Abstract
Background
This case series study evaluated the outcome and effect of portable 3D-head computed tomography (CT, MCT-I, 16 rows mobile CT made in China) navigation-guided key-hole microsurgery for supratentorial hypertensive hematomas.
Material/Methods
Thirty-five consecutive unconscious patients with a significant volume of hypertensive intracranial hemorrhages (HICH) were treated with 3D image-guided key-hole microsurgery, and the clinical features were summarized. Preoperative and postoperative hematoma volumes and reduction in midline shifts were calculated and recorded. The preoperative and postoperative (initial, discharge, and 180th day after stroke onset) neurological status was assessed by Glasgow Outcome Scale (GOS), Glasgow Coma Scale (GCS), and modified Rankin Scale (mRS) score, respectively.
Results
The range of hematoma volumes of surgical patients was 24–99 ml (median, 50 ml). The median time of CT scan (from the time of the request to navigation finish) was 11 min. Total and near-total (>90%) hematoma evacuation was achieved in 96.9% cases. Compared with the initial state of neurological assessment, there was a significant improvement in MRS and GCS at discharge (P<0.001). After 6 months, 57.1% of patients had achieved functional recovery (GOS 4–5) and 2 patients had died.
Conclusions
As a minimally invasive technique, image-guided transcortical sulci or transsylvian approach is highly effective for immediate and complete hematoma evacuation.
MeSH Keywords: Intracranial Hemorrhage, Hypertensive; Neurosurgery; Surgical Procedures, Minimally Invasive
Background
In China, the incidence of stroke is higher than that of coronary heart disease, and the proportion of hypertensive intracranial hemorrhages (HICH) is also high. In China, HICH accounts for 18.9–47.6% of all stroke cases, and patient information is derived from 3 major cities – Beijing, Shanghai, and Changsha – which is higher than that of Western countries. HICH remains the most devastating form of stroke [1], with only 48–65% survival at 1-month follow-up, and with only 10% of these patients living independently [2,3].
Surgical management of HICH is still a matter of controversy regarding indication, timing, and method [4–6]. The STICH trial was a large multicenter international randomized trial showing that traditional craniotomy does not result in significant survival improvement and reduction in patient outcomes in HICH patients compared to conservative treatment [7]. However, there were some limitations to the STICH trial, which, as a worldwide multicenter trial, involved diverse patient inclusion criteria and surgical procedures. Traditional craniotomy degrades the operative effect because of its long operation time, large surgical wound area, and more frequent postoperative complications. These disadvantages led to the development of minimally invasive technology for hematoma evacuation. Minimally invasive surgery (such as stereotaxic puncture aspiration) can only partially remove the hematoma and cannot decrease the intracranial pressure (ICP) effectively in the earlier period, resulting in a higher rate of absolute incidence of recurrent hemorrhage [2,8,9]. Endoscope assistance in evacuating hematomas has mostly shown a trend towards better outcomes than traditional craniectomy [4,10]. Key-hole surgery through natural gaps such as the Sylvian fissure and cerebral cortex to treat HICH was also advocated by some studies [11]. However, obtaining adequate imaging data is sometimes difficult in patients with a significant volume of HICH, as they may not be cooperative during CT scanning, and the plane formed by the bilateral OM lines cannot always be accessed correctly on conventional CT images. In these situations, significant errors may result from approximate radiological or surgical planning without the use of stereotactic techniques or frameless navigating systems [12].
Portable CT, in some form, has been available at many centers for several years. Sichuan Provincial People’s Hospital (Chengdu, China) is the first neurosurgical center to use the new-generation portable head computed tomography (CT) scanner (MCT-I, 16 rows mobile CT, made in China). The MCT-1 is lighter in weight and can be easily moved by 1 operator. Reliability has been high, and it provides a more modern detector system. We have performed over 10 000 CT scans, mostly at the bedside (unpublished data). In the present study, we evaluated the effectiveness of portable 3D-head CT navigation image-guided key-hole surgery for HICH.
Material and Methods
Patient selection
From January 2010 to December 2012, 557 HICH patients were admitted to the Military General Hospital of Beijing People’s Liberation Army of China (PLA), affiliated with BBH. In this study, a total of 35 patients were selected in the neurosurgery clinic who fulfilled our surgical inclusion criteria. This research was approved by the Army General Hospital Ethics Committee, approval number 2017-016. Inclusion criteria were: (a) Clinical diagnosis of non-traumatic HICH; (b) The volume of hemorrhage rangers 25–100 cm3; (c) Less than 24 h before clinical intervention (from onset of stroke to entering our hospital for treatment); (d) Receiving 3D-head CT navigation-guided key-hole surgery during January 2010 to December 2012; (e) Preoperative GCS score 5–14; (f) Age range of 40–80 years; and (g) Consent to perform surgery.
Exclusion criteria were: (a) Disturbances of blood coagulation, such as thrombocytopenia or hepatitis; (b) Traumatic intracranial hemorrhage and intracranial infection; (c) Suffering from severe diseases such as, heart, lung, renal, liver disease, or functional failure; (d) History of stroke with neurological deficits; (e) Intracranial aneurysm or arteriovenous malformation complicated with hemorrhage; and (f) Failure to provide consent.
