Abstract
Purpose of Review:
The present review highlights regenerative electrical stimulation (RES) as a potential future treatment options for patients with nerve injuries leading to urological dysfunction, such as urinary incontinence, voiding dysfunction or erectile dysfunction. Additionally, it will highlight highlights the mechanism of nerve injury and regeneration as well as similarities and differences between RES and current electrical stimulation treatments in urology, functional electrical stimulation (FES) and neuromodulation.
Recent Findings:
It has been demonstrated that RES upregulates brain derived neurotrophic factor (BDNF) and its receptor to facilitate neuroregeneration, facilitating accurate reinnervation of muscles by motoneurons. Further, RES upregulates growth factors in glial cells. Within the past two years, RES of the pudendal nerve upregulated BDNF in Onuf’s nucleus, the cell bodies of motoneurons that course through the pudendal nerve and accelerated functional recovery in an animal model of stress urinary incontinence. Additionally, ES of the vaginal tissue in an animal model of stress urinary incontinence accelerated functional recovery.
Summary:
RES has great potential but future research is needed to expand the potential beneficial effects of RES in the field of urology.
Keywords: electrical stimulation, pudendal nerve, urinary incontinence, voiding dysfunction, erectile dysfunction
Introduction
Control of the lower urinary and reproductive tract is maintained by a complex network of sympathetic, parasympathetic and somatic nervous systems. Quality of life is greatly diminished when a nerve is damaged in any one of these three systems resulting in incontinence, voiding dysfunction or erectile dysfunction [1–3]. Nerve injury can be from trauma, such as that occurs in automobile accidents, gunshot wounds, chronic exposure to nerve compression, during childbirth, or from iatrogenic causes, such as surgery [4,5]. Electrical stimulation (ES) can be used to restore function, as in functional ES (FES), modulate nervous system responses to stimuli, as by neuromodulation, or accelerate regeneration of injured peripheral or central nerves by regenerative ES (RES).
FES uses electrical stimuli of nerves to contract muscles for neurogenic lower urinary tract dysfunction, while neuromodulation uses electrical stimuli to alter neurotransmission to treat both neurogenic and non-neurogenic (i.e. myogenic) conditions [6,7]. Both have profoundly improved the understanding of neuromuscular physiology of the lower urinary tract [7,8].
RES, in contrast, promotes nerve sprouting, neuroregeneration, reinnervation of target organs, and functional recovery after nerve injury [9–14]. RES upregulates brain derived neurotrophic factor (BDNF) in motoneurons, which in turn upregulates regeneration associated genes, providing possibilities for therapy of neurogenic lower urinary tract dysfunction due to peripheral or central nerve injury, as well as other urological conditions that can result from nerve injury, such as erectile dysfunction, neurogenic bladder, and underactive bladder [15]. In this review we highlight the mechanism of nerve injury and regeneration as well as that of RES as it relates to treatment of nerve injuries in urology and highlight similarities between RES, FES and neuromodulation.
Nerve Injury
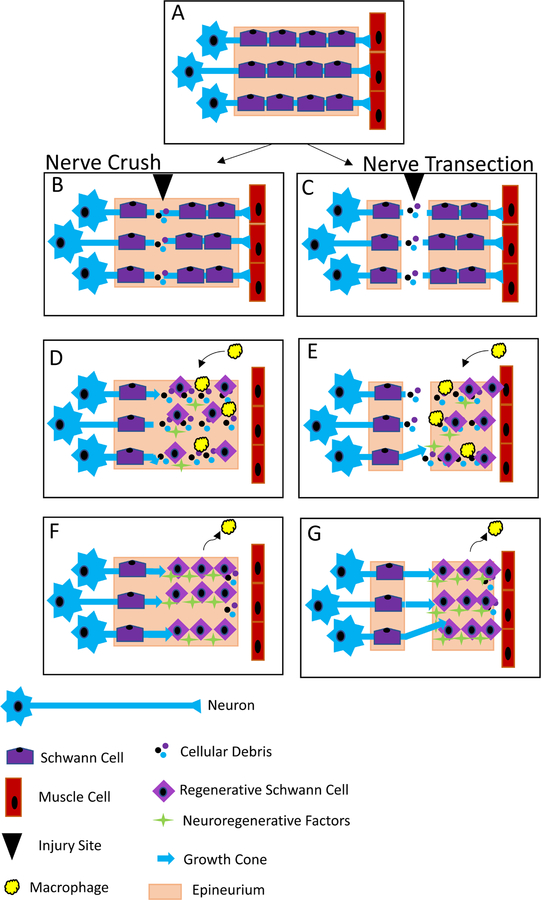
After Sunderland type II - V nerve injuries, in which the nerve is crushed or transected, the proximal nerve stump will degrade back to the first node of Ranvier while the distal nerve undergoes Wallerian degeneration (Figure 1) [16]. Within a few hours of injury, calcium ions enter the axon, activating proteases to degraded the nerve stump [17]. Myelin, myelin-associated glycoproteins (MAGs) and cellular debris are taken up by Schwann cells and macrophages; clearance of these proteins is essential for neuroregeneration [18]. MAGs inhibit proximal axon growth cones from crossing the injury site, causing delayed or staggered axon regeneration. In the central nervous system after nerve injury, MAGs and other inhibitor signals create an inhibitory environment at the injury site, preventing regeneration [19]. Peripherally, Schwann cells switch to a regenerative phenotype and start to produce pro-inflammatory cytokines and chemokines within 5 hours of injury, stimulating circulating macrophages to enter the nerve [20]. Additionally, Schwann cells will re-enter the cell cycle, dedifferentiate and line the inner surface of the endoneurium to form the band of Büngner to aid in regeneration and guide regenerating axons through the distal nerve stump [18]. Neuroregeneration requires the growth cone to follow a gradient of neurotrophic factors down the endoneurium provided by the Schwann cells of the bands of Büngner.
