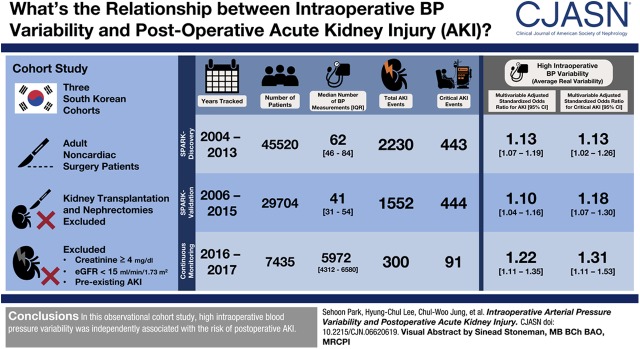
Visual Abstract
Keywords: acute kidney injury, blood pressure, variability
Abstract
Background and objectives
High BP variability may cause AKI because of inappropriate kidney perfusion. This study aimed to investigate the association between intraoperative BP variability and postoperative AKI in patients who underwent noncardiac surgery.
Design, setting, participants, & measurements
We performed a cohort study of adults undergoing noncardiac surgery in hospitals in South Korea. We studied three cohorts using the following recording windows for intraoperative BP: discovery cohort, 1-minute intervals; first validation cohort, 5-minute intervals; and second validation cohort, 2-second intervals. We calculated four variability parameters (SD, coefficient of variation, variation independent of mean, and average real variability) based on the measured mean arterial pressure values. The primary outcomes were postoperative AKI (defined by the Kidney Disease Improving Global Outcomes serum creatinine cutoffs) and critical AKI (consisting of stage 2 or higher AKI and post-AKI death or dialysis within 90 days).
Results
In the three cohorts, 45,520, 29,704, and 7435 patients were analyzed, each with 2230 (443 critical), 1552 (444 critical), and 300 (91 critical) postoperative AKI events, respectively. In the discovery cohort, all variability parameters were significantly associated with risk of AKI, even after adjusting for intraoperative hypotension. For example, average real variability was associated with higher risks of postoperative AKI (adjusted odds ratio, 1.13 per 1 SD increment; 95% CI, 1.07 to 1.19) and critical AKI (adjusted odds ratio, 1.13 per 1 SD increment; 95% CI, 1.02 to 1.26). Associations were evident predominantly among patients who also experienced intraoperative hypotension. In the validation analysis with 5-minute-interval BP records, all four variability parameters were associated with the risk of postoperative AKI or critical AKI. In the validation cohort with 2-second-interval BP records, average real variability was the only significant variability parameter.
Conclusions
Higher intraoperative BP variability is associated with higher risks of postoperative AKI after noncardiac surgery, independent of hypotension and other clinical characteristics.
Introduction
Postoperative AKI is an important complication that can cause a prolonged stay in hospital (1), higher medical costs (2), persistent kidney failure, and in-hospital death (3). Postoperative AKI is caused by many factors, the most common of which include inflammatory reactions, unstable hemodynamics, impaired volume status, and exposure to nephrotoxic agents (4). Individualized approaches with preoperative risk evaluation (5–7), early detection (8,9), and optimized supportive care may improve the prognosis of postoperative AKI (10,11).
Because decreased kidney blood perfusion can cause significant kidney injury (12), intraoperative hypotension should be avoided to reduce postoperative AKI risk (13–16). Recent studies robustly suggest that a mean arterial pressure <65 mm Hg is closely related to an increased risk of postoperative AKI in noncardiac operations (13,15,16). In addition, high BP variability may cause AKI due to inappropriate kidney perfusion. Although BP fluctuation is known to be related to several adverse outcomes (17–19), evidence of whether a higher intraoperative BP variability is associated with a higher risk of postoperative AKI has rarely been studied in patients undergoing noncardiac surgery.
This study aimed to investigate the association between intraoperative BP parameters, mainly focusing on BP variabilities, and postoperative AKI in noncardiac surgery in three cohorts with distinct characteristics. We hypothesized that patients who undergo noncardiac surgery with unstable intraoperative BP would have a higher postoperative AKI risk.
Materials and Methods
Ethical Considerations
This study was conducted in accordance with the principles of the Declaration of Helsinki. The institutional review board (IRB) of Seoul National University Hospital (SNUH; IRB number H-1306-053-495) and Seoul National University Bundang Hospital (SNUBH; IRB no. B-1706/403-101) approved the study. The IRB did not require informed consent for the retrospective cohorts because the study was based on medical record review without patient contact. The SNUH IRB also approved the use of data from the prospective continuous-monitoring cohort, allowing us to additionally review perioperative creatinine values. The prospective continuous-monitoring cohort was separately approved by the IRB (no. H-1408-101-605) and registered at www.clinicaltrials.gov (identifier: NCT02914444) (20,21). Because the prospective registry only collected clinically monitored values without additional patient contact or medical intervention, the IRB waived the requirement for informed consent.
Study Cohorts
The study was an observational study using three different cohorts from tertiary hospitals in Korea. We first investigated the cohorts from our previous study in which we developed the simple postoperative AKI risk (SPARK) index and classification for noncardiac surgery using a cohort from SNUH, which was validated in a cohort from SNUBH (5). We initially studied the “SPARK-discovery” cohort, which includes adults (age ≥18 years) who underwent noncardiac surgery from 2004 to 2013 at SNUH. We validated our findings in the “SPARK-validation” cohort, which included adults who underwent noncardiac surgery from 2006 to 2015 at SNUBH. Next, we implemented a prospective validation cohort (the “continuous-monitoring” cohort) that included continuous anesthetic monitoring of adults who underwent surgery at SNUH from 2016 to 2017. The recording windows for intraoperative BP were as follows: 1-minute intervals in the SPARK-discovery cohort, 5-minute intervals in the SPARK-validation cohort, and 2-second intervals in the continuous-monitoring cohort with intra-arterial BP measurements.
Study Population
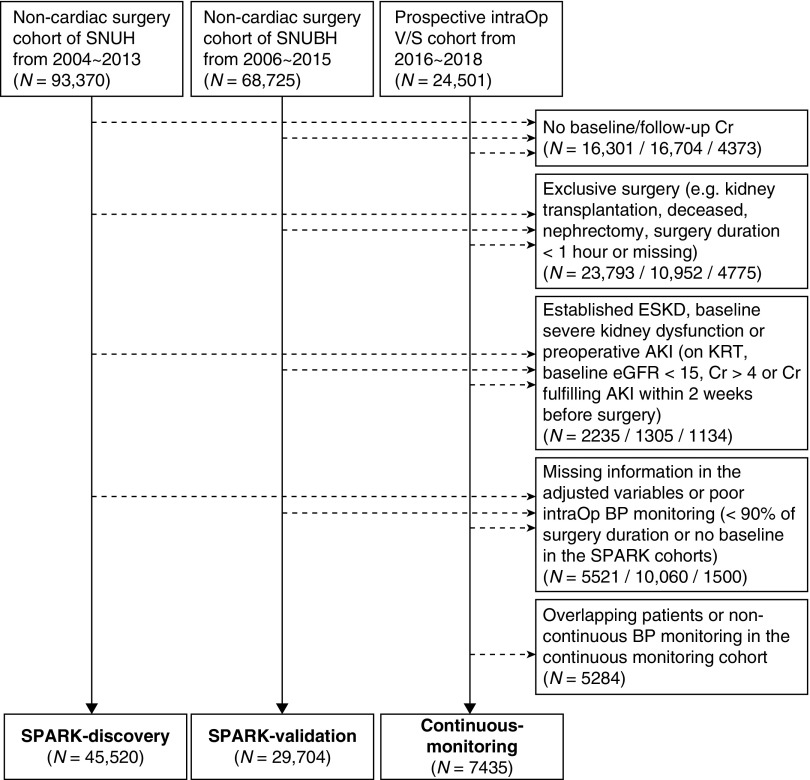
We included the first operation cases for each patient in the three cohorts during the study period. We excluded cardiac surgeries; surgeries for deceased patients (e.g., cadaver donor transplantation); partial and total nephrectomy cases or kidney transplantation, because these surgeries are directly associated with postoperative creatinine elevation; minor procedural events (surgery duration <1 hour) (13,15); those with established preoperative kidney impairment (preoperative serum creatinine ≥4 mg/dl, eGFR <15 ml/min per 1.73 m2, or a baseline serum creatinine value which fulfills AKI criteria when compared with the pre-existing minimum serum creatinine value within 3 months before surgery); those with missing baseline or follow-up serum creatinine levels within 2 weeks after surgery to identify postoperative AKI events; those with missing information on adjusted clinical variables; and those who had BP monitored for <90% of the surgery duration or those with missing baseline BP values in the SPARK cohorts. The patients in the continuous-monitoring cohort who overlapped with those in the SPARK-discovery cohort were also excluded.