Clinic assessment
We collected the patient baseline and clinical data, which included sex, age, history of hypertension and diabetes, consciousness status evaluated by GCS, hemorrhage location and side, presence of intraventricular hemorrhage, amount of ICH, degree of midline shift and time delay from admission to operation, GCS score after 24 h of hematoma evacuation, immediate CT scan after surgery showing the degree of midline shift and hematoma volume, hospital stay, and complications (occurring within 30 days, including intracranial infection, rebleeding, pulmonary infection, and digestive tract hemorrhage). The operative time was defined as an elapsed time to evacuate ICH from skin incision to skin closure and surgical approach for hematoma evacuation. We also recorded patient follow-up results, including mortality rate, functional survival rate mRS (initial, discharge, on the 180th day after stroke onset), and Glasgow Outcome Scale (GOS) (on the 180th day after stroke onset).
Surgical procedures
Setup of the portable CT scanner and navigation system
The portable CT scanner is a 16-slice CT scanner optimized for scanning any anatomy, which can be imaged in the 25-cm field of view (FOV) (Figure 1). The scanner has casters for moving it to any patient in need and scans by incremental movements on a “centipede” track system.
Figure 1.
(A–C) Photograph of the CT scanner in storage in the imaging examination room of the Neurosurgical Emergency Department.
CT findings
A 512×512 matrix with 1.5-mm slices was used for CT scans. A neuroradiologist with 15 years of experience reviewed the CT images, but we did not inform him about the purpose of the study. Then, the CT images were converted to computer files and we concealed all patient identification data. The hematoma sizes in the initial CT scans were measured; the longest diameter of the hematoma and the second diameter on the perpendicular axis were measured in the slice with the largest area of ICH. The height of the hematoma was calculated from the number of 10-mm septa slices with hematoma and we measured the third diameter. The 3 diameters were multiplied and then divided by 2 (A×B×C/2) to obtain the ICH volume. The volume of blood in the ventricles was recorded, but we did not record the hematoma volume. If present, hydrocephalus was recorded. The edema volume was defined as the low-density area around the hematoma, as described by Miller et al., and the size of the area including the hematoma was measured, and the total volume of hemorrhage and edema around the hematoma was calculated [4].
Preoperative navigation and localization
The patient was then sent to the operating room. The images were transferred to the frameless navigation system (Brainlab Kolibri workstation, Feldkirchen, Germany) in the operating room. We were careful to avoid complicated areas when choosing cortical entry points in deep hematomas. The navigation system was used to decide on the point to open Sylvian fissure or sulcus. In approaching basal ganglia hematomas, the middle transylvanian-transinsular approach was used, and the distal transylvanian-transinsular approach was also used for thalamic hematomas. We targeted the shallowest sulcus of the hematoma when it reached the cortex in lobar hematomas (Figure 2).
Figure 2.
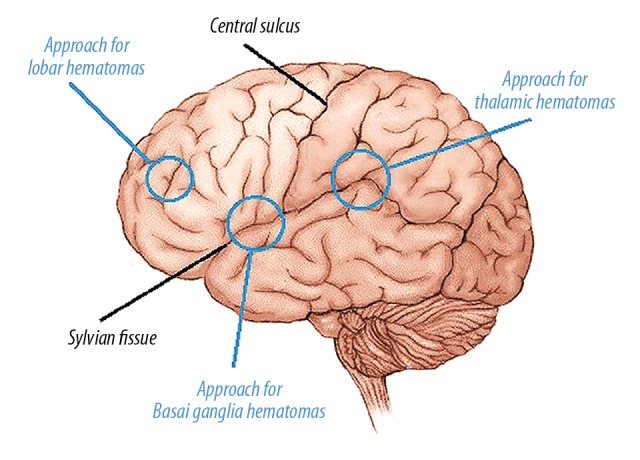
A sample of preoperative navigation to design the trajectory.
Surgical procedures
Under general anesthesia, the patient’s head was fixed in a carbon-fiber clamp. Patient registration to 3D data was done with surface matching (fiducial-free method). The skin incision was 5–6 cm long, and the bone window area was 3–3.5 cm2 under the guidance of the navigation system. Through the operating microscope, the lateral fissure or sulci was cut from the lateral side to the inside by microsurgical technique and we separated the arachnoid. The hematoma cavity was entered using a self-retaining retractor blade (length 5 or 10 mm), and brain plate use was minimized. The hematoma was removed by mild aspiration and bipolar cautery, and the hematoma cavity was continuously washed with 30°C saline. The hematoma located in the middle of the hematoma cavity was initially removed, and the remaining hematoma was transferred to the surgical area due to the pressure difference, which contributed to the complete removal of the hematoma (including large hematomas). We maintained a minimal amount of hematoma near the edge of the hematoma to avoid further damage to the adjacent healthy brain tissue. Hematoma evacuation is usually accompanied by the recovery of brain beats and significant relaxation of the brain. An intraoperative CT scan was performed prior to dural suturing to ensure complete resection of the hematoma. If a postoperative CT scan reveals a residual intraparenchymal clot, the procedure is repeated to remove the thrombus, and a CT scan is repeated to ensure complete removal of the thrombus.
Outcomes and statistical analysis
Patient outcomes were assessed using GCS and mRS scores at discharge and admission. The GOS score was determined initially and after 6 months. Data analyses were performed using the SPSS 20.0 software package (version 20.0; SPSS; Chicago, Illinois, USA). The Shapiro-Wilk test was used to explore the distribution of data. The classified data are expressed in percentages, and were analyzed by the chi-square test with Pearson’s test or Fisher’s exact test for small numbers. The continuity data are represented by means±standard, and were analyzed by using the t test or Mann-Whitney U test for variables that were not normally distributed. Multiranked data were analyzed using the Mann-Whitney U test. Differences were statistically significant at P<0.05.
