Abstract
Background:
Most U.S. hospitals publicly report 30-day risk-standardized mortality rates for pneumonia. Rates exclude severe cases, which may be assigned a secondary diagnosis of pneumonia and a principal diagnosis of sepsis or respiratory failure. By assigning sepsis and respiratory failure codes more liberally, hospitals might improve their reported performance.
Objective:
To examine the effect of the definition of pneumonia on hospital mortality rates.
Design:
Cross-sectional study.
Setting:
329 U.S. hospitals.
Patients:
Adults hospitalized for pneumonia (as a principal diagnosis or secondary diagnosis paired with a principal diagnosis of sepsis or respiratory failure) between 2007 and 2010.
Measurements:
Proportion of patients with pneumonia coded with a principal diagnosis of sepsis or respiratory failure and risk-standardized mortality rates excluding versus including a principal diagnosis of sepsis or respiratory failure.
Results:
When the definition of pneumonia was limited to patients with a principal diagnosis of pneumonia, the risk-standardized mortality rate was significantly better than the mean in 4.3% of hospitals and significantly worse in 6.4%. When the definition was broadened to include patients with a principal diagnosis of sepsis or respiratory failure, this rate was better than the mean in 11.9% of hospitals and worse in 22.8% and the outlier status of 28.3% of hospitals changed. Among hospitals in the highest quintile of proportion of patients coded with a principal diagnosis of sepsis or respiratory failure, outlier status under the broader definition improved in 7.6% and worsened in 40.9%. Among those in the lowest quintile, 20.0% improved and none worsened.
Limitation:
Only inpatient mortality was studied.
Conclusion:
Variation in use of the principal diagnosis of sepsis or respiratory failure may bias efforts to compare hospital performance regarding pneumonia outcomes.
Primary Funding Source:
Agency for Healthcare Research and Quality.
Pneumonia is the most common cause of emergency hospitalization in the United States (1). As such, it is an appropriate target for quality improvement initiatives and public reporting of hospital quality. Initial efforts at public reporting focused on processes of care, including the choice and timing of initial antibiotics, pneumococcal vaccination, and assessment of oxygenation within 24 hours of admission. However, these measures correlate only weakly with more important outcomes, such as 30-day mortality (2, 3).
In 2008, the Centers for Medicare & Medicaid Services (CMS) added hospital-level risk-standardized mortality to its Hospital Compare Web site (4). These rates reflect adjustment for patient age, sex, and comorbid conditions, and mortality estimates from the administrative prediction model have been shown to correlate well with mortality as measured by reviews of clinical records (5). Beginning in 2012, under value-based purchasing, hospital reimbursement became partly tied to 30-day mortality rates (6).
To estimate hospital 30-day risk-standardized mortality rates, CMS includes only patients with a principal diagnosis of pneumonia. The principal diagnosis is defined in the Uniform Hospital Discharge Data Set as “that condition established after study to be chiefly responsible for occasioning the admission of the patient to the hospital for care” (7). Patients who are assigned pneumonia as a secondary diagnosis are excluded because, in these cases, pneumonia may represent a complication of hospitalization rather than the reason for admission (5).
However, many patients with pneumonia, especially the sicker ones, may also have sepsis or respiratory failure, the definitions of which are subject to interpretation. For example, CMS official coding guidelines recognize 2 or more of the following as indicative of the systemic inflammatory response syndrome: a temperature more than 101 °F or less than 96.8 °F, heart rate greater than 90 beats/min, respiratory rate greater than 20 breaths/min, or leukocyte count greater than 12 × 109 cells/L or less than 4 × 109 cells/L or with greater than 10% bands. Taken together with a source of infection, such as pneumonia, these signs fulfill the definition of sepsis (8).
We have previously reported that the recent decrease in the mortality rate of patients hospitalized with pneumonia may be an artifact of the changing use of these codes, whereby the sickest patients have, over time, increasingly received a principal diagnosis of sepsis or respiratory failure. Thus, these patients are not considered in the measure of pneumonia mortality (9). Just as changes in coding over time could lead to erroneous conclusions about decreasing mortality rates, variation in coding across hospitals could lead to biased estimates of relative mortality rates.
We hypothesized that hospitals would vary in their threshold for applying the sepsis and respiratory failure codes and that those that apply these principal diagnoses more frequently would seem to have a lower pneumonia mortality rate than similar hospitals that applied the codes less frequently. On its Hospital Compare Web site and for reimbursement purposes, CMS does not emphasize the mortality rates of individual hospitals but identifies each hospital as better, worse, or no different from the national average. We therefore examined changes in hospital outlier status that would result from inclusion or exclusion of patients with a secondary diagnosis of pneumonia but a principal diagnosis of sepsis or respiratory failure in a large and diverse group of U.S. hospitals.
Methods
Setting and Participants
We included all hospitals that participated in Premier’s Perspective database between 1 July 2007 and 30 June 2010. Perspective, an administrative database used to measure quality and resource utilization, has been used extensively for quality of care and comparative effectiveness research (10, 11). Participating hospitals represent all regions of the United States and include teaching and nonteaching hospitals of various sizes located in urban or rural settings. They are generally similar to U.S. hospitals as a whole, although the data set is weighted more heavily in the South, urban locations, and teaching hospitals. Available data elements for each patient include sociodemographic information; International Classification of Diseases, Ninth Revision, Clinical Modification, diagnosis and procedure codes; and date-stamped charges for all tests and treatments done during hospitalization. The institutional review board at Baystate Medical Center (Springfield, Massachusetts) determined that the study did not constitute human subjects research.
