Visual Abstract
Keywords: Proximal tubular secretion; Tubular solute clearance; polycystic kidney disease; humans; polycystic kidney, autosomal dominant; glomerular filtration rate; cinnamoylglycine; xanthosine; pyridoxic acid; tiglyglycine; linear models; control groups; kidney; glycine; ribonucleosides; nephrons; hippurates; chronic renal insufficiency; demography; mass spectrometry
Abstract
Background and objectives
In autosomal dominant polycystic kidney disease (ADPKD), the GFR often remains normal despite significant nephron loss. Proximal tubular secretory clearance may be reduced in ADPKD before detectable changes in GFR.
Design, setting, participants, & measurements
We used targeted mass spectrometry to quantify secretory solutes from blood and urine samples from 31 patients with ADPKD and preserved GFR (mean eGFR =111±11 ml/min per 1.73 m2) and 25 healthy control individuals as well as from 95 patients with ADPKD and reduced GFR (mean eGFR =53±21 ml/min per 1.73 m2) and 92 individuals with non-ADPKD CKD. We used linear regression to compare the fractional excretion of each solute between ADPKD and control groups. Among 112 patients with ADPKD, we used linear regression to determine associations of solute fractional excretion with height-adjusted total kidney volume.
Results
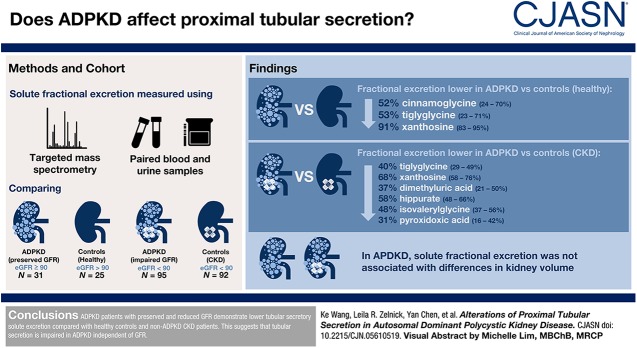
After adjusting for demographics, clinical characteristics, and kidney function measures, the fractional excretions of three secretory solutes were lower in patients with ADPKD and preserved GFR compared with healthy individuals: 52% lower cinnamoylglycine excretion (95% confidence interval, 24% to 70%), 53% lower tiglylglycine excretion (95% confidence interval, 23% to 71%), and 91% lower xanthosine excretion (95% confidence interval, 83% to 95%). In addition to lower excretions of tiglylglycine and xanthosine, patients with ADPKD and reduced GFR also demonstrated 37% lower dimethyluric acid excretion (95% confidence interval, 21% to 50%), 58% lower hippurate excretion (95% confidence interval, 48% to 66%), 48% lower isovalerylglycine excretion (95% confidence interval, 37% to 56%), and 31% lower pyridoxic acid excretion (95% confidence interval, 16% to 42%) compared with patients with non-ADPKD CKD and comparable eGFR. Among patients with ADPKD, solute fractional excretions were not associated with differences in kidney volume.
Conclusions
Patients with ADPKD and preserved and reduced GFR demonstrate lower tubular secretory solute excretion compared with healthy controls and patients with non-ADPKD CKD. Our results suggest that tubular secretion is impaired in ADPKD independent of GFR.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease and an important cause of ESKD in the United States (1). In most patients, an inherited defect in one of two polycystin genes lead to tubular epithelial proliferation and cyst formation that overtake normal kidney tissue (2). Because of compensatory glomerular hyperfiltration of noncystic nephrons, the GFR often remains normal for many years after disease onset (3,4). Therefore, the assessment of kidney functions beyond GFR has the potential to offer new insights into disease pathogenesis.
The secretion of solutes by the kidney proximal tubules is an essential mechanism for efficiently removing endogenous protein-bound substances (5,6). Unlike glomerular filtration, which is a passive process determined by size and charge selectivity, tubular secretion requires cellular energy and coordination between basolateral and apical transporters. Prior studies of ADPKD demonstrate impairments of intrinsic tubular functions before detectable changes in GFR. Patients with ADPKD with preserved GFR show a lower rate of tubular glucose reabsorption compared with healthy individuals. Patients with ADPKD also demonstrate lower urine osmolality after fluid deprivation, suggesting impaired urinary concentrating ability (4,7,8). However, little is known regarding the effect of ADPKD on proximal tubular solute clearance.
We developed targeted mass spectrometric assays to quantify candidate tubular secretory solutes in blood and urine. We used these assays to determine the kidney fractional excretion of 11 secretory solutes relative to creatinine clearance and compared tubular solute fractional excretions in patients with ADPKD with patients with non-ADPKD CKD and healthy individuals without kidney disease.