Results
This series comprised 35 patients with a median age of 60 years (range 40–86 years). Relevant clinical data and CT findings are summarized in Tables 1 and 2. Among the 35 patients with BSGs, 21 (60%) were males and 14 (40%) were females (M/F ratio 1.5: 1). The mean MAP was 144.77±26.09 mmHg. The median time of admission (TA) was 11 h, the median time of CT scan (CT time) was 11 min (range 7–32 min), and the average time of operation (T-O) was 108.49±26.61 min.
Table 1.
Summary of clinical findings in 35 patients.
| General information before admission | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General Information | Clinical outcomes | |||||||||||
| GCS | mRS | GOS | ||||||||||
| No | Age/Gender | MAP (mmHg) | TA (hou) | T-CT (min) | T-O (min) | Initial | 3rd day | Discharge | Initial | Discharge | 6th month | 6th month |
| 1 | 68/F | 140 | 21 | 11 | 105 | 13 | 15 | 15 | 4 | 2 | 2 | 3 |
| 2 | 61/M | 133 | 6.5 | 12 | 95 | 12 | 12 | 14 | 2 | 1 | 1 | 5 |
| 3 | 74/F | 136 | 13 | 21 | 105 | 9 | 13 | 15 | 5 | 4 | 3 | 4 |
| 4 | 70/M | 158 | 5 | 15 | 120 | 12 | 14 | 15 | 4 | 2 | 2 | 2 |
| 5 | 53/F | 170 | 5 | 8 | 90 | 8 | 13 | 15 | 3 | 2 | 2 | 3 |
| 6 | 70/M | 134 | 5 | 9 | 125 | 12 | 13 | 14 | 4 | 3 | 2 | 3 |
| 7 | 43/M | 160 | 4 | 16 | 160 | 9 | 14 | 15 | 5 | 3 | 3 | 4 |
| 8 | 53/M | 178 | 13.5 | 11 | 85 | 13 | 15 | 15 | 5 | 4 | 3 | 4 |
| 9 | 72/F | 146 | 5.5 | 12 | 90 | 8 | 11 | 13 | 5 | 5 | 4 | 3 |
| 10 | 40/M | 152 | 23 | 8 | 100 | 7 | 11 | 12 | 2 | 0 | 0 | 5 |
| 11 | 72/M | 150 | 13 | 11 | 95 | 7 | 12 | 12 | 4 | 5 | 3 | 3 |
| 12 | 57/F | 113 | 11 | 9 | 135 | 8 | 13 | 13 | 3 | 1 | 1 | 5 |
| 13 | 49/M | 144 | 1 | 12 | 200 | 11 | 15 | 15 | 5 | 4 | 3 | 3 |
| 14 | 75/M | 126 | 3 | 9 | 118 | 5 | 5 | – | 5 | 6 | 6 | 1 |
| 15 | 72/M | 130 | 6 | 8 | 100 | 10 | 13 | 13 | 4 | 3 | 3 | 4 |
| 16 | 44/M | 148 | 5 | 16 | 150 | 9 | 14 | 15 | 3 | 3 | 3 | 3 |
| 17 | 59/M | 110 | 14 | 7 | 110 | 12 | 12 | 12 | 4 | 3 | 3 | 3 |
| 18 | 44/F | 100 | 9 | 21 | 75 | 10 | 12 | 12 | 4 | 3 | 3 | 2 |
| 19 | 64/F | 122 | 1.5 | 26 | 95 | 8 | 9 | 11 | 3 | 3 | 6 | 1 |
| 20 | 55/F | 134 | 3 | 13 | 125 | 7 | 15 | 15 | 4 | 2 | 2 | 4 |
| 21 | 53/M | 126 | 5 | 14 | 130 | 8 | 12 | 13 | 5 | 5 | 4 | 4 |
| 22 | 56/M | 118 | 11 | 9 | 105 | 3 | 12 | 15 | 3 | 2 | 2 | 4 |
| 23 | 60/M | 108 | 16 | 11 | 96 | 7 | 11 | 14 | 5 | 4 | 4 | 3 |
| 24 | 65/F | 150 | 17 | 16 | 135 | 12 | 11 | 13 | 3 | 2 | 1 | 5 |
| 25 | 61/M | 194 | 14.5 | 7 | 120 | 9 | 11 | 12 | 3 | 3 | 2 | 4 |
| 26 | 56/M | 110 | 21 | 11 | 85 | 8 | 12 | 14 | 5 | 4 | 3 | 4 |
| 27 | 65/M | 152 | 11.5 | 8 | 90 | 11 | 12 | 14 | 5 | 5 | 4 | 3 |
| 28 | 58/F | 174 | 13.5 | 16 | 95 | 15 | 13 | 15 | 5 | 3 | 3 | 4 |
| 29 | 75/M | 192 | 22 | 21 | 90 | 7 | 15 | 15 | 4 | 3 | 2 | 5 |
| 30 | 73/M | 175 | 23.5 | 35 | 95 | 6 | 13 | 13 | 3 | 3 | 1 | 5 |
| 31 | 63/F | 130 | 17 | 13 | 80 | 7 | 12 | 14 | 4 | 3 | 3 | 4 |
| 32 | 39/F | 198 | 16.5 | 7 | 68 | 7 | 15 | 15 | 4 | 5 | 3 | 4 |
| 33 | 57/F | 186 | 3 | 11 | 135 | 9 | 13 | 15 | 4 | 3 | 3 | 4 |
| 34 | 62/F | 130 | 9.5 | 10 | 85 | 13 | 15 | 15 | 4 | 2 | 2 | 4 |
| 35 | 37/M | 140 | 4 | 13 | 110 | 14 | 15 | 15 | 5 | 4 | 4 | 2 |
TA – time of admission; T-CT – from the time of the request to transmission into PACS and navigation; T-O – time of operation; MAP – mean arterial pressure.