Figure 1. Mechanism of Nerve Injury and Neuroregeneration.
An uninjured peripheral nerve has motoneurons innervating muscle cells surrounded by Schwann cells in an intact epineurium (A). After a crush injury that does not damage the epineurium (B). After a transection injury that damages in which the epineurium is damaged (C). Regardless of the type of injury, Schwann cells switch to a regenerative phenotype and macrophages migrate into the nerve to clear cellular debris, while growth cones start to grow through the injury site (D & E). Later in the process of neuroregeneration, Schwann cells have formed bands of Büngner, while marcophages leave the nerve, and growth cones travel towards the target muscle (F & G).
Nerve Regeneration
After a nerve injury, neurons change their gene expression from an excitatory to a regenerative phenotype, decreasing production of neurofilaments and neurotransmitters and increasing tubulin and growth factors, including BDNF [18]. Several transcription factors are activated: signal transducer and activator of transcription 3 (STAT3) and transcription factors 2 and 3. Both of these in turn upregulate regeneration associated genes: neurotrophin factors, their receptors, tubulin, actin, growth associated protein 43 (GAP43), CAP23 and SCG10 [18,21]. Central nervous system neurons have a reduced regenerative capability which, when combined with the inhibitory environment at the injury site, prevents regeneration, explaining the difference in neuroregeneration between the peripheral and central nervous systems [19].
While all Schwann cells produce nerve growth factor (NGF), BDNF, neurotrophin 4/5 (NT4), glial derived neurotrophic factor (GDNF), insulin-like growth factor I and II, vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF) and pleiotrophin are produced upregulated during regeneration; some of these proteins are highly expressed only in Schwann cells associated with either sensory or motor neurons allowing for accurate reinnervation of distal targets [18,22–24]. Upregulation of neurotrophins in neurons and Schwann cells is temporary and declines to baseline within 30 days, indicating a short window for regeneration and suggesting that any therapy to increase regeneration must be given as soon as possible after injury [18,25].
To that end, several groups are investigating enhancing nerve regeneration via stem cells, BDNF, and gene therapy[26–34]. Since cavernous nerve injury during pelvic surgery results in erectile dysfunction in 50% of male patients, this is an active area of neuroregeneration research [35]. While not currently being investigated, these approaches could also be used to prevent underactive bladder symptoms, which can be caused by SCI, pelvic fractures, or nerve injury during pelvic surgery [36]. Any therapy that could increase regeneration associated genes would also help neuroregeneration after injuries or trauma when normal upregulation is either blunted or non-existent, such as occurs in childbirth which results in impaired regeneration of the pudendal nerve and reinnervation of the external urethral sphincter [37,38]. Until these therapies become available to patients, urologists have FES and neuromodulation to alleviate symptoms and treat current patients.
Functional Electrical Stimulation and Neuromodulation in Urology
Spinal cord injury (SCI) eliminates voluntary and supraspinal control of voiding, resulting in neurogenic bladder and urinary retention [39]. FES methods can be used for rehabilitation and management of complications after SCI [6]. Sacral anterior root nerve stimulation (SNS) is performed by placing unilateral percutaneous leads in the S3 foramen, stimulating the anterior roots which activate the parasympathetic efferent pathway and empty the bladder [40]. Other approaches including pudendal nerve stimulation (PNS) which activates afferent nerves and blocks efferent motor nerves to induce voiding by increasing intravesical pressure and sphincter relaxation, with different studies using frequencies ranging from 5–50 Hz, a similar frequency range to RES and neuromodulation studies [7,41].