Materials and Methods
Study Populations: The Mid-Atlantic Autosomal Dominant Polycystic Kidney Disease Cohort
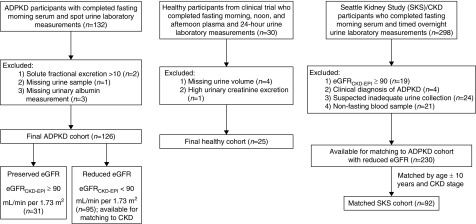
The Mid-Atlantic Autosomal Dominant Polycystic Kidney Disease Cohort is a prospective study of adults with ADPKD. From 2013 to 2016, the cohort enrolled 132 participants from the Baltimore PKD Center at the University of Maryland. The diagnosis of ADPKD was confirmed on the basis of modified Pei–Ravine criteria (9). Eligibility criteria were age >18 years old and eGFR˃15 ml/min per 1.73 m2. Major exclusions included prior kidney transplantation, pregnancy, and uncontrolled diabetes. Each participant provided a serum and spot urine sample after an overnight fast on the morning of their study visit. A subset of 112 study participants underwent abdominal magnetic resonance imaging (MRI) for determination of total kidney volume by a radiologist who was blinded to other study characteristics. For the purpose of this study, we excluded three participants who had missing urine albumin measurements and one participant who had a missing urine sample. We further excluded two participants with ADPKD who had implausibly high fractional excretions (>10) of any secretory solute: one participant had cinnamoylglycine fractional excretion of 14.7, and another participant had isovalerylgycine fractional excretion of 14.8, likely due to measurement error. Our final cohort consisted of 126 participants with ADPKD (Figure 1). All participants provided informed consent, and the institutional review board of the University of Maryland approved the study.
Figure 1.
Participant inclusions and exclusions by study cohort. ADPKD, autosomal dominant polycystic kidney disease.
Control Populations
We chose two control populations on the basis of the level of eGFR (Figure 1). For patients with ADPKD who had an eGFR calculated with the Chronic Kidney Disease Epidemiology Collaboration equation (eGFRCKD-EPI) ≥90 ml/min per 1.73 m2 (n=31), we selected a control population of 30 healthy individuals, all with eGFR≥90 ml/min per 1.73 m2, from a previously completed dietary trial (10,11). Trial eligibility criteria were a body mass index (BMI) =28–33 kg/m2 and no prior history of cardiovascular disease, diabetes mellitus, active smoking, or alcohol abuse. During the baseline trial visit, participants provided a 24-hour urine collection and multiple blood samples during the same time period. We excluded four trial participants who had missing urine data and one participant who had evidence of an overcollected urine sample on the basis of urinary creatinine excretion (12), leaving a final cohort of 25 healthy participants for comparison. For each participant, we used the three fasting blood samples obtained at 8 a.m., 12 p.m., and 5:30 p.m.; 8 a.m. blood samples was missing for three participants.
For patients with ADPKD who had an eGFR<90 ml/min per 1.73 m2 (median eGFR =110 ml/min per 1.73 m2; interquartile range, 103–120 ml/min per 1.73 m2), we selected a control population of general patients with CKD and without ADPKD from the Seattle Kidney Study (SKS), a prospective study of 691 patients with CKD recruited from outpatient nephrology clinics in Seattle, Washington. Eligibility criteria for the SKS were age >18 years old and CKD of any stage not requiring dialysis. Major exclusions were kidney transplantation or the expectation of starting RRT within 3 months. For purposes of this study, we selected 298 SKS participants who provided a timed collection of urine sample collected continuously during the night before their outpatient study visit (mean collection time =12.8±3.5 hours) and a fasting morning blood sample during morning of their study visit. We further excluded four SKS participants with a suspected clinical diagnosis of ADPKD on the basis of review of outpatient clinic notes, 19 participants with eGFRCKD-EPI≥90 ml/min per 1.73 m2, 24 participants whose timed urine samples were suspected to be under- or overcollected on the basis of urinary creatinine excretion (12), and 21 participants whose blood samples were nonfasting, leaving 230 eligible participants for matching. We then performed 1:1 matching of ADPKD cohort members to eligible SKS participants on the basis of eGFRCKD-EPI categories (<30, 30–59, and 60–89 ml/min per 1.73 m2), age within 10 years, and when possible, race and sex, with further adjustment for these characteristics in the analyses (see below). All trial and SKS participants provided informed consent, and the University of Washington’s institutional review board approved the studies.
Measurements of Secretory Solutes
We selected 16 candidate secretory solutes on the basis of the published literature demonstrating one or more of the following characteristics: affinity for basolateral proximal tubular transporters, an increase in circulating concentrations in organic anion transporter (OAT) knockout mouse models, a high reported degree of protein binding, and/or reported kidney clearances that exceed that of creatinine or GFR (13–17). We developed a targeted liquid chromatography-tandem mass spectrometry assay for these solutes in plasma and urine using labeled internal standards and single-point calibration as previously described (18,19). Supplemental Material (Supplemental Tables 1–11) provides a detailed description of our laboratory techniques. Briefly, we extracted serum or plasma solutes from bound proteins using protein precipitation with acidified acetonitrile and solid-phase extraction through phospholipid removal. We extracted urine solutes using solid-phase extraction using either a mixed cation exchange extraction plate or a hydrophilic-lipophilic binding extraction plate (Supplemental Table 1). We reconstituted dried extracts in acetonitrile/formic acid and injected solution onto a Restek PFPP chromatographic column. The column eluate was subsequently introduced into a triple quadrupole tandem mass spectrometer (Sciex 6500) (Supplemental Tables 3–8). Limits of quantification and recoveries in different matrices are shown in Supplemental Tables 9 and 10. Lipemia and low total protein did not interfere with any analyte recoveries. We used stable isotope-labeled internal standards (Supplemental Table 2) and external calibration to purchased chemicals (Supplemental Table 1) to maximize precision. To maximize the accuracy of the calibration and minimize measurement bias, we used standard addition of purified analytes that were quantified using nuclear magnetic resonance. We performed further validation experiments to maximize precision. Inter- and intraassay coefficients of variation for individual solutes in plasma and urine range from 3.4% to 14.7% (Supplemental Table 11).