We included all patients aged 18 years or older with a diagnosis of pneumonia (principal or secondary if paired with a principal diagnosis of sepsis or respiratory failure) (Appendix Table, available at www.annals.org). We excluded patients with pneumonia marked as not present on admission. In addition, all patients had to have a chest radiograph and receive antibiotic therapy within 48 hours of admission. To ensure a stable mortality estimate, we excluded hospitals with fewer than 100 admissions during the study period.
Outcomes
Our primary outcome was the hospital risk-standardized mortality rate. For each admission, we identified patient age and sex. To be consistent with the approach used by CMS in calculating a hospital’s risk-standardized mortality rate, we did not include race, marital status, or insurance type. CMS also adjusts for preexisting comorbid conditions by using the hierarchical condition categories. The CMS risk-adjustment model for hierarchical condition categories does not include comorbid conditions that may represent complications of care (5). This model requires information about outpatient diagnoses in the previous year, which is not available in Premier’s Perspective database.
Taking a similar approach, we identified comorbid conditions by using software provided by the Agency for Healthcare Research and Quality. This model, based on the work of Elixhauser and colleagues (12), uses International Classification of Diseases, Ninth Revision, Clinical Modification codes to identify relevant comorbid conditions while excluding complications or other diagnoses related to the principal diagnosis. Both models have acceptable C statistics for predicting mortality, although the hierarchical condition categories model may have better discrimination (13).
Statistical Analysis
We examined associations of patient and hospital characteristics with principal diagnosis coding (pneumonia vs. sepsis or respiratory failure) by using generalized estimating equations models with a logit link (SAS PROC GENMOD), accounting for clustering of patients within hospitals. To see whether severity of illness varied as the proportion of sepsis or respiratory failure coding increased, we evaluated Spearman correlations of hospital proportion of patients with a diagnosis of sepsis or respiratory failure with mortality rates, as well as with rates of early initiation (hospital day 1 or 2) of mechanical ventilation or vasopressors and admission to the intensive care unit of patients with these principal diagnoses. To assess for nonlinear correlations, we stratified hospitals above and below the median proportion.
Following the process that CMS uses to evaluate hospital outcomes (14, 15), we developed multivariable hierarchical generalized linear models by using SAS PROC GLIMMIX with a random effect for hospitals to predict each patient’s probability of mortality on the basis of age, sex, and comorbid conditions (Supplement, available at www.annals.org). We fit 2 models, 1 limited to admissions with pneumonia coded as a principal diagnosis and 1 including all pneumonia admissions. From each model, each hospital’s predicted mortality rate was computed as that which would be anticipated by using the hospital’s random effect, given the patient case-mix. The expected mortality rate was computed as that which would be expected if the same patient mix was treated at an “average” hospital, using the average hospital effect. For each model, a hospital risk-standardized mortality rate was computed as the ratio of predicted to expected mortality standardized by the overall unadjusted mean mortality rate for all admissions in our model.
Next, we used bootstrap methods to develop a 95% CI estimate of risk-standardized mortality for each hospital, for admission with a principal diagnosis of pneumonia, and for all pneumonia admissions. Hospitals were rated as better than average if the interval was entirely below the overall patient mean mortality and worse than average if the interval was entirely above the mean. Hospitals with intervals overlapping the mean were rated as no different than average. Finally, to see the effect of sepsis or respiratory failure coding practices on reported performance, we identified hospitals whose ratings changed when cases of sepsis or respiratory failure were included and compared the change in outlier status across the quintiles of sepsis or respiratory failure coding.
All analyses were done using SAS, version 9.3 (SAS Institute, Cary, North Carolina), and STATA, release 12 (StataCorp, College Station, Texas).
Role of the Funding Source
This study was funded by the Agency for Healthcare Research and Quality. The funding agency had no role in the design, conduct, or analysis of this study or in the decision to submit the manuscript for publication.
Results
We identified 250 907 admissions from 347 hospitals. After we excluded 18 hospitals that had fewer than 100 cases per hospital, our final data set contained 250 016 admissions. Of these, 177 514 (71.0%) had a principal diagnosis of pneumonia; the rest had a secondary diagnosis of pneumonia with a principal diagnosis of respiratory failure (8.7%) or sepsis (20.3%). The Table shows the patient characteristics.
Table.