Intraoperative Blood-Pressure Parameters
In the study cohorts, invasively monitored intra-arterial BP values were primarily used if available. In the SPARK-discovery and SPARK-validation cohorts, measurements from BP cuffs were used if the intra-arterial BP was unavailable at a time point of BP measurement. In the continuous-monitoring cohort, only the intra-arterial line BP values were included. We deleted the BP values that were possibly from artifacts (15). Further details regarding the definition of artifacts and hypotension parameters are described in Supplemental Method 1.
To quantify mean arterial pressure variability in each individual, we calculated four parameters with the measured mean-arterial-pressure values. SD is one of the most commonly used indexes of variability, but it does not take the order of the measurements into account and is largely affected by mean levels (22–24). The coefficient of variation does take the mean levels into account, and the SD is divided by the mean value in each individual. Additionally, the average real variability takes the order of individual BP measurements into account and is less sensitive to low-frequency samplings (25). The average real variability was calculated by the sum of differences between adjacent values divided by the number of gaps. Variation independent of the mean was calculated using a linear regression model in each studied cohort and, because transformation of SD is uncorrelated with means, the parameters were independent from the mean levels. The actual equations used are presented in Supplemental Method 2.
Study Outcomes
The primary study outcome was postoperative AKI. This was defined, according to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines, as serum creatinine values of 0.3 mg/dl or a 50% increase, using peak creatinine levels within 2 weeks after surgery compared with the last preoperative creatinine value within 3 months before surgery. An additional sensitivity analysis was performed with AKI outcomes defined as the peak serum creatinine values within 7 days in the SPARK-discovery cohort in patients with available laboratory values. To consider the severity and patient-centered outcomes of postoperative AKI, we additionally defined critical AKI as a combination of a twofold increase in creatinine from baseline or values >4 mg/dl within 2 weeks after surgery with a postoperative AKI and post-AKI death or dialysis within 90 days (5). Acute dialysis or death events were reviewed within the electronic health records in each hospital. To account for death and dialysis events outside our study hospitals, we reviewed the nationwide dialysis registry maintained by the Korean Society of Nephrology and the national death database from Statistics Korea (26).
Statistical Analysis
Details regarding other data collected in the study have been described previously (5). The study mainly used complete-case multivariable regression models including preoperative risk factors for postoperative AKI and, in the discovery analysis, intraoperative estimated blood loss or amounts of red blood cell transfusion. Other details are described in Supplemental Method 3.
Results
Included Patients and Measured Blood-Pressure Values
After exclusion, we included 3,179,205 measured BP values of 45,520 patients in the SPARK-discovery cohort, with a median of 62 (interquartile ranges, 46–84) measurements per patient at median time intervals of 3 (2–4) minutes (Figure 1). In the SPARK-validation cohort, we analyzed 1,353,815 BP values of 29,704 patients, with a median of 41 (31–54) measurements per patient at median time intervals of 5 (5–5) minutes. The continuous-monitoring cohort, including all patients who received continuous intra-arterial BP monitoring, included 7435 patients. The median time interval was 2 (2–2) seconds, with a total of 48,923,796 BP measurements, yielding a median of 5972 (4312–6580) BP measurements per patient. The descriptive statistics of the calculated variability parameters in each cohort are shown in Supplemental Figure 1.
Figure 1.
Study population. Cr, creatinine; intraOp, intraoperative; SNUBH, Seoul National University Bundang Hospital; SNUH, Seoul National University Hospital; SPARK, simple postoperative AKI risk; V/S, vital sign.
Clinical Characteristics of the Studied Patients
The SPARK-discovery cohort included more obstetrics and gynecology surgeries but fewer orthopedic operations than the others (Table 1). The numbers of postoperative AKI events in the SPARK-discovery and SPARK-validation cohorts were 2230 (5%) and 1552 (5%), respectively, and the numbers of critical AKI were 443 (1%) and 444 (2%), respectively. The patients in the continuous-monitoring cohort who required an invasive monitoring strategy showed worse clinical characteristics in certain aspects. The continuous-monitoring cohort had 300 (4%) and 91 (1%) patients with postoperative and critical AKI events, respectively.
Table 1.