We were unable to determine the lower limits of detection for three solutes due to isobaric interference (adipic acid, suberic acid, and succinic acid), and therefore, we excluded these solutes from further analyses. We further excluded pantothenic acid from consideration on the basis of previous studies demonstrating glomerular filtration and tubular reabsorption of this compound (20,21) and 3-hydroxy hippurate due to implausibly high kidney clearance. Subsequent protein binding studies performed in our laboratory revealed that three additional solutes (isovalerylglycine, tiglylglycine, and xanthosine) had lower binding percentages than previously reported (Supplemental Table 11). However, we retained these solutes in this study, because their kidney clearances greatly exceed eGFR, suggesting tubular secretion as the major kidney pathway of elimination.
We calculated the fractional excretion of each secretory solute (FEX) as the proportion of kidney solute clearance relative to creatinine clearance:
In this equation, UX and PX represent urine and serum/plasma concentrations of secretory solute X, respectively, and UCr and PCr represent urine and plasma concentrations of creatinine, respectively. For healthy individuals in the dietary trial, PX was calculated from the average of three fasting measurements of each solute; otherwise, we used spot serum solute concentrations to calculate fractional excretions.
Measurements of Covariates
ADPKD study coordinators obtained demographic and medical histories via patient interviews and review of medical records and measured serum creatinine concentrations using an isotope dilution mass spectrometry–traceable assay. The SKS participants provided self-reported demographic information, social habits, and prevalent medical conditions via study questionnaires administered during the study visits. In the dietary trial and the SKS, coordinators measured serum and urine concentrations of creatinine using the Modified Jaffe Method on a Beckman DXC Unicell clinical analyzer with calibration to isotope dilution mass spectroscopy. GFR was estimated using the 2009 Chronic Kidney Disease Epidemiology Collaboration equation for all study cohorts (22).
Statistical Analyses
We compared relative differences in solute fractional excretions across study cohorts using linear regression. Models included the log-transformed fractional excretion value as the dependent variable, the cohort of interest (ADPKD versus control) as the independent variable, and adjustment terms for eGFRCKD-EPI, age, sex, BMI, and urine albumin-to-creatinine ratio. Exponentiated coefficients from these models can be interpreted as the fold difference in solute fractional excretion between the cohorts under comparison holding the other model covariates constant.
We controlled for multiple comparisons using the Bonferroni correction, controlling the familywise type I error rate at 5%. For each individual test, this corresponds to a two-sided P value of 0.05/11=<0.01 for declaring statistical significance. Among the ADPKD cohort, we used linear regression to determine associations of solute fractional excretions with height-adjusted kidney volume. All statistical analyses were conducted using the statistical computing environment R version 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria) and STATA 11 (StataCorp, College Station, TX).
Results
Description of Study Populations
Among the 31 ADPKD cohort members with eGFR ≥ 90 ml/min per 1.73 m2, there were 25 healthy trial participants for comparison (Figure 1). Among the 95 ADPKD cohort members with eGFR<90 ml/min per 1.73 m2, there were 92 matches to eligible SKS participants on the basis of CKD stage and age. Participants with ADPKD and preserved eGFR and healthy individuals from the dietary trial were relatively young, predominantly white, and free of major comorbidities (Table 1). However, mean BMI was lower in the ADPKD group (26.9±5.4 versus 30.1±1.3). Among participants with ADPKD and SKS participants with reduced eGFR, age and eGFR values were similar after matching on these characteristics. Nonetheless, participants with ADPKD were more likely to be white, were women, had fewer medical comorbidities, had lower BMI, and had lower urinary albumin and creatinine excretion compared with SKS participants.
Table 1.
Patient characteristics by study cohort
| Characteristics | ADPKD, eGFR≥90 ml/min per 1.73 m2, n=31 | Healthy, n=25 | ADPKD, eGFR<90 ml/min per 1.73 m2, n=95 | CKD, n=92 |
|---|---|---|---|---|
| Age, yr | 32 (7) | 38 (13) | 50 (11) | 52 (10) |
| Women | 18 (58) | 16 (64) | 59 (62) | 37 (40) |
| Race/ethnicity | ||||
| White | 26 (84) | 25 (100) | 80 (84) | 56 (61) |
| Black | 3 (10) | 0 (0) | 11 (12) | 25 (27) |
| Other | 2 (6) | 0 (0) | 4 (4) | 11 (12) |
| Current smoking | NC | 0 (0) | NC | 27 (30) |
| Alcohol use | NC | 0 (0) | NC | 28 (30) |
| History of coronary artery disease | 0 (0) | 0 (0) | 3 (3) | 39 (42) |
| History of diabetes | 0 (0) | 0 (0) | 4 (4) | 40 (43) |
| Systolic BP, mm Hg | 122 (11) | NC | 127 (15) | 129 (24) |
| Diastolic BP, mm Hg | 77 (9) | NC | 77 (10) | 79 (15) |
| Height, cm | 172 (11) | 168 (10) | 172 (10) | 169 (10) |
| Weight, kg | 80 (17) | 85 (10) | 82 (17) | 100 (36) |
| BMI, kg/m2 | 27 (5) | 30 (1) | 27 (5) | 33 (8) |
| Serum creatinine, mg/dl | 0.8 (0.1) | 0.7 (0.1) | 1.5 (0.6) | 1.7 (0.9) |
| eGFR (CKD-EPI), ml/min per 1.73 m2a | 110 (103–120) | 112 (104–122) | 54 (37–71) | 48 (36–69) |
| Serum albumin, g/dl | 4.3 (0.3) | NC | 4.2 (0.3) | 3.8 (0.4) |
| Urine ACR mean, mg/g | 14.0 (9.2, 41.3) | 2.6 (1.7, 3.7) | 21.1 (12.0, 43.1) | 87.3 (9.4, 410.3) |
| Urine creatinine, mg/dl | 84 (40, 121) | 39 (29, 60) | 62 (41, 98) | 76 (55, 104) |
Entries are mean (SD) or N (%) except as noted. ADPKD, autosomal dominant polycystic kidney disease; NC, not collected; BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; ACR, albumin-to-creatinine ratio.
