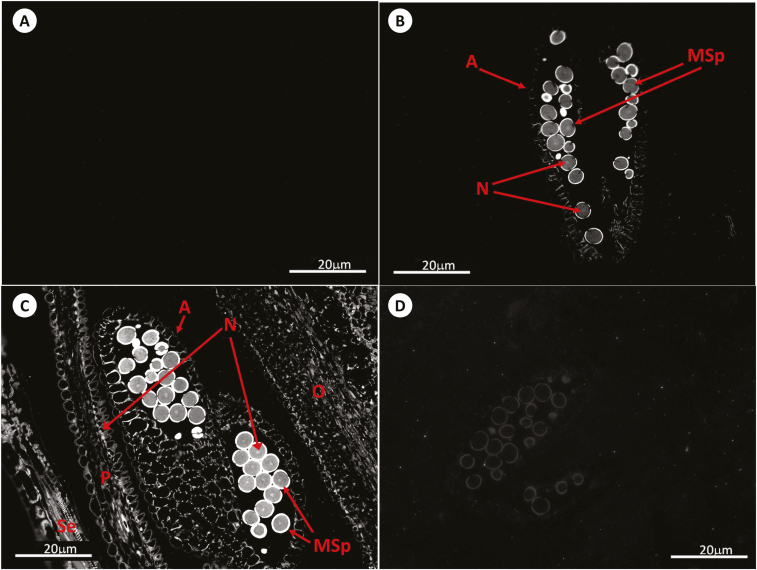
Fig. 2.
Immunolocalization of GSH by antibody-associated fluorescence microscopy in Arabidopsis anthers at stage 13. (A) Negative control; (B) WT; (C) roGFP2; (D) cad2-1/roGFP2. A, anther; MSp, microspores; N, nucleus; O, ovary; P, petal; Se, sepal.
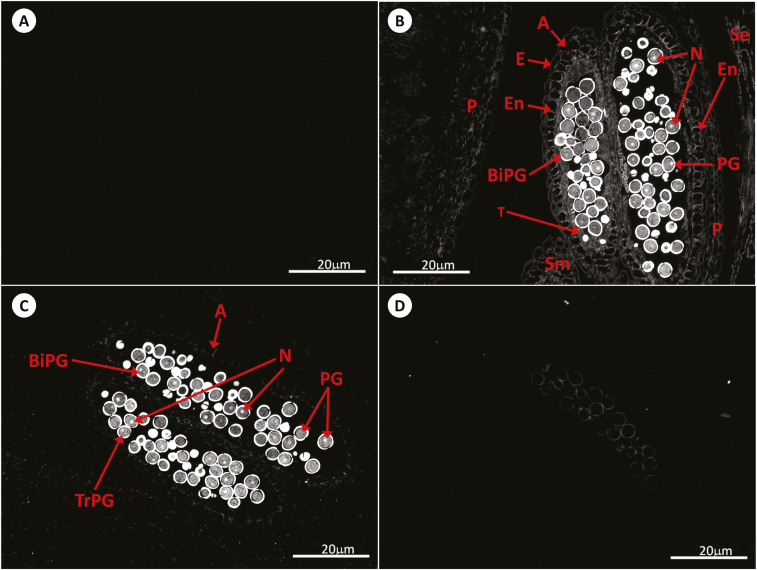
Fig. 3.
Immunolocalization of GSH by antibody-associated fluorescence microscopy in Arabidopsis anthers at stage 14. (A) Negative control; (B) WT; (C) roGFP2; (D) cad2-1/roGFP2. A, anther; BiPG, bicellular pollen grain; E, epidermis; En, endothecium; N, nucleus; P, petal; PG, pollen grain; Se, sepal; Sm, stamen; T, tapetal layer; TrPG, tricellular pollen grain.
Fig. 5.
Immunolocalization of GSH by antibody-associated fluorescence microscopy in Arabidopsis pistils at stage 13. (A) Negative control; (B) WT; (C) roGFP2; (D) cad2-1/roGFP2. N, nucleus; O, ovary; Ov, ovum.
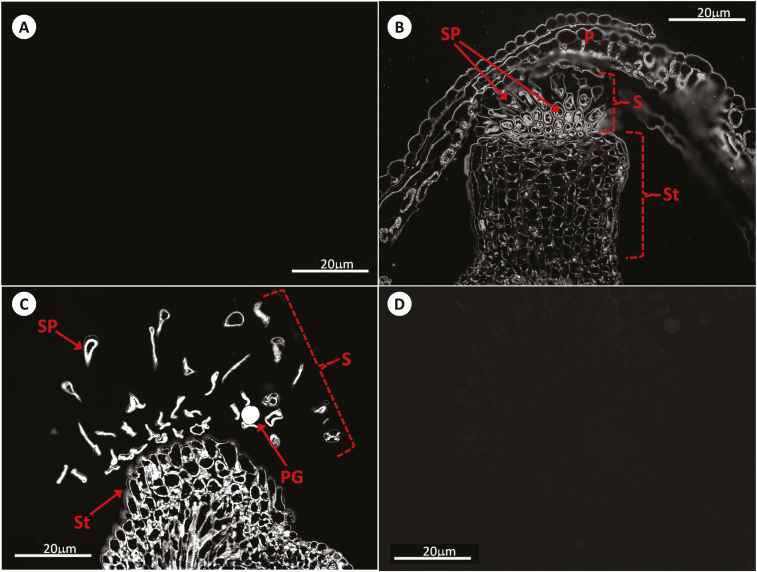
Fig. 6.
Immunolocalization of GSH by antibody-associated fluorescence microscopy in Arabidopsis pistils at stage 14. (A) Negative control; (B) WT; (C) roGFP2; (D) cad2-1/roGFP2. P, petal; PG, pollen grain; S, stigma; SP, stigmatic papillae; St, style.
