Abstract
Background
The aim of this study was to explore the effect of metformin by inducing autophagy for enhancing functional recovery of peripheral nerve in rats with sciatic nerve crush injury.
Material/Method
Autophagy was determined by electron microscopy, immunofluorescence, and Western blot analysis. Motor function recovery was studied by the footprint intensity method. Axonal growth and regeneration were detected through Western blot while axonal remyelination was analysed through immunocytochemistry. Sensory and functional recovery were assessed by reflexive motor function analysis.
Results
The present study deciphered the role of autophagy induction by metformin in motor functions and peripheral nerve regeneration following sciatic nerve crush injury in rats. The process was detected by measuring autophagosomes and the expression of microtubule-associated protein 1A/1B-light chain 3 upon metformin treatment of sciatic nerve crush-injured rats. Neurobehavioral recovery by metformin was tested by CatWalk gait analysis, and we quantified expression of myelin basic protein MBP and neurofilament NF200 at the damage sight by immunoblotting. In metformin-treated injured rats, autophagy was upregulated, by which the number of dead cells was decreased. Motor function was also recovered after metformin treatment, which was accompanied by upregulation of MBP and NF200 through autophagy induction. Surprisingly, the motor regenerative capability was reduced by treatment with 3-methyl adenine (an autophagy inhibitor) in nerve-injured rats.
Conclusions
Our study revealed that pharmacological induction of autophagy has an important and active role in the regeneration of nerve and motor function regain.
MeSH Keywords: Autophagy, Metformin, Sciatic Nerve
Background
Intrinsic ability of repair and regeneration found in the human body is limited to some organs, and the rates of these processes vary from organ to organ and in the peripheral nervous system (PNS) [1]. The generation and repair ability of the PNS is limited and functional recovery is poor [2]. The slow regeneration rate of the PNS may lead permanent damage of structure and function of organs associated with it. Before a regenerated axon re-innervates, there can be permanent complications [3,4]. Autophagy is a intracellular homeostasis mechanism which degrades cellular-damaged organelles, dysfunctional proteins, and oxidative stress through lysosomes and recycling into the cellular system [5]. Autophagy has vital roles in both disease and normal states, such as neurodegeneration, starvation, infection, and aging [6]. Under stress conditions, autophagy helps in adaption to prolong cell survival [7]. During peripheral nerve injury, initially unwanted proteins, lipids, and organelle accumulate at the damage site, generating stress that impedes the ability of Schwann cells (SC) to repair injured nerves [8]. The intracellular process known as autophagy clearance can reduce these stresses by clearing non-functional, unneeded biomolecules from the microenvironment and provide favorable conditions proper functioning and survival of SC. Various studies have assessed the neuro-protective role of autophagy in various neurodegenerative diseases [9], but little is known about its role in cerebral trauma [10], acute spinal cord injury [11], and hypoxia- ischemia brain injury [12]. There are some reports which showed autophagy has a role in preventing neurodegenerative disease in the peripheral nervous system (PNS) in animal models of neuropathy [13], but the mechanism of peripheral nervous system regeneration through autophagy is unclear. In the present study, we assessed the effect of metformin on regeneration of neurons after sciatic peripheral nerve injury. Metformin is an anti-hyperglycemia agent used to treat type II diabetes patients. A few recent reports have shown that metformin relieves neuropathic pain through induction of autophagy flux in various neuropathic model systems [14]. Interestingly, metformin treatment reduces tau hyperphosphorylation, aggregated proteins, reduced cognitive decline, and improved memory in various animal models [15]. It has been reported that metformin protects neurons against various neurotoxins and helps in rescuing neurons from neurodegeneration [16]. Metformin regulates cell survival and increases mitochondrial membrane potential, mitochondrial biogenesis, and autophagy through AMPK pathway activation [17]. These results suggest that metformin may be a potential bioactive compound which takes part in nervous system regeneration. However, to the best of our knowledge, there has been no report on whether metformin enhances functional recovery of peripheral nerves in sciatic nerve crush-injured rats by inducing autophagy.
Material and Methods
Ethics statement
All animal experiments, including electrophysiological test, surgery, tissue collection, and behavior testing were carried out with the approval of our Institutional Animal Ethics Committee (IAEC) under IAEC approval number IAEC/68/148/3/2018. All possible efforts were made to minimize animal suffering.
Chemicals and reagents
From Sigma-Aldrich, were purchased the following: 4,6-diamidino-2-phenylindole (DAPI), Phosphate-buffered saline (PBS), dimethyl sulfoxide (DMSO), BSA, sodium chloride, skimmed milk, bafilomycinA1, RIPA buffer, acrylamide, paraformaldehyde, N,N’-Methylene bis acrylamide, HEPES, sodium fluoride, 3-(4, 5, -dimethylthiazole-2-yl)-2, 5 diphenyltetrazolium bromide (MTT), tween 20, uranyl acetate, sodium orthovanadate, glycine, triton X-100, EDTA, trizma, sodium dodecyl sulphate, ammonium persulfate, TEMED, glycerol, osmium tetroxide solution, glutaraldehyde solution, and protease inhibitor cocktail. Antibodies like anti-SCG10, anti-GAP43, anti-MBP, anti-NF-200, anti-LC3B-II, anti-β-actin, scrambled siRNA, goat anti-mouse IgG Alexa fluor 555, and goat anti-mouse IgG Alexa fluor 488 secondary antibodies were purchased from Invitrogen. Polyvinylidene difluoride (PVDF) membranes were purchase from ThermoFisher Scientific. Bradford and molecular weight markers were obtained from Bio-Rad.
