Abstract
Urinary incontinence adversely affects quality of life and results in an increased financial burden for the elderly. Accumulating evidence su00ests a connection between neurotrophins, such as brain-derived neurotrophic factor (BDNF), and lower urinary tract function, particularly with regard to normal physiological function and the pathophysiological mechanisms of stress urinary incontinence (SUI) and bladder pain syndrome/interstitial cystitis (BPS/IC). The interaction between BDNF and glutamate receptors affects both bladder and external urethral sphincter function during micturition. Clinical findings indicate reduced BDNF levels in antepartum and postpartum women, potentially correlating with postpartum SUI. Experiments with animal models demonstrate that BDNF is decreased after simulated childbirth injury, thereby impeding the recovery of injured nerves and the restoration of continence. Treatment with exogenous BDNF facilitates neural recovery and the restoration of continence. Serotonin and noradrenaline reuptake inhibitors, used to treat both depression and SUI, result in enhanced BDNF levels. Understanding the neurophysiological roles of BDNF in maintaining normal urinary function and in the pathogenesis of SUI and BPS/IC could lead to future therapies based on these mechanisms.
Introduction
Brain-derived neurotrophic factor (BDNF) is the second most prevalent neurotrophin in the body, involved in neural development, the neural regulation of bladder storage and emptying, depression and pregnancy. BDNF is also essential for neuroregeneration. Because of its high prevalence, dysregulation of BDNF results in multiple disorders and can contribute to the development of stress urinary incontinence (SUI) and bladder pain syndrome/interstitial cystitis (BPS/IC). The regulation of BDNF-mediated pathways is, therefore, important for the successful treatment of voiding dysfunction and urinary incontinence.
This Review covers four major areas. First, a current summary of neuromuscular-related treatments for SUI and BPS/IC that might involve the expression of BDNF. Second, an overview of BDNF, its signalling mechanisms, and its interactions with glutamate and glutamate receptors. These interactions are related to the mechanism of action of potential pharmacological therapies for SUI and depression. Third, a review of the literature from both clinical practice and animal model data correlating BDNF with postpartum depression and urinary incontinence. BDNF has also been implicated in voiding dysfunctions, such as overactive bladder (OAB) and BPS/IC, and is thought to be involved in the mechanisms of action of both pudendal neuromodulation (PNM) and onabotulinumtoxinA (BoNT/A). The fourth section summarizes and provides insight into potential future therapeutic research directions based on these mechanisms.
Stress urinary incontinence
SUI is defined as the complaint of involuntary urinary leakage on effort or exertion, or on sneezing or coughing.1 Symptoms of SUI are reported by 10–25% ofwomen.2 The incidence of SUI in women peaks between 45 and 49 years of age,3 and white race, obesity, pregnancy and vaginal childbirth are all potential risk factors for SUI.4,5 Over US$12 billion are spent annually for the treatment of SUI in the USA.2 The quality-of-life effects of SUI include the avoidance of social and recreational activities, fear of unpleasant odour, fear of urine loss during vaginal intercourse and secondary depression, when SUI is severe.6
Injury to the urinary continence mechanism through its structural support (pelvic floor muscles and connective tissue) and/or its neuromuscular component (external urethral sphincter and pudendal nerve) can result in SUI.7 Childbirth has long been an associated risk factor for the development of SUI, and vaginal delivery confers a threefold greater risk of developing SUI than caesarean section.8 Vaginal childbirth can result in SUI through damage to ligaments, fascial support and levator ani muscles.9 Furthermore, vaginal childbirth can compress and injure the pudendal nerve as the nerve passes between the sacrospinous and sacrotuberous ligaments.10,11
Allen et al.12 demonstrated that a longer second stage of labour and a higher birthweight baby are correlated with SUI and can increase pudendal nerve terminal motor latencies, which are demonstrative of nerve injury and dysfunction. Electromyographic evidence of reinnervation of the pelvic floor muscles was found in 80% of women studied postpartum.12 Persistently prolonged pudendal nerve terminal motor latencies were observed 5 years after vaginal delivery, with evidence of partial reinnervation after pudendal nerve injury, and were more marked in women with SUI.11 Pudendal nerve damage with resultant SUI can persist ≥7 years after childbirth.13 External urethral sphincter electromyographic activity was significantly reduced in third trimester primigravidas compared to nulligravidas, and these changes persisted 6 months postpartum.14 In addition, women with SUI who have had previous anti-incontinence surgery have more significant neural injury than those without previous anti-incontinence surgery,15 implicating pudendal nerve damage as a likely aetiological factor in SUI after childbirth.
The decision to initiate treatment for SUI depends upon the degree to which the patient is bothered by the symptoms. Among currently available treatments for SUI, only a few address the neuromuscular component of the continence mechanism. Pelvic-floor-muscle exercises and behavioural modifications are neuromuscular rehabilitative therapies, and they remain first-line treatments for SUI. Miller et al.16 conducted a small, randomized trial and found that women who were trained to contract their pelvic floor muscles during a cough, sneeze or laugh had less urine loss than those women who did not contract their pelvic floor muscles. Postpartum pelvic floor physical therapy is effective in the prevention and treatment of SUI by virtue of increasing pelvic muscle strength.17 However, cure rates remain low following pelvic physical therapy years after childbirth compared with those associated with surgical treatment options, su00esting incomplete repair of the neuromuscular continence mechanism with pelvic floor physical therapy alone.
Duloxetine is a dual serotonin and norepinephrine reuptake inhibitor that acts to stimulate pudendal motor neurons and to increase both external urethral sphincter and pelvic floor muscle contractility.18 Duloxetine has some efficacy in the treatment of SUI, and decreases incontinence episode frequency compared with placebo, although it remains unapproved for this indication in the USA, in part because of the 23% incidence of nausea associated with its use.19 Mariappan et al.20 performed a meta-analysis of available randomized trial data and concluded that duloxetine decreased the frequency of SUI episodes and improved quality of life, with mild nausea noted commonly. Duloxetine appears to act in part via a BDNF-related mechanism, so the ubiquity of BDNF could account for these systemic side effects.
Imipramine has also been used to treat SUI, and is thought to reduce incontinence by increasing urethral resistance. Lin et at.21 demonstrated a 35% cure rate among women given 25 mg imipramine orally, three times a day for 3 months, and success correlated with higher urethral closure pressure. As with duloxetine, a BDNF-mediated mechanism could be involved in the pharmacological response to imipramine.
Bladder pain syndrome/interstitial cystitis
Bladder pain syndrome (BPS) is defined by the International Continence Society as suprapubic pain related to bladder filling, accompanied by other bladder storage symptoms, such as increased urinary frequency and urgency, in the absence of proven infection or other obvious pathology.1 BPS/IC represents a broader group of patients who have not only suprapubic pain but also pain or discomfort throughout the pelvis and/or lower abdomen and back.22 The urgency seen with BPS/IC is constant, and these patients void to avoid or relieve pain. The prevalence of BPS/IC in women in the USA is 3.4–7.9 million, or approximately 6.5% of all adult women.23 The diseases most commonly associated with BPS/IC include fibromyalgia (22%), chronic fatigue syndrome (20%) and irritable bowel syndrome (27%).24 These associations su00est the strong likelihood that chronic neuropathic pain persists in patients with BPS/ IC. Cystoscopy is recommended to determine if any of these patients have ulcerative interstitial cystitis, a subtype defined as having lesions that are inflamed, friable and denuded.