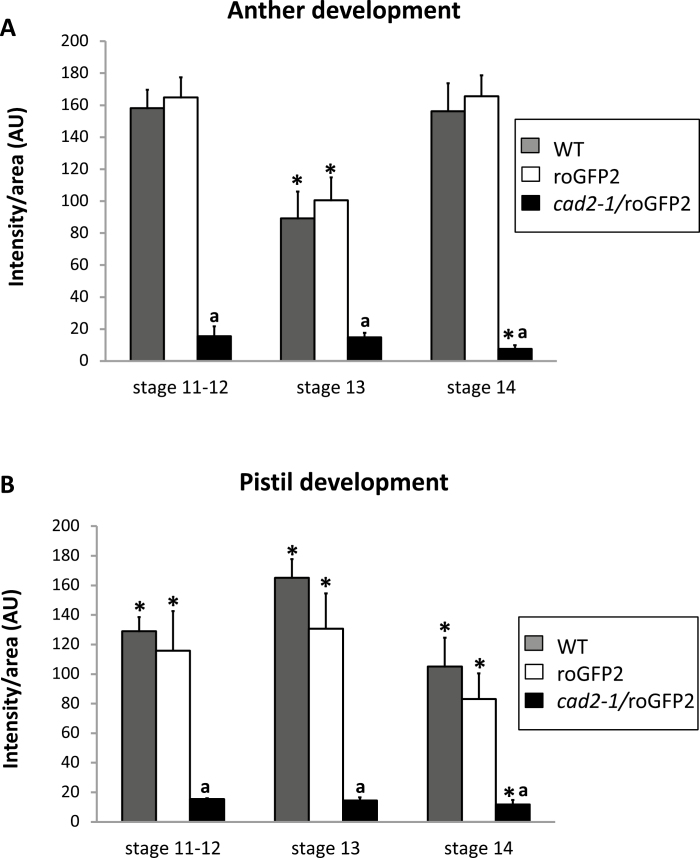
GSH was present in the nuclei, cytosol, apoplast/cell wall compartment, tapetum, and embryo sacs of these lines. GSH and GSSG are transported throughout the plant, and both metabolites are readily taken up by the plasma membrane. Low levels of GSH and GSSG have been detected in the apoplastic fluid of leaf cells (Vanacker et al., 1998), consistent with our findings. The antibody-linked fluorescence was much less intense in all the flower tissues of the cad2-1/roGFP2 plants than either the WT or roGFP2 plants. This observation reflects the lower levels of GSH present in the cad2-1/roGFP2 plants than the other lines, which had similar levels of GSH during all developmental stages, especially at the end of anther development (Fig. 7).
Fig. 7.
Relative antibody-associated fluorescence in different organs at different stages of flower development (11–12, 13, and 14) in WT, roGFP2, and cad2-1/roGFP2 plants. (A) Development of anthers. (B) Development of pistils. Asterisks indicate statistical significance (P≤0.05) among the different developmental stages within the same line (roGFP or cad2-1). a indicates statistical significance (P≤0.05) between different lines.
LC-ES/MS determination of GSH and GSSG levels
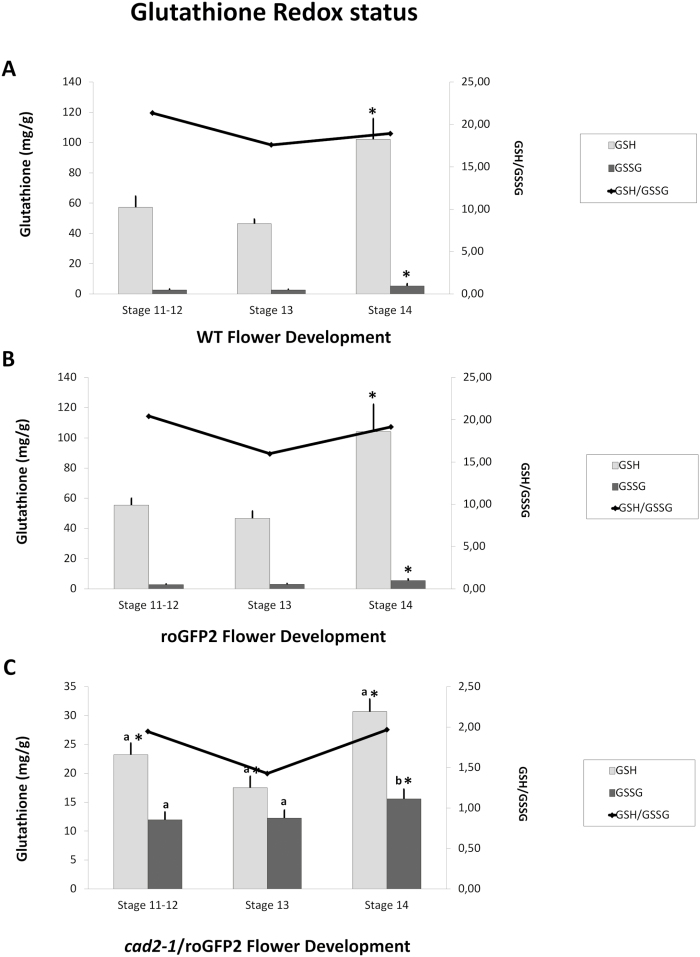
The levels of GSH and GSSG were similar in WT (Fig. 8A) and roGFP2 (Fig. 8B) flowers at all stages of development. The highest levels of GSH were determined in flowers at stage 14, with high GSH/GSSG ratios maintained throughout flower development in the WT (Fig. 8A) and roGFP2 (Fig. 8B) plants. In contrast, the flowers of cad2-1/roGFP2 plants had less than 50% of the GSH present in WT or roGFP2 lines at equivalent stages of flower development. Moreover, the levels of GSSG in cad2-1/roGFP2 flowers (Fig. 8C) were similar to or slightly higher than those detected in WT (Fig. 8A) and roGFP2 (Fig. 8B) flowers, resulting in lower GSH/GSSG ratios.
