Abstract
Bloodstream infections are an important cause of hospitalizations, morbidity, and mortality in patients receiving hemodialysis. Eliminating bloodstream infections in the hemodialysis setting has been the focus of the Centers for Disease Control and Prevention (CDC) Making Dialysis Safer for Patients Coalition and, more recently, the CDC’s partnership with the American Society of Nephrology’s Nephrologists Transforming Dialysis Safety Initiative. The majority of vascular access–associated bloodstream infections occur in patients dialyzing with central vein catheters. The CDC’s core interventions for bloodstream infection prevention are the gold standard for catheter care in the hemodialysis setting and have been proven to be effective in reducing catheter-associated bloodstream infection. However, in the United States hemodialysis catheter–associated bloodstream infections continue to occur at unacceptable rates, possibly because of lapses in adherence to strict aseptic technique, or additional factors not addressed by the CDC’s core interventions. There is a clear need for novel prophylactic therapies. This review highlights the recent advances and includes a discussion about the potential limitations and adverse effects associated with each option.
Keywords: vascular access, hemodialysis access, hemodialysis, mortality, sepsis, central venous catheter, bacteremia, humans, United States, renal dialysis, nephrology, nephrologists, central venous catheters, catheter-related infections, morbidity, Centers for Disease Control and Prevention (U.S.), hospitalization
Introduction
In the 2018 US Renal Data Report, 80% of patients initiated hemodialysis with a catheter and 21% are still in use 1 year after hemodialysis initiation (1). One of the major complications of hemodialysis catheter use is bloodstream infection, which is associated with an increased risk of systemic infectious complications, hospitalizations, and death (2–6). Hemodialysis catheter use is associated with an eight-fold higher rate of vascular access-related bloodstream infections when compared with an arteriovenous fistula (2). The definition of catheter-associated bloodstream infection used in various clinical trials lacks consistency (7–12). In 2018, a multidisciplinary group of experts formed the Kidney Health Initiative’s Catheter End Points Workgroup to establish a standardized definition of catheter-associated bloodstream infection in patients on hemodialysis (12). The workgroup proposed the following criteria for the diagnosing hemodialysis catheter–associated bloodstream infection: (1) clinical suspicion of infection (fever/temperature >37.5°C or rigors or altered mental status or new predialysis hypotension [systolic BP <90 mm Hg]), (2) confirmation of bacteremia (blood cultures growing the same organism from the hemodialysis catheter and a peripheral vein or dialysis bloodline), and (3) exclusion of any alternate sources of infection.
The accurate reporting of bloodstream infection rates in the outpatient hemodialysis setting may be jeopardized by methodological challenges. Patients who become symptomatic with fever or chills oftentimes present directly to the hospital, and not to the hemodialysis facility. Furthermore, on rare occasion, antibiotics are administered without sending blood cultures in patients who become symptomatic when in the hemodialysis unit. In the National Healthcare Safety Network reporting system, bloodstream infection events are determined solely by a positive blood culture result and does not include intravenous antibiotic starts (2,13). A determination of the completeness of capture of blood cultures for all events in which obtaining blood cultures may have been indicated is missing in this data. One proposal to mitigate inaccuracies in bloodstream infection reporting is to provide data about positive blood culture results as a percentage of all intravenous antibiotic starts in the hemodialysis facility. However, this assumes that intravenous antibiotics are ordered solely for suspected cases of bacteremia. In addition, this proposal does not account for cases of bloodstream infection in which blood cultures were obtained in the hospital and the intravenous antibiotic course was completed during the hospitalization.
In the most recent National Healthcare Safety Network Dialysis Event Surveillance report for 2014, the mean rate of all bloodstream infections in catheter-dependent patients was 2.16 per 100 patient-months and the mean rate for access-related bloodstream infection was 1.83 per 100 patient-months (combined permanent and temporary catheters) (2). This was a marked improvement compared with the previous report for 2006 when the rate of all bloodstream infections in catheter-dependent patients was 4.2 (permanent catheters) and 27.1 (temporary catheters) per 100 patient-months, and access-related bloodstream infection rates were 3.1 (permanent catheters) and 17.8 (temporary catheters) per 100 patient-months (13). Similarly, from 2003 to 2013 the hospitalization rate for vascular access infections has declined in both incident and prevalent patients on dialysis (30% and 46% reduction, respectively) (14). However, there was a simultaneous rise in the rate of hospitalization for bacteremia/sepsis in both incident (38% increase) and prevalent (40% increase) patients (14). One explanation may be a change in the frequency of coding from the diagnosis “vascular-access infection” to an increase in use of the code “bacteremia.” Further investigation into these apparent paradoxical findings would be informative. In the recent US Renal Data Systems annual report for 2018, septicemia accounted for 8% of all deaths (1). Sepsis is a potentially preventable cause of mortality in hemodialysis population, and a significant percentage is vascular access related. Interventions to reduce infections in the hemodialysis setting include protocols for catheter reduction, new tools to improve compliance with the existing Centers for Disease Control and Prevention’s core interventions for catheter care, improving patient and staff education, and the development of novel devices for preventing catheter colonization (15).
Interventions for Catheter Reduction
There are several interventions that can decrease the incidence and prevalence of catheters in patients on hemodialysis. There is evidence that early referral of patients to nephrologists, multidisciplinary teams, and vascular access coordinators who provide education to patients; implantation of early-stick grafts; and urgent-start peritoneal dialysis can play a role in decreasing catheter use.