The pathogenesis of BPS/IC is thought to result from one or more aetiologies, such as a defect in epithelial permeability, mast-cell activation and neurogenic inflammation.25 In support of neurogenic inflammation as the cause, increased sympathetic neural activity is seen in BPS/IC,26 tri00ering inflammation through the release of neurokinin A, substance P and calcitonin gene-related peptide.27 Mast-cell degranulation and urothelial injury with increased permeability have been shown to result from the actions of these neuropeptide mediators. Bladder biopsies from patients with BPS/IC demonstrate elevated levels of nerve growth factor (NGF).28 The central nervous system (CNS) upregulation and subsequent chronic neuropathic pain seen in patients with BPS/IC is thought to continue after the tissue damage resolves, and associated pelvic floor dysregulation often persists as a maladaptive mechanism.29
The American Urological Association guideline for the diagnosis and treatment of BPS/IC, published in 2011, recommends that pain management be considered throughout the various courses of treatment to maximize function and minimize pain and adverse effects.30 First-line conservative therapies include dietary restriction of caffeine and citrus fruits and behavioural modifications, such as pelvic-floor-muscle relaxation during urination and defecation, and stress reduction. Second-line treatments include pelvic-floor physical therapy, implementing myofascial release of tri00er points. In addition, oral therapies such as amitriptyline, gabapentin, hydroxyzine and pentosan polysulfate can be tried. If first-line and second-line treatments have failed to provide adequate symptomatic control, cystoscopy under anaesthesia with short-duration, low-pressure hydrodistention can be undertaken. Neuromodulation and intradetrusor BoNT/A are recommended as fourth-line and fifth-line treatments, respectively, and both BDNF and glutamate appear to have roles in the mechanism of action of these treatments.
Brain-derived neurotrophic factor
Neurotrophins, including BDNF, NGF, neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT-4/5), are a group of related proteins that share similar characteristics and biological functions.31 The actions of neurotrophins depend on interactions with the transmembrane receptor proteins p75 neurotrophin receptor (p75NTR) and tropomyosin-related kinases (Trks).32 Neurotrophins have specific binding affinities for Trk receptor isoforms: NGF binds to Trk-A, BDNF and NT-4/5 bind to Trk-B and NT-3 binds to Trk-C and, to a lesser extent, Trk-A.31 Phosphorylation of Trk receptors upon binding of neurotrophins leads to activation of three main intracellular signalling pathways: the phosphatidylinositol 3-kinase (PI3K)–Akt pathway, Ras–mitogen-activated protein kinase (MAPK) pathway and the phospholipase Cγ (PLCγ)–Ca2+ pathway.33,34 The activation of the Ras–MAPK pathway promotes cell survival, cell differentiation and synaptic plasticity through extracellular signal-regulated kinase (ERK) and MAPK/ERK kinase (MEK).34 p75NTR, a member of the tumour necrosis factor receptor family, binds all neurotrophins with similar affinity and participates in the process of cell apoptosis.35
BDNF and glutamate receptor signalling
Signalling in lower urinary tract reflexes
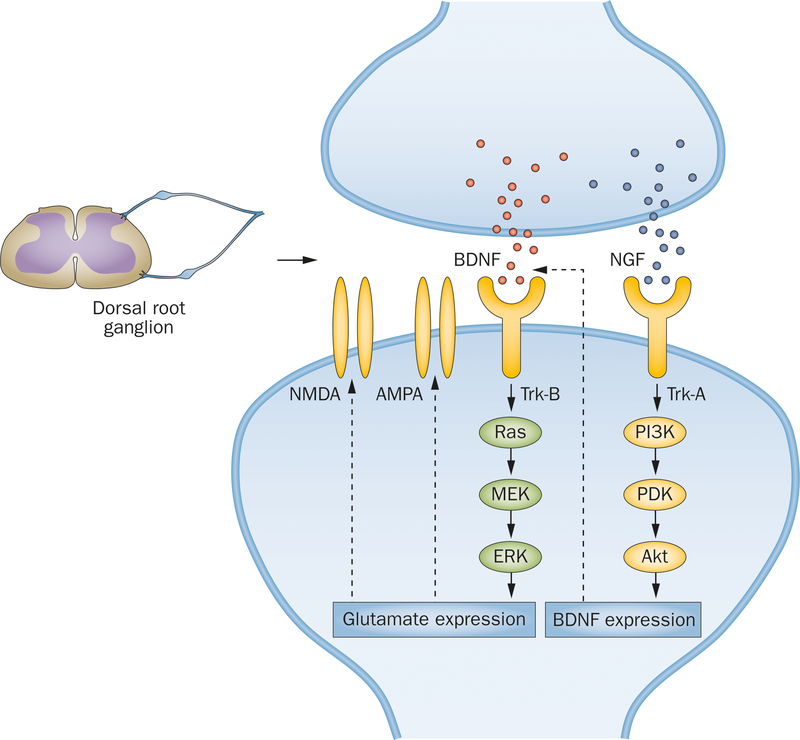
BDNF has a neuromodulatory role at the spinal cord level, enhancing glutamate-mediated responses that have a key role in maintaining lower urinary tract function (Figure 1). Frias et al.36 demonstrated that, although acute intrathecal BDNF injection induced detrusor overactivity in normal rats, treatment with a Trk-B receptor antagonist improved bladder function and relieved inflammation in rats with cyclophosphamide-induced cystitis in an animal model of BPS/IC. Similarly, ERK is upregulated in the lumbosacral spinal cord during bladder inflammation, and blocking the BDNF–Trk-B pathway via intravenous Trk-B–Ig2 treatment reduces detrusor overactivity and suppresses ERK activation in animal models of cystitis.37,38 In the rat model of cyclophosphamide-induced cystitis, phosphorylated ERK expression is strongly upregulated within the urothelium, and treatment with an ERK phosphorylation inhibitor improves bladder function.39 This finding su00ests that the BDNF–Trk-B signalling-mediated Ras–MAPK pathway exerts regulatory effects on bladder function, and that dysregulation of this mechanism can cause bladder inflammation and detrusor overactivity. Further experiments with the animal model of cyclophosphamide-induced cystitis seemingly contradict this mechanism by demonstrating that, although BDNF mRNA expression is significantly increased in bladder tissue, BDNF protein dramatically decreases.40 However, BDNF protein expression is increased in dorsal root ganglion cells of bladder afferents, su00esting that BDNF acts on bladder innervation, rather than the detrusor muscle itself, to modulate lower urinary tract reflexes.40 Upregulation of BDNF in the dorsal root ganglion is linked to the PI3K–Akt pathway, which is regulated by the NGF–Trk-A signalling cascade,41 su00esting an interaction between NGF and BDNF in their neuromodulatory function (Figure 1).
Figure 1 |.
The neuromodulatory effects of BDNF in the dorsal root ganglion, in relation to lower urinary tract function. BDNF expression is mediated by the NGF-dependent PI3K–Akt pathway. Expression of NMDA and AMPA receptors is regulated by the Ras–MAPK pathway, and modulated by BDNF–Trk-B signalling. Abbreviations: Akt, protein kinase B; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BDNF, brain-derived neurotrophic factor; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; NGF, nerve growth factor; NMDA, N-methyl-D-aspartate; PDK, phosphoinositide-dependent kinase; PI3K, phosphatidylinositol 3-kinase; Ras, Ras small GTPase family proteins; Trk, tropomyosin-related kinase.
Glutamate, a major excitatory neurotransmitter, exerts actions through both ionotropic and metabotropic glutamate receptors.42 N-methyl-D-aspartate (NMDA) receptors and a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, mediating ligand-gated, nonselective cation channels, are two major subtypes of inotropic glutamate receptors.42,43 Glutamate acts to control lower urinary tract function in the pontine micturition center, in the lumbosacral dorsal horn, in parasympathetic preganglionic neurons and in Onuf’s nucleus.44 Intrathecal administration of NMDA or AMPA receptor antagonists in unanaesthetized decerebrate rats significantly depresses bladder contractions and external urethral sphincter activity.45,46 Similar results have also been achieved using intrathecal metabotropic glutamate receptor antagonists,47–49 and intracerebroventricular injection of NMDA or AMPA receptor antagonists,50 su00esting that glutamate receptors at both spinal and supraspinal levels exert an excitatory neuromodulatory influence on both micturition and the guarding reflex.