Characteristics of adults undergoing noncardiac surgery in three cohorts of patients from South Korea
| Variables | SPARK Discovery (n=45,520) | SPARK Validation (n=29,704) | Continuous Monitoring (n=7435) |
|---|---|---|---|
| Demographics | |||
| Age | 56 (44–66) | 61 (49–70) | 62 (52–71) |
| Sex | |||
| Female | 25,503 (56%) | 14,068 (47%) | 3478 (47%) |
| Male | 20,017 (44%) | 15,636 (53%) | 3957 (53%) |
| Body mass index (kg/m2) | 23.8 (21.7–26.0) | 24.3 (22.2–26.6) | 23.7 (21.5–26.0) |
| <18.5 | 1689 (5%) | 493 (3%) | 319 (5%) |
| ≥18.5 and <30 | 40,397 (93%) | 15,924 (91%) | 5485 (90%) |
| ≥30 | 1689 (4%) | 1003 (6%) | 327 (5%) |
| Unknowna | 1745 (4%) | 12,777 (43%) | 1304 (18%) |
| Preexisting comorbidities | |||
| Heart disease | 1400 (3%) | 889 (3%) | Not collected |
| Hypertension | 8702 (19%) | 6756 (23%) | Not collected |
| Diabetes mellitus | 3472 (8%) | 2818 (10%) | 1570 (21%) |
| Surgery characteristics | |||
| Surgery departments | |||
| General surgery | 20,282 (45%) | 9865 (33%) | 3360 (45%) |
| Neurosurgery | 4234 (9%) | 2410 (8%) | 1090 (15%) |
| Obstetrics and gynecology | 7171 (16%) | 589 (2%) | 382 (5%) |
| Orthopedics | 10,331 (23%) | 13,692 (46%) | 593 (8%) |
| Urologic surgery | 3502 (8%) | 3148 (11%) | Not included |
| Thoracic surgery (lung) | Not included | Not included | 2,010 (27%) |
| Surgery duration (h) | 2.2 (1.5–3.2) | 2.5 (1.8–3.6) | 3.2 (2.2–4.3) |
| Anesthesia type | |||
| General | 39,039 (86%) | 22,491 (76%) | 7324 (99%) |
| Nongeneral | 6210 (14%) | 7213 (24%) | 111 (2%) |
| Emergency operation | 496 (1%) | 492 (2%) | 583 (8%) |
| BP before operation (mm Hg) | |||
| Systolic BP | 124 (113–136) | 128 (115–142) | Not collected |
| Diastolic BP | 77 (70–85) | 73 (64–81) | Not collected |
| Mean arterial pressure | 93 (85–101) | 91 (82–101) | Not collected |
| Preoperative renin-angiotensin-aldosterone-blockade use | 2478 (5%) | 2039 (7%) | 1,111 (15%) |
| Laboratory findings | |||
| eGFR (ml/min per 1.73 m2) | 82 (71–95) | 87 (72–98) | 91 (80–101) |
| No CKD or CKD stage 1 or 2 (≥60) | 41,943 (92%) | 26,805 (90%) | 6936 (93%) |
| CKD stage 3A (≥45 and <60) | 2873 (6%) | 2234 (8%) | 352 (5%) |
| CKD stage 3B (≥30 and <45) | 535 (1%) | 493 (2%) | 104 (1%) |
| CKD stage 4 (≥15 and <30) | 169 (0%) | 172 (1%) | 43 (1%) |
| Dipstick albuminuria (≥1+) | 3873 (9%) | 2060 (7%) | 905 (12%) |
| Hemoglobin (g/dl) | 13.3 (12.1–14.4) | 13.7 (12.6–14.9) | 13.1 (11.9–14.2) |
| Anemia (<12 for female, <13 for male) | 12,056 (27%) | 5689 (19%) | 2664 (36%) |
| Albumin (g/dl) | 4.2 (3.9–4.5) | 4.4 (4.1–4.6) | 4.2 (3.9–4.4) |
| Hypoalbuminemia (<3.5) | 4088 (9%) | 1149 (4%) | 533 (7%) |
| Sodium (mEq/L) | 141 (139–142) | 141 (139–142) | 141 (139–142) |
| Hyponatremia (<135) | 1,021 (2%) | 640 (2%) | 198 (3%) |
Continuous variables are presented as median values (interquartile ranges), and categorical variables are shown as numbers (percentages) unless stated otherwise. SPARK, simple postoperative AKI risk.
All variables except for body mass index or variables that were not collected in the continuous-monitoring cohort were not missing. The body mass index and other variables with missing values were not included in further analyses, because the analyses were performed with the complete-case method without missing information.
Characteristics of the Intraoperative Variability Parameters
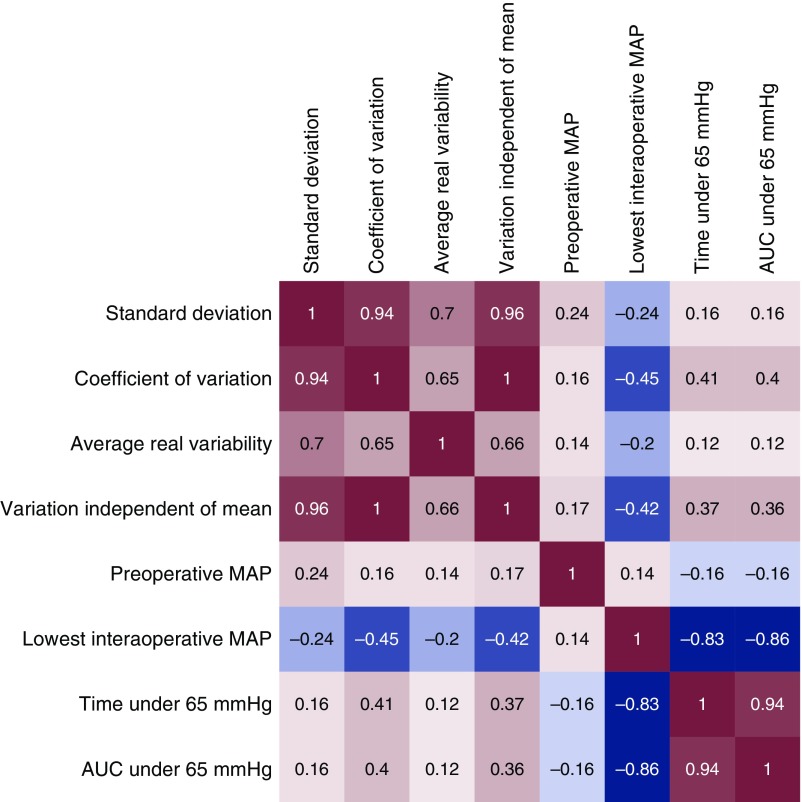
When we investigated the correlation between the BP parameters of the SPARK-discovery cohort (Figure 2), those with higher values for the lowest intraoperative mean arterial pressure had a shorter time or lower degree of hypotension during surgery and lower BP variability. On the other hand, those who had higher intraoperative BP variability showed lower minimum intraoperative mean arterial pressure and a higher baseline BP. Regarding preoperative AKI risk factors (Supplemental Figure 2), older age was prominently correlated with the variability of the intraoperative mean arterial pressure, with the exception of average real variability, which was higher in younger patients in the continuous-monitoring cohort. Additionally, those with diabetes, lower eGFR, treatment with renin-angiotensin-aldosterone system blockades, and emergency operations were more likely to have high BP variability in all three cohorts. When other surgery-related characteristics were considered (Supplemental Table 1), general surgery was the department with the largest variability parameters in the SPARK cohorts, whereas orthopedic operations had the largest variability in the continuous-monitoring cohort. Those with shorter operation duration had larger variability in most cases. Patients who received intraoperative BP-modifying agents had a relatively lower BP variability, although the absolute differences in median values were relatively small.
Figure 2.
Significant correlations were identified between the studied BP parameters in the SPARK-discovery cohort. Correlation analysis was done using the Spearman test, and the squares show the correlation index between the variables according to row and column. The significant correlations (P<0.05) are colored, with red showing positive correlations and blue showing negative correlations. AUC, area under curve; MAP, mean arterial pressure.
Usage of Absolute Mean Arterial Pressure Values To Determine Intraoperative Hypotension
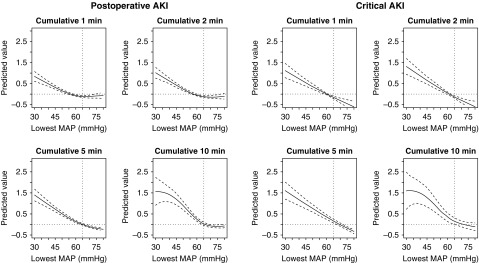
In the SPARK-discovery cohort, the risks of both postoperative and critical AKI were higher with a mean arterial pressure of <65 mm Hg (Figure 3). However, when using hypotension parameters other than the mean arterial pressure as the cutoff, there was no prominent superiority in regard to discriminating the risk of postoperative AKI or critical AKI (Supplemental Table 2). Therefore, we maintained the mean arterial pressure threshold of 65 mm Hg to determine significant intraoperative hypotension, which has been robustly studied previously (15).
Figure 3.