Expressed as median (interquartile range).
Associations among Participants with ADPKD and Preserved eGFR
Among participants with ADPKD and preserved eGFR, the fractional excretions of secretory solutes ranged from a mean of 0.09 for p-cresol sulfate to 4.53 for pyridoxic acid (Table 2). After adjusting for demographics, BMI, eGFR, and urine microalbumin-to-creatinine ratio, the fractional excretions of three secretory solutes (cinnamoylglycine, tiglylglycine, and xanthosine) were significantly lower in participants with ADPKD compared with healthy individuals. The largest difference was observed for xanthosine (91% lower; 95% confidence interval, 83% to 95% lower). The fractional excretion of kynurenic acid was higher in ADPKD; however, this difference was not statistically significant. With the exception of a substantially higher xanthosine level in ADPKD, serum/plasma concentrations were generally comparable between patients with ADPKD and healthy individuals (Supplemental Table 12, Table 2). Urinary concentrations of cinnamoylglycine, hippurate, isovalerylglycine, and trimethyluric acid were lower in participants with ADPKD compared with healthy individuals, although these differences were not statistically significant after eGFR adjustment and accounting for multiple comparisons (Supplemental Table 13).
Table 2.
Fold-difference in solute fractional excretion in autosomal dominant polycystic kidney disease with preserved eGFR versus healthy
| Solute | Serum or Plasma Concentrationa | Fractional Excretionb | Fold-Difference in Fractional Excretion ADPKD versus Healthy (95% CI) | |||
|---|---|---|---|---|---|---|
| ADPKD eGFR ≥90 ml/min per 1.73 m2, n=31 | Healthy, n=25 | ADPKD eGFR ≥90 ml/min per 1.73 m2, n=31 | Healthy, n=25 | Adjustedc | P Value | |
| Cinnamoylglycine | 16.7 (8.5–24.4) | 8.1 (4.5–14.1) | 0.56 (0.31–0.87) | 1.17 (0.89–1.39) | 0.48 (0.30 to 0.76) | 0.002d |
| Dimethyluric acid | 9.7 (2.9–27.5) | 14.6 (3.2–18.8) | 4.02 (2.80–5.06) | 4.89 (4.00–8.14) | 0.54 (0.25 to 1.18) | 0.12 |
| Hippurate | 531 (239–946) | 635 (424–892) | 3.00 (1.96–3.89) | 3.76 (3.19–4.57) | 0.65 (0.42 to 1.01) | 0.05 |
| Indoxyl sulfate | 738 (549–1021) | 455 (314–656) | 0.36 (0.28–0.48) | 0.38 (0.33–0.47) | 0.92 (0.62 to 1.38) | 0.68 |
| Isovalerylglycine | 3.8 (2.5–4.7) | 5.6 (3.7–6.3) | 2.23 (1.44–3.22) | 3.13 (2.86–3.76) | 0.55 (0.33 to 0.89) | 0.02 |
| Kynurenic acid | 8.8 (6.6–11.4) | 9.1 (8.3–10.6) | 1.41 (0.98–2.49) | 0.91 (0.73–1.19) | 1.45 (0.93 to 2.25) | 0.09 |
| P-cresol sulfate | 2846 (1434–3620) | 1942 (860–3736) | 0.09 (0.07–0.12) | 0.13 (0.10–0.15) | 0.71 (0.46 to 1.09) | 0.12 |
| Pyridoxic acid | 5.3 (3.9–10.2) | 4.7 (4.1–6.2) | 4.53 (3.10–5.86) | 4.82 (4.46–5.23) | 0.89 (0.60 to 1.32) | 0.57 |
| Tiglylglycine | 4.3 (2.8–6.1) | 3.6 (2.6–4.5) | 1.55 (0.99–2.29) | 2.94 (2.25–3.28) | 0.47 (0.29 to 0.77) | 0.004d |
| Trimethyluric acid | 0.5 (0.3–1.1) | 0.9 (0.3–1.7) | 1.93 (0.78–2.69) | 2.83 (2.39–3.76) | 0.50 (0.29 to 0.88) | 0.02 |
| Xanthosine | 28.7 (10.3–81.2) | 3.1 (2.9–3.9) | 0.12 (0.05–0.35) | 1.33 (1.26–1.77) | 0.09 (0.05 to 0.17) | <0.001d |
ADPKD, autosomal dominant polycystic kidney disease; 95% CI, 95% confidence interval.
Serum or plasma concentration (nanograms per milliliter) expressed as median (interquartile range).
Fractional excretion expressed as median (interquartile range).
Adjusted for age, sex, body mass index, eGFR calculated with the Chronic Kidney Disease Epidemiology Collaboration equation, and urine microalbumin-to-creatinine ratio.
Statistically significant after Bonferroni correction at P value threshold of <0.01.