Early referral of patients with CKD to the nephrologist results in dialysis modality choice discussions and, thereafter, leads to timely referral for permanent vascular access placement, to a transplant center for enlistment or planning for peritoneal catheter insertion. In a small study of 135 patients, early (>4 months before dialysis initiation) referral to a nephrologist was associated with a greater likelihood of starting hemodialysis using a permanent vascular access (48% early referral versus 4% late referral) (16). Similarly, in study of a large cohort of 2398 incident patients on hemodialysis, late referral to a nephrologist (<90 days of hemodialysis initiation) was associated with a 42% higher risk of initiating hemodialysis with a catheter compared with those seen by a nephrologist earlier in the course of their kidney disease (odds ratio [OR], 1.42; 95% confidence interval [95% CI], 1.17 to 1.71) (17).
There is modest evidence that when multidisciplinary teams (consisting of physicians, nurses, social workers, and dietitians) provide education to patients, either prehemodialysis or at initiation of hemodialysis, the prevalence of catheters decreases (18,19). In the Treatment Options Program, implemented by a large dialysis organization, patients were educated on modality choice and vascular access prehemodialysis. Patients undergoing hemodialysis enrolled in this program (n=2800) had a lower likelihood of initiating hemodialysis with a catheter compared with matched control patients (n=2800) (63.2% versus 75.8%; P<0.001) (18). Similar findings were reported in the Incident Management of Patients Actions Centered on Treatment program, also implemented by a large dialysis organization. When patients received education at the initiation of hemodialysis, using a multidisciplinary team, the proportion of patients with an arteriovenous fistula or graft versus a catheter was significantly higher for patients in the intervention group at 6 months (0.60 versus 0.52; P<0.001) and 1 year (0.63 versus 0.48; P<0.001) (19). Contrary to the positive results of these studies, a quality improvement initiative using a multidisciplinary vascular access team to educate patients initiating hemodialysis in Canadian units did not show a decrease in catheter-free arteriovenous fistula use at 1 year (20).
The utilization of a vascular access coordinator can significantly decrease hemodialysis initiation with a catheter. A quality improvement project in Australia reported that prehemodialysis patient education and coordination by a vascular access coordinator resulted in a significant decrease in catheter-initiated hemodialysis (from 39% to 25%) (P=0.007) and a reduction in the total number of catheter days (2833 versus 4685 days) (21). Similar findings were reported from a program in the United States, where the implementation of a comprehensive access program led by a vascular access coordinator resulted in a significant reduction in prevalent catheter use at >90 days after hemodialysis initiation (from 11% to 6%; P<0.001) (22). The use of a vascular access coordinator in the hemodialysis unit can also improve outcomes after an episode of catheter-associated bloodstream infection. A quality improvement project in the Bronx and Connecticut (n=223 episodes of catheter-associated bacteremia) reported a significant reduction in recurrent bacteremia in 3 months (from 18% to 6%; P<0.02) and death due to sepsis (6% versus 0%; P=0.05) in hemodialysis units with a vascular access coordinator (23).
In patients in need of urgent-start dialysis, the creation of an early-cannulation arteriovenous graft and placement of a peritoneal dialysis catheter for immediate use are additional options available to reduce catheters. In a randomized, controlled trial, patients in need of an urgent vascular access for hemodialysis were randomized to receive an early-cannulation arteriovenous graft (n=60) or a tunneled catheter (n=61) (24). At 6 months of follow-up, bloodstream infections developed more frequently in catheter group (16.4%) compared with the arteriovenous graft group (3.3%; P=0.02). In a retrospective study from China, 96 patients starting dialysis with a peritoneal dialysis catheter were compared with 82 patients starting with a hemodialysis catheter (25). A significantly higher incidence of bloodstream infection occurred in the hemodialysis catheter group at 30 days (11% hemodialysis catheter versus 0 peritoneal catheter; P=0.003). Although both studies comprised a single center, with small numbers of patients, they suggest that avoiding catheters can reduce bloodstream infections in patients with ESKD on dialysis.
Challenges to Adherence with the Centers for Disease Control and Prevention’s Core Interventions
Implementation of the Centers for Disease Control and Prevention’s core interventions for hemodialysis catheter care has been associated with a 20%–50% reduction in bloodstream infection rates and hospitalizations due to sepsis (Figure 1) (15,26–28). Several objective factors may potentially interfere with optimal adherence with the Centers for Disease Control and Prevention’s core interventions in outpatient hemodialysis units in the United States. Until studies are completed that quantify and account for missing blood culture results, conclusions about the effect of specific factors remain speculative. Possible factors present in the outpatient hemodialysis setting that may play a role include the high patient-to-staff ratio, the proportion of patients dependent upon dialysis catheters, and a high staff turnover. The rate of catheter use is high among new hemodialysis starts. The newly started patients are typically placed on the available late dialysis shifts at the three medical centers where the authors work. To the extent that this holds true across the United States, the result would be a disproportionately high number of catheter-dependent patients on the late shifts. Additionally, in about half of the states, only nurses but not patient care technicians are permitted to connect and disconnect catheter patients (29). The high burden of catheter-dependent patients on those late shifts is compounded by the restriction of catheter care to nurses and presents a huge burden on nursing staff. It is not unusual for one nurse to be responsible for five or more catheter-dependent patients. A high dialysis patient-to-nurse ratio has been associated with more frequent access infections (30). It is possible that this high workload may cause lapses in adhering to the Centers for Disease Control and Prevention guidelines, thereby contributing to an increased risk of catheter-associated bloodstream infections. In contrast, in Canada, Europe, and Japan, hemodialysis care is provided exclusively by nurses, and the ratio of patients to nurses is only 3–4:1, as compared with 8–12:1 in the United States.