Table 2.
Summary of the detail information on clinical data and CT findings.
| Project group | (Mean±SD)/median/N | Range or ratio | |
|---|---|---|---|
| Age (year) | 59.29±10.97 | 37–75 | |
| Gender | Male | 21(N) | 60% |
| Female | 14(N) | 40% | |
| MAP (mmHg) | 144.77±26.09 | 100–198 | |
| TA (hours) | 11(M) | 1.00–23.50 | |
| CT time (min) | 11(M) | 7–35 | |
| T-O (min) | 100 (M) | 68–200 | |
| Position | Lobar | 13(N) | 37.14% |
| Putaminal | 16(N) | 45.71% | |
| Thalamic | 6(N) | 17.15% | |
| Hematomas | Right hemisphere | 22(N) | 62.86% |
| Left hemisphere | 13(N) | 37.14% | |
| Hematoma shape | Regular | 12(N) | 34.29% |
| Irregular | 15(N) | 42.86% | |
| Uncertain | 8(N) | 22.85% | |
| Hematoma volume (ml) | Initial | 50(M) | 24–99 |
| Residual | 1.4(M) | 0.10–21.90 | |
| Midline shift (3 days after the operation) | 5–8 mm | 17(N) | 48.57% |
| 8–10 mm | 13(N) | 37.14% | |
| >10 mm | 5(N) | 14.29% | |
| GCS score | On admission | 9(N) | 3–15 |
| At discharge | 14(N) | 0–15 | |
| mRS score | On admission | 4(N) | 2–5 |
| At discharge | 3(N) | 0–6 | |
| OS rate after 6 Months | Good function | 20(N) | 57.14% |
| Disability | 10(N) | 28.57% | |
| Vegetative state | 3(N) | 8.57% | |
| Death | 2(N) | 5.72% | |
| DSA | 6(N) | 17.14% | |
M – median; N – number of patients; DSA – digital subtraction angiography.
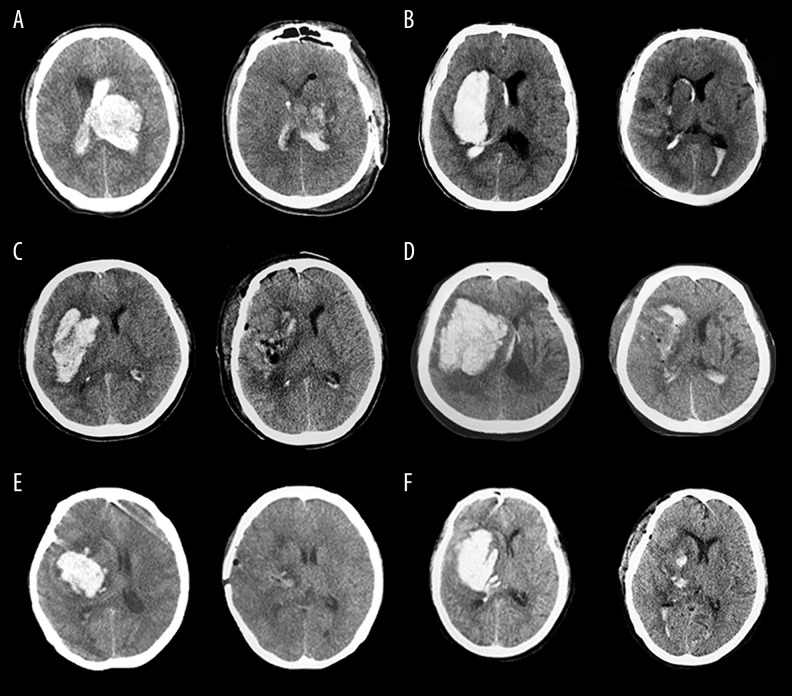
Six patients underwent digital subtraction angiography (DSA), all of which were negative. Thirteen of the 35 hematomas were lobar, 17 were putaminal, and 6 were thalamic. Twenty-two hematomas were situated in the right hemisphere of the brain and 13 were in the left hemisphere (Table 3). Regarding hematoma shape, 12 of the 35 hematomas were regular, 15 were irregular, 8 were between regular and irregular, and the volume of hematoma ranged from 24 to 99 ml (median, 50 ml). The hematoma was found enlarged in 7 patients in preoperative CT examinations. The intraoperative portable CT scan and navigation showed hematoma remains in 9 patients, and the average residue volume was 8.24±1.04 ml. Re-evaluation was performed according to navigation guidance. Postoperative CT examination (3 days after surgery) showed that 32 patients achieved total or near-total evacuation (91.43%). The mean evacuation rate was 96.9% (range 77.9–99.4%) (Figure 3). The average volume of residual hematomas was 1.4 mL (range, 0.1 to 21.9 mL). Twelve patients (37.14%) had an intraventricular extension of the hemorrhage. Partial intraventricular hematoma occurred in 6 of 12 patients with enlarged ventricular hemorrhage. For CT display of midline shift in all patients, a midline shift of 5–8 mm was found in 17 patients; 8–10 mm was found in 13 patients, and >10 mm was found in 5 patients. Three days after the operation, 17 (48.57%) patients showed no midline shift, and the rest of the patients showed lightly shift of midline (5–8 mm). The mean preoperative edema volume was 104.51±12.40 ml and the mean postoperative edema volume was 29.77±4.07ml (P=0.001) (Figure 4).
Table 3.