The lowest mean arterial pressure value of 65 mmHg was identified as a valid threshold to determine intraoperative hypotension related to a higher risk of postoperative AKI or critical AKI. We constructed generalized additive models, including preoperative and/postoperative AKI risk factors and the corresponding BP values. The predicted values are from the smoothing function of the explanatory variables and are centered to zero. The solid lines indicate the predicted values and the dotted lines indicate the 95% confidence intervals. The included preoperative/postoperative AKI risk factors were age, sex, eGFR (continuous, ml/min per 1.73 m2), diabetes mellitus, surgery duration (continuous, hours), emergency operation, usage of renin-angiotensin-aldosterone blockades, dipstick albuminuria, anemia (<13 g/dl for men, <12 g/dl for women), hypoalbuminemia (<3.5 g/dl), and hyponatremia (<135 mEq/L).
Intraoperative Hypotension and AKI
The lowest mean arterial pressure values were significantly associated with a higher risk of postoperative and critical AKI (Table 2). The meaningful hypotension (mean arterial pressure <65 mm Hg) significantly interacted (P<0.001) with the association between the lowest mean arterial pressure values and the risk of postoperative AKI. In the subgroup analysis, the lowest mean arterial pressure values were prominently associated with higher risks of the studied AKI outcomes only in patients with any mean arterial pressure <65 mm Hg.
Table 2.
Associations of intraoperative hypotension parameters with postoperative AKI in the SPARK-discovery cohort
| Outcome, Exposure, and Subgroup | Univariable Model | Multivariable Modela | ||
|---|---|---|---|---|
| sOR (95% CI) | P Value | Adjusted sOR (95% CI) | P Value | |
| Postoperative AKI | ||||
| Mean arterial pressure (per 10.3 mm Hg lower value) | 1.68 (1.61 to 1.76) | <0.001 | 1.18 (1.13 to 1.25) | <0.001 |
| In patients with hypotensionb | 2.00 (1.88 to 2.12) | <0.001 | 1.36 (1.26 to 1.46) | <0.001 |
| In patients without hypotensionb | 1.02 (0.89 to 1.18) | 0.80 | 1.00 (0.87 to 1.17) | 0.95 |
| Time with mean arterial pressure <65 mm Hg (per 37.6 min) | 1.64 (1.60 to 1.68) | <0.001 | 1.23 (1.19 to 1.27) | <0.001 |
| Area under curve <65 mm Hg (per 319.5 min × mm Hg) | 1.59 (1.65 to 1.65) | <0.001 | 1.19 (1.15 to 1.24) | <0.001 |
| Critical AKI | ||||
| Mean arterial pressure (per 10.3 mm Hg lower value) | 2.11 (1.92 to 2.32) | <0.001 | 1.43 (1.29 to 1.59) | <0.001 |
| In patients with hypotensionb | 2.36 (2.10 to 2.64) | <0.001 | 1.55 (1.35 to 1.77) | <0.001 |
| In patients without hypotensionb | 1.36 (0.91 to 2.16) | 0.16 | 1.30 (0.87 to 2.06) | 0.24 |
| Time with mean arterial pressure <65 mm Hg (per 37.6 min) | 1.49 (1.44 to 1.55) | <0.001 | 1.18 (1.12 to 1.23) | <0.001 |
| Area under curve <65 mm Hg (per 319.5 min × mm Hg) | 1.33 (1.28 to 1.37) | <0.001 | 1.12 (1.09 to 1.16) | <0.001 |
All BP parameters were included in the regression model as continuous variables, and standardized odds ratios per 1-SD higher are presented; the 1-SD values were 10.3 mm Hg for lowest mean arterial pressure values, 37.6 min for time under 65 mm Hg, and 319.5 min × mm Hg for area under curve under 65 mm Hg, respectively. The numbers of postoperative AKI events were 2230 (5%), the number of critical AKI was 443 (1%) in the SPARK-discovery cohort with 45,520 patients. SPARK, simple postoperative AKI risk; sOR, standardized odds ratio.
Multivariable model was adjusted for age, sex, eGFR, diabetes mellitus, surgery duration (continuous, hours), emergency operation, usage of renin-angiotensin-aldosterone system blockades, dipstick albuminuria, anemia (<13 g/dl for male, <12 g/dl for female), hypoalbuminemia (<3.5 g/dl), and hyponatremia (<135 mEq/L), without missing values.
Subgroups were divided according to presence of any mean arterial pressure <65 mm Hg during surgery. The subgroup with hypotension included 32,101 patients with 1827 (6%) AKI and 383 (1%) critical AKI events, respectively. The subgroup without hypotension included 13,419 patients with 403 (3%) AKI and 60 (0%) critical AKI events, respectively.
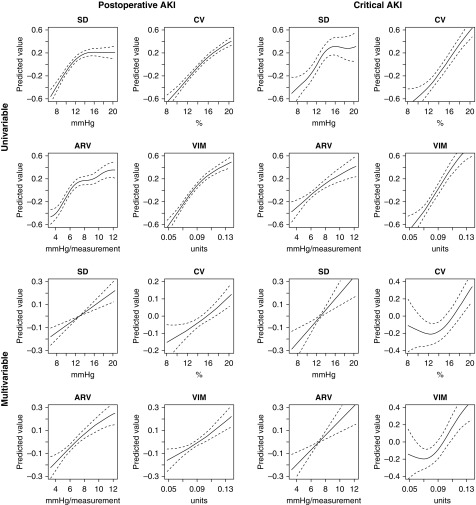
Intraoperative Blood-Pressure Variability and AKI
The risks of postoperative and critical AKI events were higher in patients with high intraoperative BP variability (Figure 4). These associations remained significant even when we adjusted for intraoperative hypotension or blood loss parameters in the multivariable model (Table 3). Additionally, the presence of intraoperative hypotension (mean arterial pressure <65 mm Hg) had a significant interaction with all variability parameters and on the risk of postoperative AKI (interaction P: for SD 0.02, for coefficient of variation <0.001, for average real variability 0.003, for variation independent of mean <0.001). In the subgroup analysis, high mean arterial pressure variability was more prominently associated with the risk of postoperative AKI or critical AKI in those with mean arterial pressure <65 mm Hg. The results were similarly reproduced even when the time window to define AKI was restricted to 7 days after surgery (Supplemental Table 3).
Figure 4.
Higher intraoperative BP variability parameters were associated with higher risks of postoperative AKI or critical AKI. We constructed generalized additive models, including preoperative postoperative AKI risk factors, hypotensive parameters, and the corresponding BP values. The predicted values are from the smoothing function of the explanatory variables and are centered to zero. The solid lines indicate the predicted values and the dotted lines indicate the 95% confidence intervals. The analysis included variability ranges from the fifth to the 95th percentiles of each parameter. The adjusted variables were age, sex, eGFR (continuous, ml/min per 1.73 m2), diabetes mellitus, surgery duration (continuous, hours), emergency operation, usage of renin-angiotensin-aldosterone blockades, dipstick albuminuria, anemia (<13 g/dl for male, <12 g/dl for female), hypoalbuminemia (<3.5 g/dl), hyponatremia (<135 mEq/L), the lowest mean arterial pressure values, and area under curve under the mean arterial pressure of 65 mm Hg. ARV, average real variability; CV, coefficient of variation; VIM, variation independent of mean.
Table 3.