Static nerve injury model development and drug administration
We utilized female C57BL/C mice (n=15 per group, weight=18–20 g, 6–8 weeks old) for establishment of the sciatic nerve crush injury model, as previously described [18,19]. All mice received intraperitoneal injection of sodium pentobarbital as anesthesia at a concentration of 50 mg/kg body weight. In the left leg, the sciatic nerve was exposed with sterilized forceps immediately after incision of lateral skin along the femur length. Without disturbing the nerve, wounds were closed or subjected to sciatic injury from distal to notch. A smooth hemostat (tip width 1 mm) was used to deform the nerve for 2 min, and the crush area was marked with 9-o nylon suture, as previously described [20]. Nerve-injured mice were randomly grouped into M and saline groups. After surgery, M group animals received metformin solution (5 mg/kg) daily, while the saline group received normal saline and served as control. All animals were housed under standard laboratory conditions and received proper postoperative care. The animals were provided with a 12/12 h dark/light cycle and had free access to standard water and food. Five mice were killed on the third day after injury (dpi) and regeneration of the injured axonal nerve was identified through immunoblotting and immunohistochemistry of superior cervical ganglion 10 (SCG10), growth-associated protein 43 (GAP43), and autophagic marker protein LC3-II. At 28 dpi, the other 10 mice of group M were subjected to behavioral assessment and electrophysiological testing. Later, the gastrocnemius muscle and the sciatic nerve were collected, stained with hematoxylin, and examined by electron microscopy and immunohistochemistry.
Drug treatment
Drug treatments were performed by dividing mice into 4 groups: sham+vehicle (V), crush+V, crush+3MA, and crush+metformin. Drugs were dissolved in dimethylsulfoxide (DMSO), and 50 mg/kg of 3-MA were given by intraperitoneal injections. At 5 days after surgery, 5 mg/kg of metformin was given to the crush+metformin group and the DMSO to crush+V group for 10 days after injury. The drug dosage was selected based on results from a preliminary experiment.
Tissue preparation
At 1 and 6 weeks after injury, crushed sciatic nerves (5 animals in each group) were dissected and harvested at each time period. Animals were sacrificed and were perfused with cold normal saline via the left ventricle, followed by 4% paraformaldehyde fixation. Later, sciatic nerves were harvested and frozen at −80°C for Western blot analysis and immunohistochemistry.
Behavior testing
Behavioral testing was performed in all groups, with and without drug treatment. Motor function was measured by taking readings of foot print time and standing time using the CatWalk system. Animals were allowed to cross the walkway with an illuminated glass floor fitted with high-speed video camera assembled with 8.5-mm wide-angle lenses below the walkway to record animal paw prints as the animal moved across the floor.
Gait analysis
A Rotarod device was used to measure motor performance. Briefly, we gave the mice 2 days of pre-training on an automated Rotarod unit. Training was given according to a previously described method. Mice were placed on a rod rotating at a speed of 20 rotations per minute (RPM) and we recorded the length of time each mouse was able to stay on the rod before falling off.
Hot plate test
An electric hot plate was used for determination of sensory function and injured nerve recovery. Before the actual test, mice were acclimated to tolerate standing on the hot plate set at 20°C. For testing, the temperature of the hot plate was set at 55±0.5°C, the mouse was placed on it, and withdrawal latency was measured by time (seconds) until jumping or licking.
Electrophysiological analysis
Each mouse was anesthetized and processed for electrophysiological analysis as described earlier. Briefly, upon re-exposing the sciatic nerve, stimulating electrodes (13 mm long, 0.5 mm diameter) were inserted into the foot muscle to measure compound muscle action potential using an electrophysiological recorder. For each test, latency and amplitude were accessed to measure conduction of nerve strength and speed.
Tissue collection
Two time points were taken for analysis of neuronal regeneration in injured nerves. At first, 3 dpi sciatic nerve samples were monitored for immunohistochemistry. Furthermore, 28 dpi sciatic nerve-injured mice were immediately taken for electrophysiological recording. Phosphate-buffered saline (0.1 M) was used for intracardial perfusion for all mice at 10 min, followed by fixation for 20 min with 5% paraformaldehyde. Gastrocnemius muscles and sciatic nerves were taken for further assessment.