Characteristics of Patients With a Principal Diagnosis of Pneumonia or Sepsis/Respiratory Failure
| Characteristic | Principal Diagnosis, n (%) | P Value* | |
|---|---|---|---|
| Pneumonia | Sepsis/Respiratory Failure | ||
| Median age, y† | 72 (57–82) | 71 (59–81) | 0.005 |
| Sex | <0.001 | ||
| Male | 81 639 (46.0) | 35 136 (48.5) | |
| Female | 95 875 (54.0) | 37 366 (51.5) | |
| Comorbid conditions | |||
| Congestive heart failure | 33 115 (18.7) | 16 191 (22.3) | <0.001 |
| Valvular disease | 11 295 (6.4) | 4451 (6.1) | 0.35 |
| Pulmonary circulation disease | 7874 (4.4) | 4419 (6.1) | <0.001 |
| Peripheral vascular disease | 9829 (5.5) | 4295 (5.9) | 0.060 |
| Hypertension | 88 085 (49.6) | 28 081 (38.7) | <0.001 |
| Paralysis | 4110 (2.3) | 2474 (3.4) | <0.001 |
| Other neurologic disorders | 17 812 (10.0) | 7741 (10.7) | 0.002 |
| Chronic pulmonary disease | 85 491 (48.2) | 35 974 (49.6) | 0.020 |
| Diabetes | 43 101 (24.3) | 16 391 (22.6) | <0.001 |
| Hypothyroidism | 21 726 (12.2) | 7005 (9.7) | <0.001 |
| Liver disease | 3215 (1.8) | 1679 (2.3) | <0.001 |
| Ulcer | 38 (0.02) | 14 (0.02) | 0.98 |
| AIDS | 17 (0.01) | 51 (0.07) | <0.001 |
| Lymphoma | 2304 (1.3) | 843 (1.2) | 0.070 |
| Metastatic cancer | 4489 (2.5) | 2160 (3.0) | <0.001 |
| Solid tumor without metastasis | 5091 (2.9) | 1895 (2.6) | <0.001 |
| Rheumatoid arthritis/collagen vascular disease | 6063 (3.4) | 1930 (2.7) | <0.001 |
| Obesity | 14 488 (8.2) | 5827 (8.0) | 0.030 |
| Weight loss | 8781 (5.0) | 7058 (9.7) | <0.001 |
| Chronic blood loss anemia | 1011 (0.6) | 631 (0.9) | <0.001 |
| Deficiency anemias | 38 830 (21.7) | 16 721 (23.1) | 0.020 |
| Alcohol abuse | 4231 (2.4) | 2006 (2.8) | 0.001 |
| Drug abuse | 3389 (1.9) | 1358 (1.9) | 0.120 |
| Psychoses | 7212 (4.1) | 2822 (3.9) | 0.004 |
| Depression | 19 842 (11.2) | 6289 (8.7) | <0.001 |
| Renal failure | 21 552 (12.1) | 10 443 (14.4) | <0.001 |
| Hospital characteristics | |||
| Bed size | <0.001 | ||
| ≤200 beds | 37 290 (21.0) | 11 919 (16.4) | |
| 201–400 beds | 68 349 (38.5) | 29 298 (40.4) | |
| ≥401 beds | 71 875 (40.5) | 31 285 (43.2) | |
| Rural/urban status | <0.001 | ||
| Rural | 24 936 (14.1) | 7914 (10.9) | |
| Urban | 152 578 (86.0) | 64 588 (89.1) | |
| Teaching status | 0.090 | ||
| Teaching | 60 342 (34.0) | 26 393 (36.4) | |
| Nonteaching | 117 172 (66.0) | 46 109 (63.6) | |
| Region | 0.140 | ||
| Northeast | 30 949 (17.4) | 9900 (13.7) | |
| Midwest | 37 967 (21.4) | 17 281 (23.8) | |
| West | 29 299 (16.5) | 13 036 (18.0) | |
| South | 79 299 (44.7) | 32 285 (44.5) | |
| In-hospital mortality | 6297 (3.6) | 11 742 (16.2) | <0.001 |
| Total | 177 514 (71.0) | 72 502 (29.0) | |
Accounting for clustering of patients within hospitals.
Values in parentheses are 25th, 75th percentiles.