Associations of intraoperative BP variability parameters with postoperative AKI in the SPARK-discovery cohort
| Subgroup, Outcome, and Exposure | Univariable Model | Multivariable Model 1a | Multivariable Model 2b | |||
|---|---|---|---|---|---|---|
| sOR (95% CI) | P Value | Adjusted sOR (95% CI) | P Value | Adjusted sOR (95% CI) | P Value | |
| All SPARK-discovery patientsc | ||||||
| Postoperative AKI | ||||||
| SD | 1.2 (1.16 to 1.25) | <0.001 | 1.11 (1.05 to 1.17) | <0.001 | 1.12 (1.06 to 1.19) | <0.001 |
| Coefficient of variation | 1.39 (1.33 to 1.44) | <0.001 | 1.14 (1.08 to 1.2) | <0.001 | 1.15 (1.08 to 1.22) | <0.001 |
| Average real variability | 1.2 (1.15 to 1.25) | <0.001 | 1.13 (1.08 to 1.18) | <0.001 | 1.13 (1.07 to 1.19) | <0.001 |
| Variation independent of mean | 1.36 (1.3 to 1.41) | <0.001 | 1.13 (1.07 to 1.2) | <0.001 | 1.15 (1.08 to 1.22) | <0.001 |
| Critical AKI | ||||||
| SD | 1.29 (1.19 to 1.41) | <0.001 | 1.14 (1.02 to 1.27) | 0.02 | 1.18 (1.04 to 1.32) | 0.01 |
| Coefficient of variation | 1.59 (1.46 to 1.72) | <0.001 | 1.2 (1.07 to 1.34) | <0.001 | 1.23 (1.09 to 1.39) | <0.001 |
| Average real variability | 1.24 (1.14 to 1.35) | <0.001 | 1.13 (1.03 to 1.25) | 0.01 | 1.13 (1.02 to 1.26) | 0.02 |
| Variation independent of mean | 1.54 (1.42 to 1.67) | <0.001 | 1.19 (1.06 to 1.33) | <0.001 | 1.22 (1.08 to 1.38) | <0.001 |
| In patients with hypotensiond | ||||||
| Postoperative AKI | ||||||
| SD | 1.17 (1.12 to 1.22) | <0.001 | 1.16 (1.09 to 1.23) | <0.001 | 1.17 (1.09 to 1.24) | <0.001 |
| Coefficient of variation | 1.35 (1.29 to 1.42) | <0.001 | 1.2 (1.12 to 1.27) | <0.001 | 1.20 (1.12 to 1.29) | <0.001 |
| Average real variability | 1.19 (1.14 to 1.25) | <0.001 | 1.19 (1.13 to 1.25) | <0.001 | 1.18 (1.11 to 1.25) | <0.001 |
| Variation independent of mean | 1.32 (1.26 to 1.38) | <0.001 | 1.19 (1.12 to 1.27) | <0.001 | 1.20 (1.12 to 1.28) | <0.001 |
| Critical AKI | ||||||
| SD | 1.28 (1.16 to 1.4) | <0.001 | 1.22 (1.08 to 1.36) | <0.001 | 1.24 (1.09 to 1.4) | <0.001 |
| Coefficient of variation | 1.56 (1.42 to 1.71) | <0.001 | 1.27 (1.13 to 1.44) | <0.001 | 1.29 (1.13 to 1.47) | <0.001 |
| Average real variability | 1.23 (1.13 to 1.34) | <0.001 | 1.19 (1.07 to 1.31) | <0.001 | 1.17 (1.04 to 1.31) | 0.01 |
| Variation independent of mean | 1.51 (1.38 to 1.65) | <0.001 | 1.26 (1.12 to 1.42) | <0.001 | 1.28 (1.12 to 1.46) | <0.001 |
| In patients without hypotensiond | ||||||
| Postoperative AKI | ||||||
| SD | 1.11 (1.01 to 1.22) | 0.03 | 1.00 (0.89 to 1.11) | 0.95 | 1.02 (0.90 to 1.15) | 0.79 |
| Coefficient of variation | 1.12 (1.00 to 1.25) | 0.04 | 1.00 (0.88 to 1.14) | 0.94 | 1.03 (0.89 to 1.19) | 0.71 |
| Average real variability | 1.00 (0.90 to 1.11) | 0.96 | 0.94 (0.84 to 1.05) | 0.31 | 0.97 (0.85 to 1.10) | 0.62 |
| Variation independent of mean | 1.12 (1.01 to 1.24) | 0.04 | 1.00 (0.88 to 1.14) | 0.96 | 1.03 (0.89 to 1.18) | 0.72 |
| Critical AKI | ||||||
| SD | 0.96 (0.74 to 1.23) | 0.75 | 0.81 (0.60 to 1.09) | 0.18 | 0.83 (0.58 to 1.16) | 0.29 |
| Coefficient of variation | 1.01 (0.76 to 1.33) | 0.97 | 0.82 (0.58 to 1.15) | 0.26 | 0.85 (0.57 to 1.26) | 0.44 |
| Average real variability | 0.91 (0.68 to 1.18) | 0.49 | 0.84 (0.61 to 1.13) | 0.27 | 0.86 (0.60 to 1.2) | 0.39 |
| Variation independent of mean | 1.00 (0.75 to 1.31) | 0.99 | 0.82 (0.58 to 1.14) | 0.24 | 0.85 (0.57 to 1.24) | 0.41 |
All variability parameters were included in the regression model as continuous variables, and standardized odds ratios per 1 SD higher are presented; the 1-SD values were 4.2 mm Hg, 4.7%, 2.8 mm Hg/measurement, and 0.03 unit for SD, coefficient of variation, average real variability, and variation independent of mean parameters, respectively. SPARK, simple postoperative AKI risk; sOR, standardized odds ratio.
Multivariable model 1 was adjusted for preoperative risk factors: age, sex, eGFR (continuous, ml/min per 1.73 m2), diabetes mellitus, surgery duration (continuous, hours), emergency operation, usage of renin-angiotensin-aldosterone system blockades, dipstick albuminuria, anemia (<13 g/dl for male, <12 g/dl for female), hypoalbuminemia (<3.5 g/dl), hyponatremia (<135 mEq/L), and hypotensive variables, including area under curve (minutes multiplied by subtraction [mm Hg×min]) of mean arterial pressure <65 mm Hg, and the lowest mean arterial pressure value during surgery, without missing values.
In multivariable model 2, estimated blood loss (ml) and red blood cell transfusion amount (ml) during surgery were added to multivariable model 1. The model was constructed with 32,446 patients with existing estimated blood loss records.
The numbers of postoperative AKI events were 2230 (5%) and the number of critical AKI events was 443 (1%) in the SPARK-discovery cohort with 45,520 patients.
Subgroups were divided according to presence of any mean arterial pressure <65 mm Hg during surgery. The subgroup with hypotension included 32,101 patients with 1827 (6%) AKI and 383 (1%) critical AKI events, respectively. The subgroup without hypotension included 13,419 patients with 403 (3%) AKI and 60 (0%) critical AKI events, respectively.
We further divided subgroups according to the presence of significant intraoperative hypotension (mean arterial pressure <65 mm Hg) and high BP variability (average real variability in the upper-quartile range) and investigated the risk of AKI outcomes within the subgroups (Table 4). Those without intraoperative hypotension did not have a higher risk of postoperative AKI, even though they had high BP variability. In the multivariable analysis, those with intraoperative hypotension did not have significantly higher risk of postoperative AKI or associated outcomes in the absence of intraoperative high BP variability. Meanwhile, those who experienced the two BP instability events, hypotension and high variability, at the same time had a significantly higher risk of postoperative AKI and associated outcomes when compared with those who did not.