In the 1970s, researchers experimented with sacral root stimulation in paraplegic patients with urinary incontinence (20Hz, 0.1mS, amplitude not indicated) [8]. These early experiments created the concept of neuromodulation and while the exact mechanism of neuromodulation is not completely understood, two reflexes play key roles in modulating bladder function, the guarding reflex and the bladder afferent loop reflex [42]. The guarding reflex prevents urine leakage from increased abdominal pressure during coughing or laughing. Bladder pain and fullness signals are sent via bladder afferent nerves to the brain through the sacral root, which in turn initiates the micturition reflex [43]. In neurologic or inflammatory bladder disorders, the previously silent C fibers may dominate and trigger the micturition reflex. Hence, treatment with neuromodulation by blockade of this pathway will suppress detrusor overactivity (DO) [44].
Neuromodulation is based on the theory that selective activation of afferent fibers to cause inhibition at the spinal or supraspinal levels [42,45]. Experimental neuromodulation methods include ES of the bladder, pudendal nerve, transcutaneous posterior tibial nerve, dorsal genital nerve, urethral or SNS which use low frequencies (5–30Hz), according to their site of stimulation [45–53]. Interestingly, early sacral neuromodulation after a complete spinal cord injury prevented lower urinary tract dysfunction [53]. A recent rodent study demonstrated similar results with sacral neuromodulation within 7 days of an incomplete SCI could improve bladder function [54]. The stimulation parameters (30 minutes/day, 20 Hz, pulse width of 0.1msec) used in the rodent study are similar to those used in RES studies, suggesting a dual mechanism of both neuromodulation and RES could be working to improve function. Clinical neuromodulation has been approved for both bladder storage dysfunctions, including urgency/frequency and urgency urinary incontinence, and bladder empty dysfunctions, including nonobstructive urinary retention [55,56].
Regenerative Electrical Stimulation (RES)
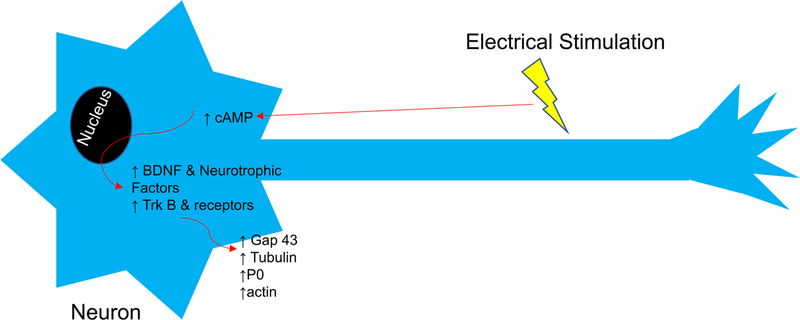
While it has been known since the 1950s that ES can facilitate increased axonal sprouting, it has only been shown within the last 20 years that ES upregulates BDNF and its receptor tyrosine kinase B (TrkB) in both sensory and motoneurons (Figure 2) [57–59]. BDNF expression via ES is significantly increased compared to injury alone, while transgenic mice experiments have shown that BDNF, NT4 and TrkB expression are vital to ES-induced regeneration [60–62]. BDNF secreted at the growth cone binds to TrkB receptors on the growth cones of either the neuron or neighboring neurons working in a paracrine or autocrine fashion, to facilitate regeneration [63]. While BDNF, TrkB and NT4 involvement in vivo are known, the involvement of other neurotrophins is unclear and warrants further investigation, since in vitro studies of ES on cultured neuronal cell lines has shown an increase in NGF, NT3, VEGF and platelet-derived growth factor (PDGF) expression, suggesting their involvement in RES [64]. RES also upregulates other growth associated genes in neurons: tubulin, actin, myelin protein zero (P0), and Gap-43, which are vital to the nerve regeneration process [58,65,66].
Figure 2. Mechanism of Facilitated Regeneration via Regenerative Electrical Stimulation.
Electrical stimulation of a neuron at the right frequency increases cAMP, which increases expression of brain derived neurotrophic factor (BDNF) and other neurotrophic factors and their receptors, including tyrosine kinase B (Trk B), which in turn increase expression of regeneration associated proteins: growth associated protein 43 (Gap 43), tubulin, actin, and myelin protein zero (P0).
ES causes calcium ions to enter the neuron, increasing cAMP, which overcomes inhibitory signals (MAGs) in the distal nerve stump, to promote regeneration [60]. RES upregulation and nerve regeneration are dependent on retrograde conduction of action potentials to the perikaryon as tetrodotoxin administered proximal to the stimulation site inhibits effects of ES [58].
ES of cultured Schwann cells leads to a significant increase in NGF, BDNF, GDNF, and peripheral myelin protein 22 [67]. A study of oscillating RES in SCI rats showed increased number of oligodendrocyte precursor cells compared to non-stimulated animals, indicating that RES has beneficial effects after SCI [68]. Müller cells, glial cells of the retina, have been shown to increase production of ciliary derived neurotrophic factor and Bcl-2, an anti-apoptotic factor, after stimulation, indicating that RES aids in regeneration of glial cells [69]. These results show that RES not only has an effect on neurons but also has a pro-regenerative effect on glial cells of the peripheral and central nervous systems, suggesting that stimulation of these glial cells, has an additive effect on ES recovery.