Western blotting
At 3 dpi sciatic nerve of 2 mice per group were scarified and samples of 1-cm sciatic nerve were taken from the injured site after chilled in liquid nitrogen for 30 s. Protein lysates were prepared by homogenizing tissue samples in RIPA lysis buffer containing protease inhibitor cocktail, 0.25 mMNAOV4, 75 mM NaF, and 2 mM PMSF. The protein samples were subjected to SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. Protein membranes were blocked with 5% skimmed milk for 1 h and primary antibody (LC3II) was added overnight at 4°C. Membranes were washed 3 times with blocking buffer, and secondary antibodies (HRP-conjugated) were added into each membrane for 2 h at room temperature. The protein expression was detected by ECL substrate on X-ray film or Chemidoc (Bio-Rad). Densitometry of Western blots was performed using Image J Pro Plus 6.0 software.
Immunohistochemistry
Transverse sections of frozen sciatic nerve were mounted on slides and were blocked at room temperature with blocking buffer (10% goat serum and 3% BSA) for 1 h followed by incubation with primary antibodies LC3-II, MPB, and NF200 overnight at 4°C. These samples were further incubated with Alexa Fluor-555 and Alexa Fluor-488 at room temperature for 1 h, along with DAPI straining for 15 min. The images were captured under a fluorescence microscope.
Transmission electron microscopy
We processed 28 dpi sciatic nerves dissected for transmission electron microscopy (TEM). Segments 2 mm long were taken 4–6 mm from the injury site, followed by fixation for 24 h with glutaraldehyde (2.5%), paraformaldehyde (2%), and 2% osmium tetroxide at 4°C for 2 h. Samples were dehydrated and embedded in resin for ultrathin sectioning (70 nm). We used 2% lead citrate and uranyl acetate for staining these embedded samples. Images were taken using a transmission electron microscope JEM-1010 (Jeol, Tokyo, Japan).
TUNEL assay
Dead cells in frozen section of sciatic nerve was detecting by DNA fragmentation through terminal deoxynucleotidyl transferase UTP nick-end labelling (TUNEL). Moreover, stained samples were counter-stained with DAPI, and TUNEL-positive cells were counted under a fluorescence microscope.
Histomorphometry of gastrocnemius muscle
Regeneration and nerve injury define the intensity of myoatrophy in the target muscle. Therefore, histomorphometry of gastrocnemius muscle was performed at 28 dpi. Briefly, trimmed midbellies of gastrocnemius muscles of perfused mice were fixed at 4°C with PFA 4% for 24 h.
Tissues embedded in OCT-freeze medium were transversely cut into 10-μm sections and then stained with hematoxylin to demarcate the myofibers, which were then calculated [21]. Seven random fields from 4 sections were captured, and quantification was done using Image J software.
Primary cell culture
The dorsal root ganglia neurons were cultured from 1-day-old neonate rats and were digested with 0.2% papain at 37°C for 30 min. The suspended single cells were seeded into poly-L-lysine-coated coverslips in neurobasal media containing 5% B27 and 1 mM GlutaMax for 21 days. These cells were treated in the presence or absence of bafilomycin for 24 h, followed by fixation with 4% paraformaldehyde. These samples were used for immunocytochemistry to analyze Tuj1 and RhoA.
Primary Schwann cell culture
Sprague-Dawley rats (SD rats) were euthanized at postnatal day 3, and Schwann cells were isolated from the spinal nerves. The nerves were incubated for 30 min in 0.3% papain at 37°C to make single-cell suspensions. Cells were cultured in DMEM/F12 media containing 10% FBS in poly-L-lysine-coated culture dishes. On the next day, we added cytosine arabinoside (10 μM) to cultured cells for 48 h to remove fibroblasts. Cells were then cultured in normal (DMEM/F12+3% FBS, 3 μM forskolin), 10 neuregulin, and 100 mg/ml penicillin-streptomycin to culture cells.
Primary microphage culture
Microphages were isolated from the peritoneal cavity from anesthetized adult SD rats and were grown to a density 4×106 into 60-mm dishes in DMEM media supplemented with 10% FBS. The non-adherent cells were removed by changing media regularly every 2 h. Later, adherent macrophages were cultured according to a previously described protocol [22].
Migration assay
Macrophages and Schwann cells were assessed by Transwell migration assay with 6.5-mm Transwell chambers (8-μm pore size). Transwell chambers were pre-treated with 10 ug/ml Matrigel solution or culture media with 1×105 macrophage or Schwann cells in 100 ul DMEM/F12 media per well in upper chambers and 600 ul DMEM/F12 media supplemented with 10% FBS were added into lower chambers. Migration time of Schwann cells and macrophages was stopped at 4 and 24 h, respectively, followed by fixation with 4% paraformaldehyde for 20 min. Cells from the upper surface of wells were removed by cotton swabs and the remaining cells in the wells were stained with crystal violet for 20 min. Cells were washed 3 times with PBS and images were taken under an inverted microscope at 5 different areas per well.
Statistical analysis
Data are presented as mean±standard error of mean. Group comparisons were made by one-way analysis of variance (ANOVA) and between-group comparisons were done by one-way ANOVA with Bonferroni correction with multiple comparison. p≤0.005 was considered to be significant. All measurements were done using GraphPad Pro software.