Table 4.
Combined associations of hypotension and intraoperative BP variability parameters with postoperative AKI in the SPARK-discovery cohort
| Outcome and Exposure | Outcome/Patient (N) | Univariable Model | Multivariable Modela | ||
|---|---|---|---|---|---|
| sOR (95% CI) | P Value | Adjusted sOR (95% CI) | P Value | ||
| Postoperative AKIb | |||||
| Intraoperative hypotension (+), high variability (+) | 612/8831 (7%) | 2.40 (2.09 to 2.76) | <0.001 | 1.26 (1.09 to 1.46) | 0.002 |
| Intraoperative hypotension (+), high variability (−) | 1215/23,270 (5%) | 1.78 (1.57 to 2.01) | <0.001 | 1.00 (0.87 to 1.14) | 0.97 |
| Intraoperative hypotension (−), high variability (+) | 76/2549 (3%) | 0.99 (0.76 to 1.27) | 0.94 | 0.98 (0.87 to 1.27) | 0.91 |
| Intraoperative hypotension (−), high variability (−) | 327/10,870 (3%) | Reference | Reference | ||
| Critical AKIb | |||||
| Intraoperative hypotension (+), high variability (+) | 136/8831 (2%) | 3.19 (2.34 to 4.43) | <0.001 | 1.57 (1.13 to 2.20) | 0.008 |
| Intraoperative hypotension (+), high variability (−) | 247/23,270 (1%) | 2.19 (1.64 to 2.98) | <0.001 | 1.20 (0.88 to 1.65) | 0.25 |
| Intraoperative hypotension (−), high variability (+) | 7/2549 (0%) | 0.56 (0.23 to 1.16) | 0.15 | 0.54 (0.22 to 1.12) | 0.13 |
| Intraoperative hypotension (−), high variability (−) | 53/10,870 (1%) | Reference | Reference | ||
Intraoperative hypotension was defined as any experience of mean arterial pressure <65 mm Hg. Presence of high variability was determined by an average real variability value in the upper-quartile range (≥8.5605 mm Hg/measurement in the SPARK-discovery cohort). SPARK, simple postoperative AKI risk; sOR, standardized odds ratio.
Multivariable model was adjusted for age, sex, eGFR, diabetes mellitus, surgery duration (continuous, hours), emergency operation, usage of renin-angiotensin-aldosterone system blockades, dipstick albuminuria, anemia (<13 g/dl for male, <12 g/dl for female), hypoalbuminemia (<3.5 g/dl), and hyponatremia (<135 mEq/L), without missing values.
The number of postoperative AKI events was 2230 (5%) and the number of critical AKI events was 443 (1%) in the SPARK-discovery cohort of 45,520 patients.
Lastly, when the analysis was limited to those who experienced intraoperative hypotension, the addition of intraoperative BP-variability parameters to a prediction model for postoperative AKI yielded significant improvement in discriminative power when compared with a model that included both preoperative AKI risk factors and hypotension-related variables (Supplemental Table 4).
Validation Study in the Simple Postoperative AKI Risk–Validation Cohort
In the SPARK-validation cohort, the lowest mean arterial pressure was significantly associated with the risk of postoperative AKI only in patients with significant intraoperative hypotension (Supplemental Table 5). Moreover, the variability parameters were significantly associated with the risk of postoperative AKI (Table 5), and the association was particularly significant in those who experienced intraoperative mean arterial pressure <65 mm Hg (Supplemental Table 6). However, the addition of the variability parameters only resulted in significant improvement in the predictive power of the multivariable models for postoperative AKI risks in those without intraoperative hypotension (Supplemental Table 4).
Table 5.
Associations of intraoperative BP variability with postoperative AKI in validation cohorts
| Cohort, Outcome, and Exposure | Univariable Model | Multivariable Model 1a | ||
|---|---|---|---|---|
| sOR (95% CI) | P Value | Adjusted sOR (95% CI) | P Value | |
| SPARK-validation cohortb | ||||
| Postoperative AKI | ||||
| SD | 1.16 (1.10 to 1.21) | <0.001 | 1.11 (1.05 to 1.18) | <0.001 |
| Coefficient of variation | 1.20 (1.15 to 1.26) | <0.001 | 1.13 (1.07 to 1.21) | <0.001 |
| Average real variability | 1.17 (1.11 to 1.22) | <0.001 | 1.10 (1.04 to 1.16) | <0.001 |
| Variation independent of mean | 1.19 (1.14 to 1.25) | <0.001 | 1.13 (1.06 to 1.20) | <0.001 |
| Critical AKI | ||||
| SD | 1.14 (1.04 to 1.24) | <0.001 | 1.08 (0.97 to 1.20) | 0.13 |
| Coefficient of variation | 1.22 (1.12 to 1.33) | <0.001 | 1.09 (0.97 to 1.23) | 0.12 |
| Average real variability | 1.23 (1.13 to 1.34) | <0.001 | 1.18 (1.07 to 1.30) | <0.001 |
| Variation independent of mean | 1.19 (1.09 to 1.30) | <0.001 | 1.09 (0.98 to 1.22) | 0.12 |
| Continuous-monitoring cohortc | ||||
| Postoperative AKI | ||||
| SD | 1.03 (0.92 to 1.15) | 0.63 | 1.06 (0.93 to 1.2) | 0.38 |
| Coefficient of variation | 1.13 (1.01 to 1.26) | 0.03 | 1.06 (0.93 to 1.21) | 0.37 |
| Average real variability | 1.27 (1.15 to 1.39) | <0.001 | 1.22 (1.11 to 1.35) | <0.001 |
| Variation independent of mean | 1.13 (1.01 to 1.26) | 0.03 | 1.06 (0.93 to 1.21) | 0.37 |
| Critical AKI | ||||
| SD | 0.95 (0.76 to 1.16) | 0.61 | 0.88 (0.7 to 1.1) | 0.27 |
| Coefficient of variation | 1.20 (0.98 to 1.44) | 0.06 | 0.96 (0.77 to 1.2) | 0.75 |
| Average real variability | 1.40 (1.21 to 1.61) | <0.001 | 1.31 (1.11 to 1.53) | <0.001 |
| Variation independent of mean | 1.19 (0.98 to 1.44) | 0.07 | 0.96 (0.77 to 1.2) | 0.74 |
All variability parameters were included in the regression model as continuous variables, and standardized odds ratios per 1-SD higher are presented. In the SPARK-validation cohort, the 1-SD values were 3.7 mm Hg, 3.7%, 3.1 mm Hg/measurement, and 0.08 unit for SD, coefficient of variation, average real variability, and variation independent of mean parameters, respectively. In the continuous-monitoring cohort, the 1-SD values were 3.0 mm Hg, 3.4%, 0.5 mm Hg/measurement, and 0.005 unit for SD, coefficient of variation, average real variability, and variation independent of mean parameters, respectively. sOR, standardized odds ratio; SPARK, simple postoperative AKI risk.
Multivariable model 1 was adjusted for preoperative risk factors; age, sex, eGFR (continuous, ml/min per 1.73 m2), diabetes mellitus, surgery duration (continuous, hours), emergency operation, usage of renin-angiotensin-aldosterone system blockades, dipstick albuminuria, anemia (<13 g/dl for male, <12 g/dl for female), hypoalbuminemia (<3.5 g/dl), hyponatremia (<135 mEq/L), and hypotensive variables, including area under curve (minutes multiplied by subtraction [mm Hg×min]) of mean arterial pressure <65 mm Hg, and the lowest mean arterial pressure value during surgery, without missing values.