Fig. 8.
GSH and GSSG contents and GSH/GSSG ratios of flowers at different stages of development. (A) WT; (B) roGFP2; (C) cad2-1/roGFP2. Asterisks indicate statistical significance (P≤0.05) between different developmental stages. a and b indicate statistical significance at P≤0.01 and P≤0.05, respectively, between different lines.
Redox state of the glutathione pool in vivo
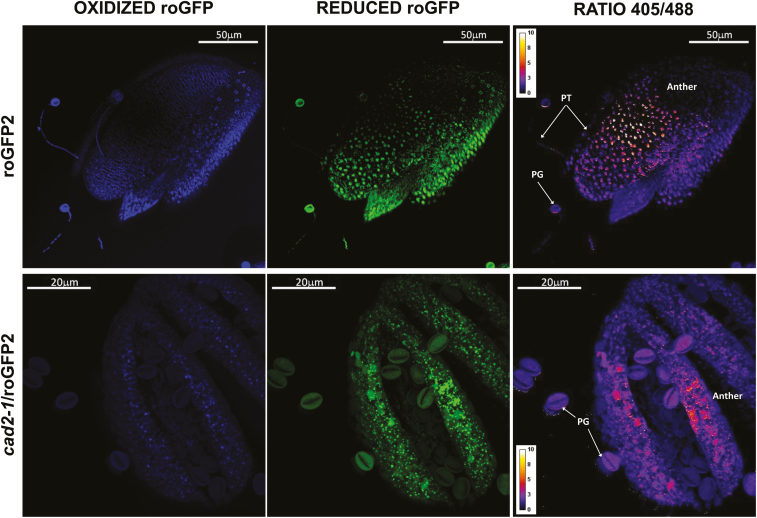
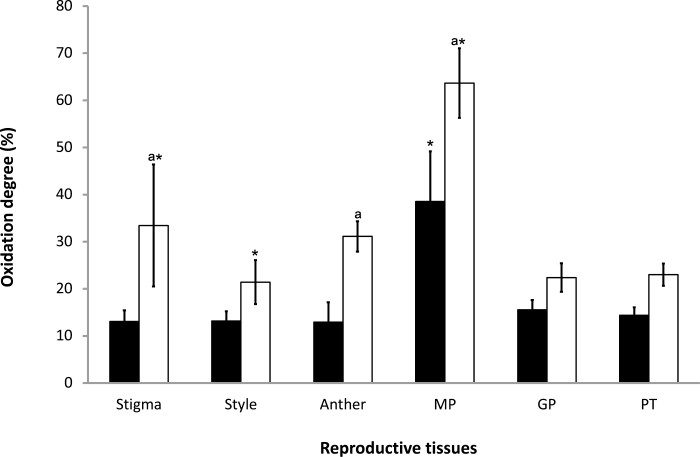
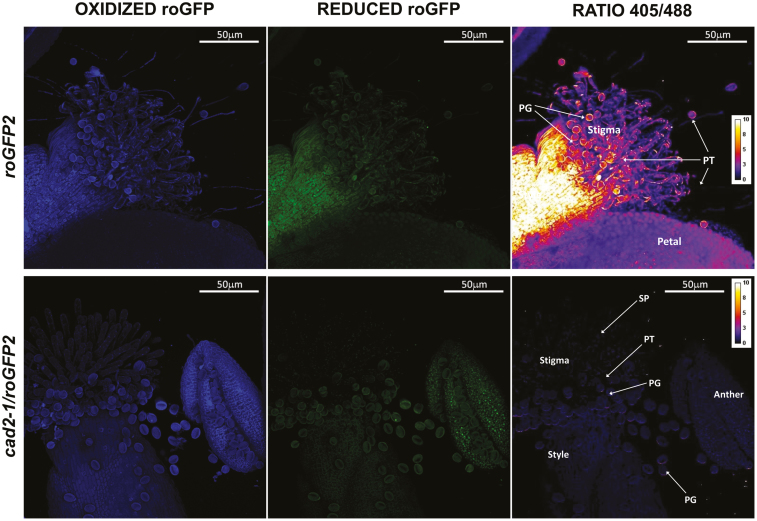
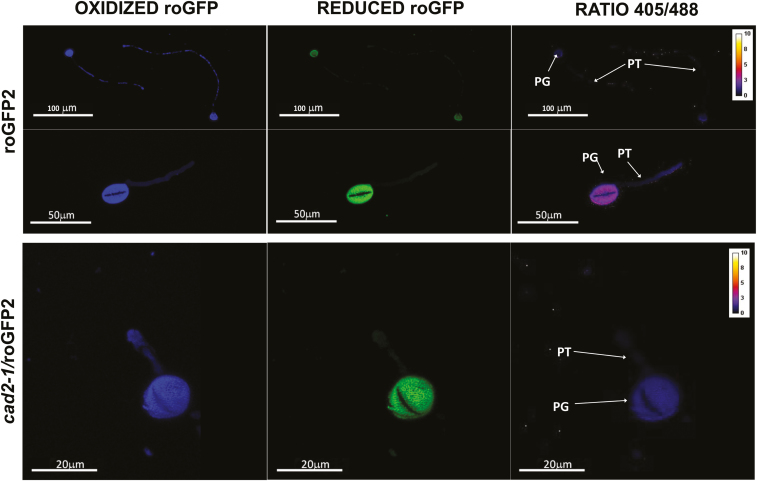
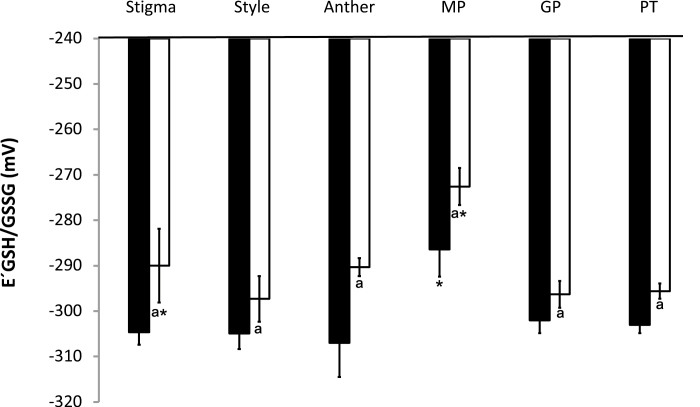
roGFP fluorescence was readily detected in the anthers, pistils, and germinated pollen grains of roGFP2 and cad2-1/roGFP2 plants (Figs 9–11). The degree of oxidation was low in the stigma, style, anther, germinated pollen grains, and pollen tubes of roGFP2 flowers (Fig. 12), suggesting that the glutathione pool is highly reduced in these tissues. This finding is in agreement with the data obtained by biochemical analysis of the tissues described above. In contrast, the ungerminated pollen grains were significantly more oxidized than the germinated pollen grains (Fig. 12). The degree of oxidation was also higher in the ungerminated pollen grains of cad2-1/roGPF2 flowers compared with germinated pollen grains and other tissues (Fig. 12). All tissues (stigma, style, anther, mature and germinated pollen grains, and pollen tubes) of the cad2-1/roGFP2 flowers tended to be more oxidized than the respective tissues of roGFP2 flowers (Fig. 12). However, these differences were statistically significant only for stigma, anther, and mature pollen.
Fig. 9.
roGFP fluorescence in anthers with pollen grains at dehiscence (stage 14). The first column shows fluorescence resulting from excitation at 405 nm, the second shows fluorescence resulting from excitation at 488 nm, and the last column shows the ratio of fluorescence at 405/488 nm. PG, pollen grain; PT, pollen tube.
Fig. 12.
Degree of oxidation in the stigma, style, anther, ungerminated pollen grains (MP), germinated pollen grains (GP), and pollen tube (PT) in roGFP2 (black bars) and cad2-1/roGFP2 (white bars). Asterisks indicate statistical significance (P≤0.05) among the different tissues within the same line. a indicates statistical significance (P≤0.05) between the two lines.
Fig. 10.