Electrical Stimulation Parameters
Both direct current and alternating current stimulation have been shown to upregulate regeneration associated factors in cell culture and improve functional recovery in SCI animal models, while pulsed current is used in peripheral nerve regeneration studies with a standard pulse width of 0.1ms, and amplitude either ~3 volts (V) or 0.3 mAmps [9,57,60,61,67,70–72]. Excess current can damage the nerve and surrounding tissue, but this can be mediated by altering the frequency of stimulation [73]. Frequencies ranging from 5 – 200 Hz have been used for RES, similar to those reported for neuromodulation and FES, suggesting that FES and neuromodulation could be having the unintended effect of upregulating neuroregenerative genes [9,11,60,65,74]. Twenty Hz, which is most widely used in RES experiments, was first selected because it is the mean spontaneous frequency of motoneuron firing, but other frequencies can have therapeutic benefits [60]. A recent study investigated the difference between 0 (control), 2, 20, and 200 Hz in a nerve transected repair diabetic rat model and found that 20 and 200 Hz had a beneficial effect on regeneration, suggesting that future investigation is needed with other frequencies to determine which frequency is most beneficial; however, this could be nerve and injury specific [74].
The parameters that vary most widely between RES studies are duration of stimulation, the number of stimulation sessions, and the delay until ES treatment, all of which can have dramatic effects on regeneration outcome. Duration and interval of stimulation differ greatly between studies, with intervals ranging from once to repeatedly for weeks after the injury with lengths as short at 20 minutes to continuous [9,10,60,65]. Sensory neurons need short stimulation durations (≤ 1 hour); whereas increased durations are more beneficial for motoneurons [60,65]. While 1 hour of stimulation is the most common, investigations to optimize duration of RES will allow for better clinical translation [9,60,65].
While starting RES immediately after injury is ideal when treating nerve injuries, it is not always possible clinically, so investigating the effects of delayed RES treatment is required for most clinical situations. Rodent studies have investigated delayed RES treatments, but because of conflicting results, it is unclear if a delayed treatment of greater than one month would be beneficial; however, delaying treatment decreased regeneration potential compared to immediate treatment [75,76].
Regenerative Electrical Stimulation in Urology
While FES and neuromodulation are beneficial for patients after SCI, and RES in SCI models has shown accelerated regeneration and recovery of function, none of these studies investigated urinary or sexual function [7,77]. RES increases the number of precursor glial cells after SCI, which could aid regeneration, potentially preventing underactive bladder which can occur after SCI, pelvic fractures, or nerve injury during pelvic surgery [5,74]. During surgery, injured nerves could be stimulated to accelerate regeneration and functional recovery, improving patient quality of life, with only a small increase in surgery time. In situations where nerve injury in surgery is inevitable, RES of the nerves before surgery would enhance regeneration after the injury since RES before an injury creates a conditioning lesion, enhancing nerve regeneration [78].
Pelvic surgery and treatments for prostate cancer can injure the cavernous nerve, resulting in erectile dysfunction [35]. While many groups are researching methods to enhance nerve regeneration, none are currently available for patients [35]. RES is a possible treatment for cavernous nerve injury since it enhances the intrinsic regeneration process without administering exogenous growth factors, a preferable approach in cancer patients in whom recurrence is a concern [35].
In preclinical animal studies, RES upregulated BDNF in Onuf’s nucleus, the cell body of the pudendal nerve, and accelerated functional recovery in a stress urinary incontinence model [9,10]. While RES is not currently used clinically for regeneration in urology, FES of the pudendal nerve has been used in post-menopausal women, suggesting implantation of electrodes for RES to treat stress urinary incontinence is a possibility. Nonetheless, other routes of stimulation could be beneficial and should be investigated [79,80]. Min et al. demonstrated that vaginal ES accelerates recovery from stress incontinence in a mouse model, which resulted from upregulation of transforming growth factor β1 [81]. While they showed both 20 and 50 Hz had beneficial effects, 50 Hz had a greater effect than 20 Hz [81].
Conclusion
Nerve injuries that result in voiding dysfunction, incontinence and erectile dysfunction can be treated with FES and neuromodulation to improve quality of life. However, ES can also be used to enhance the intrinsic neuroregenerative process through RES, which promotes nerve sprouting, neuroregeneration, reinnervation of target organs, and functional recovery after nerve injury [9,10]. RES upregulates BDNF in the nerve cell body, which in turn upregulates regeneration associated genes, providing possibilities for therapy of neurogenic lower urinary tract dysfunction due to peripheral nerve injury as well as other urological conditions that can result from nerve injury, such as erectile dysfunction, neurogenic bladder, and underactive bladder [15].
Key Points.
FES and Neuromodulation are used to treat urologic conditions, but electrical stimulation can also be used to accelerated nerve regeneration after injury.
RES upregulates BDNF and TrkB in the neurons as well as regenerative associated proteins in Schwann cells, aiding in regeneration.