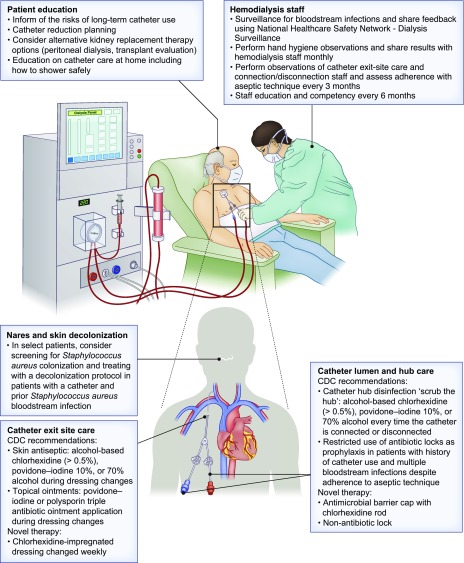
Figure 1.
Potential strategies for the prevention of bloodstream infections in patients undergoing dialysis. Educating patients and hemodialysis staff about the risks of long-term catheter use and about optimal catheter care are key components to reduce bloodstream infections. Attention to proper catheter exit-site care and hub disinfection using recommended antiseptic agents, and the use of recommended topical ointments during exit site dressing changes are important core interventions. Novel therapies, including chlorhexidine containing hub devices, and chlorhexidine dressings may further reduce bloodstream infections in catheter-dependent hemodialysis patients. In select patients, novel non-antibiotic locking solutions and Staphylococcus decolonization protocols may also be considered. CDC, Centers for Disease Control and Prevention.
A recent observational study reported the counterintuitive finding that the frequency of catheter-associated bloodstream infection in a given hemodialysis unit was inversely related to the proportion of patients using a catheter at that unit (31). In other words, the greater the proportion of catheter-dependent patients, the lower the frequency of catheter-associated bloodstream infection. One possible explanation for this surprising finding may be that in hemodialysis units where a greater proportion (>20%) of patients use a catheter, there may be a higher percentage of healthier patients, who are therefore less prone to infection. One can speculate that these healthier patients may be suitable for an arteriovenous fistula or graft, and might not have a catheter if they were dialyzing in units with lower catheter percentages (<10%). Catheter-dependent patients in facilities with a lower catheter percentages (<10%) may have more comorbidities and unsuitable for other forms of vascular accesses, and therefore be at higher risk for infection. Consistent with this hypothesis is the finding that catheter-associated bloodstream infection rates are particularly low in Alberta, Canada (0.19 per 1000 days or 0.57 per 100 patient-months), despite the fact that in Canada, 45% of patients on hemodialysis are catheter-dependent (32,33). An alternative explanation is that in high-catheter-percentage units, experienced staff, who are frequently performing catheter care may better adhere to core infection prevention techniques.
It is possible that lapses in adherence with the Centers for Disease Control and Prevention core interventions is a factor contributing to the relatively high catheter-associated bloodstream infection rate in the United States. There is clearly a need for studies on the use of catheter care audit checklists to assess whether they improve adherence with the Centers for Disease Control and Prevention guidelines. In addition, in an effort to reduce catheter-associated bloodstream infection, novel interventions have been developed to reduce exit-site and catheter lumen bacterial colonization. These measures are described in following sections.
Patient Self-Care and Showering
Frequent and consistent patient education about the associated infectious risks of prolonged hemodialysis catheter use and on self-care of the catheter are important for engaging patients to be an active participant in improving safety (34). Historically, showering was discouraged in catheter-dependent patients on hemodialysis; however, showering is important from a quality-of-life perspective. The Centers for Disease Control and Prevention now recommends showering be permitted, using exit-site and hub protection with an impermeable cover to reduce the likelihood of introducing organisms into the hemodialysis catheter. Sheet and pouch products have been designed to prevent water contamination of the hemodialysis catheter during showering, but may be cost-prohibitive. There is insufficient evidence to determine whether showering with a protocol that includes hemodialysis catheter hub protection or after-shower exit-site care decreases catheter-associated bloodstream infection rates (35,36). In a small study, comparable bloodstream infection rates were reported in patients on hemodialysis trained in after-shower exit-site care and a control group for which standard catheter care was performed by hemodialysis nurses (35). Similarly, another small trial compared showering without an exit-site dressing (with catheter hub protection) to a no-showering policy. There was no difference in bloodstream infection rates between the groups, suggesting that showering without an exit-site dressing may be safe (36). Further investigation with a large, randomized clinical trial is warranted.
Interventions Targeting the Catheter Exit Site
Ointments
Topical antimicrobial ointments that are applied to the catheter exit site are recommended at time of catheter insertion and at each hemodialysis session (Table 1) (37–42). The Centers for Disease Control and Prevention recommends using polysporin triple antibiotic ointment (bacitracin/gramicidin/polymyxin B) or povidone iodine ointment, which have been shown to be associated with a 75%–93% reduction in catheter-associated bloodstream infection (15,37,40). Polysporin ointment was also associated with a significant reduction in mortality, and long-term follow-up over 6 years was not associated with a change in microbiologic isolates over time (40,41). Unfortunately, gramicidin is not available in the United States, although polysporin ointments containing bacitracin/zinc/polymyxin B are in clinical use, but have not been rigorously studied for catheter-associated bloodstream infection prophylaxis. Mupirocin has also been associated with an 85% reduction in the bloodstream infection rate; however, there are reports of developing resistant microbes with the long-term routine use of mupirocin (38,39). Medicinal honey has been shown to be similar in efficacy to mupirocin in a small study (42). The advantage of medicinal honey is that it has a low likelihood for selecting resistant strains and is effective against antibiotic-resistant microorganisms. Well designed, adequately powered studies are needed before medicinal honey can be recommended for catheter-associated bloodstream infection prophylaxis. When using any ointment, checking catheter compatibility is mandatory, and a chart is available on the Centers for Disease Control and Prevention’s website (15).