roGFP fluorescence in flowers (pistil, anther, and germinated pollen) (stage 14) on germination medium. The first column shows fluorescence resulting from excitation at 405 nm, the second shows fluorescence resulting from excitation at 488 nm, and the last column shows the ratio of fluorescence at 405/488 nm. PG, pollen grain; PT, pollen tube; SP, stigmatic papillae.
Fig. 11.
GFP fluorescence in germinated pollen (stage 14). The first column shows fluorescence resulting from excitation at 405nm, the second shows fluorescence resulting from excitation at 488 nm, and the last column shows the ratio of fluorescence at 405/488 nm. PG, pollen grain; PT, pollen tube.
The glutathione redox potentials were similar in the stigma, style, anther, germinated pollen grains, and pollen tubes of the roGFP2 flowers (Fig. 13). However, the glutathione redox potential of the ungerminated pollen grains was significantly lower than that of the germinated pollen grains of roGFP2 (Fig. 13). The degree of oxidation was lower in the stigma, style, anther, germinated pollen grains, and pollen tubes of cad2-1/roGFP2 flowers than in roGFP2 plants (Fig. 13). The most positive glutathione redox potentials were determined in the ungerminated pollen grains. These values were significantly more positive than those calculated for the roGFP2 plants (Fig. 13).
Fig. 13.
Redox potential in the stigma, style, anther, ungerminated pollen grains (MP), germinated pollen grains (GP), and pollen tube (PT) in roGFP2 (black bars) and cad2-1/roGFP2 (white bars). Asterisks indicate statistical significance (P≤0.05) among the different tissues within the same line. a indicates statistical significance (P≤0.05) between the two lines.
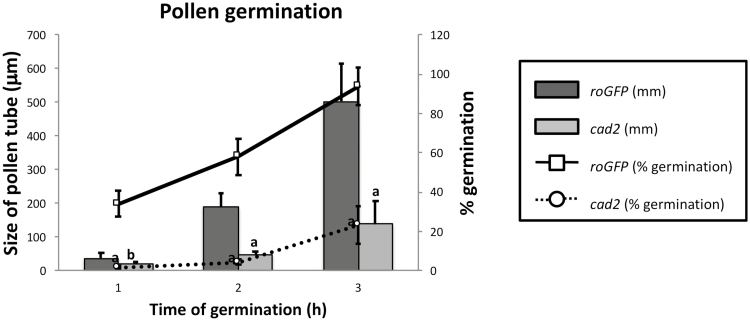
Germination was significantly slower in cad2-1/roGFP2 pollen than in roGFP2 pollen (Fig. 14). The germination percentage was also lower in the cad2-1/roGFP2 pollen. Pollen tube growth arrested earlier in cad2-1/roGFP2 pollen compared with roGFP2 pollen (Fig. 14). Viability experiments demonstrated that the non-germinated pollen grains were alive (data not shown).
Fig. 14.
Differences in in vitro pollen germination between freshly opened flowers of roGFP2 and cad2-1/roGFP2. The primary axis (on the left and with bars) shows the elongation of the pollen tube during in vitro pollen germination. The secondary axis (on the right and with lines) corresponds to the percentage pollen germination measured at the different times of germination. a and b indicate statistical significance at P≤0.01 and P≤0.05, respectively.