RES has been shown to upregulate BDNF and Beta II tubulin in Onuf’s nucleus in the motoneuron cell bodies of the pudendal nerve in a stress urinary incontinence model.
Acknowledgements
Financial support and sponsorship
This work was supported in part by grants from the Rehabilitation Research and Development Service of the Department of Veterans Affairs, (I01 RX001262A1 and F9261-L to M.S.D.), the Cleveland Clinic, the National Natural Science Foundation of China (grant number 81700669 to K.D.), the Natural Science Foundation of Hubei Province, People’s Republic of China (grant number 2016CFB217 to K.D.), and the China Scholarship Council.
Footnotes
Conflicts of Interest
None
References
- 1.Birder L, De Groat W, Mills I, Morrison J, Thor K, Drake M. Neural Control of the Lower Urinary Tract: Peripheral and Spinal Mechanisms. Neurourol Urodyn 2010;29(1):128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Groat WC, Yoshimura N. Anatomy and physiology of the lower urinary tract In: Handbook of Clinical Neurology,Neurology of Sexual and Bladder Disorders. 2015. p. 61–108. [DOI] [PubMed]
- 3.Yoshimura N Lower urinary tract symptoms (LUTS) and bladder afferent activity. Neurourol Urodyn 2007;26:908–913. [DOI] [PubMed] [Google Scholar]
- 4.Allen RE, Hosker GL, Smith AR, Warrell DW. Pelvic floor damage and childbirth: a neurophysiological study. Br J Obstet Gynaecol 1990. September;97(9):770–9. [DOI] [PubMed] [Google Scholar]
- 5.Hohenfellner R Nerve injuries in urological surgery. Georgian Med News. 2007. February;(143):7–11. [PubMed] [Google Scholar]
- 6.Ho CH, Triolo RJ, Elias AL, Kilgore KL, DiMarco AF, Bogie K, et al. Functional electrical stimulation and spinal cord injury. Phys Med Rehabil Clin N Am 2014;25(3):631–54, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGee MJ, Amundsen CL, Grill WM. Electrical stimulation for the treatment of lower urinary tract dysfunction after spinal cord injury. J Spinal Cord Med 2015. March;38(2):135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brindley GS. Emptying the bladder by stimulating sacral ventral roots. J Physiol 1974. March;237(2):15P–16P. [PubMed] [Google Scholar]
- 9.Jiang H-HH, Gill BC, Dissaranan C, Zutshi M, Balog BM, Lin D, et al. Effects of acute selective pudendal nerve electrical stimulation after simulated childbirth injury. Am J Physiol Ren Physiol 2012/11/16 2013;304(3):F239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang H, Song Q, Gill BC, Balog BM, Juarez R, Cruz Y, et al. Electrical stimulation of the pudendal nerve promotes neuroregeneration and functional recovery from stress urinary incontinence in a rat model. Am J Physiol Ren Physiol 315. 2019;315:1555–64.** Demonstrated twice weekly RES upregulated BDNF and βII tubulin and accelerated functional recovery after in a stress urinary incontinence model.
- 11.Samiee F, Zarrindast M-R. Effect of electrical stimulation on motor nerve regeneration in sciatic nerve ligated-mice. Vol. 27, European journal of translational myology. Italy; 2017. p. 6488.* Demonstrated RES accelerated recovery in a sciatic nerve ligation model.
- 12.Deng Y, Xu Y, Liu H, Peng H, Tao Q, Liu H, et al. Electrical stimulation promotes regeneration and re-myelination of axons of injured facial nerve in rats. Neurol Res 2018. March;40(3):231–8.* Demonstrated RES accelrated reinnervation and recovery in a facial nerve transection model.