Regulatory mechanisms
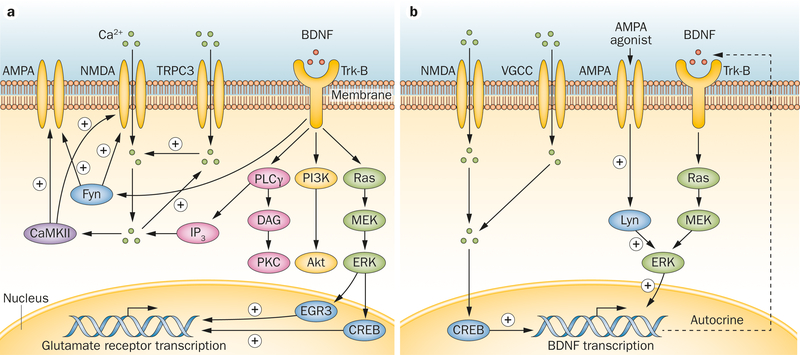
In the CNS, the regulatory mechanisms involving BDNF and glutamate receptors have been extensively investigated (Figure 2). Presynaptic BDNF–Trk-B signalling in glutamatergic terminals increases Ca2+ concentration, facilitates glutamate release and elevates NMDA receptor activity.51–53 BDNF activates postsynaptic Trk-B receptors and leads to increased tyrosine phosphorylation and the activation of NMDA receptors through the Src family nonreceptor-type tyrosine-protein kinase Fyn.54–56 Furthermore, in cultured neocortical neurons of wild type mice, BDNF increases AMPA protein expression, but in the neocortical neurons of homozygous Fyn knockout mice, BDNF treatment does not affect AMPA protein expression, su00esting that Fyn plays a key role in BDNF-mediated AMPA regulation.57
Figure 2 |.
Schematic diagram of the interaction between BDNF and glutamate receptors. BDNF binds to the extracellular domain of Trk-B receptor, activating an intracellular signalling cascade that includes PI3K–Akt, Ras–MAPK and the PLCγ–Ca2+ pathways. a | BDNF can facilitate NMDA receptor and AMPA receptor signalling through Fyn and PLCγ-Ca2+ and CaMKII pathways. NMDA and AMPA receptor expression is enhanced through the BDNF-mediated MAPK pathway. b | Glutamate receptors modulate BDNF signalling through Lyn and calcium signalling, which further induces BDNF expression and secretion in an autocrine fashion. Abbreviations: Akt, protein kinase B; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BDNF, brain-derived neurotrophic factor; CaMKII, calcium/calmodulin-dependent protein kinase II; CREB, cAMP-responsive element-binding protein; DAG, diacylglycerol; EGR3, early growth response protein 3; ERK, extracellular signal-regulated kinase; Fyn, tyrosine-protein kinase Fyn; IP3, inositol trisphosphate; Lyn, tyrosine-protein kinase Lyn; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; NmDA, N-methyl-D-aspartate; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; PLCγ, phospholipase Cγ; Ras, Ras small GTPase family proteins; Trk-B, tropomyosin-related kinase B; TRPC3, short transient receptor potential channel 3; VGCC, voltage-gated calcium channel.
The PLCγ pathway of BDNF-Trk-B signalling promotes the influx of Ca2+ through transient receptor potential (TRP) channels, followed by the increased inward flow of Ca2+ through NMDA receptors.58 The latter can further activate calcium/calmodulin-dependent protein kinase II (CaMKII), which modulates the activity and trafficking of both NMDA and AMPA receptors.59,60 Recent findings su00est that the Ras–MAPK pathway mediates the transcription of NMDA receptor subunits by activating cAMP-responsive element-binding protein (CREB) and early growth response protein 3 (EGR3) in the nucleus, increasing NMDA receptor expression.61
Activation of glutamate receptors can in turn modulate BDNF expression, and is thought to result in neuroprotection (Figure 2). Stimulation of AMPA receptors activates the Src family nonreceptor tyrosine-protein kinase Lyn, which subsequently activates ERK in the Ras–MAPK pathway and promotes BDNF gene transcription.62,63 BDNF can further activate Trk-B receptors in an autocrine fashion.63 AMPA receptor agonists increase the influx of Ca2+ through NMDA receptors and/ or activation of L-type voltage-gated Ca2+ channels, followed by increased BDNF mRNA expression through a CREB-dependent mechanism.64,65
Modulation of CNS disorders
The interaction between BDNF and glutamate receptors is associated with both physiological and pathophysiological activities within the CNS. Regulated by BDNF–Trk-B signalling, NMDA and AMPA receptors participate in the synaptic plasticity of long-term potentiation and long-term psychological depression.66 Abnormalities in this regulatory mechanism could contribute to neuronal dysfunction and neurodegeneration.67 For example, in the hippocampus of patients with seizure disorders, a high level of BDNF and Trk-B expression was found, which is thought to induce increased NMDA receptor currents.68
Antidepressants for depression and SUI
The role of BDNF and glutamate signalling
An insufficiency of BDNF is thought to have a critical role in the pathophysiology of depression: BDNF is significantly decreased in the hippocampus, serum and plasma of patients with depression, as well as in animal models of depression,69,70 and the local application of exogenous BDNF to the hippocampus produces antidepressant effects.71 Furthermore, antidepressant treatment with selective serotonin (5-hydroxytryptamine) reuptake inhibitors (SSRIs) significantly enhances BDNF levels, and BDNF blockade attenuates the effects of antidepressant therapy.72–76 Treatment with SSRIs can increase extracellular serotonin concentration and activate glutamate receptors, which is followed by the influx of Ca2+, which further induces CREB-mediated BDNF transcription.77 The increased BDNF expression that results in turn enhances Ras–MAPK and PI3K–Akt signalling cascades, which are implicated in the treatment response to SSRIs in patients with depression.77–79
The mechanism of action of duloxetine
Duloxetine is used for the treatment of depression, and also has a therapeutic effect in the treatment of lower urinary tract disorders (Figure 3).80–82 Serotonin and norepinephrine terminals within the spinal cord are associated with lower urinary tract reflex pathways, as urine storage and emptying are both modified by serotonin and norepinephrine agonists and antagonists.83–85 Serotonin and norepinephrine receptors have been found in Lissauer’s tract and the sacral parasympathetic nucleus, which contain bladder primary afferent fibres and preganglionic neurons, respectively.86 In general, serotonin relaxes detrusor muscle and increases bladder capacity during urine storage, possibly by enhancing AMPA and NMDA signalling in the dorsal root ganglion, which facilitates the sympathetic storage reflex.86,87 Thus, duloxetine has been studied in animal models of urinary incontinence and has been used clinically to treat mixed urinary incontinence.88–90 Although duloxetine decreases bladder excitation, it has no effect on bladder contraction amplitude or duration, indicating that the mechanism of action is via afferent modulation within the dorsal horn of the spinal cord.18
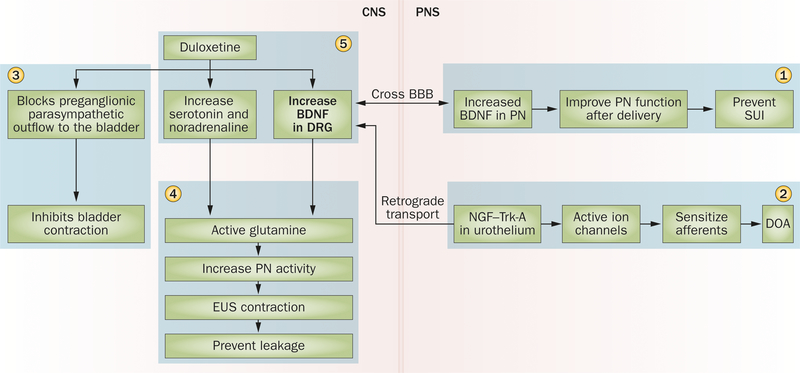
Figure 3 |.