Table 1.
Topical ointments and dressings used for hemodialysis catheter exit-site application
| Study | N | Methods | Standard Definition of Bloodstream Infection Used | Definition Used | Ointment | Control | Outcome | Rate Ratio (95% CI) | P Value | Concerns |
|---|---|---|---|---|---|---|---|---|---|---|
| Levin et al. (37) | 129 | Randomized, double-blind, placebo-controlled trial | No | Positive peripheral blood culture without another demonstrable focus | Povidone-iodine | No ointment | Bloodstream infection | 0.07 (0.06 to 0.24) | <0.01 | Nontunneled catheters included |
| Sesso et al. (38) | 136 | Randomized, prospective trial | No | (1) One or more peripheral blood cultures with Staphylococcus aureus; (2) fever >37.8°C, with or without other signs of infection | Mupirocin | Povidone-iodine | Staphylococcus aureus bloodstream infection | 0.14 (0.03 to 0.63) | <0.001 | Potential for resistance with long-term use |
| Johnson et al. (39) | 50 | Prospective, randomized, controlled, open-label trial | No | (1) A single positive culture together with a positive culture from the catheter tip or exit site with same organism; (2) two or more positive cultures with no evidence of other infection source | Mupirocin | No ointment | Bloodstream infection | 0.15 (0.03 to 0.80) | <0.01 | Potential for resistance with long-term use |
| Lok et al. (40) | 169 | Double-blind, placebo-controlled, randomized trial | Yes | Health Canada guidelines (7) | Bacitracin-gramicidin-polymyxin B (polysporin triple ointment) | Placebo | a) Bloodstream infection b) Death | a) 0.25 (0.19 to 0.34) b) 0.22 (0.07 to 0.74) | a) <0.001 b) 0.004 | Gramicidin containing polysporin triple ointment is not available in United States |
| Battistella et al. (41) | 1478 | Quality improvement report | Yes | Health Canada guidelines (7) | Bacitracin-gramicidin-polymyxin B (polysporin triple ointment) | Historical controls | Bloodstream infection | <1.0/1000 catheter-d | — | Gramicidin containing polysporin triple ointment is not available in United States |
| Johnson et al. (42) | 101 | Prospective, open-label, randomized, controlled trial | Yes | Health Canada guidelines (7) | Antibacterial honey | Mupirocin | Bloodstream infection | 0.94 (0.27 to 3.24) | 0.92 | Underpowered to prove equivalence |
| Camins et al. (46) | 121 | Prospective, nonblinded, crossover intervention trial | Yes | CDC/NNIS (9) | Chlorhexidine-impregnated sponge | Transparent dressing | Bloodstream infection | 1.22 (0.75 to 1.97) | 0.46 | Lack of efficacy |
| Apata et al. (47) | N/A | Quality improvement report | No | Bacteremia in any patient with a tunneled catheter or tunnel site infection | Chlorhexidine-impregnated transparent dressing | Dry gauze dressing plus antibiotic (not specified) ointment | Bloodstream infection | 0.5 95% CI, N/A <1.0/1000 catheter-d | 0.04 | Local allergic reaction/rash, cost |
95% CI, 95% confidence interval; CDC, Centers for Disease Control and Prevention; NNIS, National Nosocomial Infections Surveillance System; N/A, not available.
Antimicrobial Dressings
In the 2017 Centers for Disease Control and Prevention’s updated guidelines, chlorhexidine-impregnated sponge dressings are recognized as an alternative to ointments for prophylactic use in short-term, nontunneled catheters (43). These recommendations were on the basis of studies performed in hospitalized patients not on hemodialysis. Chlorhexidine is a nonantibiotic antimicrobial agent; therefore, the risk of selection for resistant organisms is minimal. Chlorhexidine-impregnated dressings were associated with a 70% reduction in bloodstream infection rates (44,45). Data from studies performed in catheters used for hemodialysis are conflicting (Table 1) (46,47). A study comparing chlorhexidine-impregnated sponge dressing with a transparent dressing at the catheter exit site found no difference in bloodstream infection (46). In contrast, in a recent quality improvement project a 50% reduction in bloodstream infection was reported using chlorhexidine-impregnated transparent dressings (changed weekly) compared with the control group using dry gauze dressings with antibiotic ointment applied to the catheter exit site (changed three times weekly) (47). The weekly cost per patient of the chlorhexidine dressing regimen was twice that of the standard dressing, which may be offset by overall cost-savings from a reduction in bloodstream infections. Unfortunately, although a reduction in bloodstream infection saves overall health care costs, the savings are not realized by the dialysis provider.
Interventions Targeting the Catheter Hub and Lumen
Hub Devices
Data regarding catheter hub devices on bloodstream infection outcomes are provided in Table 2 (48–51). There are two published studies using a neutral-valve catheter hub connector, which is changed weekly and locked with either saline or heparin. The first was a small study in which bloodstream infection rates did not statistically differ between neutral-valve connector with saline lock and standard catheter caps locked with citrate (concentration 46.7%) (48). The second study was a large, retrospective trial that reported a small reduction in the use of intravenous antibiotics with the neutral-valve connector, but no difference in bloodstream infection rates (49).
Table 2.