- 13.Shapira Y, Sammons V, Forden J, Guo GF, Kipp A, Girgulis J, et al. Brief Electrical Stimulation Promotes Nerve Regeneration Following Experimental In-Continuity Nerve Injury. Neurosurgery 2018. June; [DOI] [PubMed]
- 14.Mendez A, Hopkins A, Biron VL, Seikaly H, Zhu LF, Cote DWJ. Brief electrical stimulation and synkinesis after facial nerve crush injury: a randomized prospective animal study. J Otolaryngol Head Neck Surg 2018. March;47(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Majed AA, Tam SL, Gordon T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell Mol Neurobiol 2004/06/23 2004;24(3):379–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menorca RM, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin 2013;29(3):317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int 2014;(2314–6141 (Electronic)):698256. [DOI] [PMC free article] [PubMed]
- 18.Gordon T The Biology, Limits, and Promotion of Peripheral Nerve Regenration in Rats and Humans In: Nerves and Nerve Injuries, Vol 2 Elsevier LTD.; 2015. p. 993–1019. [Google Scholar]
- 19.Curcio M, Bradke F. Axon Regeneration in the Central Nervous System: Facing the Challenges from the Inside. Annu Rev Cell Dev Biol 2018;34(1):495–521. [DOI] [PubMed] [Google Scholar]
- 20.Mietto BS, Mostacada K, Martinez AM. Neurotrauma and inflammation: CNS and PNS responses. Mediat Inflamm 2015;2015(1466–1861 (Electronic)):251204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeFrancesco-Lisowitz A, Lindborg JA, Niemi JP, Zigmond RE. The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience [Internet]. 2015;302:174–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, et al. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci 2006;26(38):9646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michalski B, Bain JR, Fahnestock M. Long-term changes in neurotrophic factor expression in distal nerve stump following denervation and reinnervation with motor or sensory nerve. J Neurochem 2008;105(4):1244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brushart TM, Aspalter M, Griffin JW, Redett R, Hameed H, Zhou C, et al. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Exp Neurol 2013;247:272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon T, Chan KM, Sulaiman OA, Udina E, Amirjani N, Brushart TM. Accelerating axon growth to overcome limitations in functional recovery after peripheral nerve injury. Neurosurgery 2009/12/16 2009;65(4 Suppl):A132–44. [DOI] [PubMed] [Google Scholar]
- 26.Deng K, Lin DL, Hanzlicek B, Balog B, Penn MS, Kiedrowski MJ, et al. Mesenchymal stem cells and their secretome partially restore nerve and urethral function in a dual muscle and nerve injury stress urinary incontinence model. 2019;44195:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shan H, Chen F, Zhang T, He S, Xu L, Wei A. Stem cell therapy for erectile dysfunction of cavernous nerve injury rats: a systematic review and meta-analysis. PLoS One. 2015;10(4):e0121428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett NE, Kim JH, Wolfe DP, Sasaki K, Yoshimura N, Goins WF, et al. Improvement in erectile dysfunction after neurotrophic factor gene therapy in diabetic rats. J Urol 2005. May;173(5):1820–4. [DOI] [PubMed] [Google Scholar]
- 29.Kato R, Wolfe D, Coyle CH, Huang S, Wechuck JB, Goins WF, et al. Herpes simplex virus vector-mediated delivery of glial cell line-derived neurotrophic factor rescues erectile dysfunction following cavernous nerve injury. Gene Ther 2007. September;14(18):1344–52. [DOI] [PubMed] [Google Scholar]
- 30.Bond CW, Angeloni N, Harrington D, Stupp S, Podlasek CA. Sonic Hedgehog regulates brain-derived neurotrophic factor in normal and regenerating cavernous nerves. J Sex Med 2013. March;10(3):730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin GN, Choi MJ, Kim WJ, Kwon M-H, Song K-M, Park J-M, et al. Inhibition of Ninjurin 1 restores erectile function through dual angiogenic and neurotrophic effects in the diabetic mouse. Proc Natl Acad Sci U S A. 2014. July;111(26):E2731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnett AL, Sezen SF, Hoke A, Caggiano AO, Iaci J, Lagoda G, et al. GGF2 is neuroprotective in a rat model of cavernous nerve injury-induced erectile dysfunction. J Sex Med 2015. April;12(4):897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sezen SF, Hoke A, Burnett AL, Snyder SH. Immunophilin ligand FK506 is neuroprotective for penile innervation. Vol. 7, Nature medicine. United States; 2001. p. 1073–4. [DOI] [PubMed] [Google Scholar]