Discussion
The redox state of cells is a crucial regulator of metabolism, signalling, and function. While very few tools are available to measure the redox state of living cells, the roGFP probes have proved to be reliable and widely applicable (Meyer et al., 2007; Schwarzländer et al., 2008, 2009; Aller et al., 2013), especially when they incorporate a glutaredoxin (Fernandes et al., 2004; Meyer et al., 2007; Gutscher et al., 2008). The data obtained from roGFP probes therefore provides reliable information concerning the abundance and oxidation state of the glutathione pool in situ and in vivo. Moreover, roGFP probes have been expressed in a wide variety of cells and organisms, where they can be targeted to specific organelles (Hanson et al., 2004; Birk et al., 2013). The effectiveness of the roGFP2 probes in measuring the redox status of the glutathione pool has previously been demonstrated in studies on other species, such as Drosophila (Liu et al., 2012; Aller et al., 2013). The data presented here show that all the tissues of the cad2-1/roGFP2 flowers had a lower GSH/GSSG ratio (Fig. 8) and a higher degree of oxidation than the roGFP2 flowers (i.e. in the WT background; Figs 12 and 13). These findings contrast with data from leaves: the level of total glutathione was decreased in the leaves of cad2-1 mutants but the GSH/GSSG ratios were similar to the WT (Schnaubelt et al., 2015). Taken together, these findings suggest that the flowers of cad2-1 mutants have a lower capacity to keep the cellular glutathione pool reduced and maintain high GSH/GSSG ratios than the leaves.
A strong association between the extent of glutathione accumulation and flowering time has been demonstrated (Ogawa et al., 2001; Yanagida et al. 2004). Flowering was accelerated in the cad2-1/roGFP2 mutants compared to the WT (Kocsy et al., 2013). Moreover, glutathione is required for the initiation of the floral meristem (Bashandy et al., 2010, Kocsy et al., 2013) and also pollen tube germination (Zechmann et al., 2011b). The data presented here builds on this firm foundation and reveals the presence of a low degree of oxidation in all the flower parts (stigma, style, anther, germinated pollen grains and pollen tubes) except the ungerminated pollen. Ungerminated pollen exists in a highly oxidized state that is similar to that found in other quiescent cells (Schippers et al., 2016). In contrast to ungerminated pollen, germinated pollen grains and pollen tubes have a similar degree of oxidation to the other flower parts. These findings suggest that the glutathione pool in pollen is either increased or becomes more reduced (or both) once metabolism is triggered by germination. This process appears to be essential for germination and pollen tube growth because these processes are significantly impaired in the cad2-1/roGFP2 pollen, which maintained a high degree of oxidation compared with the roGFP2 controls.
The pollen germination rates measured in the WT in the present study were comparable to values in the literature (~71%; Boavida et al., 2007). Germination rates were decreased to ~5–20% in the pollen grains of the cad2-1/roGFP2 mutants, which have a restricted capacity for glutathione synthesis (Cobbett et al., 1998; Meyer et al., 2007; Maughan et al., 2010; Zechmann et al., 2011a, b; Aller et al., 2013). In addition, the growth rates were much lower in the pollen grains of the mutants. Calculations of glutathione redox potentials based on the roGFP2 measurements confirmed that all the flower parts apart from ungerminated pollen are maintained in a highly reduced state, as is the case for the germinated pollen and pollen tubes. The highly reduced states measured in the germinated pollen show that the endogenous antioxidant systems have sufficient capacity to deal with the large amounts of ROS and reactive nitrogen species produced by the mitochondria (Traverso et al., 2013; Jiménez-Quesada et al., 2016, 2017; Zafra et al., 2018). Interestingly, the mitochondria in leaf cells were found to have the highest concentrations of glutathione of any of the intracellular compartments, even more than the chloroplasts (Zechmann et al., 2008). A roGFP probe targeted specifically to the mitochondria may be useful in future studies of the role of the glutathione pool in pollen tube mitochondria (Rosenwasser et al., 2010).
The finding that the glutathione levels were highest in mature flowers, with a gradual increase in glutathione accumulation during flower development, is interesting. Increasing accumulation ROS during flower development may lead to the oxidative activation of γ-ECS in the plastids, resulting in increased glutathione synthesis and accumulation (Gromes et al., 2008). ROS accumulate during leaf senescence and during the orchestration of programmed cell death because of alterations in the expression of enzymes involved in the antioxidant system and changes in mitochondrial metabolism (Marí et al., 2009; Cui et al., 2012). However, the concept that ROS are pro-life signals generated by aerobic metabolism is widely accepted (Foyer et al., 2018). Open flowers contain mature pollen, which will have already interacted with the pistil to trigger the signalling cascade that results in the germination of the pollen tube. A flow cytometry approach to determine the viability of mature tomato and Arabidopsis pollen related to ROS accumulation revealed that two types of pollen polulations could be identified, namely ‘low‐ROS’ and ‘high‐ROS’ populations, representing low or high metabolic activity, respectively (Luria et al., 2019). The high‐ROS pollen germinated with a much higher frequency than the low‐ROS pollen (Luria et al., 2019). The metabolically active high‐ROS pollen would require high levels of antioxidant activity, particularly high activities of enzymes such as glutathione reductase in order to maintain high GSH/GSSG ratios. Exposure to high temperatures resulted in an increase in the pollen ROS levels and a decrease in germination (Luria et al., 2019). Such stress-induced increases in ROS signals can modify metabolism and cell signalling, particularly if there is no compensating increase in antioxidant capacity. The accumulation of GSSG that was observed in the cad2-1/roGFP2 flowers (Figs 8, 12, 13) suggests that the pollen cells were in an oxidized state, which would have a negative impact on pollen–pistil metabolism and pollen tube germination in the mature flowers. Cellular GSSG levels are usually kept at very low levels because this metabolite is not compatible with metabolism. In mutants such as the catalase-deficient cat2, where GSSG accumulates, the metabolite is sequestered in the vacuoles (Queval et al., 2011; Noctor et al., 2013). It is therefore likely that much of the GSSG accumulated in the cad2-1/roGFP2 flowers is localized in the vacuole and not the cytoplasm. Mature flowers begin to degenerate, undergoing programmed cell death processes, which involve high ROS concentrations (Marí et al., 2009; Ayer et al., 2010). The restriction of glutathione accumulation in the cad2-1/roGFP2 flowers and the lower GSH/GSSG ratios may therefore result in faster flower senescence.