Hub devices used for hemodialysis catheters
| Study | N | Methods | Standard Definition of Bloodstream Infection Used | Definition of Bloodstream Infection | Hub Device | Control | Outcome | Rate Ratio (95% CI) | P Value | Concerns |
|---|---|---|---|---|---|---|---|---|---|---|
| Bonkain et al. (48) | 66 | Prospective, nonblinded, randomized trial | No | Same organism in two blood samples from catheter with clinical suspicion | Neutral valve connector+saline lock | Standard cap+trisodium citrate (46.7%) lock | Composite of catheter dysfunction or bloodstream infection | 0.16 (0.02 to 1.38) | 0.06 | No difference, small underpowered study |
| Brunelli et al. (49) | 17,145 | Retrospective, observational analysis | No | (1) IV antibiotics started; (2) receipt of antibiotics course; (3) positive blood cultures | Neutral valve connector+saline lock | Standard cap+heparin lock | a) IV Antibiotic start b) Bloodstream infection | a) 0.87 (0.82 to 0.93); b) 0.95 (0.88 to 1.09) | a) <0.001; b) 0.34 | Retrospective study |
| Hymes et al. (50) | 2470 | Prospective, cluster-randomized, comparative effectiveness trial | No | Positive blood cultures reported | Chlorhexidine cap+heparin lock | Standard cap+heparin lock | a) Bloodstream infection rate b) IV Antibiotic start | a) 0.44 (0.23 to 0.83); b) 0.9 (0.74 to 1.19) | a) 0.01 b) 0.6 | Cost |
| Brunelli et al. (51) | 1671 | Prospective, cluster-randomized, open-label trial | Yes | NHSN (2) | Chlorhexidine cap+heparin lock | NVC+70% isopropanol alcohol cap | Bloodstream infection | 0.37 (0.20 to 0.68) | 0.003 | Cost |
95% CI, 95% confidence interval; IV, intravenous; NHSN, National Healthcare Safety Network.
Another novel hub device contains a chlorhexidine-coated rod that extends into the catheter lumen (50,51). It is changed with each hemodialysis session and used with a heparin lock. In a prospective, cluster-randomized trial, the use of the chlorhexidine cap was associated with a significant reduction in the rate of positive blood culture episodes (56%) and in hospital admissions for bloodstream infections (40%) over a 1-year follow-up period, when compared with standard caps (50). A recently published, prospective, cluster-randomized trial compared bloodstream infection rates between hemodialysis facilities using the chlorhexidine cap to a neutral-valve connector with disinfecting caps containing 70% isopropyl alcohol (51). The use of the chlorhexidine cap was associated with a 63% reduction in bloodstream infection compared with the neutral-valve connector plus 70% isopropyl alcohol cap. The chlorhexidine cap has been approved by the US Food and Drug Administration for use in hemodialysis catheters, and has recently replaced the neutral-valve connector hub device in some units operated by large dialysis organizations in the United States. The cost of chlorhexidine-coated rod hub devices varies according to the volume purchased, and is $6 per pair if purchased directly in small quantities. Hymes et al. (50) argue that the upfront cost of the chlorhexidine-coated rod hub device may be offset by reducing the total economic effect of catheter-associated bloodstream infections, and project that the estimated savings to dialysis providers and Medicare would be $2400 and $16,000 per episode, respectively.
Antimicrobial Lock Solutions
Antimicrobial lock solutions are highly concentrated antiseptic agents that are instilled in the catheter when the catheter is not in use, thereby targeting the intraluminal route of entry (52–86). An antiseptic agent is needed to prevent colonization and biofilm formation, thereby reducing catheter-associated bloodstream infections. Antiseptic catheter locking agents may consist of an antibiotic or a nonantibiotic solution, and both types of catheter locks have been shown to effectively reduce the incidence of catheter-associated bloodstream infections in clinical trials (Table 3). The routine use of an antibiotic-containing catheter lock for prevention of bloodstream infections in hemodialysis may result in the emergence of resistant organisms, and has led to the development of safer, nonantibiotic locks. The second component added to most antimicrobial locks is an anticoagulant, used to prevent catheter dysfunction. Heparin promotes biofilm formation, whereas citrate in concentrations in concentrations ≥0.2% prevents biofilm formation, making citrate advantageous (87).
Table 3.