BDNF regulation of lower urinary tract function. BDNF facilitates PN function and could increase EUS muscle tone (1). Retrograde transport of BDNF from the bladder to the DRG has a role in DOA (2). BDNF-mediated glutamate signalling participates in bladder and EUS contractility (3). Duloxetine promotes BDNF expression and has a critical role in maintaining continence (4). The mechanism of duloxetine in treating overactive bladder is to promote the sympathetic storage reflex and increase bladder capacity during urine storage (5). Abbreviations: BBB, blood–brain barrier; BDNF, brain-derived neurotrophic factor; CNS, central nervous system; DOA, detrusor overactivity; DRG, dorsal root ganglion; EUS, external urethral sphincter; NGF, nerve growth factor; PN, pudendal nerve; PNS, peripheral nervous system; SUI, stress urinary incontinence; Trk-A, tropomyosin-related kinase A.
Onuf’s nucleus, the location of pudendal motor neurons, is densely populated with serotonin and norepinephrine terminals.91 During urine storage, duloxetine treatment leads to increased levels of serotonin and norepinephrine within the sacral spinal cord, potentiating excitation of glutamate signalling and activating the pudendal nerve to prevent urine leakage.92 In contrast, during urinary expulsion, serotonin and norepinephrine have little effect upon the voiding phase, owing to the absence of excitatory glutamatergic activation.92 Consequently, duloxetine has been used to successfully treat SUI by increasing urethral outlet resistance without impairing bladder emptying.
Importantly, the effects of increased serotonin and norepinephrine, caused by duloxetine treatment, are not mediated through direct muscle contraction, but rather through the regulation of other receptors.86,92 Because duloxetine treatment significantly elevates BDNF expression and facilitates glutamate signalling, mechanisms which increase BDNF within the spinal cord could be another therapeutic avenue for the treatment of SUI, without the side effects of duloxetine.
BDNF and postpartum SUI
BDNF levels decrease in pregnancy
Clinical studies have demonstrated that BDNF levels are significantly decreased between the thirtieth and thirty-seventh weeks of pregnancy, and that they can remain low for 2–3 months postpartum.93,94 An investigation ofwomen pregnant for ≥28 weeks confirmed that serum BDNF levels are half those observed in nonpregnant women.95 Garcés et al.96 demonstrated in both rats and humans that, although BDNF mRNA expression is significantly increased with advancing gestation, serum protein levels remain low throughout almost the entire pregnancy. Dysregulation of BDNF expression during gestation is associated with multiple disorders, including, anxiety and depression,97,98 preeclampsia,99 intrauterine growth restriction,100 gestational diabetes and preterm deliveries.96,101
The mechanism underlying the decrease in peripheral BDNF during gestation is unclear. Although depression can lead to decreased BDNF in both the CNS and serum, serum BDNF was also found to be decreased in pregnant women without psychiatric conditions.95 Nearly all the BDNF in serum is transported and released by platelets. Platelet counts decrease in pregnancy,102–104 su00esting that low platelets could be a mechanism for decreased BDNF during gestation. However, Lommatzsch et al.93 failed to show a significant correlation between BDNF and platelet counts in pregnant women. Alternative mechanisms for the decrease in BDNF during pregnancy, none of which provides a full explanation, include fetal sequestration, haemodilution and decreased gonadal steroids.105–107
Reduced levels of BDNF are seen in both the central and peripheral nervous systems in depression, so the downregulated serum BDNF levels observed during and after pregnancy could put women at risk for developing mood disorders. An investigation of 40 pregnant and 40 nonpregnant women demonstrated that the reduced BDNF levels in pregnant women correlated with depression during and after pregnancy.93 The results of a cross-sectional study of 190 postpartum women su00ested that women with decreased BDNF expression were more vulnerable to postpartum mood disorders.98 Vega et al.94 further demonstrated that significantly increased BDNF levels during and after pregnancy were detected during aerobic exercise, which could reduce the incidence of depression in these women.
Changes in BDNF levels in animal models of SUI
Animal models have provided valuable insights into the likely mechanisms of SUI-related maternal injuries during vaginal delivery. Bilateral pudendal nerve crush (PNC) in rats produces a reversible model for postpartum SUI that replicates the pudendal nerve injury in Alcock’s canal during vaginal childbirth.108–110 In this model, BDNF expression increases in the external urethral sphincter 1 day after PNC,111 promoting pudendal neuroregeneration via the retrograde transport of BDNF from the external urethral sphincter to its innervating motor neuron cell bodies within Onuf’s nucleus.112 Similarly, BDNF expression is enhanced in the target muscle after other peripheral nerve injuries, such as in the gastrocnemius muscle following sciatic nerve injury.113
Vaginal distension, another animal model of SUI, leads to hypoxia and overstretching of the external urethral sphincter and urethral smooth muscle, mimicking the urethrovaginal damage that occurs during the second phase of labour.114 In contrast to PNC, BDNF is downregulated within the external urethral sphincter following vaginal distension.111 Although BDNF promotes the regeneration and survival of injured peripheral nerves, it inhibits agrin-induced acetylcholine receptor clustering, which is considered to be essential for development and repair of neuromuscular junctions.115,116 In addition, BDNF is dramatically reduced during myogenic differentiation in cultured myoblasts. Knocking down the BDNF gene leads to enhanced myogenic differentiation of myoblasts, implying that BDNF is inhibitory to myogenic regeneration and differentiation.117 Thus, external urethral sphincter recovery after vaginal delivery could be maximized by reduction of BDNF production in the external urethral sphincter, despite the need for BDNF upregulation in pudendal nerve cell bodies for pudendal neuroregeneration after injury in childbirth.
Combining PNC and vaginal distension in the same animal produces a more persistent and durable injury to the continence mechanism, and is considered to be a more clinically relevant SUI model than either injury alone.111,114,118 In this combination injury model, BDNF is only mildly upregulated compared with uninjured controls, and upregulation is much reduced compared with PNC alone.111 The inadequate enhancement of BDNF after combined PNC and vaginal distension could facilitate external urethral sphincter restoration, but at the same time impair pudendal nerve regeneration.109 This mechanism could partly explain the persistent external urethral sphincter denervation, despite normal electromyography, that is observed 9 weeks after the combined injury.119 Insufficient pudendal nerve regeneration could also explain the prolonged pudendal nerve latencies that are observed in patients with SUI 7 years after vaginal delivery.11,13 Consistent with the hypothesis that pudendal nerve regeneration is mediated by BDNF, the direct application of BDNF to the pudendal nerve after PNC with vaginal distension in rats enhanced recovery of the pudendal nerve and restoration of external urethral sphincter anatomy and function.120
Taken together with reduced BDNF levels during late pregnancy and postpartum, data from animal models su00est that postpartum BDNF levels are insufficient for complete pudendal nerve regeneration after vaginal delivery even if SUI resolves. We hypothesize that, with a partially denervated external urethral sphincter, women might or might not have SUI symptoms postpartum. However, innervation of the external urethral sphincter probably does not completely recover. As a result, the effects of ageing, with changing perimenopausal oestrogen levels, predispose women to develop SUI because of insufficient pudendal neuroregeneration, as supported by epidemiological research.121,122
BDNF and pudendal neuromodulation