Preoperative and immediate postoperative CT findings.
| No | HL | HS | HP | IVH | Initial HV (cm3) | Residual HV (cm3) | ER (100%) | Pre-o MS (mm) | Post-o MS (mm) | Pre-o EV (cm3) | Post-o EV (cm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Putaminal | 1 | R | 2 | 31 | 0.2 | 99.4 | ++ | − | 48 | 13 |
| 2 | Lobar | 1 | R | 1 | 72 | 1.0 | 98.6 | ++ | + | 115 | 35 |
| 3 | Lobar | 1 | L | 1 | 53 | 2.1 | 96.3 | +++ | + | 63 | 12 |
| 4 | Putaminal | 3 | R | 2 | 64 | 1.5 | 97.7 | + | − | 74 | 24 |
| 5 | Lobar | 1 | R | 2 | 50 | 0.6 | 98.8 | + | + | 89 | 32 |
| 6 | Lobar | 2 | R | 1 | 32 | 1.0 | 96.9 | + | + | 53 | 12 |
| 7 | Thalamic | 3 | R | 1 | 38 | 0.8 | 97.9 | ++ | − | 72 | 25 |
| 8 | Lobar | 3 | L | 1 | 70 | 0.8 | 98.9 | + | − | 225 | 65 |
| 9 | Putaminal | 1 | R | 1 | 37 | 1.0 | 97.3 | ++ | − | 41 | 9 |
| 10 | Putaminal | 2 | R | 1 | 33 | 2.0 | 93.9 | + | + | 48 | 16 |
| 11 | Thalamic | 2 | R | 1 | 57 | 0.7 | 98.8 | ++ | + | 73 | 20 |
| 12 | Lobar | 2 | L | 1 | 28 | 2.5 | 91.1 | + | − | 32 | 19 |
| 13 | Putaminal | 2 | L | 1 | 33 | 1.5 | 95.5 | + | + | 105 | 21 |
| 14 | Thalamic | 3 | R | 2 | 99 | 21.9 | 77.9 | +++ | + | 321 | 104 |
| 15 | Putaminal | 2 | L | 3 | 50 | 1.3 | 97.4 | + | + | 96 | 31 |
| 16 | Putaminal | 2 | R | 1 | 63 | 0.7 | 98.9 | + | − | 114 | 31 |
| 17 | Lobar | 1 | L | 2 | 30 | 2.8 | 90.7 | +++ | + | 70 | 14 |
| 18 | Putaminal | 3 | L | 4 | 60 | 2.6 | 95.7 | ++ | + | 143 | 23 |
| 19 | Thalamic | 3 | L | 2 | 58 | 1.1 | 98.1 | +++ | + | 72 | 26 |
| 20 | Putaminal | 1 | R | 1 | 36 | 2.2 | 93.9 | + | − | 68 | 31 |
| 21 | Putaminal | 2 | R | 1 | 35 | 0.8 | 97.7 | ++ | − | 47 | 32 |
| 22 | Putaminal | 2 | L | 2 | 33 | 1.1 | 96.7 | + | + | 100 | 24 |
| 23 | Thalamic | 1 | R | 1 | 27 | 0.3 | 98.9 | ++ | − | 41 | 10 |
| 24 | Lobar | 1 | R | 1 | 24 | 0.1 | 97.1 | ++ | + | 37 | 11 |
| 25 | Lobar | 2 | R | 1 | 74 | 3.0 | 95.9 | + | + | 108 | 21 |
| 26 | Lobar | 2 | L | 1 | 28 | 1.6 | 94.3 | + | − | 53 | 12 |
| 27 | Putaminal | 2 | R | 1 | 32 | 0.8 | 97.5 | + | − | 88 | 18 |
| 28 | Putaminal | 3 | R | 2 | 70 | 3.2 | 95.4 | + | + | 185 | 37 |
| 29 | Putaminal | 3 | R | 2 | 90 | 5.4 | 94.0 | ++ | − | 306 | 116 |
| 30 | Lobar | 1 | R | 2 | 86 | 5.0 | 94.2 | ++ | + | 203 | 37 |
| 31 | Lobar | 2 | R | 2 | 53 | 2.6 | 95.1 | + | − | 73 | 22 |
| 32 | Putaminal | 2 | L | 2 | 75 | 1.8 | 97.6 | + | − | 156 | 25 |
| 33 | Lobar | 1 | L | 4 | 90 | 15.5 | 82.8 | +++ | − | 221 | 69 |
| 34 | Thalamic | 2 | L | 4 | 24 | 1.3 | 94.6 | + | − | 57 | 31 |
| 35 | Putaminal | 1 | R | 1 | 55 | 1.4 | 97.5 | ++ | + | 61 | 14 |
R – right; L – left; IVH – intraventricuar hemorrhage; HS – hematoma shape; HP – hematoma position; HL – hematoma location; Pre-o – preoperative; Post-o – post-operative; MS – midline shifting. Post-operative midline shifting indicating 24 hours post operation; Post-operative cisternal status indicating 72 hours post operation; Post operative GCS indicating 1 week post operation. IVH: 1=none; 2=single lateral ventricle; 3=bilateral ventricles; 4=all ventricles, including 3rd and 4th ventricle; Hematoma Shape: 1=regular; 2=irregular; 3=between 1 and 2 grade. Midline shifting: − normal, + 5–8 mm, ++ 8–10 mm, +++ >10 mm; EV – edema volume.
Figure 3.
Preoperative and immediate postoperative CT images in image-guided key-hole evacuation of representative SICH patients (A–F).
Figure 4.