Antimicrobial locking solutions used for hemodialysis catheters
| Study | N | Methods | Standard Definition of Bloodstream Infection Used | Definition of Bloodstream Infection | Antimicrobial Locking Solution | Control | Outcome | Rate/1000 Catheter-Days | P Value | Concerns |
|---|---|---|---|---|---|---|---|---|---|---|
| Pervez et al. (52) | 36 | Prospective, randomized study | No | Fever or chills without an alternate source of infection | Gentamicin 20 mg/ml+citrate 4.67% | Heparin 1000 U/ml | Bloodstream infection | 0.62 versus 2.11 | N/A | Resistance not measured |
| Dogra et al. (53) | 83 | Double-blind, randomized trial | Yes | CDC/IDSA (10) | Gentamicin 27 mg/ml+citrate 1% | Heparin 5000 U/ml | Bloodstream infection | 0 0.3 versus 4.2 | <0.001 | Vestibular toxicity, resistance not measured |
| McIntyre et al. (54) | 50 | Prospective, randomized, controlled trial | Yes | CDC/IDSA (10) | Gentamicin 5 mg/ml+heparin 5000 U/ml | Heparin 5000 U/ml | Bloodstream infection | 0.3 versus 4 | 0.02 | Resistance not measured |
| Nori et al. (55) | 30 | Prospective, open-label, randomized, controlled trial | Yes | CDC/IDSA (10) | Gentamicin 4 mg/ml+citrate 3.13% | Heparin 5000 U/ml | Bloodstream infection | 0 versus 4 | 0.008 | Resistance not measured |
| Venditto et al. (56) | 265 | Prospective, observational trial | No | Two positive blood cultures from peripheral or catheter in a symptomatic patient with fever >38°C with no other apparent source of infection | Gentamicin (N/A)+heparin | Heparin (units N/A) | Bloodstream infection | 0.4 versus 2.9 | 0.06 | Resistance not measured |
| Landry et al. (57) | 1410 | Retrospective chart review after initiation of a unit protocol | Yes | CDC/IDSA (12) | Gentamicin 4 mg/ml+heparin 5000 U/ml | Historical controls before introduction of locks | Bloodstream infection | 0.83–1.2 versus 17.0 | N/A | 4 yr study, gentamicin resistance observed in 32%. Bloodstream infection (coagulase-negative staphylococci n=13, Enterococcus n=7, Streptococcus n=2, Staphylococcus aureus n=1) |
| Fernández-Gallego et al. (58) | 101 | Prospective, observational study | No | Clinical improvement after antibiotics in patients with a fever and with positive blood cultures taken from the circuit, excluding other possible infection sites | Gentamicin 5 mg/lumen (approximately 1.5 mg/ml)+heparin 100 U | None | Bloodstream infection and bacterial resistance to gentamicin | 0.11 | N/A | 7 yr study, no resistance for pathogens “normally sensitive to gentamicin”, two cases of MRSA resistant to gentamicin |
| Moran et al. (59) | 303 | Randomized, prospective, single-blinded, multicenter trial | No | Presence of a positive blood culture obtained from catheter, associated with fever or chills or hypotension | Gentamicin 0.32 mg/ml+citrate 4% | Heparin 1000 U/ml | Bloodstream infection | 0.28 versus 0.91 | 0.003 | 4.5 yr study, no change in gentamicin resistance |
| Moore et al. (60) | 555 | Prospective, multicenter, observational cohort study | Yes | CDC/IDSA (12) | Gentamicin 0.32 mg/ml+citrate 4% | Heparin 1000 U/ml | a) Bloodstream infection b) Mortality | a) 0.45 versus 1.68 b) 10% versus 18% HR 0.32 (0.14–0.75) | a) 0.001 b) 0.001 | 3 yr study, reduction in gentamicin resistance by approximately 50% (P=0.01) |
| Goh et al. (61) | 64 | Single-center, retrospective cohort study | Yes | NHSN (2) | a) Gentamicin 5 mg/lumen (approximately 1.5 mg/ml) + Heparin 1000 U/ml b) Gentamicin +Citrate | a) Heparin 1000 U/ml b) Heparin 1000 U/ml | Bloodstream infection | a) 0.66 versus 1.42 RR 0.46 (0.30 to 0.72) b) 0.16 versus 1.42 RR 0.11 (0.05 to 0.22) | 0.001 | Resistance not measured |
| Onder et al. (62) | 43 | Single-center, retrospective cohort study | No | Positive blood culture from the catheter with or without positive peripheral blood culture with systemic symptoms (fever, chills, vomiting, hypotension) and no other identified source of infection | Tobramycin 5 mg/dl+tissue plasminogen activator 1 mg/ml | Heparin 5000 U/ml | Bloodstream infection | 6.2 versus 16.8 | 0.2 | Resistance not measured |
| Bleyer et al. (63) | 60 | Single-center, double-blind, randomized, controlled trial | No | Catheter colonization plus a peripheral blood culture growing the same organism | Minocycline 3 mg/ml+EDTA 30 mg/ml | Heparin (dose N/A) | Bloodstream infection | 0 versus 0.47 | 0.35 | Resistance not measured |
| Campos et al. (64) | 204 | Multicenter, open-label, randomized, controlled trial | Yes | KDOQI and CDC (8,10) | Minocycline 3 mg/ml+EDTA 30 mg/ml | Heparin 5000 U/ml | Bloodstream infection | 1.1 versus 4.3 | 0.005 | Resistance not measured |
| Saxena and Panhotra (65) | 96 | Prospective, case-control study | Yes | CDC (9) | Cefotaxime 10 mg/ml+heparin 5000 U/ml | Heparin 5000 U/ml | Bloodstream infection | 1.65 versus 3.13 | N/A | Resistance not measured |
| Saxena et al. (66) | 113 | Single-center, double-blind, randomized, controlled trial | Yes | CDC (9) | Cefotaxime 10 mg/ml+heparin 5000 U/ml | Heparin 5000 U/ml | Bloodstream infection | 1.44 versus 3.15 | <0.001 | Resistance not measured |
| Al-Hwiesh and Abdul-Rahman (67) | 63 | Single-center, randomized, controlled trial | Yes | CDC (9) | Vancomycin 25 mg/ml+gentamicin 40 mg/ml+heparin5000 U/ml | Heparin 5000 U/ml | Bloodstream infection | 4.54 versus 13.11 | 0.05 | Resistance not measured |
| Kim et al. (68) | 120 | Single-center, double-blind, randomized, controlled trial | Yes | CDC (10) | Cefazolin 10 mg/ml+gentamicin 5 mg/ml+heparin 1000 U/ml | Heparin 1000 U/ml | Bloodstream infection | 0.44 versus 3.12 | 0.03 | Resistance not measured |