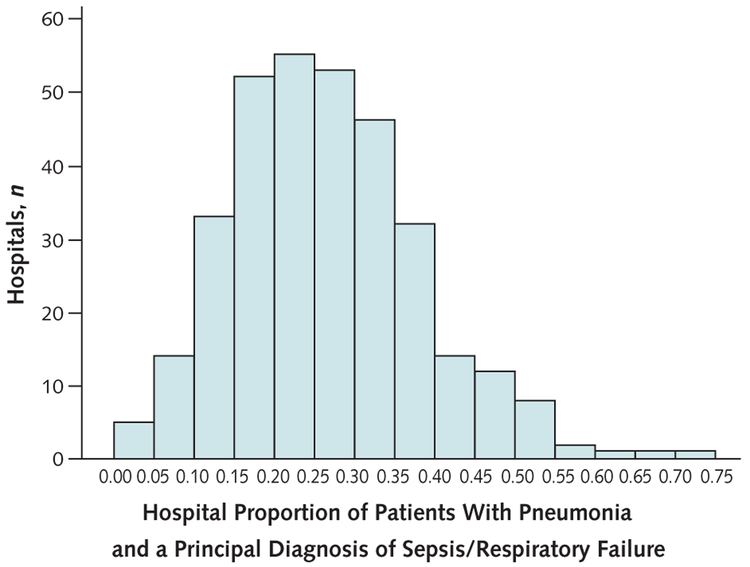
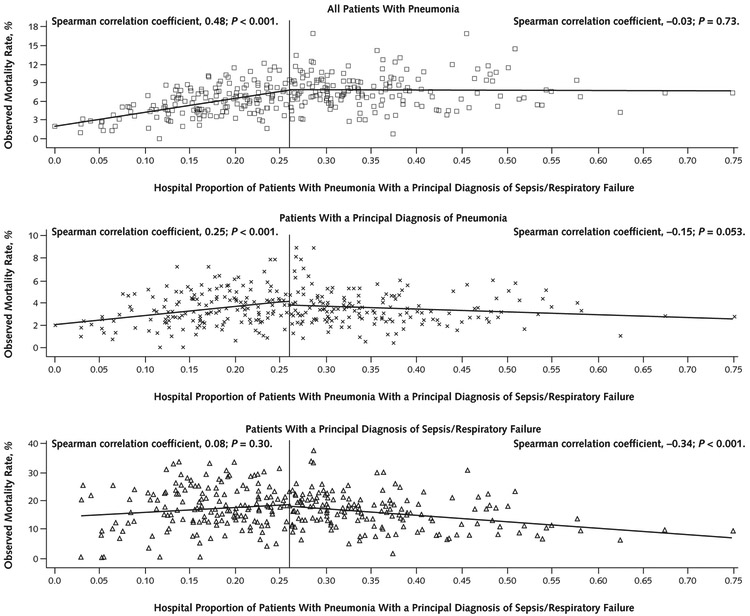
Across the included hospitals, the proportion of patients with pneumonia who received a principal diagnosis of sepsis or respiratory failure varied from 0.00 to 0.75 (median, 0.26 [interquartile range {IQR}, 0.18 to 0.34]) (Figure 1). Observed mortality rates for all patients with pneumonia at individual hospitals ranged from 0.0% to 16.9% (median, 6.8% [IQR, 4.9% to 8.5%]). The median hospital mortality rate was 3.3% (IQR, 2.5% to 4.4%) for patients with a principal diagnosis of pneumonia and 16.4% (IQR, 12.2% to 21.0%) for patients with pneumonia with a principal diagnosis of sepsis or respiratory failure. Figure 2 shows the association of in-hospital mortality rates with the proportion of a hospital’s patients with pneumonia who received a code of sepsis or respiratory failure stratified at the median.
Figure 1. Variation in hospital rate of coding a principal diagnosis of sepsis/respiratory failure among patients with pneumonia.
Figure 2. Hospital pneumonia mortality rates and proportion of pneumonia cases with a principal diagnosis of sepsis/respiratory failure.
Hospitals are divided into 2 equal groups at the median proportion of sepsis/respiratory failure cases.
As the proportion of patients coded with sepsis or respiratory failure increased, the overall pneumonia mortality rate initially increased, reflecting that the hospital had more septic patients. However, among the 50% of hospitals with the highest proportions of sepsis or respiratory failure, a higher proportion of sepsis or respiratory failure was not associated with higher mortality (Spearman r = −0.03; P = 0.73). In contrast, for these same hospitals a higher proportion of sepsis or respiratory failure was associated with decreased mortality for the subset of patients with a principal diagnosis of pneumonia (Spearman r = −0.15; P = 0.053) and those with a principal diagnosis of sepsis or respiratory failure (Spearman r = −0.34; P < 0.001).
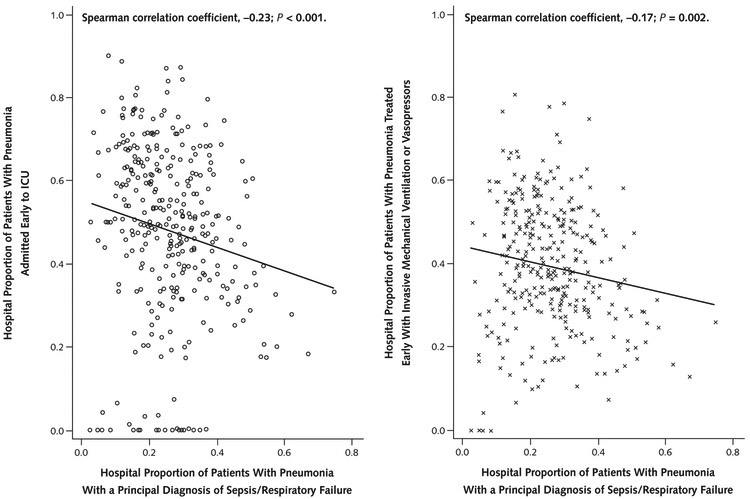
To determine whether this finding was due to a lower threshold to code sepsis or respiratory failure at hospitals with a higher proportion of cases designated as these diagnoses or improved quality of care at these institutions, we examined the association of rates of early use of vasopressors or mechanical ventilation and initial admission to the intensive care unit among patients with these diagnoses with the proportion of cases coded as these diagnoses (Figure 3). Both rates tended to decrease with an increasing proportion of sepsis or respiratory failure coding, suggesting that, at these hospitals, the codes were used more often among patients who were, on average, less sick on admission.
Figure 3. Early admission to the ICU or treatment with invasive mechanical ventilation or vasopressors among patients with pneumonia and a principal diagnosis of sepsis/respiratory failure.