Taken together, the data presented here confirm that maintenance of a highly reduced glutathione pool is essential for pollen germination and tube growth (Zechmann et al., 2008). The transition from the oxidized quiescent state to the metabolically active germinated state that facilitates pollen growth therefore requires a large change in cellular redox homeostasis that involves both increased glutathione accumulation and extensive reduction of the glutathione pool. The data presented here show that the capacity to regenerate GSH from GSSG is restricted in cad2-1/roGFP2 flowers, as is the ability to synthesize glutathione. These findings demonstrate the importance of cellular redox control in pollen germination and tube growth. Further work is required to demonstrate the nature of the mechanisms involved.
Future studies using combinations of techniques such as roGFP2, fluorochromatic methods (Heslop-Harrison and Heslop-Harrison, 1970), and propidium iodide staining (Jimenez-Quesada et al., 2017) are required to investigate the mechanisms involved in oxidation-induced losses of viability and function. However, current methods that are able to determine the viability of individual pollen grains are difficult to use simultaneously with roGFP2 analysis of redox properties. For example, fluorochromatic detection interferes with the roGFP2 signal because both methods use similar excitation and emission wavelengths.
Pollen grains require a robust antioxidant system to germinate and penetrate through the stigma (Creissen et al., 1999, Zechmann, 2008); further studies are required to determine how much of this defence is constitutive and how much is induced upon alleviation of the quiescent state. The data presented here demonstrate that glutathione is an essential component of this antioxidant defence and perhaps also pollen–stigma signalling through thiol-disulfide exchange mechanisms, protein gluathionylation, and S-nitrosoglutathione-mediated processes (Corpas et al., 2013; Zafra et al., 2016). Future work using a range of other mutants and oxidative stress markers is required to elucidate the redox differences between ungerminated and germinated pollen and to provide a more complete picture of the involvement of GSH metabolism in pollen germination.
Acknowledgements
We thank the Scientific Instrumentation Service (Estación Experimental del Zaidín, CSIC, Granada) for assistance in GC-MS analysis. We also thank the Microscopy Service (Estación Experimental del Zaidín, CSIC, Granada) for help during the confocal microscopy. This work was supported by European Regional Development Fund-co-funded projects BFU2016-77243-P, P2011-CVI7487, RTC2016-4824-2, and RTC2017-6654-2. EGQ thanks the Spanish Ministry of Economy and Competitiveness for “Formación del Personal Investigador” program grant funding.
References
- Airaki M, Sánchez-Moreno L, Leterrier M, Barroso JB, Palma JM, Corpas FJ. 2011. Detection and quantification of S-nitrosoglutathione (GSNO) in pepper (Capsicum annuum L.) plant organs by LC-ES/MS. Plant & Cell Physiology 52, 2006–2015. [DOI] [PubMed] [Google Scholar]
- Aller I, Rouhier N, Meyer AJ. 2013. Development of roGFP2-derived redox probes for measurement of the glutathione redox potential in the cytosol of severely glutathione-deficient rml1 seedlings. Frontiers in Plant Science 4, 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla ER, Benítez M, Corvera-Poiré A, et al. 2010. Flower development. The Arabidopsis Book 8, e0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer A, Tan SX, Grant CM, Meyer AJ, Dawes IW, Perrone GG. 2010. The critical role of glutathione in maintenance of the mitochondrial genome. Free Radical Biology & Medicine 49, 1956–1968. [DOI] [PubMed] [Google Scholar]
- Ball L, Accotto GP, Bechtold U, et al. 2004. Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. The Plant Cell 16, 2448–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, Reichheld JP. 2010. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. The Plant Cell 22, 376–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk J, Meyer M, Aller I, Hansen HG , Odermatt A, Dick TP, Meyer AJ, Appenzeller-Herzog C. 2013. Endoplasmic reticulum: reduced and oxidized glutathione revisited. Journal of Cell Science 126, 1604–1617. [DOI] [PubMed] [Google Scholar]
- Boavida LC, McCormick S. 2007. Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. The Plant Journal 52, 570–582. [DOI] [PubMed] [Google Scholar]
- Cairns NG, Pasternak M, Wachter A, Cobbett CS, Meyer AJ. 2006. Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiology 141, 446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B. 1998. The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. The Plant Journal 16, 73–78. [DOI] [PubMed] [Google Scholar]
- Considine MJ, Foyer CH. 2014. Redox regulation of plant development. Antioxidants & Redox Signaling 21, 1305–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, Alché JD, Barroso JB. 2013. Current overview of S-nitrosoglutathione (GSNO) in higher plants. Frontiers in Plant Science 4, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creissen G, Firmin J, Fryer M, et al. 1999. Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. The Plant Cell 11, 1277–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Kong Y, Zhang H. 2012. Oxidative stress, mitochondrial dysfunction, and aging. Journal of Signal Transduction 2012, 646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Simone A, Hubbard R, de la Torre NV, Velappan Y, Wilson M, Considine MJ, Soppe WJJ, Foyer CH. 2017. Redox changes during the cell cycle in the embryonic root meristem of Arabidopsis thaliana. Antioxidants & Redox Signaling 27, 1505–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LM, Wang YF, Wang H, Wu WH. 2001. In vitro Arabidopsis pollen germination and characterization of the inward potassium currents in Arabidopsis pollen grain protoplasts. Journal of Experimental Botany 52, 1603–1614. [PubMed] [Google Scholar]