Results
Metformin induced autophagy at early stage of peripheral nerve injury
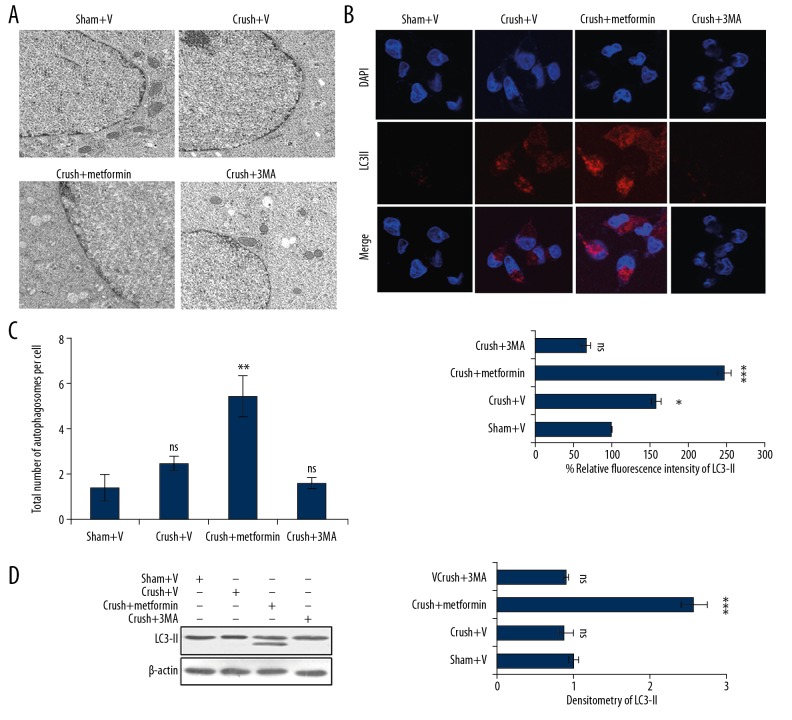
Sciatic nerves were collected after nerve crush injury from different groups of mice treated with or without metformin. The samples were analysed by electron microscopy for induction of autophagy by measuring the number of autophagosomes formed. In metformin-treated groups, the number of autophagosomes were higher than in DMSO-treated groups (Figure 1A, 1C). Conversely, inhibiting autophagy using 3-methyl adenine strongly inhibited the formation of autophagosomes (Figure 1A, 1C). These results were further corroborated by immunofluorescence, in which the autophagic marker showed significant enhancement of LC3-II fluorescence in metformin-treated samples (Figure 1B). Similar results were obtained through Western blot analysis in which the expression of LC3-II was increased compared with crush+ V and 3-methyl adenine-treated groups (Figure 1D).
Figure 1.
Metformin induces autophagy in sciatic nerve following peripheral nerve injury. (A) Electron microscopy represents induction of formation of autophagosome and lysosomes in the axons of rat sciatic nerve in different groups – sham+V group (DMSO treated), crush+V group (processed to NCI and injected with DMSO), crush+metformin (exposed to NCI and injected with metformin 5 mg/kg), crush+3-MA (exposed to NCI and treated with 50 mg/kg 3-MA). All groups were compared with sham+V group. (B) Immunofluorescence of LC3-II in the above treated groups in which metformin groups have high intensity of LC3-II. (C) Quantification of the total number of autophagosomes per cell of Figure 1A through ImageJ software. (D) Western blot analysis of the above-mentioned samples depicts that LC3-II expression is enhanced in metformin treated groups. * p<0.05, ** p<0.01, *** p<0.001, ns – non-significance.
Metformin accelerates motor function recovery in peripheral nerve injury
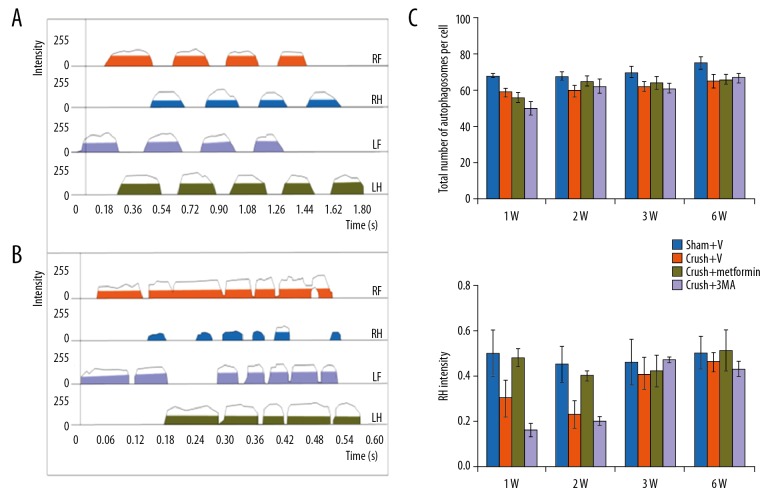
Motor functional recovery was analysed by use of the CatWalk system. At 1, 2, 4, and 6 weeks after injury, we assessed the standing time and footprint density of the operated limb. The mean standing times of metformin-treated groups were 1.8-fold higher compared with DMSO-treated groups (Crush+V group). The foot print intensities were different between groups (Figure 2A–2C). Moreover, animals treated with metformin (a known autophagy inducer) were better able to support their body weight compared with the 3-Methyl adenine group, in which the mean standing time was shorter (18%) and the mean foot print intensity was 25–35% lower at 2–3 weeks after injury as compared to the sham group (p<0.05) (Figure 2A–2C).