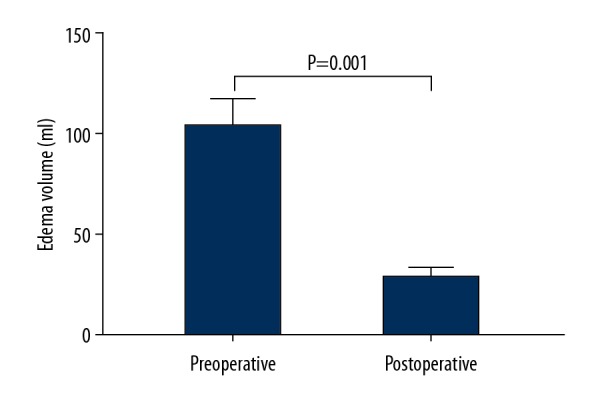
Mean preoperative edema volume and mean postoperative edema volume.
On admission, the median GCS score for the patients was 9 (range, 3–15), and median mRS scores were 4 (range, 2–5). On the third postoperative day, all patients had no neurological deterioration; the GCS score did not improve (p=0.001), and there was no rebleeding. Thirty-three patients were discharged to their homes. The GCS scores (median, 14; range, 0–15) and the mRS scores (median, 3; range, 0–6) were significantly improved compared to admission scores (P<0.001). After 6 months, mRS scores had improved compared to initial admission (P<0.001). The overall 6-month patient survival rate was 94.3% (33/35), including 20 patients (57.1%) with useful function (GOS 4–5), 10 patients (28.6%) with disability (GOS 3), and 3 patients (8.6%) with a vegetative state (GOS 2). The patient mortality rate was 5.7% (2/35). One patient died because of pulmonary infection, and another patient died due to de novo HICH.
Three of 35 patients experienced postoperative pulmonary infection (8.5%, 3/35), and 4 of them had gastrointestinal hemorrhage (14.3%, 5/35). Other postoperative complications included recurrence of HICH (2.9%, 1/35) and seizures (2.9%, 1/35) (Table 4).
Table 4.
Incidence of complications.
| Complications | n (%) |
|---|---|
| Pulmonary infection | 3 (8.5) |
| Bleeding recurrence | 1 (2.9) |
| Digestive tract hemorrhage | 5 (14.3) |
| Epilepsy | 1 (2.9) |
Discussion
Although traditional craniotomy can immediately remove the hematoma, it exposes the brain to the surrounding environment and to irrigation fluid [11,13]. Therefore, surgical tissue trauma may be one of the essential reasons for the craniotomy not being proved beneficial in the treatment of HICH. In addition, deep hematoma requires a significant incision into the cortex, and long-term surgery and contraction of white matter can cause further trauma to the brain, which is a serious adverse effect of surgical treatment after hemorrhage [7,11]. Because of a lack of validated therapeutic options for this form of stroke, the role of minimally invasive surgery (MIS) in the treatment of HICH has gained importance and several different surgical methods have emerged over the past decade.
The main advantages of CT-guided hematoma evacuation in patients with hypertensive thalamic hemorrhage include: (1) This method is simple and can be performed at the bedside without special equipment required by other methods. The surgery is performed under local anesthesia and can be applied to elderly patients who cannot tolerate general anesthesia. (2) Short preparation time for surgery can quickly remove hematoma decompression. The minimally invasive group has a shorter operation time after admission compared with the craniotomy group. Thus, the method can avoid the possibility of the patient getting worse or even cerebral palsy in the long-term routine surgical preparation. (3) The effect of depressurization is sufficient. Yan et al. [14] reported that there are many residual hematomas with drilling drainage compared with craniotomy and endoscopic surgery. However, according to the intracranial pressure-volume curve, a small amount of hematoma can be removed, which can significantly reduce intracranial pressure (P<0.05). Xiao et al. show that the decompression effect can be achieved by aspirating 40–50% of the hematoma [15]. In our study, total and near-total (>90%) hematoma evacuation was achieved in 96.9% cases, and there was a significant improvement in the assessment of MRS and GCS at discharge (P<0.001). (4) It avoids the need for a craniotomy to open the bone flap for a long time and pull the normal brain tissue when entering the hematoma cavity. Therefore, the intraoperative time is shorter than that of the craniotomy group (P<0.05), and the traumatic impact is small. The patient’s intraoperative blood loss was small (P<0.05), which was conducive to postoperative recovery and reduced postoperative complications. A study of fully endoscopic free-hand evacuation of spontaneous supratentorial intraparenchymal hemorrhage [16] showed that the mean operative time (mean, 96 min vs. 11 min), the 6-month GOS (mean, 2.67 vs. 3.53), and the 6-month outcome of mRS (mean, 4.17 vs. 2.74) were worse than in our study. Image-guided key-hole evacuation, using microsurgical techniques, has been described by some authors. Barlas et al. [17] reported that, by using CT navigation and neuro-endoscopy, the average hematoma volume was reduced in 97.5% (range 92.9–100%), and they demonstrated significant improvements in both radiological and clinical data. Lin et al. [18] reported that an 82% average hematoma evacuation rate was achieved by using 3D reconstructed CT images combining neuro-endoscopy. Endoscopy is minimally invasive to the brain tissue, but it has some limitations. Trephination use in endoscopic surgery may damage the underlying brain tissue, such as the speech center on the left hemisphere, which may cause further neurological deficits or significant vessel injuries [12]. Further research is needed to overcome the shortcomings of limited visualization and limited working channels [19,20].