- 34.Gill BC, Balog BM, Dissaranan C, Jiang HH, Steward JB, Lin DL, et al. Neurotrophin therapy improves recovery of the neuromuscular continence mechanism following simulated birth injury in rats. Neurourol Urodyn 2012/05/15 2013;32(1):82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell JD, Burnett AL. Neuroprotective and nerve regenerative approaches for treatment of erectile dysfunction after cavernous nerve injury. Int J Mol Sci 2017;18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Liao LM, Chen GQ, Wang ZX, Lu TJ, Deng H. Clinical and urodynamic characteristics of underactive bladder. Med (United States). 2018;97(3):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan HQ, Kerns JM, Lin DL, Sypert D, Steward J, Hoover CR, et al. Dual simulated childbirth injury delays anatomic recovery. Am J Physiol Ren Physiol 2009;296(2):F277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang HH, Gustilo-Ashby AM, Salcedo LB, Pan HQ, Sypert DF, Butler RS, et al. Electrophysiological function during voiding after simulated childbirth injuries. Exp Neurol 2008/12/06 2009;215(2):342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson CE, Chamberlain JD, Jordan X, Kessler TM, Luca E, Mohr S, et al. Bladder emptying method is the primary determinant of urinary tract infections in patients with spinal cord injury: results from a prospective rehabilitation cohort study. BJU Int 2019. February;123(2):342–52. [DOI] [PubMed] [Google Scholar]
- 40.Brindley GS. The first 500 patients with sacral anterior root stimulator implants: general description. Paraplegia 1994. December;32(12):795–805. [DOI] [PubMed] [Google Scholar]
- 41.Kennelly MJ, Bennett ME, Grill WM, Grill JH, Boggs JW. Electrical stimulation of the urethra evokes bladder contractions and emptying in spinal cord injury men: case studies. J Spinal Cord Med 2011;34(3):315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Compr Physiol 2015;5:327–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng CL, Ma CP, de Groat WC. Effects of capsaicin on micturition and associated reflexes in rats. Am J Physiol 1993. July;265(1 Pt 2):R132–8. [DOI] [PubMed] [Google Scholar]
- 44.Maggi CA, Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol 1988;19(1):1–43. [DOI] [PubMed] [Google Scholar]
- 45.Zhang F, Zhao S, Shen B, Wang J, Nelson DE, Roppolo JR, et al. Neural pathways involved in sacral neuromodulation of reflex bladder activity in cats. Am J Physiol Renal Physiol 2013. March;304(6):F710–7. [DOI] [PubMed] [Google Scholar]
- 46.Ammi M, Chautard D, Brassart E, Culty T, Azzouzi AR, Bigot P. Transcutaneous posterior tibial nerve stimulation: evaluation of a therapeutic option in the management of anticholinergic refractory overactive bladder. Int Urogynecol J 2014. August;25(8):1065–9. [DOI] [PubMed] [Google Scholar]
- 47.Chen ML, Chermansky CJ, Shen B, Roppolo JR, de Groat WC, Tai C. Electrical stimulation of somatic afferent nerves in the foot increases bladder capacity in healthy human subjects. J Urol 2014. April;191(4):1009–13. [DOI] [PubMed] [Google Scholar]
- 48.Opisso E, Borau A, Rijkhoff NJM. Subject-controlled stimulation of dorsal genital nerve to treat neurogenic detrusor overactivity at home. Neurourol Urodyn 2013. September;32(7):1004–9. [DOI] [PubMed] [Google Scholar]
- 49.Peters KM, Killinger KA, Boguslawski BM, Boura JA. Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol Urodyn 2010. September;29(7):1267–71. [DOI] [PubMed] [Google Scholar]
- 50.Yoo PB, Horvath EE, Amundsen CL, Webster GD, Grill WM. Intraurethral activation of excitatory bladder reflexes in persons with spinal cord injury. Conf Proc. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2009;2009:6781–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willand MP, Nguyen MA, Borschel GH, Gordon T. Electrical Stimulation to Promote Peripheral Nerve Regeneration. Neurorehabil Neural Repair. 2015;30(5):490–6. [DOI] [PubMed] [Google Scholar]
- 52.Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol 2006. January;197(1):225–34. [DOI] [PubMed] [Google Scholar]
- 53.Sievert K-D, Amend B, Gakis G, Toomey P, Badke A, Kaps HP, et al. Early sacral neuromodulation prevents urinary incontinence after complete spinal cord injury. Ann Neurol 2010. January;67(1):74–84. [DOI] [PubMed] [Google Scholar]
- 54.Lee YJ, Yoon CY, Lee MS, Song B Do, Lee SW, Jeong SJ. Effect of Early Sacral Neuromodulation on Bladder Function in a Rat Model of Incomplete Spinal Cord Injury Due to Focal Contusion. Neuromodulation 2018. December;** Demonstrated early sacral neuromodulation had a beneficial effect incontience in a rodent model of spinal cord injury
- 55.Khunda A, McCormick C, Ballard P. Sacral neuromodulation and sexual function: a systematic review and meta-analysis of the literature. Int Urogynecol J 2019. March;30(3):339–52. [DOI] [PubMed] [Google Scholar]
- 56.Tahseen S Role of sacral neuromodulation in modern urogynaecology practice: a review of recent literature. Int Urogynecol J 2018. August;29(8):1081–91. [DOI] [PubMed] [Google Scholar]
- 57.Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol 2007;205(2):347–59. [DOI] [PubMed] [Google Scholar]
- 58.Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci 2000;12:4381–90. [PubMed] [Google Scholar]
- 59.HOFFMAN H Acceleration and retardation of the process of axon-sprouting in partially devervated muscles. Aust J Exp Biol Med Sci 1952;30(6):541–66. [DOI] [PubMed] [Google Scholar]