| Rijnders et al. (69) | 270 | Multicenter, open-label, evaluator-blinded, randomized, controlled trial | No | Clinical signs or symptoms of systemic infection and one of the following: positive blood culture in combination with temperature >38°C, temperature >37.5°C (during dialysis), rigors, chills, malaise, altered mental status, or hypotension unresponsive to fluids on dialysis | Trimethoprim 5 mg/ml+ethanol 25%+EDTA 3% | Heparin 5000 U/ml | Bloodstream infection | 0.09 versus 0.41 | <0.03 | Catheter dysfunction |
| Bueloni et al. (70) | 145 | Multicenter, open-label, nonrandomized trial | No | Presence of at least one of the signs or symptoms of infection, such as fever, tremors or hypotension without another apparent focus of infection, with a positive culture if one is performed | Cefazolin 12 mg/ml+gentamicin 7 mg/ml+heparin 500 U/ml | Taurolidine 1.35%+citrate 4%+heparin 500 U/ml | a) Bloodstream infection b) Catheter removal | a) 0.79 versus 1.1 b) 49% versus 53% | 0.01 0.85 | Significantly higher rate of MRSA exit-site infections with cefazolin+gentamin lock |
| Allon (71) | 50 | Prospective, case-control study | No | Positive peripheral blood cultures in a febrile patient | Taurolidine 1.35%+ citrate 4% | Heparin 5000 U/ml | Bloodstream infection | 0.6 versus 5.9 | <0.001 | Catheter dysfunction |
| Betjes and van Agteren (72) | 58 | Single-center, randomized, controlled trial | No | Symptomatic patient with a positive bacterial blood culture drawn from the dialysis catheter with no other apparent source of infection | Taurolidine 1.35%+ citrate 4% | Heparin 5000 U/ml | Bloodstream infection | 0 versus 2.1 | 0.05 | Catheter dysfunction |
| Solomon et al. (73) | 110 | Multicenter, double-blind, randomized, controlled trial | No | A single positive blood culture | Taurolidine 1.35%+ citrate 4% | Heparin 5000 U/ml | Bloodstream infection | 1.4 versus 2.4 | 0.1 | Catheter dysfunction |
| Solomon et al. (74) | 174 | Prospective, cohort study compared with historical controls | No | A single positive blood culture | Taurolidine 1.35%+citrate 4%+heparin 5000 U/ml | a) Heparin 5000 U/ml b) Taurolidine 1.35%+ Citrate 4% | a1) Bloodstream infection a2) First use of thrombolytic b1) Blood stream infection b2) First use of thrombolytic | a1) 1.33 versus 3.25 a2) RR 1.4 (0.5 to 3.9) b1) 1.33 versus 1.22 b2) RR 0.2 (0.06 to 0.5) | a1) <0.001 a2) 0.5 b1) <0.001 b2) <0.001 | Cost |
| Winnicki et al. (75) | 106 | Multicenter, randomized, controlled trial | No | A positive bacterial blood culture drawn from the dialysis catheter in a symptomatic patient with fever or chills associated with dialysis and no apparent other source of infection | Taurolidine 1.35%+citrate 4%+heparin 5000 U/ml twice a wk with taurolidine 1.35%+citrate 4%+urokinase 25,000 U once a wk | Citrate 4% | a) Bloodstream infection b) Catheter dysfunction | a) 0.67 versus 2.7 b) 18.7 versus 44.3 | 0.003 0.001 | Cost |
| Al-Ali et al. (76) | 164 | Multicenter, single-blinded, randomized, controlled trial | No | Same organism obtained from blood aspirated through the catheter hub and from blood sample obtained from peripheral vein with no other identifiable cause of infection | Taurolidine 1.35%+citrate 4%+heparin 5000 U/ml twice a wk with taurolidine 1.35%+citrate 4%+urokinase 25,000 U once a wk | Taurolidine 1.35%+citrate 4%+heparin 5000 U/ml three times a wk | a) Catheter removal for bloodstream infection b) Catheter removal for dysfunction c) Need for tissue plasminogen activator use | a) 0 versus 3 b) 1 versus 4 c) 5 versus 12 | 0.08 0.17 0.61 | Cost |
| Weijmer et al. (77) | 291 | Multicenter, double-blind, randomized, controlled trial | No | Fever or cold chills not during a dialysis treatment and at least one positive blood culture and no other obvious cause of infection | Trisodium citrate 30% | Heparin 5000 U/ml | Bloodstream infection | 1.1 versus 4.1 | <0.001 | —- |
| Winnett et al. (78) | 413 | Multicenter, case-control study | No | Fever and one positive blood culture result with no other obvious source of infection | Trisodium citrate 46.7% | Heparin 5000 U/ml | Bloodstream infection | 0.81 versus 2.13 | <0.001 | Not FDA approved, excessive overfill may result in death |
| Power et al. (79) | 232 | Single-center, randomized, controlled trial | No | Symptomatic febrile patient with positive blood cultures | Trisodium citrate 46.7% | Heparin 5000 U/ml | Bloodstream infection | 0.7 versus 0.7 | 0.9 | Lack of efficacy |
| Venditto et al. (56) | 265 | Single-center, prospective, cohort study compared with historical controls | Yes | CDC (10) | Trisodium citrate 46% | Heparin (n/a) | Bloodstream infection | 3.4 versus 2.9 | 0.6 | Lack of efficacy |
| Correa Barcellos et al. (80) | 464 | Single-center, double-blind, randomized, controlled trial | Yes | CDC (10) | Trisodium citrate 30% | Heparin 5000 U/ml | Bloodstream infection | Rate ratio 1.53 (95% CI, 0.9–2.58) | — | Lack of efficacy |
| Hemmelgarn et al. (81) | 225 | Multicenter, double-blind, randomized, controlled trial | Yes | Health Canadian 1997 Guidelines (7) | Recombinant tissue plasminogen activator once a wk +heparin 5000 U/ml twice a wk | Heparin 5000 U/ml | Bloodstream infection | 0.4 versus 1.37 | 0.02 | Cost |