Although not currently approved by the FDA, clinical studies have su00ested that pudendal neuromodulation could be an effective treatment for patients with lower urinary tract symptoms including urgency/frequency and urge urinary incontinence.123 A prospective, single-blinded, randomized crossover trial, involving 30 patients with voiding dysfunction, compared the symptom reduction rate between pudendal neuromodulation and sacral neuromodulation, an FDA-approved treatment for urgency/frequency and urge urinary incontinence. The overall reduction in voiding symptoms was 63% with pudendal neuromodulation and 46% with sacral neuromodulation.124 Peters et al.125 further demonstrated that, among 84 patients with BPS/IC or OAB, 71% reported a ≥50% level of symptom improvement with pudendal neuromodulation. Patients who fail sacral neuromodulation can alternatively respond to pudendal neuromodulation.125
The exact mechanism of the effects of pudendal neuromodulation on lower urinary tract function remains unclear. Using a model of bladder overactivity in cats, Mally et al.126 demonstrated that metabotropic glutamate receptor 5 (mGluR5) antagonism significantly decreases the bladder inhibition effects of pudendal neuromodulation. The same group also showed that serotonergic 5-HT3 receptors are involved in the mechanism of action of pudendal neuromodulation.127 Moreover, using single time-point pudendal nerve motor-branch electrical stimulation, bladder contractility was temporarily inhibited, and the expression of BDNF in Onuf’s nucleus was significantly enhanced. This response su00ests that BDNF could also be involved in determining the efficacy of pudendal neuromodulation, and implicates the synergetic effects of BDNF, glutamate and serotonin in the mechanism of action of pudendal neuromodulation.128
BDNF treatment of voiding dysfunctions
Neurotrophins, especially NGF, have been found in high levels in the urine of patients with OAB and BPS/IC.129,130 The urinary NGF:creatinine ratio is elevated in women with BPS/IC and correlated with severity of pain.131,132 Likewise, recent evidence su00ests that the urinary BDNF level is upregulated in OAB patients.133 Thus, both NGF and BDNF have been recognized as promising biomarkers for the diagnosis of OAB.134
Intratrigonal injection of BoNT/A has been used to treat OAB, neurogenic detrusor overactivity and BPS/IC.135 In a 12-month follow-up study, Pinto et al.136 demonstrated that intravesical BoNT/A treatment relieved BPS/IC symptoms and significantly suppressed urinary levels of NGF and BDNF. The same group reported that patients with ulcerative and nonulcerative BPS/IC responded to BoNT/A injections with dramatically improved bladder function and reduced urinary NGF, BDNF and glial-cell-derived neurotrophic factor levels.137 BoNT/A decreases acetylcholine release from parasympathetic fibres, thereby reducing detrusor overactivity.138 Bladder sensory activity is also reduced by BoNT/A treatment and this effect is thought to be caused by suppression of calcitonin gene-related peptide and transient receptor potential vanilloid-1 receptors and purinergic receptors.139–142 BoNT/A injections also prevent glutamate neurotransmitter release in bladder dorsal root ganglion cells, su00esting a probable connection to the BDNF-mediated signalling pathway.143
Conclusions and future directions
BDNF is involved in both normal and abnormal lower urinary tract function. Clinical evidence demonstrates a connection between BDNF and SUI, OAB and BPS/ IC.134 Investigations with animal models demonstrate that increased BDNF levels facilitate external urethral sphincter reinnervation, and that BDNF is essential for normal sphincter function.109 In addition, BDNF activates glutamate receptors in the dorsal root ganglion, thereby participating in the regulation of bladder contraction.36,44,77 Modulating BDNF could have a role in the treatment of SUI and other lower urinary tract dysfunctions.
Nonetheless, much research remains to be completed before these findings can be applied to clinical practice. A systematic review published in 2013 summarized eight case-control studies of the accuracy of diagnosis of OAB using urinary NGF levels, but did not perform a meta-analysis because of the inconsistency of the reported data. Thus, the use of urinary NGF levels was not recommended as an adjunct for OAB diagnosis.144 Moreover, although the dysregulation of BDNF-mediated glutamate receptor signalling might lead to dysfunction of micturition and continence, the mechanism underlying this process has not been fully elucidated. How the interaction between BDNF and glutamate receptors within the spinal cord and dorsal root ganglion results in normal or abnormal voiding is not well known. Future translational research into the role of BDNF could impact our understanding of the mechanism of OAB and BPS/IC development and pave the way for development of more effective treatments.
Further research is also needed both in animal models and clinical studies linking pregnancy, vaginal delivery, BDNF regulation and the development of SUI. For example, clinical trials could be performed to determine if the reduction in BDNF levels during or after pregnancy is associated with postpartum SUI. Animal studies could be used to test alternative methods of upregulating BDNF in pudendal nerve cell bodies without causing an increase in the external urethral sphincter. Electrical stimulation is one such potential treatment, as animal experiments have demonstrated that a single subthreshold pudendal nerve stimulation session can upregulate BDNF in Onuf’s nucleus for up to one week.128 Despite the great need for future research, initial studies in animals have set the stage for the modulation of BDNF as a treatment for SUI and other lower urinary tract dysfunctions, and future work will focus and clarify this concept in preparation for translation to the clinic.
Key points
-
■
Interactions between brain-derived neurotrophic factor (BDNF) and glutamate receptors participate in regulation of lower urinary tract function
-
■
BDNF-mediated glutamate signalling could have a role in the pharmacological mechanism of action of duloxetine
-
■
BDNF helps to maintain the continence mechanism by facilitation of pudendal nerve restoration after injury during childbirth
-
■
Both pudendal neuromodulation and onabotulinumtoxinA injection demonstrate a possible BDNF-related mechanism of action
-
■
Manipulation of BDNF levels, by electrical stimulation or other indirect methods, is a potential therapeutic strategy in the management of lower urinary tract symptoms
Review criteria
The primary literature was obtained by searching the MEDLINE database for the keywords “BDNF” and/or “bladder” in combination with the additional keywords: “stress urinary incontinence”, “overactive bladder”, “bladder pain syndrome/interstitial cystitis”, “neurogenic bladder”, “glutamate”, “depression”, “duloxetine”, “sacral neuromodulation” and “onabotulinum toxin-A”. Cited references are peer-reviewed full-text manuscripts published in English.
Acknowledgements
This work was supported in part by the Rehabilitation Research & Development Service of the US Department of Veterans Affairs Office of Research and Development and The Cleveland Clinic.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Abrams P et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 21, 167–178 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Chong EC, Khan AA & Anger JT The financial burden of stress urinary incontinence among women in the United States. Curr. Urol. Rep. 12, 358–362 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Hannestad YS, Rortveit G, Sandvik H & Hunskaar S A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. J. Clin. Epidemiol. 53, 1150–1157 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Sze EH, Jones WP, Ferguson JL, Barker CD & Dolezal JM Prevalence of urinary incontinence symptoms among black, white, and Hispanic women. Obstet. Gynecol. 99, 572–575 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Subak LL et al. Weight loss: a novel and effective treatment for urinary incontinence. J. Urol. 174, 190–195 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milsom I et al. in incontinence: 4th International Consultation on incontinence 4th edn (eds Abrams P, Cardozo L, Khoury S &Wein A) 35–112 (Health Publication, 2009). [Google Scholar]
- 7.DeLancey JO et al. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J. Urol. 179, 2286–2290 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rortveit G, Daltveit AK, Hannestad YS & Hunskaar S Urinary incontinence after vaginal delivery or cesarean section. N. Engl. J. Med. 348, 900–907 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Tunn R et al. MRI morphology of the levator ani muscle, endopelvic fascia, and urethra in women with stress urinary incontinence. Eur. J. Obstet. Gynecol. Reprod. Biol. 126, 239–245 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Lien KC, Morgan DM, DeLancey JO & Ashton-Miller JA Pudendal nerve stretch during vaginal birth: a 3D computer simulation. Am. J. Obstet. Gynecol. 192, 1669–1676 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Snooks SJ, Swash M, Mathers SE & Henry MM Effect of vaginal delivery on the pelvic floor: a 5-year follow-up. Br. J. Surg. 77, 1358–1360 (1990). [DOI] [PubMed] [Google Scholar]