Figure 2.
Motor function recovery through metformin in peripheral nerve injury: (A, B) Catwalk analysis of 1 sham (Sham+V) operated groups and post operated group. Groups already defined in Figure 1. Lh, Rh, Lf and Rf, represents left, right hand limb, left, right foot. (C) Represents foot print intensity of stand time of 1, 2, 3 and 6 week after injury (1W, 2W, 3W and 6W respectively). * p<0.05, ** p<0.01, *** p<0.001, ns – no significance.
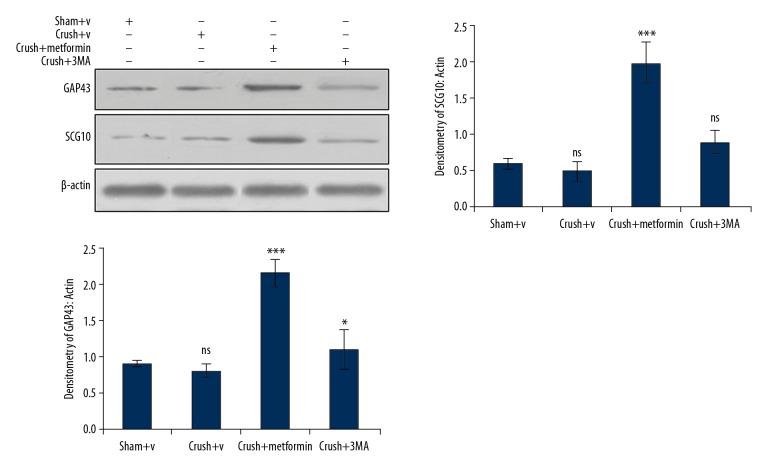
Metformin upregulates axonal regeneration of injured nerves at early stages
After nerve injury, the axonal growth and regeneration were detected by the widely used markers growth associated protein 43 (GAP43) and superior cervical ganglion 10 (SCG10). The expression of GAP43 and SCG10 was significantly enhanced in metformin-treated groups, as detected by Western blot. In metformin-treated groups, the expressions of GAP43 and SCG10 were significantly increased from crush site to distal trunk of metformin-treated groups compared with normal saline groups, whereas the 3-Methyl adenine group had effects opposite those in the metformin-treated group (Figure 3).
Figure 3.
Metformin enhances the expression of axonal growth and regeneration: The expression of axonal growth and regeneration proteins GAP43 and SCG10 was increased in metformin treated groups, while as the expression of GAP43 and SCG10 was decreased in 3-methyl adenine groups. * p<0.05, *** p<0.001, ns – no significance.
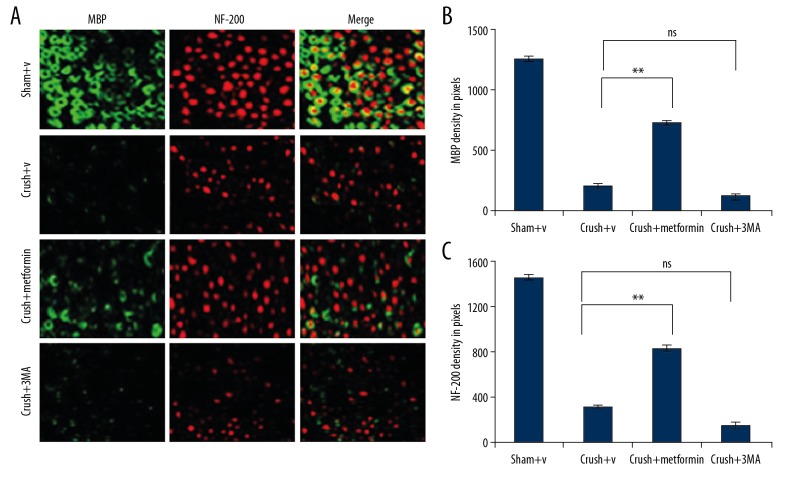
Metformin enhances axonal regeneration and increases remyelination of damaged nerves
Myelin basic protein (MBP) and neurofilament 200 (NF200) have vital roles in the process of myelin regeneration. Therefore, we assessed whether metformin can increase the regeneration of axons and enhance the remyelination of damaged nerves. Moreover, in metformin-treated groups, the digital portions of injured nerve neurofilament-positive axons were present and were protected with myelin basic protein. MBP is an important component of the myelin sheath, produced by SCs in PNS and NF200. To evaluate the effect of metformin on nerve regeneration, expressions of MBP and NF200 through autophagy were visualized through immunocytochemistry at different time points after injury. The expression of nerve regenerative proteins MBP and NF200 at 1 week after injury was 3–4 times higher in the crush+ metformin group compared with the crush+ DMSO or crush+3MA group (Figure 4A–4C).