- 60.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci 2000;20(7):2602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X, Xin N, Tong L, Tong XJ. Electrical stimulation enhances peripheral nerve regeneration after crush injury in rats. Mol Med Rep 2013;7(5):1523–7. [DOI] [PubMed] [Google Scholar]
- 62.Kim IS, Song YM, Cho TH, Pan H, Lee TH, Kim SJ, et al. Biphasic electrical targeting plays a significant role in schwann cell activation. Tissue Eng Part A. 2011/01/18 2011;17(9–10):1327–40. [DOI] [PubMed] [Google Scholar]
- 63.English AW, Wilhelm JC, Ward PJ. Exercise, Neurotrophins, and Axon Regeneration in the PNS. Physiology 2014;29(6):437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamamoto K, Yamamoto T, Honjo K, Ichioka H, Oseko F, Kishida T, et al. Electrical stimulation with periodic alternating intervals stimulates neuronal cells to produce neurotrophins and cytokines through activation of mitogen-activated protein kinase pathways. Eur J Oral Sci 2015;123(6):403–8. [DOI] [PubMed] [Google Scholar]
- 65.Sharma N, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation and testosterone differentially enhance expression of regeneration-associated genes. Exp Neurol 2010;223(1):183–91. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X, Xin N, Tong L, Tong XJ. Electrical stimulation enhances peripheral nerve regeneration after crush injury in rats. Mol Med Rep 2013;7(5):1523–7. [DOI] [PubMed] [Google Scholar]
- 67.Kim IS, Song YM, Cho TH, Pan H, Lee TH, Kim SJ, et al. Biphasic electrical targeting plays a significant role in schwann cell activation. Tissue Eng Part A. 2011;17(9–10):1327–40. [DOI] [PubMed] [Google Scholar]
- 68.Zhang C, Zhang G, Rong W, Wang A, Wu C, Huo X. Oscillating field stimulation promotes spinal cord remyelination by inducing differentiation of oligodendrocyte precursor cells after spinal cord injury. Biomed Mater Eng 2014;24(6):3629–36. [DOI] [PubMed] [Google Scholar]
- 69.Ni Y qin, Gan D kang, Xu H dong, Xu G zhi, Da C di. Neuroprotective effect of transcorneal electrical stimulation on light-induced photoreceptor degeneration. Exp Neurol 2009;219(2):439–52. [DOI] [PubMed] [Google Scholar]
- 70.Erskine L, McCaig CD. Growth cone neurotransmitter receptor activation modulates electric field-guided nerve growth. Dev Biol 1995;171(2):330–9. [DOI] [PubMed] [Google Scholar]
- 71.Rajnicek AM, Foubister LE, McCaig CD. Temporally and spatially coordinated roles for Rho, Rac, Cdc42 and their effectors in growth cone guidance by a physiological electric field. J Cell Sci 2006;119(Pt 9):1723–35. [DOI] [PubMed] [Google Scholar]
- 72.Kobelt LJ, Wilkinson AE, McCormick AM, Willits RK, Leipzig ND. Short Duration Electrical Stimulation to Enhance Neurite Outgrowth and Maturation of Adult Neural Stem Progenitor Cells. Ann Biomed Eng 2014;40(10):2164–78. [DOI] [PubMed] [Google Scholar]
- 73.Nag S, Thakor NV. Implantable neurotechnologies: electrical stimulation and applications. Med Biol Eng Comput 2016. January;54(1):63–76. [DOI] [PubMed] [Google Scholar]
- 74.Kao CH, Chen JJ, Hsu YM, Bau DT, Yao CH, Chen YS. High-frequency electrical stimulation can be a complementary therapy to promote nerve regeneration in diabetic rats. PLoS One. 2013;8(11):e79078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang J, Zhang Y, Lu L, Hu X, Luo Z. Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats. Eur J Neurosci 2013;38(12):3691–701. [DOI] [PubMed] [Google Scholar]
- 76.Xu C, Kou Y, Zhang P, Han N, Yin X, Deng J, et al. Electrical stimulation promotes regeneration of defective peripheral nerves after delayed repair intervals lasting under one month. PLoS One. 2014;9(9):e105045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamid S, Hayek R. Role of electrical stimulation for rehabilitation and regeneration after spinal cord injury: An overview. Eur Spine J 2008;17(9):1256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Senger JLB, Verge VMK, Macandili HSJ, Olson JL, Chan KM, Webber CA. Electrical stimulation as a conditioning strategy for promoting and accelerating peripheral nerve regeneration. Exp Neurol 2018. April;302:75–84.* Demonstrated ES of a nerve act as a conditioning lension enhancing regeneration, suggesting RES could be done before surgeries to a nerve incase of injury during surgery.
- 79.Wang S, Zhang S. Simultaneous perineal ultrasound and vaginal pressure measurement prove the action of electrical pudendal nerve stimulation in treating female stress incontinence. BJU Int 2012;110:1338–43. [DOI] [PubMed] [Google Scholar]
- 80.Wang S, Lv J, Feng X, Wang G, Lv T. Efficacy of electrical pudendal nerve stimulation in treating female stress incontinence. Urology 2016;91:64–9. [DOI] [PubMed] [Google Scholar]
- 81.Min J, Li B, Liu C, Hong S, Tang J, Hu M, et al. Therapeutic effect and mechanism of electrical stimulation in female stress urinary incontinence. Urology 2019;104:45–51.** Demonstrated ES of vaginal tissue in a stress urinary incontinence model can accelerate regeneration, suggesting altervative stimulatation location as a possible for RES regeneration for stress incontinence.