ICU = intensive care unit.
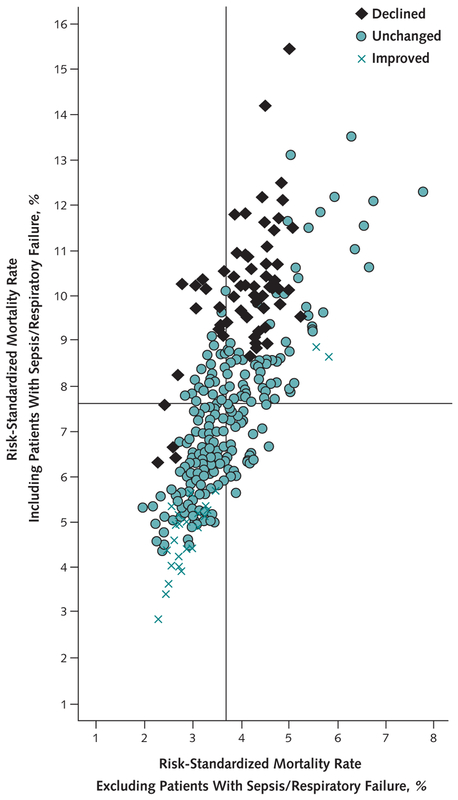
Figure 4 shows the correlation between hospital risk-standardized mortality rates when the case definition for pneumonia was limited to those with a principal diagnosis of pneumonia (x-axis) compared with a definition that included patients with sepsis or respiratory failure (y-axis). When cases of sepsis or respiratory failure were included, the outlier status did not change in 236 hospitals (71.7% of the total), improved in 32 hospitals, and declined in 61 hospitals.
Figure 4. Risk-standardized mortality rates for each hospital including or excluding patients with a principal diagnosis of sepsis/respiratory failure.
The vertical line represents the mean risk-standardized mortality rate excluding sepsis/respiratory failure. The horizontal line represents the mean risk-standardized mortality rate including sepsis/respiratory failure. Including patients with sepsis/respiratory failure causes a hospital’s outlier status to improve, worsen, or remain the same.
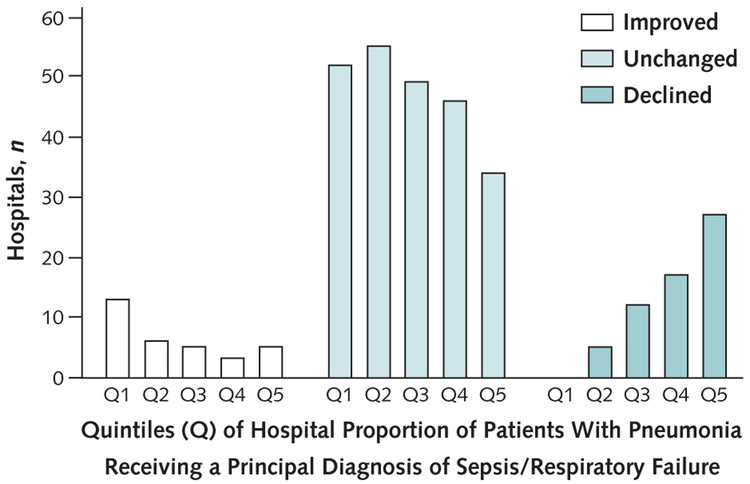
Figure 5 shows changes in outlier status according to quintile of sepsis or respiratory failure coding. In the quintile of hospitals with the highest proportion of patients coded with a principal diagnosis of sepsis or respiratory failure, 7.6% improved outlier status under the broader definition and 40.9% worsened. In the quintile with the lowest proportion, 20.0% improved and none worsened.
Figure 5. Number of hospitals whose performance improved, remained the same, or declined when sepsis/respiratory failure cases were included in the definition of pneumonia.
Discussion
As the U.S. government tries to foster value-based care, CMS reimbursement strategies increasingly incorporate financial incentives tied to hospital performance. The value-based purchasing component of the Patient Protection and Affordable Care Act uses 30-day risk-standardized mortality rates for patients diagnosed with pneumonia as 1 criterion for rewarding or penalizing hospitals (6). In this study of 329 U.S. hospitals, we found that when risk-standardized mortality rates for patients with pneumonia were used to evaluate and compare the outcomes of hospitals, the approach to case definition substantially affected hospital ratings.
Specifically, we found that whether patients with pneumonia and a principal diagnosis of sepsis or respiratory failure were included led to a change in the performance ranking of 28.3% of the hospitals studied. The risk-standardized mortality rate tended to increase when sepsis or respiratory failure was included in a broader definition of pneumonia in hospitals that assigned these codes to a greater proportion of patients and to decrease in hospitals that applied the codes to a smaller proportion of cases.
Recently, we reported on the association between temporal trends in the use of sepsis and respiratory failure codes and pneumonia mortality in the Nationwide Inpatient Sample (9). We found that, over time, cases with principal diagnoses of sepsis and, to a lesser extent, respiratory failure increased, whereas cases with a principal diagnosis of pneumonia decreased. In essence, the sickest of those patients who historically had been given a principal diagnosis of pneumonia were increasingly being given a diagnosis of sepsis or respiratory failure, decreasing the average mortality in the pneumonia group (because the sickest patients had been removed) and the sepsis or respiratory failure group (because the newly added patients were less sick than the average patient with sepsis or respiratory failure). These events gave the false impression that pneumonia outcomes had improved more than they had.