Total number of patients in the SPARK-validation cohort was 29,704, including 1552 (5%) postoperative AKI and 444 (2%) critical AKI events.
Total number of patients in the continuous-monitoring cohort was 7435, including 300 (4%) postoperative AKI and 91 (1%) critical AKI events.
Validation Study in the Continuous-Monitoring Cohort
In the continuous-monitoring cohort, time or area under curve of mean arterial pressure of <65 mm Hg was significantly associated with a higher risk of postoperative AKI or critical AKI (Supplemental Table 7). In this cohort, only 84 (1%) patients did not experience any mean arterial pressure <65 mm Hg because hypotension for a few seconds was frequent; thus, no additional subgroup analysis according to the presence of significant hypotension was performed. When we assessed the variability parameters (Table 5), the average real variability showed significant association with the risk of postoperative AKI or critical AKI. SD, coefficient variation, or variation independent of the mean were not significantly associated with an increased risk of the studied outcomes. The addition of the variability parameters resulted in a significant improvement of the predictive power of the multivariable models to predict risk of postoperative AKI (Supplemental Table 4).
Discussion
In this observational study, we found that high intraoperative BP variability was associated with the risk of postoperative AKI, independent of the confounding factors including absolute BP levels and hypotension. A mean arterial pressure of 65 mm Hg was found to be an acceptable threshold for defining significant intraoperative hypotension. We found that the mean arterial pressure variability parameters were associated with increased risk of AKI, particularly in patients experiencing significant intraoperative hypotension.
Intraoperative hemodynamic instability is considered to be one of the most important causes of postoperative AKI (27). The postoperative AKI risk in cardiac surgery that requires heart bypass is critically high (28,29) because of possible ischemic insult during the operation. In noncardiac surgery, recent studies revealed a mean arterial pressure of 65 mm Hg to be a threshold for determining significant BP decrement (13,15,16). Furthermore, whether greatly fluctuating BP is associated with the risk of postoperative AKI for patients who undergo noncardiac surgery has rarely been reported, although it has been suspected (17,18). The strengths of this study are the following: we reported a significant association between high BP variability and the risk of postoperative AKI using multiple variability parameters; we validated our findings in two additional large cohorts; we included patient-oriented outcomes; and we considered intraoperative hypotension, which is considered to be a major determinant of kidney injury during surgery. Because high mean arterial pressure variability was a significant risk factor for both postoperative and critical AKI in all studied cohorts, we suggest that BP fluctuation be reduced, along with the avoidance of hypotension, particularly in patients with other preoperative clinical risk factors.
Kidneys adapt to changes in blood perfusion using appropriate neurohormonal responses to maintain adequate perfusion status (12,30,31). Because the neurohormonal balance changes over time, a rapid alteration of hemodynamic status, not only its absolute level, would likely be beyond the capacity of such kidney adaptation (17,18). The average real variability, which is calculated from the difference between adjacent BP values, showed a prominent association with postoperative AKI risk when intra-arterial mean arterial pressure was measured in seconds. The superiority of average real variability in reflecting the order and changes of BP in short time intervals can be easily understood with some examples (Supplemental Figure 3). This suggests that such BP variability within a momentary time frame, rather than the overall amount of BP fluctuation, may be the factor that causes ischemic injury. Moreover, adding the BP-variability parameters to the prediction models for postoperative AKI modestly improved their predictability, particularly when the BP values were measured in short time intervals. Therefore, high mean arterial pressure variability may be regarded as an independent risk factor for postoperative AKI that requires clinicians’ real-time monitoring.
Another interesting finding was that high mean arterial pressure variability was not prominently associated with the risk of AKI and AKI-related outcomes in patients without significant intraoperative hypotension. This suggests that fluctuations in BP that are over the threshold levels for significant kidney hypoperfusion may be benign or only cause easily reversible, mild creatinine elevation. On the contrary, the importance of BP variability was even more obvious in patients with significant intraoperative hypotension; hence, a rapid decrease in BP below the threshold level is the primary event to avoid. In addition, because intraoperative hypotension without high BP variability was not significantly associated with risk of postoperative AKI in the multivariable models, a flat BP marginally below the threshold may have minimal significance or be within the adaptable range in patients without other major risk factors. Further prospective studies should confirm whether securing adequate perioperative patient volume status or appropriate usage of BP-modifying agents would be helpful in avoiding such an event. Particularly, clinicians may consider implementing additional monitoring or intervention strategies for patient groups who are at risk of impaired adaptation to BP changes leading to a possible higher BP variability (e.g., impaired kidney function, diabetes mellitus) (32,33). Still, because the effect of BP variability was fairly modest, BP variability may be a supplementary target along with other major postoperative AKI determinants (including intraoperative bleeding or hypotension) that should be added to current guidelines or care bundles for postoperative AKI (11).
Our study had several limitations. First, because of its observational nature, our study did not provide any actual strategy to decrease the BP variability and consequently ameliorate the postoperative AKI risk. An appropriate management principle to minimize intraoperative hypotension and variability may be tested in future studies and suggested in guidelines. Second, because the creatinine values were reviewed retrospectively, the timing of assessment for kidney function was not unified. The possible inclusion of undiagnosed preoperative kidney injury and considerable exclusion of patients without available perioperative creatinine levels or those who died before the complete assessment of kidney injury might have caused selection bias. Furthermore, the lack of urine output information due to scarce availability is another limitation. Lastly, the effects of using BP-modifying agents have not been studied in detail due to their complexity. A clinical trial or a study with clear classification of the types and timing of the usage of such agents may give important evidence for a clinical practice to maintain stable intraoperative BP.
In conclusion, we found that high intraoperative BP variability was independently associated with postoperative AKI in patients who underwent noncardiac surgery. Clinicians may consider BP variability as a significant parameter of intraoperative hemodynamic stability in addition to hypotension. Future studies may test the benefits of reducing BP variability and intraoperative hypotension in decreasing the critical burden of postoperative AKI in patients who undergo noncardiac operations.
Data Sharing Statement
Data are available from the corresponding author for reasonable requests.
Disclosures
All authors have nothing to disclose.
Funding
This research was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant numbers HI16C2221 and HI18C1604). The research received a grant from the National Research Foundation, Republic of Korea (2019R1A2C1085411). The funders did not have any role in study design, collection, analysis, and interpretation of data, writing the report, or the decision to submit the report for publication.
Supplementary Material
Acknowledgments
We acknowledge Hyunjin Cho for her assistance in data collection.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06620619/-/DCSupplemental.
Supplemental Table 1. Variation parameters according to intraoperative and surgery-related characteristics.
Supplemental Table 2. The discriminative powers of the multivariable logistic regression models with inclusion of intraoperative hypotension parameters with different thresholds in SPARK-discovery cohort.
Supplemental Table 3. Sensitivity analysis for the associations between intraoperative BP variability and postoperative AKI risk with defining AKI with peak serum creatinine level within 7 days in the SPARK-discovery cohort.
Supplemental Table 4. Comparisons of area-under-the-curve values of the postoperative AKI predictive models according to addition of BP parameters.
Supplemental Table 5. Validation analysis regarding intraoperative hypotensive parameters in the SPARK-validation cohort.
Supplemental Table 6. Validation analysis results in the subgroups of SPARK-validation cohort with the variability parameters.