Associations among Participants with ADPKD and Reduced eGFR
Among participants with ADPKD and reduced eGFR, the fractional excretions of secretory solutes ranged from 0.11 for p-cresol sulfate to 4.52 for pyridoxic acid (Table 3). After full covariate adjustment, the fractional excretions of six secretory solutes were lower in patients with ADPKD compared with general patients with CKD: dimethyluric acid, hippurate, isovalerylglycine, pyridoxic acid, tiglylglycine, and xanthosine. The largest difference in fractional secretory clearance was again observed for xanthosine (68% lower; 95% confidence interval, 58% to 76% lower). With the exception of marked elevated xanthosine level in ADPKD, the serum/plasma concentrations of most solutes were comparable between ADPKD and general CKD (Supplemental Table 12, Table 3). However, urinary solute fractional excretion was consistently lower in ADPKD compared with general CKD (Supplemental Table 13).
Table 3.
Fold-difference in solute fractional excretion in autosomal dominant polycystic kidney disease with reduced eGFR versus CKD
| Solute | Serum or Plasma Concentrationa | Fractional Excretionb | Fold-Difference in Fractional Excretion ADPKD versus CKD (95% CI)c | |||
|---|---|---|---|---|---|---|
| ADPKD eGFR <90 ml/min per 1.73 m2, n=95 | CKD, n=92 | ADPKD eGFR <90 ml/min per 1.73 m2, n=95 | CKD, n=92 | Adjustedc | P Value | |
| Cinnamoylglycine | 16.9 (5.5–55.9) | 14.5 (8.5–24.7) | 0.54 (0.34–0.78) | 0.75 (0.35–1.36) | 0.79 (0.59 to 1.08) | 0.14 |
| Dimethyluric acid | 17.6 (4.1–49.3) | 20.7 (7.2–47.4) | 3.78 (2.75–4.84) | 5.33 (4.05–7.32) | 0.63 (0.50 to 0.79) | <0.001d |
| Hippurate | 790 (454–1466) | 526 (295–1022) | 3.05 (2.19–3.80) | 6.68 (3.63–10.75) | 0.42 (0.34 to 0.52) | <0.001d |
| Indoxyl sulfate | 1615 (1060–2334) | 1981 (1073–2021) | 0.44 (0.30–0.55) | 0.39 (0.29–0.51) | 1.02 (0.86 to 1.22) | 0.76 |
| Isovalerylglycine | 6.7 (4.9–9.7) | 5.7 (4.4–7.5) | 2.19 (1.59–3.10) | 3.30 (2.32–4.99) | 0.52 (0.44 to 0.63) | <0.001d |
| Kynurenic acid | 15.2 (11.3–26.6) | 14.1 (10.6–21.7) | 1.53 (1.08–1.95) | 1.23 (0.98–1.47) | 1.13 (0.97 to 1.31) | 0.12 |
| P-cresol sulfate | 5646 (3521–9650) | 8990 (5255–15,618) | 0.11 (0.08–0.15) | 0.12 (0.09–0.15) | 0.87 (0.73 to 1.03) | 0.12 |
| Pyridoxic acid | 12.5 (7.0–31.5) | 13.3 (8.2–23.9) | 4.52 (3.29–5.97) | 6.39 (5.01–8.51) | 0.69 (0.58 to 0.84) | <0.001d |
| Tiglylglycine | 8.4 (5.8–14.8) | 6.8 (4.8–10.2) | 1.48 (1.16–2.10) | 2.64 (2.04–3.70) | 0.60 (0.51 to 0.71) | <0.001d |
| Trimethyluric acid | 0.81 (0.30–2.17) | 1.50 (0.65–3.03) | 1.81 (1.22–2.57) | 1.99 (1.26–3.51) | 0.82 (0.59 to 1.12) | 0.21 |
| Xanthosine | 47.8 (21.6–87.6) | 17.6 (11.4–33.4) | 0.23 (0.10–0.43) | 0.82 (0.51–1.20) | 0.32 (0.24 to 0.42) | <0.001d |
ADPKD, autosomal dominant polycystic kidney disease; 95% CI, 95% confidence interval.
Serum or plasma concentration (nanograms per milliliter) expressed as median (interquartile range).
Fractional excretion expressed as median (interquartile range).
Adjusted for age, sex, body mass index, GFR calculated with the Chronic Kidney Disease Epidemiology Collaboration equation, and urine microalbumin-to-creatinine ratio.
Statistically significant after Bonferroni correction at P value threshold of <0.01.
Association of Solute Fractional Excretion with Total Kidney Volume in ADPKD
Among 112 participants with ADPKD who underwent abdominal MRI, lower fractional secretory clearances tended to track with relatively greater height-adjusted kidney volumes. However, none of these associations were statistically significant (Table 4).
Table 4.
Association between solute fractional excretion and total kidney volume in autosomal dominant polycystic kidney disease (n=112)
| Solute | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| Difference in Kidney Volume (95% CI) | P Value | Difference in Kidney Volume (95% CI) | P Value | |
| Cinnamoylglycine | −27 (−418 to 362) | 0.88 | −60 (−388 to 269) | 0.72 |
| Dimethyluric acid | −50 (−176 to 75) | 0.43 | −4 (−111 to 104) | 0.94 |
| Hippurate | 42 (−415 to 500) | 0.85 | 23 (−360 to 408) | 0.90 |
| Indoxyl sulfate | −43 (−214 to 129) | 0.62 | −55 (−201 to 90) | 0.45 |
| Isovalerylglycine | −121 (−348 to 105) | 0.29 | −138 (−329 to 53) | 0.15 |
| Kynurenic acid | −45 (−191 to 101) | 0.55 | −59 (−183 to 65) | 0.35 |
| p-cresol sulfate | −33 (−200 to 133) | 0.69 | −58 (−199 to 82) | 0.41 |
| Pyridoxic acid | −89 (−282 to 102) | 0.36 | −51 (−213 to 110) | 0.53 |
| Tiglylglycine | −68 (−244 to 107) | 0.44 | −85 (−236 to 65) | 0.26 |
| Trimethyluric acid | 193 (−132 to 519) | 0.24 | 106 (−170 to 382) | 0.45 |
| Xanthosine | 175 (−166 to 517) | 0.31 | 62 (−261 to 385) | 0.70 |
Differences in kidney volume per SD difference in solute fractional excretion. 95% CI, 95% confidence interval.