Figure 4.
The expression of MBP and NF-200 were detected by immunocytochemistry: (A) In experimental groups (defined in figure caption 1) the fluorescence intensity of MBP (green) and NF-200 (red) was elevated which was detected by immunocytochemistry. (B, C) the fluorescence intensity was measured by integrated density pixel. * p<0.05, ** p<0.01, *** p<0.001, ns – no significance.
Metformin upregulated sensory and functional recovery
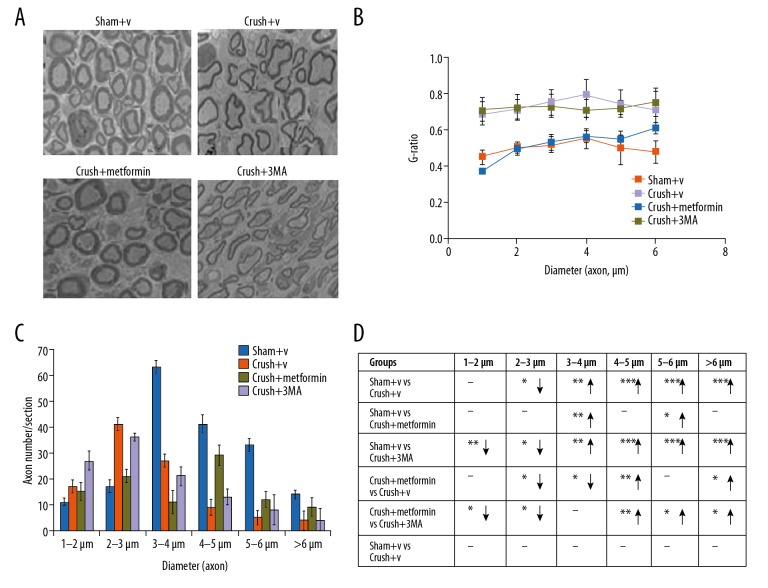
After sciatic nerve injury, SFI perimeters were used for the measurement of reflexive motor function. In the peripheral nerve injury group, there was a decrease in SFI value, which was reversed with metformin as compared with the sham group. The balance and coordination were monitored by rotarod test. Interestingly, the animals treated with metformin stayed on the rotating rod longer with animals in the normal saline group. The longer latency in sensory functional recovery depicts slow sensory function in the tested foot, which was analysed by hot plate test. Sciatic nerve injury resulted in peak latency in the saline group as compared with the metformin group (Figure 5A–5D). These data showed that metformin treatment increased sensory and functional recovery.
Figure 5.
Metformin enhances the nerve regeneration and remylination tracked by peripheral nerve injury: (A) Electron microscopy of sciatic nerve axon in sham+V, crush+V, crush+metformin and crush+3MA. (B, C) Total number of axons quantification for each group. (D) G-ratio comparison were shown upword and downword arrow represent increase and decrease expression and dash (−) represent no significant difference. * p<0.05, ** p<0.01, *** p<0.001, ns – no significance. Scale bar=20 μm.
Metformin promotes nerve conduction recovery
The latency and amplitude of the compound muscle action potential (CMAP) were measured by electrophysiology. Diminished latency depicts fast nerve conduction, which is due to fine nerve myelination. Higher amplitude showed a substantial number of regenerated axons and greater level of reinnervation of measured muscles. Compound muscle action potential imaging showed that the latency in the metformin group was less than in the normal saline group, while the amplitude of the metformin group was higher than in the saline group (Figure 6A, 6B). These results suggest that metformin administration enhances recovery of motor and sensory function.
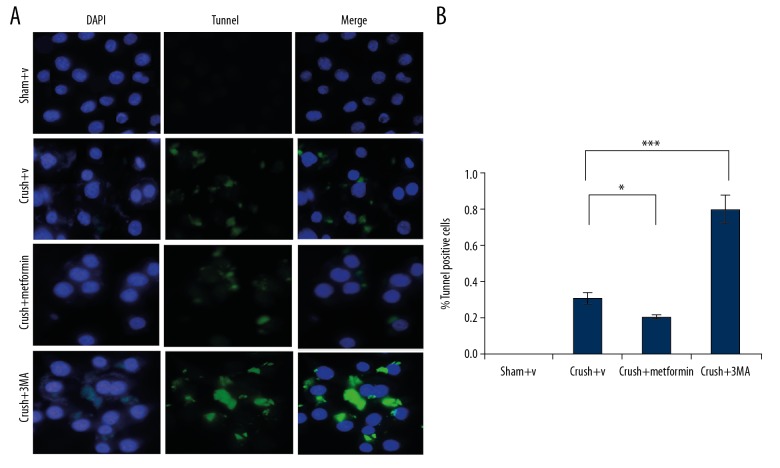
Figure 6.