This study extends those findings by showing that, when the case definition of pneumonia was restricted to patients with a principal diagnosis of pneumonia, differences in hospital coding practices (specifically the application of sepsis and respiratory failure codes as the principal diagnosis) may bias hospital performance measurement efforts, resulting in 28.3% of hospitals being misclassified. This observation suggests that using a broader case definition might improve the validity of the public reporting of pneumonia mortality as currently practiced on Hospital Compare and elsewhere.
Could hospitals that assigned the principal diagnosis of sepsis or respiratory failure to a higher percentage of patients have been justified because their patients were sicker? This is unlikely for 2 reasons. If all hospitals used the same coding practices and treated patients in a similar fashion, as the proportion of diagnoses of sepsis or respiratory failure increased overall mortality should have increased, reflecting the fact that a larger proportion of the patients were sicker. At the same time, mortality within the specific diagnoses should have remained constant.
We observed this pattern among the 50% of hospitals with the lowest proportion of patients coded with a principal diagnosis of sepsis or respiratory failure. However, among the 50% of hospitals with the highest proportion of patients coded with these diagnoses, the overall mortality did not increase as the proportion of patients coded with these diagnoses increased, whereas the mortality for patients with a principal diagnosis of pneumonia and of sepsis or respiratory failure actually decreased. This finding suggests that, in these hospitals, patients that might have received a principal diagnosis of pneumonia elsewhere were instead being coded with a principal diagnosis of sepsis or respiratory failure and a secondary diagnosis of pneumonia. This is further supported by the fact that patients with a principal diagnosis of sepsis or respiratory failure at these hospitals were the least likely to be admitted to an intensive care unit or receive vasopressors or mechanical ventilation within the first 2 hospital days.
This increase in principal diagnoses of sepsis and respiratory failure is not surprising. Reimbursement for either diagnosis is substantially higher than that for pneumonia. Because many patients with pneumonia fit the definition of the systemic inflammatory response syndrome on the basis of vital signs or laboratory values, hospitals that wish to maximize reimbursement should use these codes aggressively (16). In addition, national quality improvement initiatives, such as the Surviving Sepsis Campaign, have raised provider awareness about sepsis and may have led to changes in documentation.
One of the goals of measuring risk-standardized mortality is to enable patients and payers to identify hospitals that are better or worse than average. Our findings suggest that altering the definition of pneumonia may represent an opportunity to improve discrimination. When we restricted our analysis to patients with a principal diagnosis of pneumonia, 4.3% of hospitals were statistically better than the mean and 6.4% were statistically worse.
These rates are similar to the percentage of hospitals considered outliers on the CMS Web site Hospital Compare, which reports 30-day mortality rates. On this site, 4% of hospitals are considered better than the national mean and 5% are considered worse. However, when we included all pneumonia cases, 11.9% were considered statistically better than the mean and 22.8% were considered statistically worse.
The reasons for this difference may be the larger number of patients when sepsis or respiratory failure is included and that variation in the quality of care for the sickest patients may have the greatest effect on mortality. However, it could also be due to the wide variation in acuity of patients receiving a principal diagnosis of sepsis or respiratory failure. Because our adjustment method, like that of CMS, did not account for acuity of illness beyond existing comorbid conditions, it may unfairly penalize hospitals whose patients present with more severe illness. In fact, a recent study of stroke mortality showed that, without adjustment for stroke severity, 26% of hospitals could be misclassified with regard to whether they differed from the mean (17).
Patients with a principal diagnosis of sepsis or respiratory failure are included in the current specifications manual for CMS process measures of pneumonia quality (for example, the current specifications manual includes the percentage of patients whose antibiotics were administered within 6 hours of arrival). This is possible because adherence to process measures is assessed through manual review of medical records, whereas outcome measures are calculated using only claims data. In the latter context, including patients with a secondary diagnosis of pneumonia may inadvertently include those in whom pneumonia represented a complication of hospitalization rather than the reason for admission. Including such patients would also probably increase a hospital’s risk-standardized mortality rate because the outcomes of such patients are generally worse than average. Present-on-admission codes, which were not available when the current pneumonia measures were developed, could help to overcome this problem. However, early experience showed only moderate accuracy of these codes for secondary diagnoses (18). Before such a change could be implemented on a national level, validation studies would be necessary.
Our study has limitations. First, although we included a large sample of U.S. hospitals, our results may not reflect nationwide coding practices. Second, we examined only in-hospital mortality, which others have found does not correlate perfectly with the 30-day mortality reported on Hospital Compare (19). In fact, use of inpatient mortality introduces its own set of biases related to variations in length of stay (15). Nevertheless, the coding issues that we have identified will probably also affect 30-day mortality rates. Third, our method for estimating risk-standardized mortality rates was not identical to that used by CMS. We did not have access to diagnoses recorded during prior encounters. Even so, we adjusted for a similar number of comorbid conditions and our model performed slightly better than that used by CMS.