Adjusted for age, sex, body mass index, GFR calculated with the Chronic Kidney Disease Epidemiology Collaboration equation, and urine microalbumin-to-creatinine ratio.
Discussion
We observed lower fractional excretions of several proximal tubular secretory solutes among individuals with ADPKD compared with general patients with CKD and healthy control individuals after adjusting for GFR and accounting for multiple comparisons. Secretory solute fractional excretions were not associated with total kidney volume assessed by MRI. Our findings suggest that impairment of tubular secretory solute clearance in ADPKD occurs before and is independent of detectable changes in GFR.
Several studies have assessed the clearance of p-aminohippurate (PAH) in ADPKD (8,23,24). PAH is a protein-bound organic acid that is avidly eliminated through tubular secretion and a protype solute for assessing kidney blood flow (25). ADPKD and wild-type mice demonstrate similar values of transport maximum of PAH, or the highest rate that PAH is secreted given complete saturation of tubular transporters (24). However, lower transport maximum of PAH is observed in patients with ADPKD and preserved eGFR compared with healthy controls (8). In a recent metabolomic study, Grams et al. (26) demonstrated higher serum concentrations of hippurate and kynurenate, a conjugate of kynurenic acid, in individuals with ADPKD compared with individuals with other etiologies of CKD. The kidney handling of uric acid is complex, with glomerular filtration, tubular reabsorption, and tubular secretion all contributing to homeostasis. Uric acid is secreted via basolateral OATs and an apical ATP-dependent urate transporter (27). Mice with double knockout of OAT1/OAT3 demonstrate impaired urate secretion (28). However, data in ADPKD are more conflicting. Several small cross-sectional studies have shown that uric acid fractional and secretory clearance rates do not differ between patients with ADPKD with both preserved and depressed GFR compared with healthy individuals and patients with CKD (29,30).
Prior studies have also demonstrated impairments of diverse tubular functions in ADPKD before detectable changes in glomerular filtration. For example, patients with ADPKD with both preserved and reduced GFR are unable to maximally concentrate urine to the same extent as healthy controls after water deprivation (7,31). Analogously, lower ammonium excretion after acid loading was also observed in patients with ADPKD compared with healthy controls with comparable GFR, suggesting that ADPKD is associated with functional defects related to proximal tubular ammonia production, medullary ammonia transport, or distal tubular ammonia excretion (23,32).
Several potential mechanisms may compromise tubular solute transport in ADPKD. Obstruction of normal tubular structures by enlarging cysts may physically disrupt epithelial cells of the proximal tubule. Abnormalities of kidney hemodynamics in ADPKD may also contribute toward impairment of tubular solute clearance. Young patients with ADPKD and preserved GFR demonstrate markedly decreased kidney blood flow and increased filtration fraction compared with age-matched healthy controls (33). In the setting of increased filtration fraction, more blood is filtered through the glomerulus, which in turn, decreases blood flow through the peritubular capillaries. Such changes would compromise delivery of solutes to the tubular epithelial cells and decrease solute clearance (1,34,35).
Alterations of metabolic pathways in ADPKD are another potential explanation for the observed difference in tubular solute excretion. Cysts in ADPKD undergo dedifferentiation and uncontrolled proliferation as a consequence of cellular reprogramming. PKD1 mutated cells isolated from ADPKD mice and humans with ADPKD preferentially metabolize glucose through glycolysis rather than the tricarboxylic acid cycle (36). Metabolomic analyses of urine and kidney tissue obtained from polycystic kidney disease mice reveal alterations in pathways of fatty acid oxidation and nucleotide synthesis (37–39). Cyst-lining cells in kidney tissue of ADPKD mice also demonstrate higher expression of advanced glycation end product–specific receptor, which binds advanced glycation end products (40). Advanced glycation end products are derived from nonenzymatic glycation reactions between reducing sugars and other compounds, and their interactions with advanced glycation end product–specific receptor activate intracellular signaling pathways of inflammation and cellular proliferation (41). Downregulation of advanced glycation end product–specific receptor is associated with smaller cyst size and kidney volume in ADPKD mice (42).
Our study has several strengths. We studied fractional excretions rather than plasma levels of secretory solutes to focus specifically on the kidney removal of these substances and avoid conflation with potential differences in production. We utilized targeted mass spectrometry assays with external calibrators, the concentrations of which were determined using gold standard nuclear magnetic resonance–characterized purified compounds, to quantify secretory solute concentrations. We also obtained samples from a well characterized clinical cohort of individuals with ADPKD. One limitation of our study is the possibility that differences in other characteristics between individuals with ADPKD and individuals without ADPKD may have confounded the observed associations with secretory solute clearances. Furthermore, differences in blood and urine collection procedures across the three cohorts may affect the secretory measurements and potentially bias our results. Blood (serum in patients with ADPKD and the SKS and plasma in healthy individuals) and urine (spot collection in patients with ADPKD, timed overnight collection in the SKS, and 24-hour collection in healthy individuals) collection techniques did differ across cohorts. A third limitation is the relatively small sample size within each cohort, thereby limiting power to detect differences in solute fractional excretion across cohorts. Finally, the cross-sectional nature of our study precludes assessment of timing of changes in tubular solute excretion relative to glomerular filtration during course of ADPKD.