Metformin induces autophagy decreases cell death following sciatic peripheral nerve injury. (A) Death in Schwann cells were observed in each group (mentioned in Figure 1A) by double staining DAPI (blue) and Tunnel (green). (B) Quantification of cell death tunnel positive cell. * p<0.05, *** p<0.001. Scale bar=20 μm.
Discussion
The interactions between the axonal connections of peripheral nerves are important for maintaining the integrity of the nervous system. In periphery nerve injury, which is the commonest form of trauma, the disruption in axonal connections are the major culprit [23]. Previous reports suggest that the mammalian target of rapamycin (mTORC1) pathway has important roles in functional recovery and regenerative response following trauma by coordinating the metabolism, death, and proliferation of cells and plays a major role in maintaining autophagy [24]. The autophagy pathway has been found to play a vital role in brain injury, neonatal hypoxia ischemia induced brain injury, and spinal cord injury [10–12]. Further studies have shown that mTOR inhibition, via potent inhibitors like rapamycin, upregulates autophagy. The phenomenon of autophagy removes damaged organelles, unfolding proteins to maintain proper functioning of cells for growth and development [25]. The present study deciphered the role of metformin in inducing lysosomal mediated autophagy in injured rat sciatic nerves. Metformin strongly induces autophagy by upregulating the expression of LC3-II following injury. In addition, autophagy induced by metformin reduced the cell mortality at the lesion site of rats. It enhances the nerve tissue regeneration as assessed by increased expression of axonal growth and differentiating markers GAP43 and SCG10 as compared to the control group. Metformin also helps axons to stabilize their structural integrity by remyelinating the axons, as identified by the increased proportion of basic component of myelin sheath MBP after the injury. In the presence of 3-MA, autophagy inhibitor nerve cells start to degenerate and there is a loss of motor function. These findings suggest that autophagy activation upregulates nerve homeostasis after sciatic NCI. Furthermore, electron microscopy was used to detect the metformin-induced autophagosome formation, as this method is considered the criterion standard method for autophagy detection [26]. Further validation of metformin-induced autophagy is provided by the increased protein expression of the well-known autophagy marker LC3-II through immunoblotting [27]. Myelination is an important characteristic of axons that plays a crucial role in nerve regeneration after nerve or spinal injury, and Schwan cells are the primary source of the myelin sheath [28]. The present study showed metformin treatment in rats after sciatic nerve injury showed upregulated Schwan cells and improvement in remyelination at 1–6 weeks after injury when compared to the DMSO-treated group. Therefore, our data revealed that autophagy activation by metformin improves Schwann cell survival and proliferation and, ultimately, nerve recovery. Autophagy has a protective role during oxidative stress, inflammatory response, and ischemia [29]. Autophagy was enhanced in metformin-treated groups, in which there is an improvement time and re-establishment of motor function in the long term. The process of myelination and motor functional recovery was delayed in the presence of autophagy inhibitor 3-MA compared with metformin. In conclusion, our study provides evidence that metformin is crucial for autophagy induction in nerve regeneration and motor function recovery in peripheral nerve injury, and it can provide a new therapeutic approach to improve the regenerative and functional recovery of nerves through pharmacological induction of autophagy.
Conclusions
In conclusion, we explored the potential of metformin for induction of autophagy, nerve regeneration, motor function recovery, and axonal regeneration, finding that it increased remyelination and promotes nerve conduction recovery. Based on these data, we plan to study the detailed mechanism by which metformin promotes nerve regeneration through autophagy in knockout animal models. This work may help in catalyzing research on metformin and other natural products for their possible use as autophagy inducers. We believe that the data presented in this study will strengthen the idea of targeting autophagy for treatment of nerve regeneration and functional recovery.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Sallin P. Regeneration versus scarring in vertebrate appendages and heart. J Pathol. 2016;238(2):233–46. doi: 10.1002/path.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silver J, Schwab ME, Popovich PG. Central nervous system regenerative failure: Role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb Perspect Biol. 2014;7(3):a020602. doi: 10.1101/cshperspect.a020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reier PJ, Zholudeva LV, Lane MA. Conn’s Translational Neuroscience. Elsevier; 2017. Axonal degeneration and regeneration in the peripheral and central nervous systems; pp. 553–75. [Google Scholar]