Public profiling of hospital performance represents 1 step toward improving the value of care delivered to patients. Even if patients do not generally make decisions on the basis of hospital performance, hospital administrators and boards take the information seriously and the media may punish outliers (20). Value-based care initiatives may potentiate these effects.
Efforts to broaden the scope of hospital performance measures from the initial set of measures based on processes to those focused on patient outcomes are laudable, but caution is required. Misclassification could harm individual hospitals and weaken confidence in public reporting. Our analysis reveals 1 important way that hospitals could be misclassified. The solution may be as simple as a change in case definition, but further study is needed to validate alternative approaches to cohort selection and identify whether other conditions may be subject to similar biases related to coding practices.
Supplementary Material
Context
Hospital risk-standardized mortality rates for pneumonia are publicly reported but exclude more severe cases of pneumonia, which are coded as sepsis or respiratory failure with pneumonia as a secondary diagnosis.
Contribution
A sample of U.S. hospitals varied widely in the proportion of all pneumonia cases coded as sepsis or respiratory failure with a secondary diagnosis of pneumonia, even after adjustment for indicators of disease severity.
Caution
Sampled hospitals may not be fully representative of all U.S. hospitals.
Implication
Variation among hospitals in risk-standardized rates of mortality from pneumonia may be related to variation in coding practices rather than the quality of care delivered.
Acknowledgments
Grant Support: By the Agency for Healthcare Research and Quality (R01HS018723).
Appendix
Appendix Table.
ICD-9-CM Codes Used in the Definition of Pneumonia, Sepsis, and Respiratory Failure
| ICD-9-CM Code | Description |
|---|---|
| Pneumonia | |
| 481 | Pneumococcal pneumonia (Streptococcus pneumoniae pneumonia) |
| 482 | Pneumonia due to Klebsiella pneumoniae |
| 482.1 | Pneumonia due to Pseudomonas |
| 482.2 | Pneumonia due to Haemophilus influenzae |
| 482.3 | Pneumonia due to streptococcus unspecified |
| 482.31 | Pneumonia due to group A streptococcus |
| 482.32 | Pneumonia due to group B streptococcus |
| 482.39 | Pneumonia due to other streptococcus |
| 482.4 | Pneumonia due to staphylococcus unspecified |
| 482.41 | Pneumonia due to Staphylococcus aureus |
| 482.42 | Methicillin-resistant pneumonia due to Staphylococcus aureus |
| 482.49 | Other staphylococcus pneumonia |
| 482.81 | Pneumonia due to anaerobes |
| 482.82 | Pneumonia due to Escherichia coli |
| 482.83 | Pneumonia due to other gram-negative bacteria |
| 482.84 | Pneumonia due to Legionnaires disease |
| 482.89 | Pneumonia due to other specified bacteria |
| 482.9 | Bacterial pneumonia unspecified |
| 483.1 | Pneumonia due to chlamydia |
| 483.8 | Pneumonia due to other specified organism |
| 484.8 | Pneumonia with other infectious diseases classified elsewhere |
| 485 | Bronchopneumonia organism unspecified |
| 486 | Pneumonia organism unspecified |
| 487.0 | Influenza with pneumonia |
| 487.1 | Influenza with respiratory manifestations |
| 487.8 | Influenza with other manifestations |
| 488.0 | Influenza due to identified avian influenza virus |
| 488.1 | Influenza due to H1N1 influenza virus identified in 2009 |
| Sepsis | |
| 038.0 | Streptococcal septicemia |
| 038.10 | Staphylococcal septicemia, unspecified |
| 038.11 | Methicillin-susceptible Staphylococcus aureus septicemia |
| 038.12 | Methicillin-resistant Staphylococcus aureus septicemia |
| 038.19 | Other staphylococcal septicemia |
| 038.2 | Pneumococcal septicemia (Streptococcus pneumoniae septicemia) |
| 038.3 | Septicemia due to anaerobes |
| 038.40 | Gram-negative organism unspecified |
| 038.41 | Haemophilus influenzae |
| 038.42 | Escherichia coli |
| 038.43 | Pseudomonas |
| 038.44 | Serratia |
| 038.49 | Other |
| 038.8 | Other specified septicemias |
| 038.9 | Unspecified septicemia |
| 785.52 | Septic shock |
| 790.7 | Bacteremia |
| 995.91 | Sepsis |
| 995.92 | Severe sepsis |
| Respiratory failure | |
| 518.81 | Acute respiratory failure |
| 518.82 | Other pulmonary insufficiency, not elsewhere classified |
| 518.84 | Acute and chronic respiratory failure |
| 799.1 | Respiratory arrest |
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
Footnotes
From Medicine Institute, Cleveland Clinic, Cleveland, Ohio; Center for Quality of Care Research, Baystate Medical Center, Springfield, Massachusetts; Tufts University School of Medicine, Boston, Massachusetts; and University of Massachusetts, Amherst, Massachusetts.
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M13-1419.
Reproducible Research Statement: Study protocol: Available from Dr. Rothberg (rothbem@ccf.org). Statistical code: Available from Dr. Pekow (penelope.pekow@bhs.org). Data set: Not available.
Current author addresses and author contributions are available at www.annals.org.