- 12.Allen RE, Hosker GL, Smith AR & Warrell DW Pelvic floor damage and childbirth: a neurophysiological study. Br. J. Obstet. Gynaecol. 97, 770–779 (1990). [DOI] [PubMed] [Google Scholar]
- 13.Dolan LM, Hosker GL, Mallett VT, Allen RE & Smith AR Stress incontinence and pelvic floor neurophysiology 15 years after the first delivery. BJOG 110, 1107–1114 (2003). [PubMed] [Google Scholar]
- 14.Weidner AC, South MM, Sanders DB & Stinnett SS Change in urethral sphincter neuromuscular function during pregnancy persists after delivery. Am. J. Obstet. Gynecol. 201, 529.e1–529.e6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenton K, Mahajan S, Fitzgerald MP & Brubaker L Recurrent stress incontinence is associated with decreased neuromuscular function in the striated urethral sphincter. Am. J. Obstet. Gynecol. 194, 1434–1437 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Miller JM, Ashton-Miller JA & DeLancey JO A pelvic muscle precontraction can reduce cough-related urine loss in selected women with mild SUI. J. Am. Geriatr. Soc. 46, 870–874 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Morkved S & Bo K Effect of postpartum pelvic floor muscle training in prevention and treatment of urinary incontinence: a one-year follow up. BJOG 107, 1022–1028 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Thor KB & Katofiasc MA Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J. Pharmacol. Exp. Ther. 274, 1014–1024 (1995). [PubMed] [Google Scholar]
- 19.Dmochowski RR et al. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J. Urol. 170, 1259–1263 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Mariappan P, Alhasso A, Ballantyne Z, Grant A & N’Dow J Duloxetine, a serotonin and noradrenaline reuptake inhibitor (SNRI) for the treatment of stress urinary incontinence: a systematic review. Eur. Urol. 51, 67–74 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Lin HH, Sheu BC, Lo MC & Huang SC Comparison of treatment outcomes for imipramine for female genuine stress incontinence. Br. J. Obstet. Gynaecol. 106, 1089–1092 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Warren JW et al. Evidence-based criteria for pain of interstitial cystitis/painful bladder syndrome in women. Urology 71, 444–448 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry SH et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J. Urol. 186, 540–544 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren JW et al. Antecedent nonbladder syndromes in case-control study of interstitial cystitis/painful bladder syndrome. Urology 73, 52–57 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Elbadawi A Interstitial cystitis: a critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis. Urology 49, 14–40 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Dimitrakov J, Tchitalov J, Zlatanov T, Dikov D & Rawadi G Corticotropin-releasing hormone perturbations in interstitial cystitis patients: evidence for abnormal sympathetic activity. Urology 57, 128 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Elbadawi AE & Light JK Distinctive ultrastructural pathology of nonulcerative interstitial cystitis: new observations and their potential significance in pathogenesis. Urol. Int. 56, 137–162 (1996). [DOI] [PubMed] [Google Scholar]
- 28.Lowe EM et al. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br. J. Urol. 79, 572–577 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Zermann DH, Wunderlich H, Schubert J & Ishigooka M Re: The diagnosis of interstitial cystitis revisited: lessons learned from the National Institutes of Health Interstitial Cystitis Database Study. J. Urol. 162, 807 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Hanno PM et al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J. Urol. 185, 2162–2170 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao MV Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 4, 299–309 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Kaplan DR & Miller FD Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 10, 381–391 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Minichiello L TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 10, 850–860 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Segal RA Selectivity in neurotrophin signaling: theme and variations. Annu. Rev. Neurosci. 26, 299–330 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Schweigreiter R The dual nature of neurotrophins. Bioessays 28, 583–594 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Frias B et al. Brain-derived neurotrophic factor, acting at the spinal cord level, participates in bladder hyperactivity and referred pain during chronic bladder inflammation. Neuroscience 234, 88–102 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Lai HH et al. Activation of spinal extracellular signal-regulated kinases (ERK) 1/2 is associated with the development of visceral hyperalgesia of the bladder. Pain 152, 2117–2124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinto R et al. Sequestration of brain derived nerve factor by intravenous delivery of TrkB-Ig2 reduces bladder overactivity and noxious input in animals with chronic cystitis. Neuroscience 166, 907–916 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Corrow KA & Vizzard MA Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R125–R134 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Murray E, Malley SE, Qiao LY, Hu VY & Vizzard MA Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J. Urol. 172, 2434–2439 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Qiao LY, Yu SJ, Kay JC & Xia CM In vivo regulation of brain-derived neurotrophic factor in dorsal root ganglia is mediated by nerve growth factor-tri00ered Akt activation during cystitis. PLoS ONE 8, e81547 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traynelis SF et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62, 405–496 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaczor AA & Matosiuk D Molecular structure of ionotropic glutamate receptors. Curr. Med. Chem. 17, 2608–2635 (2010). [DOI] [PubMed] [Google Scholar]
- 44.de Groat WC & Yoshimura N Pharmacology of the lower urinary tract. Annu. Rev. Pharmacol. Toxicol. 41, 691–721 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Yoshiyama M, Roppolo JR & de Groat WC Effects of LY215490, a competitive alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor antagonist, on the micturition reflex in the rat. J. Pharmacol. Exp. Ther. 280, 894–904 (1997). [PubMed] [Google Scholar]
- 46.Yoshiyama M, Roppolo JR & de Groat WC Effects of MK-801 on the micturition reflex in the rat—possible sites of action. J. Pharmacol. Exp. Ther. 265, 844–850 (1993). [PubMed] [Google Scholar]
- 47.Tanaka H, Kakizaki H, Shibata T, Ameda K & Koyanagi T Effects of a selective metabotropic glutamate receptor agonist on the micturition reflex pathway in urethane-anesthetized rats. Neurourol. Urodyn. 22, 611–616 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Hu Y et al. The role of metabotropic glutamate receptor mGlu5 in control of micturition and bladder nociception. Neurosci. Lett. 450, 12–17 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Guarneri L et al. Effect of selective antagonists of group I metabotropic glutamate receptors on the micturition reflex in rats. BJU Int. 102, 890–898 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Yoshiyama M & de Groat WC Supraspinal and spinal alpha-amino-3-hydroxy-5-methylisoxaz ole-4-propionic acid and N-methyl-D-aspartate glutamatergic control of the micturition reflex in the urethane-anesthetized rat. Neuroscience 132, 1017–1026 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto T et al. Brain-derived neurotrophic factor enhances depolarization-evoked glutamate release in cultured cortical neurons. J. Neurochem. 79, 522–530 (2001). [DOI] [PubMed] [Google Scholar]
- 52.Poo MM Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2, 24–32 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Tyler WJ, Perrett SP & Pozzo-Miller LD The role of neurotrophins in neurotransmitter release. Neuroscientist 8, 524–531 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin SY et al. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res. Mol. Brain Res. 55, 20–27 (1998). [DOI] [PubMed] [Google Scholar]