- Fernandes AP, Holmgren A. 2004. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxidants & Redox Signaling 6, 63–74. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2005. Oxidant and antioxidant signalling in plants: a re‐evaluation of the concept of oxidative stress in a physiological context. Plant, Cell & Environment 28, 1056–1071. [Google Scholar]
- Foyer CH, Noctor G. 2009. Redox regulation and photosynthetic organisms: signaling, acclimation, and practical implications. Antioxidants & Redox Signaling 11, 861–905. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2011. Ascorbate and glutathione: the heart of the redox hub. Plant Physiology 155, 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Theodoulou FL, Delrot S. 2001. The functions of inter- and intracellular glutathione transport systems in plants. Trends in Plant Science 6, 486–492. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Wilson M, Wright M. 2018. Redox regulation of cell proliferation: Bioinformatics and redox proteomics approaches to identify redox-sensitive cell cycle regulators. Free Radical Biology and Medicine 122, 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galant A, Preuss ML, Cameron JC, Jez JM. 2011. Plant glutathione biosynthesis: diversity in biochemical regulation and reaction products. Frontiers in Plant Science 2, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Quirós E, Carmona R, Zafra D, Claros MG, Alché JD. 2017. Identification and in silico analysis of glutathione reductase transcripts expressed in Olive (Olea europaea L.) pollen and pistil. In: Rojas I, Ortuño F, eds. Bioinformatics and biomedical engineering: 5th International Work-Conference, IWBBIO 2017, Granada, Spain, April 26–28, 2017, Proceedings, Part II. Cham: Springer, 185–195. [Google Scholar]
- Gigolashvili T, Kopriva S. 2014. Transporters in plant sulfur metabolism. Frontiers in Plant Science 5, 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromes R, Hothorn M, Lenherr ED, Rybin V, Scheffzek K, Rausch T. 2008. The redox switch of γ‐glutamylcysteine ligase via a reversible monomer–dimer transition is a mechanism unique to plants. Plant Journal 54, 1063–1075. [DOI] [PubMed] [Google Scholar]
- Gutscher M, Pauleau AL, Marty L, Brach T, Wabnitz GH, Samstag Y, Meyer AJ, Dick TP. 2008. Real-time imaging of the intracellular glutathione redox potential. Nature Methods 5, 553–559. [DOI] [PubMed] [Google Scholar]
- Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. 2004. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. Journal of Biological Chemistry 279, 13044–13053. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. 1970. Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technology 45, 115–120. [DOI] [PubMed] [Google Scholar]
- Jimenez-Quesada MJ, Carmona R, Lima-Cabello E, Traverso JÁ, Castro AJ, Claros MG, Alché JD. 2017. Generation of nitric oxide by olive (Olea europaea L.) pollen during in vitro germination and assessment of the S-nitroso- and nitro-proteomes by computational predictive methods. Nitric Oxide 68, 23–37. [DOI] [PubMed] [Google Scholar]
- Jiménez-Quesada MJ, Traverso JÁ, Alché JD. 2016. NADPH oxidase-dependent superoxide production in plant reproductive tissues. Frontiers in Plant Science 7, 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsy G, Tari I, Vanková R, Zechmann B, Gulyás Z, Poór P, Galiba G. 2013. Redox control of plant growth and development. Plant Science 211, 77–91. [DOI] [PubMed] [Google Scholar]
- Li X. 2011. Arabidopsis pollen tube germination. Bio-101, e73. doi: 10.21769/BioProtoc.73 [DOI] [Google Scholar]
- Liu Z, Celotto AM, Romero G, Wipf P, Palladino MJ. 2012. Genetically encoded redox sensor identifies the role of ROS in degenerative and mitochondrial disease pathogenesis. Neurobiology of Disease 45, 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Mirabal HR, Winther JR. 2008. Redox characteristics of the eukaryotic cytosol. Biochimica et Biophysica Acta 1783, 629–640. [DOI] [PubMed] [Google Scholar]
- Luria G Rutley N Lazar I Harper JF Miller G.. 2019. Direct analysis of pollen fitness by flow cytometry: implications for pollen response to stress. The Plant Journal 98, 942–952. [DOI] [PubMed] [Google Scholar]
- Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC. 2009. Mitochondrial glutathione, a key survival antioxidant. Antioxidants & Redox Signaling 11, 2685–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty L, Siala W, Schwarzländer M, Fricker MD, Wirtz M, Sweetlove LJ, Meyer Y, Meyer AJ, Reichheld JP, Hell R. 2009. The NADPH-dependent thioredoxin system constitutes a functional backup for cytosolic glutathione reductase in Arabidopsis. Proceedings of the National Academy of Sciences, USA 106, 9109–9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan S, Foyer CH. 2006. Engineering and genetic approaches to modulating the glutathione network in plants. Physiologia Plantarum 126, 382–397. [Google Scholar]
- Maughan SC, Pasternak M, Cairns N, et al. 2010. Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proceedings of the National Academy of Sciences, USA 107, 2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A, Anderson ME. 1983. Glutathione. Annual Review of Biochemistry 52, 711–760. [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Brach T, Marty L, Kreye S, Rouhier N, Jacquot JP, Hell R. 2007. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. The Plant Journal 52, 973–986. [DOI] [PubMed] [Google Scholar]