- 4.Cattin AL, Lloyd AC. The multicellular complexity of peripheral nerve regeneration. Curr Opin Neurobiol. 2016;39:38–46. doi: 10.1016/j.conb.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Levine B, Klionsky DJ. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–77. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 6.Choi AMK, Ryter SW, Levine B. Autophagy in human health and disease. New N Engl J Med. 2016;368(7):651–62. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann A. Autophagy and cell death: No longer at odds. Cell. 2007;131(6):1032–34. doi: 10.1016/j.cell.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira JA, Lebrun-Julien F, Suter U. Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci. 2012;35(2):123–13. doi: 10.1016/j.tins.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Wani A, Gupta M, Ahmad M, et al. Alborixin clears amyloid-β by inducing autophagy through PTEN-mediated inhibition of the AKT pathway. Autophagy. https://www.tandfonline.com/loi/kaup20. [DOI] [PMC free article] [PubMed]
- 10.Clark RS, Bayir H, Chu CT, et al. Autophagy is increased in mice after traumatic brain injury and is detectable in human brain after trauma and critical illness. Autophagy. 2008;4(1):88–90. doi: 10.4161/auto.5173. [DOI] [PubMed] [Google Scholar]
- 11.Wang ZY, Liu WG, Muharram A, et al. Neuroprotective effects of autophagy induced by rapamycin in rat acute spinal cord injury model. Neuroimmunomodulation. 2014;21(5):257–67. doi: 10.1159/000357382. [DOI] [PubMed] [Google Scholar]
- 12.Carloni S, Albertini MC, Galluzzi L, et al. Increased autophagy reduces endoplasmic reticulum stress after neonatal hypoxia “ischemia: Role of protein synthesis and autophagic pathways. Exp Neurol. 2014;255:103–12. doi: 10.1016/j.expneurol.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Qu L, Liang X, Gu B, Liu W. Quercetin alleviates high glucose-induced Schwann cell damage by autophagy. Neural Regen Res. 2014;9(12):1195–203. doi: 10.4103/1673-5374.135328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng W, Yao C, Poonit K, et al. Metformin relieves neuropathic pain after spinal nerve ligation via autophagy flux stimulation. J Cell Mol Med. 2019;23(2):1313–24. doi: 10.1111/jcmm.14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotermund C, Machetanz G, Fitzgerald JC. The therapeutic potential of metformin in neurodegenerative diseases. Front Endocrinol. 2018;9:400. doi: 10.3389/fendo.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boland B, Yu WH, Corti O, et al. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2018;17(9):660–88. doi: 10.1038/nrd.2018.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho K, Chung JY, Cho SK, et al. Antihyperglycemic mechanism of metformin occurs via the AMPK/LXRα/POMC pathway. Sci Rep. 2015;5:8145. doi: 10.1038/srep08145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Li Y, Fan Z, et al. Ascorbic acid facilitates neural regeneration after sciatic nerve crush injury. Front Cell Neurosci. 2019;13:108. doi: 10.3389/fncel.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raducan A, Mirica S, Duicu O, et al. Morphological and functional aspects of sciatic nerve regeneration after crush injury. Rom J Morphol Embryol. 2013;54:735–39. [PubMed] [Google Scholar]
- 20.Kappos EA, Sieber PK, Engels PE, et al. Validity and reliability of the CatWalk system as a static and dynamic gait analysis tool for the assessment of functional nerve recovery in small animal models. Brain Behav. 2017;7(7):e00723. doi: 10.1002/brb3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali A, Derar D, El-Manakhly ESM, et al. A congenital case of intermediate-type fetal rhabdomyoma in an Ardi goat kid. Journal of Histotechnology. 2016;39(1):17–20. [Google Scholar]
- 22.Hassanpour P, Amirfarhangi A, Hosseini-Fard SR, et al. Interleukin 6 may be related to indoleamine 2, 3-dioxygense function in M2 macrophages treated with small dense LDL particles. Gene. 2017;626:442–46. doi: 10.1016/j.gene.2017.05.063. [DOI] [PubMed] [Google Scholar]
- 23.Sta M, Cappaert NL, Ramekers D, et al. The functional and morphological characteristics of sciatic nerve degeneration and regeneration after crush injury in rats. J Neurosci Methods. 2014;222:189–98. doi: 10.1016/j.jneumeth.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(20):3589–94. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunlop EA, Tee AR. mTOR and autophagy: A dynamic relationship governed by nutrients and energy. Semin Cell Dev Biol. 2014;36:121–29. doi: 10.1016/j.semcdb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Klionsky DJ, Cuervo AM, Seglen PO. Methods for monitoring autophagy from yeast to human. Autophagy. 2007;3(3):181–206. doi: 10.4161/auto.3678. [DOI] [PubMed] [Google Scholar]
- 27.Mizushima N. Methods for monitoring autophagy using GFP LC3 transgenic mice. Methods Enzymol. 2009;452:13–23. doi: 10.1016/S0076-6879(08)03602-1. [DOI] [PubMed] [Google Scholar]
- 28.Stassart RM, Fledrich R, Velanac V, et al. A role for Schwann cell derived neuregulin-1 in remyelination. Nat Neurosci. 2013;16(1):48–54. doi: 10.1038/nn.3281. [DOI] [PubMed] [Google Scholar]
- 29.Ma S, Wang Y, Chen Y, Cao F. The role of the autophagy in myocardial ischemia/reperfusion injury. Biochim Biophys Acta. 2015;1852(2):271–76. doi: 10.1016/j.bbadis.2014.05.010. [DOI] [PubMed] [Google Scholar]