In conclusion, we observed lower fractional excretions of proximal tubular secretory solutes among individuals with ADPKD compared with general CKD patients and healthy individuals independent of eGFR. Our findings suggest that tubular secretory clearance may be compromised before detectable changes in GFR and that assessment of secretory clearance could potentially expand the assessment of disease severity among patients with ADPKD. Future directions include larger prospective studies to assess associations of secretory function with clinical outcomes in patients with ADPKD. Animal studies can also help to further probe potential molecular mechanisms underlying altered tubular transport in ADPKD.
Disclosures
Dr. Hoofnagle reports receiving a grant from Waters outside of the submitted work. Dr. Kestenbaum reports receiving speaker fees for a talk given for Sanofi in 2016. Dr. Seliger reports receiving consulting fees from Tricida outside of the submitted work. Dr. Watnick and Dr. Seliger report receiving grants from Kadmon Corporation, Palladio Biosciences, Reata Pharmaceuticals, and Sanofi outside of the submitted work.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01 DK107931 and an unrestricted fund from the Northwest Kidney Centers. Dr. Wang is supported by the American Society of Nephrology Ben J. Lipps Research Fellowship. Dr. Zelnick is supported by a grant from NIDDK. Dr. Hoofnagle and the University of Washington Nutrition and Obesity Research Center are supported by NIDDK grant P30 DK035816. Dr. Watnick and Dr. Seliger are supported by NIDDK grant P30-DK090868. Dr. Kestenbaum is supported by NIDDK grant R01 DK103986.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05610519/-/DCSupplemental.
Supplemental Material Methods.
Supplemental Table 1. Analyte and internal standard vendors and solid-phase extraction used for urine.
Supplemental Table 2. Internal standards.
Supplemental Table 3. Chromatographic parameters.
Supplemental Table 4. Chromatographic program.
Supplemental Table 5. Mass spectrometric parameters (part 1).
Supplemental Table 6. Mass spectrometric parameters (part 2).
Supplemental Table 7. Mass spectrometric parameters (part 3).
Supplemental Table 8. Pamoic acid internal nuclear magnetic resonance standard integrals and assignments.
Supplemental Table 9. Limits of quantification for each analyte.
Supplemental Table 10. Recoveries in different matrices.
Supplemental Table 11. Detection limits, laboratory variability, and characteristics of candidate secretory solutes.
Supplemental Table 12. Serum or plasma solute concentrations by cohort.
Supplemental Table 13. Urine concentrations by cohort.
References
- 1.Grantham JJ, Mulamalla S, Swenson-Fields KI: Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 7: 556–566, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Wu G, D’Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H Jr., Kucherlapati R, Edelmann W, Somlo S: Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93: 177–188, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Franz KA, Reubi FC: Rate of functional deterioration in polycystic kidney disease. Kidney Int 23: 526–529, 1983 [DOI] [PubMed] [Google Scholar]
- 4.Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH: Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41: 1311–1319, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Smith HW, Goldring W, Chasis H: The measurement of the tubular excretory mass, effective blood flow and filtration rate in the normal human kidney. J Clin Invest 17: 263–278, 1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nigam SK: What do drug transporters really do? Nat Rev Drug Discov 14: 29–44, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Maldonado M, Yium JJ, Eknoyan G, Suki WN: Adult polycystic kidney disease: Studies of the defect in urine concentration. Kidney Int 2: 107–113, 1972 [DOI] [PubMed] [Google Scholar]
- 8.Pabico RC, McKenna BA, Freeman RB: Renal tubular dysfunction in patients with cystic disease of the kidneys. Urology 51[Suppl]: 156–160, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D: Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20: 205–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kratz M, Callahan HS, Yang PY, Matthys CC, Weigle DS: Dietary n-3-polyunsaturated fatty acids and energy balance in overweight or moderately obese men and women: A randomized controlled trial. Nutr Metab (Lond) 6: 24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kratz M, Swarbrick MM, Callahan HS, Matthys CC, Havel PJ, Weigle DS: Effect of dietary n-3 polyunsaturated fatty acids on plasma total and high-molecular-weight adiponectin concentrations in overweight to moderately obese men and women. Am J Clin Nutr 87: 347–353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ix JH, Wassel CL, Stevens LA, Beck GJ, Froissart M, Navis G, Rodby R, Torres VE, Zhang YL, Greene T, Levey AS: Equations to estimate creatinine excretion rate: The CKD epidemiology collaboration. Clin J Am Soc Nephrol 6: 184–191, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush KT, Wu W, Lun C, Nigam SK: The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut-liver-kidney axis. J Biol Chem 292: 15789–15803, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, Yang Q, Cheng S, Pierce K, Deik A, Souza AL, Farrell L, Domos C, Yeh RW, Palacios I, Rosenfield K, Vasan RS, Florez JC, Wang TJ, Fox CS, Gerszten RE: A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol 24: 1330–1338, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sirich TL, Aronov PA, Plummer NS, Hostetter TH, Meyer TW: Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 84: 585–590, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wikoff