- Mittler R. 2017. ROS are good. Trends in Plant Science 22, 11–19. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. 2004. Reactive oxygen gene network of plants. Trends in Plant Science 9, 490–498. [DOI] [PubMed] [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH. 2002. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. Journal of Experimental Botany 53, 1283–1304. [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH. 2012. Glutathione in plants: an integrated overview. Plant, Cell & Environment 35, 454–484. [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Foyer CH. 2016. Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant, Cell & Environment 39, 1140–1160. [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Queval G, Foyer CH. 2013. Regulating the redox gatekeeper: vacuolar sequestration puts glutathione disulfide in its place. Plant Physiology 163, 665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Queval G, Mhamdi A, Chaouch S, Foyer CH. 2011. Glutathione. The Arabidopsis Book 9, e0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Tasaka Y, Mino M, Tanaka Y, Iwabuchi M. 2001. Association of glutathione with flowering in Arabidopsis thaliana. Plant & Cell Physiology 42, 524–530. [DOI] [PubMed] [Google Scholar]
- Pasternak M, Lim B, Wirtz M, Hell R, Cobbett CS, Meyer AJ. 2008. Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development. The Plant Journal 53, 999–1012. [DOI] [PubMed] [Google Scholar]
- Queval G, Jaillard D, Zechmann B, Noctor G. 2011. Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant, Cell & Environment 34, 21–32. [DOI] [PubMed] [Google Scholar]
- Queval G, Thominet D, Vanacker H, Miginiac-Maslow M, Gakière B, Noctor G. 2009. H2O2-activated up-regulation of glutathione in Arabidopsis involves induction of genes encoding enzymes involved in cysteine synthesis in the chloroplast. Molecular Plant 2, 344–356. [DOI] [PubMed] [Google Scholar]
- Rosenwasser S, Rot I, Meyer AJ, Feldman L, Jiang K, Friedman H. 2010. A fluorometer-based method for monitoring oxidation of redox-sensitive GFP (roGFP) during development and extended dark stress. Physiologia Plantarum 138, 493–502. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. 2001. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Biology & Medicine 30, 1191–1212. [DOI] [PubMed] [Google Scholar]
- Schippers JH, Foyer CH, van Dongen JT. 2016. Redox regulation in shoot growth, SAM maintenance and flowering. Current Opinion in Plant Biology 29, 121–128. [DOI] [PubMed] [Google Scholar]
- Schnaubelt D, Queval G, Dong Y, Diaz-Vivancos P, Makgopa ME, Howell G, De Simone A, Bai J, Hannah MA, Foyer CH. 2015. Low glutathione regulates gene expression and the redox potentials of the nucleus and cytosol in Arabidopsis thaliana. Plant, Cell & Environment 38, 266–279. [DOI] [PubMed] [Google Scholar]
- Schnaubelt D, Schulz P, Hannah MA, Yocgo RE, Foyer CH. 2013. A phenomics approach to the analysis of the influence of glutathione on leaf area and abiotic stress tolerance in Arabidopsis thaliana. Frontiers in Plant Science 4, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzländer M, Fricker MD, Müller C, Marty L, Brach T, Novak J, Sweetlove LJ, Hell R, Meyer AJ. 2008. Confocal imaging of glutathione redox potential in living plant cells. Journal of Microscopy 231, 299–316. [DOI] [PubMed] [Google Scholar]
- Schwarzländer M, Fricker MD, Sweetlove LJ. 2009. Monitoring the in vivo redox state of plant mitochondria: effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Biochimica et Biophysica Acta 1787, 468–475. [DOI] [PubMed] [Google Scholar]
- Sugiyama A, Nishimura J, Mochizuki Y, Inagaki K, Sekiya J. 2004. Homoglutathione synthesis in transgenic tobacco plants expressing soybean homoglutathione synthetase. Plant Biotechnology 21, 79–83. [Google Scholar]
- Traverso JA, Pulido A, Rodríguez-García MI, Alché JD. 2013. Thiol-based redox regulation in sexual plant reproduction: new insights and perspectives. Frontiers in Plant Science 4, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker H, Harbinson J, Ruisch J, Carver TLW, Foyer CH. 1998. Antioxidant defences of the apoplast. Protoplasma 205, 129–140. [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, et al. 2000. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. The Plant Cell 12, 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter A, Wolf S, Steininger H, Bogs J, Rausch T. 2005. Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. The Plant Journal 41, 15–30. [DOI] [PubMed] [Google Scholar]
- Yanagida M, Mino M, Iwabuchi M, Ogawa K. 2004. Reduced glutathione is a novel regulator of vernalization-induced bolting in the rosette plant Eustoma grandiflorum. Plant & Cell Physiology 45, 129–137. [DOI] [PubMed] [Google Scholar]
- Zafra A, Castro AJ, Alché JD. 2018. Identification of novel superoxide dismutase isoenzymes in the olive (Olea europaea L.) pollen. BMC Plant Biology 18, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra A, Rejón JD, Hiscock SJ, Alché Jde D. 2016. Patterns of ROS accumulation in the stigmas of angiosperms and visions into their multi-functionality in plant reproduction. Frontiers in Plant Science 7, 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra A, Rodríguez-García MI, Alché Jde D. 2010. Cellular localization of ROS and NO in olive reproductive tissues during flower development. BMC Plant Biology 10, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B. 2014. Compartment-specific importance of glutathione during abiotic and biotic stress. Frontiers in Plant Science 5, 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B, Koffler BE, Russell SD. 2011b. Glutathione synthesis is essential for pollen germination in vitro. BMC Plant Biology 11, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B, Liou LC, Koffler BE, Horvat L, Tomašić A, Fulgosi H, Zhang Z. 2011a. Subcellular distribution of glutathione and its dynamic changes under oxidative stress in the yeast Saccharomyces cerevisiae. FEMS Yeast Research 11, 631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B, Mauch F, Sticher L, Müller M. 2008. Subcellular immunocytochemical analysis detects the highest concentrations of glutathione in mitochondria and not in plastids. Journal of Experimental Botany 59, 4017–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B, Müller M. 2010. Subcellular compartmentation of glutathione in dicotyledonous plants. Protoplasma 246, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]