- 55.Levine ES, Crozier RA, Black IB & Plummer MR Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc. Natl Acad. Sci. USA 95, 10235–10239 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizuno M, Yamada K, He J, Nakajima A & Nabeshima T Involvement of BDNF receptor TrkB in spatial memory formation. Learn. Mem. 10, 108–115 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Narisawa-Saito M et al. Growth factor-mediated Fyn signaling regulates alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor expression in rodent neocortical neurons. Proc. Nati Acad. Sci. USA 96, 2461–2466 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuczewski N, Porcher C, Lessmann V, Medina I & Gaiarsa JL Activity-dependent dendritic release of BDNF and biological consequences. Moi. Neurobioi. 39, 37–49 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caldeira MV et al. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Moi. Ceii. Neurosci. 35, 208–219 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Caldeira MV et al. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J. Bioi. Chem. 282, 12619–12628 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Kim JH et al. Brain-derived neurotrophic factor uses CREB and Egr3 to regulate NMDA receptor levels in cortical neurons. J. Neurochem. 120, 210–219 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayashi T, Umemori H, Mishina M & Yamamoto T The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. Nature 397, 72–76 (1999). [DOI] [PubMed] [Google Scholar]
- 63.Wu X et al. AMPA protects cultured neurons against glutamate excitotoxicity through a phosphatidylinositol 3-kinase-dependent activation in extracellular signal-regulated kinase to upregulate BDNF gene expression. J. Neurochem. 90, 807–818 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ & Greenberg ME Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20, 709–726 (1998). [DOI] [PubMed] [Google Scholar]
- 65.O’Neill MJ, Bleakman D, Zimmerman DM & Nisenbaum ES AMPA receptor potentiators for the treatment of CNS disorders. Curr. Drug Targets CNS Neuroi. Disord. 3, 181–194 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Collingridge GL, Peineau S, Howland JG & Wang YT Long-term depression in the CNS. Nat. Rev. Neurosci. 11, 459–473 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Mattson MP Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann. N. Y.Acad.Sci. 1144, 97–112 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casillas-Espinosa PM, Powell KL & O’Brien TJ Regulators of synaptic transmission: roles in the pathogenesis and treatment of epilepsy. Epiiepsia 53 (Suppl. 9), 41–58 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Hashimoto K, Shimizu E & lyo M Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res. Brain Res. Rev. 45, 104–114 (2004). [DOI] [PubMed] [Google Scholar]
- 70.Lee BH, Kim H, Park SH & Kim YK Decreased plasma BDNF level in depressive patients. J. Affect. Disord. 101, 239–244 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Shirayama Y, Chen AC, Nakagawa S, Russell DS & Duman RS Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 22, 3251–3261 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ball S, Marangell LB, Lipsius S & Russell JM Brain-derived neurotrophic factor in generalized anxiety disorder: results from a duloxetine clinical trial. Prog. Neuropsychopharmacoi. Bioi. Psychiatry 43, 217–221 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Calabrese F et al. Long-Term duloxetine treatment normalizes altered brain-derived neurotrophic factor expression in serotonin transporter knockout rats through the modulation of specific neurotrophin isoforms. Moi. Pharmacol. 77, 846–853 (2010). [DOI] [PubMed] [Google Scholar]
- 74.Duman RS&Monteggia LM. A neurotrophic model for stress-related mood disorders. Bioi. Psychiatry 59, 1116–1127 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Schmidt HD & Duman RS The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav. Pharmacol. 18, 391–418 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Sen S, Duman R & Sanacora G Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Bioi. Psychiatry 64, 527–532 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duman RS & Voleti B Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid- acting agents. Trends Neurosci. 35, 47–56 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dwivedi Y et al. Reduced activation and expression of ERK1/2 MAP kinase in the postmortem brain of depressed suicide subjects. J. Neurochem. 77, 916–928 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Hsiung SC et al. Attenuated S-HT1A receptor signaling in brains of suicide victims: involvement of adenylyl cyclase, phosphatidylinositol 3-kinase, Akt and mitogen- activated protein kinase. J. Neurochem. 87, 182–194 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Burgard EC, Fraser MO & Thor KB Serotonergic modulation of bladder afferent pathways. Urology 62, 10–15 (2003). [DOI] [PubMed] [Google Scholar]
- 81.Ghoniem GM et al. A randomized controlled trial of duloxetine alone, pelvic floor muscle training alone, combined treatment and no active treatment in women with stress urinary incontinence. J. Uroi. 173, 1647–1653 (2005). [DOI] [PubMed] [Google Scholar]
- 82.Millard RJ, Moore K, Rencken R, Yalcin I & Bump RC Duloxetine vs placebo in the treatment of stress urinary incontinence: a four-continent randomized clinical trial. BJU Int. 93, 311–318 (2004). [DOI] [PubMed] [Google Scholar]
- 83.Espey MJ, Downie JW & Fine A Effect of S-HT receptor and adrenoceptor antagonists on micturition in conscious cats. Eur. J. Pharmacol. 221, 167–170 (1992). [DOI] [PubMed] [Google Scholar]
- 84.Miyazato M et al. Effect of duloxetine, a norepinephrine and serotonin reuptake inhibitor, on sneeze-induced urethral continence reflex in rats. Am. J. Physiol. Renal Physiol. 295, F264–F271 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshimura N, Sasa M, Yoshida O & Takaori S Mediation of micturition reflex by central norepinephrine from the locus coeruleus in the cat. J. Uroi. 143, 840–843 (1990). [DOI] [PubMed] [Google Scholar]
- 86.Thor KB Targeting serotonin and norepinephrine receptors in stress urinary incontinence. Int. J. Gynaecol. Obstet. 86 (Suppl. 1), S38–S52 (2004). [DOI] [PubMed] [Google Scholar]
- 87.Jost W & Marsalek P Duloxetine: mechanism of action at the lower urinary tract and Onuf’s nucleus. Clin. Auton. Res. 14, 220–227 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Di Rezze S et al. Duloxetine for the treatment of overactive bladder syndrome in multiple sclerosis: a pilot study. Clin. Neuropharmacoi. 35, 231–234 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Schwen Z et al. Inhibition of bladder overactivity by duloxetine in combination with foot stimulation or WAY-10063S treatment in cats. Am. J. Physioi. Renai Physioi. 305, F1663–F1668 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bent AE et al. Duloxetine compared with placebo for the treatment of women with mixed urinary incontinence. Neurouroi. Urodyn. 27, 212–221 (2008). [DOI] [PubMed] [Google Scholar]
- 91.Ramage AG The role of central 5-hydroxytryptamine (5-HT, serotonin) receptors in the control of micturition. Br. J. Pharmacol. 147 (Suppl. 2), S120–S131 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schuessler B What do we know about duloxetine’s mode of action? Evidence from animals to humans. BJOG 113 (Suppl. 1), 5–9 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Lommatzsch M et al. Maternal serum concentrations of BDNF and depression in the perinatal period. Psychoneuroendocrinology 31, 388–394 (2006). [DOI] [PubMed] [Google Scholar]
- 94.Vega SR et al. Responses of serum neurotrophic factors to exercise in pregnant and postpartum women. Psychoneuroendocrinology 36, 220–227 (2011). [DOI] [PubMed] [Google Scholar]
- 95.Kim DR, Gonzalez JM, Sammel MD, Parry S & Epperson CN Brain derived neurotrophic factor is altered in human pregnancy. Clinical Neuropsychiatry 9, 207–211 (2012). [Google Scholar]
- 96.Garcés MF et al. Brain-Derived Neurotrophic Factor is expressed in rat and human placenta and its serum levels are similarly regulated throughout pregnancy in both species. Clin. Endocrinol. (Oxf.) 81, 141–151 (2014). [DOI] [PubMed] [Google Scholar]
- 97.Aksu I et al. Maternal treadmill exercise during pregnancy decreases anxiety and increases prefrontal cortex VEGF and BDNF levels of rat pups in early and late periods of life. Neurosci. Lett. 516, 221–225 (2012). [DOI] [PubMed] [Google Scholar]
- 98.Pinheiro RT et al. Brain-derived neurotrophic factor levels in women with postpartum affective disorder and suicidality. Neurochem. Res. 37, 2229–2234 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Fujita K et al. Differential expression and the anti-apoptotic effect of human placental neurotrophins and their receptors. Placenta 32, 737–744 (2011). [DOI] [PubMed] [Google Scholar]