WR, Nagle MA, Kouznetsova VL, Tsigelny IF, Nigam SK: Untargeted metabolomics identifies enterobiome metabolites and putative uremic toxins as substrates of organic anion transporter 1 (Oat1). J Proteome Res 10: 2842–2851, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu W, Bush KT, Nigam SK: Key role for the organic anion transporters, OAT1 and OAT3, in the in vivo handling of uremic toxins and solutes. Sci Rep 7: 4939, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suchy-Dicey AM, Laha T, Hoofnagle A, Newitt R, Sirich TL, Meyer TW, Thummel KE, Yanez ND, Himmelfarb J, Weiss NS, Kestenbaum BR: Tubular secretion in CKD. J Am Soc Nephrol 27: 2148–2155, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivara MB, Zelnick LR, Hoofnagle AN, Newitt R, Tracy RP, Kratz M, Weigle DS, Kestenbaum BR: Diurnal and long-term variation in plasma concentrations and renal clearances of circulating markers of kidney proximal tubular secretion. Clin Chem 63: 915–923, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbarat B, Podevin RA: Pantothenate-sodium cotransport in renal brush-border membranes. J Biol Chem 261: 14455–14460, 1986 [PubMed] [Google Scholar]
- 21.Karnitz LM, Gross CJ, Henderson LM: Transport and metabolism of pantothenic acid by rat kidney. Biochim Biophys Acta 769: 486–492, 1984 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preuss H, Geoly K, Johnson M, Chester A, Kliger A, Schreiner G: Tubular function in adult polycystic kidney disease. Nephron 24: 198–204, 1979 [DOI] [PubMed] [Google Scholar]
- 24.Tanner GA, Gretz N, Shao Y, Evan AP, Steinhausen M: Organic anion secretion in polycystic kidney disease. J Am Soc Nephrol 8: 1222–1231, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Smith HW, Finkelstein N, Aliminosa L, Crawford B, Graber M: The renal clearances of substituted hippuric acid derivatives and other aromatic acids in dog and man. J Clin Invest 24: 388–404, 1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grams ME, Tin A, Rebholz CM, Shafi T, Köttgen A, Perrone RD, Sarnak MJ, Inker LA, Levey AS, Coresh J: Metabolomic alterations associated with cause of CKD. Clin J Am Soc Nephrol 12: 1787–1794, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodward OM, Köttgen A, Coresh J, Boerwinkle E, Guggino WB, Köttgen M: Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A 106: 10338–10342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eraly SA, Vallon V, Rieg T, Gangoiti JA, Wikoff WR, Siuzdak G, Barshop BA, Nigam SK: Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol Genomics 33: 180–192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mejías E, Navas J, Lluberes R, Martínez-Maldonado M: Hyperuricemia, gout, and autosomal dominant polycystic kidney disease. Am J Med Sci 297: 145–148, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Kaehny WD, Tangel DJ, Johnson AM, Kimberling WJ, Schrier RW, Gabow PA: Uric acid handling in autosomal dominant polycystic kidney disease with normal filtration rates. Am J Med 89: 49–52, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Gabow PA, Kaehny WD, Johnson AM, Duley IT, Manco-Johnson M, Lezotte DC, Schrier RW: The clinical utility of renal concentrating capacity in polycystic kidney disease. Kidney Int 35: 675–680, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Torres VE, Keith DS, Offord KP, Kon SP, Wilson DM: Renal ammonia in autosomal dominant polycystic kidney disease. Kidney Int 45: 1745–1753, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Meijer E, Rook M, Tent H, Navis G, van der Jagt EJ, de Jong PE, Gansevoort RT: Early renal abnormalities in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 5: 1091–1098, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambert PP: Polycystic disease of the kidney; a review. Arch Pathol (Chic) 44: 34–58, 1947 [PubMed] [Google Scholar]
- 35.Ward JN, Draper JW, Lavengood RW Jr.: A clinical review of polycystic kidney disease in 53 patients. J Urol 98: 48–53, 1967 [DOI] [PubMed] [Google Scholar]
- 36.Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F, Pei Y, Musco G, Boletta A: Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 19: 488–493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang VJ, Kim J, Rand A, Yang C, Sturdivant S, Hammock B, Bell PD, Guay-Woodford LM, Weiss RH: The cpk model of recessive PKD shows glutamine dependence associated with the production of the oncometabolite 2-hydroxyglutarate. Am J Physiol Renal Physiol 309: F492–F498, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor SL, Ganti S, Bukanov NO, Chapman A, Fiehn O, Osier M, Kim K, Weiss RH: A metabolomics approach using juvenile cystic mice to identify urinary biomarkers and altered pathways in polycystic kidney disease. Am J Physiol Renal Physiol 298: F909–F922, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menezes LF, Lin CC, Zhou F, Germino GG: Fatty acid oxidation is impaired in an orthologous mouse model of autosomal dominant polycystic kidney disease. EBioMedicine 5: 183–192, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park EY, Seo MJ, Park JH: Effects of specific genes activating RAGE on polycystic kidney disease. Am J Nephrol 32: 169–178, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Stinghen AE, Massy ZA, Vlassara H, Striker GE, Boullier A: Uremic toxicity of advanced glycation end products in CKD. J Am Soc Nephrol 27: 354–370, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park EY, Kim BH, Lee EJ, Chang E, Kim DW, Choi SY, Park JH: Targeting of receptor for advanced glycation end products suppresses cyst growth in polycystic kidney disease. J Biol Chem 289: 9254–9262, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.