Supplemental Table 7. Validation analysis regarding intraoperative hypotensive parameters in the continuous-monitoring cohort.
Supplemental Figure 1. Distribution of the variability parameters and their descriptive statistics.
Supplemental Figure 2. Correlation between preoperative clinical variables and variability parameters in the study population.
Supplemental Figure 3. Example cases showing the advantage of average real variability parameter which reflects the orders and differences between the adjacent values.
Supplemental Method 1. The studied mean arterial pressure parameters.
Supplemental Method 2. Equations for calculation of the variability parameters from measured n number of BP values (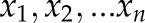 ) with mean value (
) with mean value (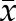 ).
).
Supplemental Method 3. Details of the statistical analysis.
References
- 1.Abelha FJ, Botelho M, Fernandes V, Barros H: Determinants of postoperative acute kidney injury. Crit Care 13: R79, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, Thottakkara P, Efron PA, Moore FA, Moldawer LL, Segal MS, Bihorac A: Cost and mortality associated with postoperative acute kidney injury. Ann Surg 261: 1207–1214, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biteker M, Dayan A, Tekkeşin AI, Can MM, Taycı İ, İlhan E, Şahin G: Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 207: 53–59, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Bellomo R, Kellum JA, Ronco C: Acute kidney injury. Lancet 380: 756–766, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Park S, Cho H, Park S, Lee S, Kim K, Yoon HJ, Park J, Choi Y, Lee S, Kim JH, Kim S, Chin HJ, Kim DK, Joo KW, Kim YS, Lee H: Simple postoperative AKI risk (SPARK) classification before noncardiac surgery: A prediction index development study with external validation. J Am Soc Nephrol 30: 170–181, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kheterpal S, Tremper KK, Heung M, Rosenberg AL, Englesbe M, Shanks AM, Campbell DA Jr.: Development and validation of an acute kidney injury risk index for patients undergoing general surgery: Results from a national data set. Anesthesiology 110: 505–515, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Park S, Lee H: Acute kidney injury prediction models: Current concepts and future strategies. Curr Opin Nephrol Hypertens 28: 552–559, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Soares DM, Pessanha JF, Sharma A, Brocca A, Ronco C: Delayed Nephrology consultation and high mortality on acute kidney injury: A meta-analysis. Blood Purif 43: 57–67, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Park S, Baek SH, Ahn S, Lee KH, Hwang H, Ryu J, Ahn SY, Chin HJ, Na KY, Chae DW, Kim S: Impact of electronic Acute Kidney Injury (AKI) alerts with automated nephrologist consultation on detection and Severity of AKI: A quality improvement study. Am J Kidney Dis 71: 9–19, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Göcze I, Jauch D, Götz M, Kennedy P, Jung B, Zeman F, Gnewuch C, Graf BM, Gnann W, Banas B, Bein T, Schlitt HJ, Bergler T: Biomarker-guided intervention to prevent acute kidney injury after major surgery: The prospective randomized BigpAK study. Ann Surg 267: 1013–1020, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, Zarbock A: Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensive Care Med 43: 1551–1561, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munshi R, Hsu C, Himmelfarb J: Advances in understanding ischemic acute kidney injury. BMC Med 9: 11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, Cywinski J, Thabane L, Sessler DI: Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension. Anesthesiology 119: 507–515, 2013 [DOI] [PubMed] [Google Scholar]
- 14.KDIGO Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 6, 2012 [Google Scholar]
- 15.Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, Kurz A: Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: A retrospective cohort analysis. Anesthesiology 126: 47–65, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Wesselink EM, Kappen TH, Torn HM, Slooter AJC, van Klei WA: Intraoperative hypotension and the risk of postoperative adverse outcomes: A systematic review. Br J Anaesth 121: 706–721, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Collange O, Jazaerli L, Lejay A, Biermann C, Caillard S, Moulin B, Chakfe N, Severac F, Schaeffer M, Mertes PM, Steib A: Intraoperative pleth variability index is linked to delayed graft function after kidney transplantation. Transplant Proc 48: 2615–2621, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Mascha EJ, Yang D, Weiss S, Sessler DI: Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology 123: 79–91, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Xie Z, Liao X, Yin W, Kang Y, Guo J, Lu M: Relationship between short-term blood pressure variability and incidence of acute kidney injury in critically ill patients. Kidney Blood Press Res 42: 1238–1246, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Lee HC, Jung CW: Vital Recorder-a free research tool for automatic recording of high-resolution time-synchronised physiological data from multiple anaesthesia devices. Sci Rep 8: 1527, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HC, Ryu HG, Chung EJ, Jung CW: Prediction of bispectral index during target-controlled infusion of propofol and remifentanil: A deep learning approach. Anesthesiology 128: 492–501, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, Inoue R, Hoshi H, Hashimoto J, Totsune K, Satoh H, Imai Y: Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: The Ohasama study. Hypertension 52: 1045–1050, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van Der Niepen P, O’Brien E; Office versus Ambulatory Pressure Study Investigators : Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med 348: 2407–2415, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR: Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 375: 895–905, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Pierdomenico SD, Di Nicola M, Esposito AL, Di Mascio R, Ballone E, Lapenna D, Cuccurullo F: Prognostic value of different indices of blood pressure variability in hypertensive patients. Am J Hypertens 22: 842–847, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Jin DC, Yun SR, Lee SW, Han SW, Kim W, Park J, Kim YK: Current characteristics of dialysis therapy in Korea: 2016 registry data focusing on diabetic patients. Kidney Res Clin Pract 37: 20–29, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ: Incidence of intraoperative hypotension as a function of the chosen definition: Literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology 107: 213–220, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Garg AX, Devereaux PJ, Yusuf S, Cuerden MS, Parikh CR, Coca SG, Walsh M, Novick R, Cook RJ, Jain AR, Pan X, Noiseux N, Vik K, Stolf NA, Ritchie A, Favaloro RR, Parvathaneni S, Whitlock RP, Ou Y, Lawrence M, Lamy A; CORONARY Investigators : Kidney function after off-pump or on-pump coronary artery bypass graft surgery: A randomized clinical trial [Published correction appears in JAMA 312: 97, 2014]. JAMA 311: 2191–2198, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Shroyer AL, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E, Lucke JC, Baltz JH, Novitzky D; Veterans Affairs Randomized On/Off Bypass (ROOBY) Study Group : On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med 361: 1827–1837, 2009. 19890125 [Google Scholar]
- 30.Bonventre JV, Weinberg JM: Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Guazzi MD, Barbier P, Loaldi A, Montorsi P, Polese A, Tosi E, Fiorentini C: Intrarenal beta-receptor and renal baroreceptor interaction in the control of the renin response to transient reduction of the renal perfusion pressure in man. J Hypertens 3: 39–45, 1985 [DOI] [PubMed] [Google Scholar]
- 32.Zhou TL, Kroon AA, Reesink KD, Schram MT, Koster A, Schaper NC, Dagnelie PC, van der Kallen CJH, Sep SJS, Stehouwer CDA, Henry RMA: Blood pressure variability in individuals with and without (pre)diabetes: The Maastricht Study. J Hypertens 36: 259–267, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Sarafidis PA, Ruilope LM, Loutradis C, Gorostidi M, de la Sierra A, de la Cruz JJ, Vinyoles E, Divisón-Garrote JA, Segura J, Banegas JR: Blood pressure variability increases with advancing chronic kidney disease stage: A cross-sectional analysis of 16 546 hypertensive patients. J Hypertens 36: 1076–1085, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.