Contributor Information
Michael B. Rothberg, Department of Medicine, Medicine Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195..
Penelope S. Pekow, Center for Quality of Care Research, Baystate Medical Center, 280 Chestnut Street, Third Floor, Springfield, MA 01199..
Aruna Priya, Center for Quality of Care Research, Baystate Medical Center, 280 Chestnut Street, Third Floor, Springfield, MA 01199..
Peter K. Lindenauer, Center for Quality of Care Research, Baystate Medical Center, 280 Chestnut Street, Third Floor, Springfield, MA 01199..
References
- 1.Elixhauser A, Owens P . Reasons for Being Admitted to the Hospital through the Emergency Department, 2003 Healthcare Cost and Utilization Project Statistical Brief 2. Rockville, MD: Agency for Healthcare Research and Quality; 2006. Accessed at www.hcup-us.ahrq.gov/reports/statbriefs/sb2.pdf on 19 August 2013. [PubMed] [Google Scholar]
- 2.Werner RM, Bradlow ET. Relationship between Medicare’s hospital compare performance measures and mortality rates. JAMA. 2006;296:2694–702. [PMID: 17164455] [DOI] [PubMed] [Google Scholar]
- 3.Ryan AM, Burgess JF Jr, Tompkins CP, Wallack SS. The relationship between Medicare’s process of care quality measures and mortality. Inquiry. 2009; 46:274–90. [PMID: 19938724] [DOI] [PubMed] [Google Scholar]
- 4.Centers for Medicare & Medicaid Services. 30-Day Death and Readmission Measures. 2012. Accessed at https://data.medicare.gov/Hospital-Compare/Hospital-Outcome-Of-Care-Measures/rcw8-6swd on 19 August 2013.
- 5.Bratzler DW, Normand SL, Wang Y, O’Donnell WJ, Metersky M, Han LF, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLoS One. 2011;6:e17401. [PMID: 21532758] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kupfer JM. The morality of using mortality as a financial incentive: unintended consequences and implications for acute hospital care. JAMA. 2013;309: 2213–4. [PMID: 23736729] [DOI] [PubMed] [Google Scholar]
- 7.Health information policy council; 1984 revision of the Uniform Hospital Discharge Data Set—HHS. Notice. Fed Regist 1985;50:31038–40. [PMID: 10272121] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. ICD-9-CM Official Guidelines for Coding and Reporting. 2011. Accessed at www.cdc.gov/nchs/data/icd/icd9cm_guidelines_2011.pdf on 20 August 2013.
- 9.Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003-2009. JAMA. 2012;307:1405–13. [PMID: 22474204] [DOI] [PubMed] [Google Scholar]
- 10.Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144:894–903. [PMID: 16785478] [DOI] [PubMed] [Google Scholar]
- 11.Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035–42. [PMID: 20501925] [DOI] [PubMed] [Google Scholar]
- 12.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [PMID: 9431328] [DOI] [PubMed] [Google Scholar]
- 13.Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10:245. [PMID: 20727154] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krumholz H, Normand S, Galusha D, Mattera J, Rich A, Wang Y, et al. Risk-adjustment Models for AMI and HF 30-Day Mortality. 2007. Accessed at www.qualitynet.org/dcs/BlobServer?blobkey=id&blobnocache=tme&blobwhere=1228861777994&blobheader=multipart%2Foctet-stream&blobheadername1=Content-Disposition&blobheadervalue1=attachment%3Bfilename%3DYale_AMI-HF_Report_7-13-05%2C0.pdf&blobcol=urldata&blobtable=MungoBlobs on 20 August 2013.
- 15.Drye EE, Normand SL, Wang Y, Ross JS, Schreiner GC, Han L, et al. Comparison of hospital risk-standardized mortality rates calculated by using in-hospital and 30-day models: an observational study with implications for hospital profiling. Ann Intern Med. 2012;156:19–26. [PMID: 22213491] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahn AC. Shore up your clinical documentation. Strategies to improve reimbursements through more precise and specific inpatient documentation. Med Econ. 2011;88:26–8. [PMID: 21800510] [PubMed] [Google Scholar]
- 17.Fonarow GC, Pan W, Saver JL, Smith EE, Reeves MJ, Broderick JP, et al. Comparison of 30-day mortality models for profiling hospital performance in acute ischemic stroke with vs without adjustment for stroke severity. JAMA. 2012;308:257–64. [PMID: 22797643] [DOI] [PubMed] [Google Scholar]
- 18.Goldman LE, Chu PW, Osmond D, Bindman A. The accuracy of present-on-admission reporting in administrative data. Health Serv Res. 2011;46:1946–62. [PMID: 22092023] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borzecki AM, Christiansen CL, Chew P, Loveland S, Rosen AK. Comparison of in-hospital versus 30-day mortality assessments for selected medical conditions. Med Care. 2010;48:1117–21. [PMID: 20978451] [DOI] [PubMed] [Google Scholar]
- 20.Ettinger WH, Hylka SM, Phillips RA, Harrison LH Jr, Cyr JA, Sussman AJ. When things go wrong: the impact of being a statistical outlier in publicly reported coronary artery bypass graft surgery mortality data. Am J Med Qual. 2008;23:90–5. [PMID: 18245577] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.