Stereotactic aspiration and drainage is another minimally invasive surgery performed with or without the use of thrombolysis. CT-guided stereotactic aspiration, which was introduced in 1978 by Backlund and Holst [21], was advocated with the early evacuation of hematoma, with much less invasiveness [22]. In 2005, Vespa et al. [23] published a study describing frameless stereotactic catheter aspiration with the use of a tissue plasminogen activator. In their study of 28 patients, the authors reported a mean reduction of hematoma volume of 77±13% and a significant improvement in NIH Stroke Scale score at discharge compared with the original score. This strategy can be used at the bedside in the ICU, thereby avoiding the risks of general anesthesia. However, the average hematoma evacuation rate by stereotactic and simple aspiration is not satisfactory, and a higher rebleeding rate was encountered when early surgical intervention was performed [24]. The risk of rebleeding was reported to be as high as 5% to 13.3% [25–27].
Some authors found that microsurgical evacuation requires more cortical exposure and brain retraction [28]. Here, by the image guidance of 3D-head CT navigation, a restricted burr hole (about 20 mm in diameter) was made, and the natural gap such as the cerebral sulcus or Sylvian fissure was used as the most accurate, shortest, and safest trajectory to the hematoma center, thus minimizing brain damage. Lack of expansion of perivascular edema after complete emptying in the early stage indicates that the burden on brain tissue has not increased. While rebleeding is a concern in minimally invasive hematoma evacuation, imaging revealed no episodes of recurrent bleeding after surgery in our patients.
Our report is the first study to evaluate the invasiveness of the portable CT (MCT-1) navigation and the key-hole approach with the help of microscopic methods. The portable CT is beneficial for the intraoperative navigation guide recalculation and scanning the lesion to assess how much remains during surgery. For the hematoma residues in 9 patients (8.24±1.04 ml), re-evaluation was performed, and the hematoma was obliterated. However, postoperative CT examination (3 days after surgery) showed 3 patients did not reach total or near-total evacuation (evacuation rate >90%). The shapes in all 3 patients were irregular type. Rebleeding may contribute to the instability of a hematoma with intermittent nature. Whether the hematoma shape affects the prognosis needs further study. Additionally, HICH usually requires frequent CTs of the head before, in the perioperative stage, and after the operation [29], or even during surgery, often at short notice. However, most of these patients are ventilated with multiple infusion lines, and it is difficult to transfer such patients for CT scans to the Radiology Department. It is not uncommon to have some mishaps, including endotracheal tube dislodgement, ventilator failure, and oxygen supply issues, which can occur while doing such transfers. In such a scenario, the availability of a mobile CT scan is invaluable, as shown in this study [30].
Our preliminary results showed some dramatic improvements in patients with poor neurological condition. Evaluation of results might stimulate a re-evaluation of the efficacy of such less invasive surgical procedures for ICH in the future, even for ICH with impending herniation.
Conclusions
The 3D reconstructed CT scan image-guided minimally invasive microscopic method is minimally invasive as well as highly effective in obtaining immediate and complete hematoma evacuation.
Footnotes
Source of support: This work was supported by the Natural Scientific Research funds of China (No. 81371345) for Dr. Hong-Tian Zhang and the Twelfth Five-Year Key Military Medical Plan of the Army (No. BWS12J010) for Prof. Ru-Xiang Xu
Conflict of interest
None.
References
- 1.Zhou H, Zhang Y, Liu L, et al. A prospective controlled study: Minimally invasive stereotactic puncture therapy versus conventional craniotomy in the treatment of acute intracerebral hemorrhage. BMC Neurol. 2011;11:76. doi: 10.1186/1471-2377-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grotta JC. Management of primary hypertensive hemorrhage of the brain. Curr Treat Options Neurol. 2004;6(6):435–42. doi: 10.1007/s11940-004-0001-z. [DOI] [PubMed] [Google Scholar]
- 3.Kojima S, Omura T, Wakamatsu W, et al. Prognosis and disability of stroke patients after 5 years in Akita, Japan. Stroke. 1990;21(1):72–77. doi: 10.1161/01.str.21.1.72. [DOI] [PubMed] [Google Scholar]
- 4.Miller CM, Vespa P, Saver JL, et al. Image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. Surg Neurol. 2008;69(5):441–46. doi: 10.1016/j.surneu.2007.12.016. discussion 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Wang J, Zhao H, et al. Clinical analysis and treatment of symptomatic intracranial hemorrhage after deep brain stimulation surgery. Br J Neurosurg. 2016;31(2):217–22. doi: 10.1080/02688697.2016.1244252. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Zhou N, Wang C. Minimally invasive surgery for patients with hypertensive intracerebral hemorrhage with large hematoma volume: A retrospective study. World Neurosurg. 2017;105:348–58. doi: 10.1016/j.wneu.2017.05.158. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes HM, Gregson B, Siddique S, et al. Surgery in intracerebral hemorrhage. The uncertainty continues. Stroke. 2000;31(10):2511–16. doi: 10.1161/01.str.31.10.2511. [DOI] [PubMed] [Google Scholar]