- 100.Mayeur S et al. Placental BDNF/TrkB signaling system is modulated by fetal growth disturbances in rat and human. Placenta 31, 785–791 (2010). [DOI] [PubMed] [Google Scholar]
- 101.Dhobale M, Mehendale S, Pisal H, D’Souza V & Joshi S Association of brain- derived neurotrophic factor and tyrosine kinase B receptor in pregnancy. Neuroscience 216, 31–37 (2012). [DOI] [PubMed] [Google Scholar]
- 102.Karege F et al. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol. Psychiatry 57, 1068–1072 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Fujimura H et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 87, 728–734 (2002). [PubMed] [Google Scholar]
- 104.Burrows RF & Kelton JG Thrombocytopenia at delivery: a prospective survey of 6715 deliveries. Am. J. Obstet. Gynecol. 162, 731–734 (1990). [DOI] [PubMed] [Google Scholar]
- 105.Franklin TB & Perrot-Sinal TS Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology 31, 38–48 (2006). [DOI] [PubMed] [Google Scholar]
- 106.Kodomari I, Wada E, Nakamura S & Wada K Maternal supply of BDNF to mouse fetal brain through the placenta. Neurochem. Int. 54, 95–98 (2009). [DOI] [PubMed] [Google Scholar]
- 107.Whittaker PG, Macphail S & Lind T Serial hematologic changes and pregnancy outcome. Obstet. Gynecol. 88, 33–39 (1996). [DOI] [PubMed] [Google Scholar]
- 108.Damaser MS, Broxton-King C, Ferguson C, Kim FJ & Kerns JM Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J. Urol. 170, 1027–1031 (2003). [DOI] [PubMed] [Google Scholar]
- 109.Gill BC, Damaser MS, Vasavada SP & Goldman HB Stress incontinence in the era of regenerative medicine: reviewing the importance of the pudendal nerve. J. Urol. 190, 22–28 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kerns JM et al. Effects of pudendal nerve injury in the female rat. Neurourol. Urodyn. 19, 53–69 (2000). [DOI] [PubMed] [Google Scholar]
- 111.Pan HQ et al. Dual simulated childbirth injury delays anatomic recovery. Am. J. Physiol. Renal Physiol. 296, F277–F283 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gordon T The role of neurotrophic factors in nerve regeneration. Neurosurg. Focus 26, E3 (2009). [DOI] [PubMed] [Google Scholar]
- 113.Omura T et al. Different expressions of BDNF NT3, and NT4 in muscle and nerve after various types of peripheral nerve injuries. J. Peripher. Nerv. Syst. 10, 293–300 (2005). [DOI] [PubMed] [Google Scholar]
- 114.Gill BC, Moore C & Damaser MS Postpartum stress urinary incontinence: lessons from animal models. Expert Rev. Obstet. Gynecol. 5, 567–580 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sakuma K et al. A possible role for BDNF, NT-4 and TrkB in the spinal cord and muscle of rat subjected to mechanical overload, bupivacaine injection and axotomy. Brain Res. 907, 1–19 (2001). [DOI] [PubMed] [Google Scholar]
- 116.Wells DG, McKechnie BA, Kelkar S & Fallon JR Neurotrophins regulate agrin-induced postsynaptic differentiation. Proc. Natl Acad. Sci. USA 96, 1112–1117 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mousavi K & Jasmin BJ BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J. Neurosci. 26, 5739–5749 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jiang HH et al. Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function. Neurourol. Urodyn. 28, 229–235 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Song QX et al. Long-term effects of simulated childbirth injury on function and innervation of the urethra. Neurourol. Urodyn. 10.1002/nau.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gill BC et al. Neurotrophin therapy improves recovery of the neuromuscular continence mechanism following simulated birth injury in rats. Neurourol. Urodyn. 32, 82–87 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fritel X, Ringa V, Quiboeuf E & Fauconnier A Female urinary incontinence, from pregnancy to menopause: a review of epidemiological and pathophysiological findings. Acta Obstet. Gynecol. Scand. 91, 901–910 (2012). [DOI] [PubMed] [Google Scholar]
- 122.Ashton-Miller JA & DeLancey JO Functional anatomy of the female pelvic floor. Ann. N. Y. Acad. Sci. 1101, 266–296 (2007). [DOI] [PubMed] [Google Scholar]
- 123.Bartley J, Gilleran J & Peters K Neuromodulation for overactive bladder. Nat. Rev. Urol. 10, 513–521 (2013). [DOI] [PubMed] [Google Scholar]
- 124.Peters KM, Feber KM & Bennett RC Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol. Urodyn. 24, 643–647 (2005). [DOI] [PubMed] [Google Scholar]
- 125.Peters KM, Killinger KA, Boguslawski BM & Boura JA Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol. Urodyn. 29, 1267–1271 (2010). [DOI] [PubMed] [Google Scholar]
- 126.Mally AD et al. Role of opioid and metabotropic glutamate 5 receptors in pudendal inhibition of bladder overactivity in cats. J. Urol. 189, 1574–1579 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schwen Z et al. Involvement of 5-HT3 receptors in pudendal inhibition of bladder overactivity in cats. Am. J. Physiol. Renal Physiol. 305, F663–F671 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang HH et al. Effects of acute selective pudendal nerve electrical stimulation after simulated childbirth injury. Am. J. Physiol. Renal Physiol. 304, F239–F247 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cruz CD Neurotrophins in bladder function: what do we know and where do we go from here? Neurourol. Urodyn. 33, 39–45 (2014). [DOI] [PubMed] [Google Scholar]
- 130.Kuo HC, Liu HT & Chancellor MB Urinary nerve growth factor is a better biomarker than detrusor wall thickness for the assessment of overactive bladder with incontinence. Neurourol. Urodyn. 29, 482–487 (2010). [DOI] [PubMed] [Google Scholar]
- 131.Liu HT, Tyagi P, Chancellor MB & Kuo HC Urinary nerve growth factor but not prostaglandin E2 increases in patients with interstitial cystitis/bladder pain syndrome and detrusor overactivity. BJU Int. 106, 1681–1685 (2010). [DOI] [PubMed] [Google Scholar]
- 132.Kim SW et al. Urinary nerve growth factor correlates with the severity of urgency and pain. Int. Urogynecol. J 10.1007/s00192-014-2424-8. [DOI] [PubMed] [Google Scholar]
- 133.Wang LW, Han XM, Chen CH, Ma Y & Hai B Urinary brain-derived neurotrophic factor: a potential biomarker for objective diagnosis of overactive bladder. Int. Urol. Nephrol. 46, 341–347 (2014). [DOI] [PubMed] [Google Scholar]
- 134.Bhide AA, Cartwright R, Khullar V & Digesu GA Biomarkers in overactive bladder. Int. Urogynecol. J. 24, 1065–1072 (2013). [DOI] [PubMed] [Google Scholar]
- 135.Seth J, Khan MS, Dasgupta P & Sahai A Botulinum toxin-what urologic uses does the data support? Curr. Urol. Rep. 14, 227–234 (2013). [DOI] [PubMed] [Google Scholar]
- 136.Pinto R et al. Trigonal injection of botulinum toxin A in patients with refractory bladder pain syndrome/interstitial cystitis. Eur. Urol. 58, 360–365 (2010). [DOI] [PubMed] [Google Scholar]
- 137.Pinto R et al. Ulcerative and nonulcerative forms of bladder pain syndrome/interstitial cystitis do not differ in symptom intensity or response to onabotulinum toxin A. Urology 83, 1030–1034 (2014). [DOI] [PubMed] [Google Scholar]
- 138.Chancellor MB et al. Drug Insight: biological effects of botulinum toxin A in the lower urinary tract. Nat. Clin. Pract. Urol. 5, 319–328 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Apostolidis A et al. Decreased sensory receptors P2X3and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J. Urol. 174, 977–982 (2005). [DOI] [PubMed] [Google Scholar]
- 140.Chuang YC, Yoshimura N, Huang CC, Chiang PH & Chancellor MB Intravesical botulinum toxin a administration produces analgesia against acetic acid induced bladder pain responses in rats. J. Urol. 172, 1529–1532 (2004). [DOI] [PubMed] [Google Scholar]
- 141.Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N & Ferrer-Montiel A Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J. Biol.Chem. 279, 25665–25672 (2004). [DOI] [PubMed] [Google Scholar]
- 142.Rapp DE, Turk KW, Bales GT & Cook SP Botulinum toxin type a inhibits calcitonin gene-related peptide release from isolated rat bladder. J. Urol. 175, 1138–1142 (2006). [DOI] [PubMed] [Google Scholar]
- 143.Duggan MJ et al. Inhibition of release of neurotransmitters from rat dorsal root ganglia by a novel conjugate of a Clostridium botulinum toxin A endopeptidase fragment and Erythrina cristagalli lectin. J. Biol. Chem. 277, 34846–34852 (2002). [DOI] [PubMed] [Google Scholar]
- 144.Rachaneni S, Arya P & Latthe P Urinary nerve growth factor: a biomarker of detrusor overactivity? A systematic review. Int. Urogynecol. J. 24, 1603–1609 (2013). [DOI] [PubMed] [Google Scholar]