- 8.Kim CH, Choi JH, Park HS. Safety and efficacy of minimally invasive stereotactic aspiration with multicatheter insertion compared with conventional craniotomy for large spontaneous intracerebral hemorrhage (≥50 mL) World Neurosurg. 2019 doi: 10.1016/j.wneu.2019.04.258. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Zhou S, Zhang Q, et al. Stereotactic aspiration for hypertensive intracerebral haemorrhage in a Chinese population: A retrospective cohort study. 2019;4(1):14–21. doi: 10.1136/svn-2018-000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakano T, Ohkuma H, Ebina K, et al. Neuroendoscopic surgery for intracerebral haemorrhage – comparison with traditional therapies. Minim Invasive Neurosurg. 2003;46(5):278–83. doi: 10.1055/s-2003-44451. [DOI] [PubMed] [Google Scholar]
- 11.Barlas O, Karadereler S, Bahar S, et al. Image-guided key-hole evacuation of spontaneous supratentorial intracerebral hemorrhage. Minim Invasive Neurosurg. 2009;52(2):62–68. doi: 10.1055/s-0028-1104610. [DOI] [PubMed] [Google Scholar]
- 12.Lin HL, Lo YC, Liu YF, et al. Endoscopic evacuation of hypertensive putaminal hemorrhage guided by the 3D reconstructed CT scan: A preliminary report. Clin Neurol Neurosurg. 2010;112(10):892–96. doi: 10.1016/j.clineuro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Reisch R, Perneczky A. Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery. 2005;57(4 Suppl):242–55. doi: 10.1227/01.neu.0000178353.42777.2c. [DOI] [PubMed] [Google Scholar]
- 14.Yan RM, Li AM, Zhang ZW, et al. [Comparison of efficacies of three minimally invasive surgical methods for basal ganglia hemorrhage]. Chinese Journal of Minimally Invasive Neurosurgery. 2007;2:59–61. [in Chinese] [Google Scholar]
- 15.Xiao B, Zhu ZA, Zhang H, et al. [Microinvasive drilling drainage to rescue basal ganglia hemorrhage with cerebral palsy]. Chin J Emerg Med. 2004;13(9):634–36. [in Chinese] [Google Scholar]
- 16.Angileri FF, Esposito F, Priola SM, et al. Fully endoscopic free-hand evacuation of spontaneous supratentorial intraparenchymal hemorrhage. World Neurosurg. 2016;94:268–72. doi: 10.1016/j.wneu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Barlas O, Karadereler S, Bahar S, et al. Image-guided keyhole evacuation of spontaneous supratentorial intracerebral hemorrhage. Minim Invasive Neurosurg. 2009;52(02):62–68. doi: 10.1055/s-0028-1104610. [DOI] [PubMed] [Google Scholar]
- 18.Kuo LT, Chen CM, Li CH, et al. Early endoscope-assisted hematoma evacuation in patients with supratentorial intracerebral hemorrhage: Case selection, surgical technique, and long-term results. Neurosurg Focus. 2011;30(4):E9. doi: 10.3171/2011.2.FOCUS10313. [DOI] [PubMed] [Google Scholar]
- 19.Nishihara T, Nagata K, Tanaka S, et al. Newly developed endoscopic instruments for the removal of intracerebral hematoma. Neurocrit Care. 2005;2(1):67–74. doi: 10.1385/NCC:2:1:067. [DOI] [PubMed] [Google Scholar]
- 20.Kim IS, Son BC, Lee SW, et al. Comparison of frame-based and frameless stereotactic hematoma puncture and subsequent fibrinolytic therapy for the treatment of supratentorial deep seated spontaneous intracerebral hemorrhage. Minim Invasive Neurosurg. 2007;50(2):86–90. doi: 10.1055/s-2007-982503. [DOI] [PubMed] [Google Scholar]
- 21.Backlund EO, von Holst H. Controlled subtotal evacuation of intracerebral haematomas by stereotactic technique. Surg Neurol. 1978;9(2):99–101. [PubMed] [Google Scholar]
- 22.Matsumoto K, Hondo H. CT-guided stereotaxic evacuation of hypertensive intracerebral hematomas. J Neurosurg. 1984;61(3):440–48. doi: 10.3171/jns.1984.61.3.0440. [DOI] [PubMed] [Google Scholar]
- 23.Vespa P, Mcarthur D, Miller C, et al. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care. 2005;2(3):274–81. doi: 10.1385/NCC:2:3:274. [DOI] [PubMed] [Google Scholar]
- 24.Nehls DG, Mendelow DA, Graham DI, et al. Experimental intracerebral hemorrhage: Early removal of a spontaneous mass lesion improves late outcome. Neurosurgery. 1990;27(5):674–82. [PubMed] [Google Scholar]
- 25.Niizuma H, Shimizu Y, Yonemitsu T, et al. Results of stereotactic aspiration in 175 cases of putaminal hemorrhage. Neurosurgery. 1989;24(6):814–19. doi: 10.1227/00006123-198906000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Niizuma H, Suzuki J. Stereotactic aspiration of putaminal hemorrhage using a double track aspiration technique. Neurosurgery. 1988;22(2):432–36. doi: 10.1227/00006123-198802000-00031. [DOI] [PubMed] [Google Scholar]
- 27.Thiex R, Rohde V, Rohde I, et al. Frame-based and frameless stereotactic hematoma puncture and subsequent fibrinolytic therapy for the treatment of spontaneous intracerebral hemorrhage. J Neurol. 2004;251(12):1443–50. doi: 10.1007/s00415-004-0554-5. [DOI] [PubMed] [Google Scholar]
- 28.Krayenbühl N, Oinas M, Erdem E, et al. The impact of minimizing brain retraction in aneurysm surgery: Evaluation using magnetic resonance imaging. Neurosurgery. 2011;69(2):344–48. doi: 10.1227/NEU.0b013e31821819a0. [DOI] [PubMed] [Google Scholar]
- 29.McCunn M, Mirvis S, Reynolds N, et al. Physician utilization of a portable computed tomography scanner in the intensive care unit. Crit Care Med. 2000;28(12):3808–13. doi: 10.1097/00003246-200012000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Agrawal D, Sahoo S, Satyarthee GD, et al. Initial experience with mobile computed tomogram in neurosurgery intensive care unit in a level 1 trauma center in India. Neurol India. 2011;59(5):739–42. doi: 10.4103/0028-3886.86551. [DOI] [PubMed] [Google Scholar]