| Maki et al. (82) | 407 | Multicenter, open-label, randomized, controlled trial | No | Definite catheter-related: fever with concordant positive blood cultures drawn from the catheter and a peripheral vein or a peripheral blood culture and a concordant exit-site culture; concordant catheter-related bloodstream infection: two concordant positive blood cultures but with temperature not exceeding 38.0°C; probable catheter-related bloodstream infection: fever with one positive blood culture | Citrate 7%+methylene blue+methylparabens/propylparaben | Heparin 5000 U/ml | Bloodstream infection | 0.24 versus 0.82 | 0.005 | Not FDA approved |
| Broom et al. (83) | 49 | Multicenter, open-label, randomized, controlled trial | Yes | KDOQI (8) | Ethanol 70% once a wk+heparin 1000 U/ml twice a wk | Heparin 1000 U/ml | Bloodstream infection | 0.28 versus 0.85 | 0.12 | Small proof-of-concept study. potential symptoms, hepatotoxicity and mechanical changes in catheter polymer using high-dose ethanol |
| Sofroniadou et al. (84) | 103 | Single-center, randomized, controlled trial | Yes | CDC/IDSA (11) | Ethanol 70%+heparin 2000 U/ml | Heparin 2000 U/ml | Bloodstream infection | 2.53 versus 6.7 | 0.04 | Short-term study, potential symptoms, hepatotoxicity and mechanical changes in catheter polymer using high-dose ethanol |
| Vercaigne et al. (85) | 40 | Multicenter, randomized, controlled trial | No | Two or more positive blood cultures of the same organism from any source (peripheral or intravascular device cultures) from a patient with no other source of infection | Ethanol 30%+citrate 4% | Heparin 1000 U/ml | Bloodstream infection | 0 versus 0.75 | 0.12 | Small pilot study |
| El-Hennawy et al. (86) | 452 | Single-center, open-label, randomized, controlled trial | Yes | CDC/IDSA (10) | Sodium bicarbonate (7.5% or 8.4%) | Normal saline 0.9% | a) Bloodstream infection b) Catheter loss due to thrombosis c) Catheter loss due to bloodstream infection | a) 0.17 versus 2.6 b) 0.4% versus 0.6% c) 0.4% versus 6.6% | 0.01 <0.001 <0.001 |
N/A, not available; CDC, Centers for Disease Control and Prevention; IDSA, Infectious Disease Society of America; MRSA, methicillin resistant Staphylococcus aureus; NHSN, National Healthcare Safety Network; KDOQI, Kidney Disease Outcomes Quality Initiative; FDA, Food and Drug Administration.
Antibiotic Locks
A variety of antibiotic-containing locking agents have been studied for the prevention of catheter-associated bloodstream infection in the hemodialysis setting (Table 3) (52–70). The current Centers for Disease Control and Prevention recommendations are for the limited use of prophylactic antimicrobial lock solutions in catheter-dependent patients on hemodialysis who have a history of multiple bloodstream infections (15). The prophylactic use of combination antibiotic-anticoagulant catheter lock solutions is associated with a significant reduction in bloodstream infections (range 50%–100% reduction). The antibiotics used as a catheter locking-solution in these trials were gentamicin, tobramycin, minocycline, cefotaxime, vancomycin, cefazolin, and trimethoprim, with gentamicin being the most commonly studied. Early trials, which used a relatively high-dose gentamicin (4–27 mg/ml) lock, reported that gentamicin alone was as effective as other antibiotic combinations and had a broad spectrum of activity against both Gram-positive (including Staphylococcus aureus) and Gram-negative bacteria at drug levels achieved in the catheter lumen. The emergence of gentamicin resistant strains of Enterococcus and Staphylococcus have been associated with serious episodes of bloodstream infection, and one death has been reported using a gentamicin (4 mg/ml) lock (57). In more recent studies, using a lower concentration of gentamicin lock (range 0.32–1.7 mg/ml), no gentamicin resistance has been observed over long follow-up periods, and in one study the rate of resistance declined, although an explanation for this finding is unclear (58–60). In one study, a low-dose gentamicin (0.32 mg/ml)-citrate (4%) lock was associated with significant reduction in bloodstream infections, infection-related hospitalizations, and patient mortality (60). If a gentamicin lock is used, a low-dose formulation (0.32 mg/ml) is recommended. In a recent, retrospective, cost-effectiveness analysis from New Zealand, the routine use of a gentamicin-containing catheter lock was associated with significantly lower rates of catheter-associated bloodstream infection, which translated to significant cost-savings (61). Inpatient costs associated with the management of catheter-associated bloodstream infection were NZ$27,792 per 1000 catheter-days (heparin-only lock) versus NZ$10,608 (gentamicin-heparin) and NZ$1898 (gentamicin-citrate). Concerns about antibiotic resistance associated with prophylactic use of antibiotic catheter lock solutions have prevented their adoption by consensus guidelines.
Nonantibiotic Locks
In an attempt to avoid the development of antimicrobial resistance, more recent focus has been on the development of nonantibiotic catheter lock solutions (Table 3) (56,70–86). Taurolidine is a broad spectrum, antimicrobial agent that reduces biofilm formation and has a low-risk bacterial resistance. Although taurolidine-citrate 4% antimicrobial lock is associated with a reduction in bloodstream infections, when compared with heparin lock, the need for thrombolytic therapy is increased (71–73). Newer preparations, which have added heparin or a thrombolytic agent to taurolidine-citrate 4%, are associated with improved rates of catheter dysfunction and bloodstream infections (70,74–76) A taurolidine-based, nonantibiotic lock (taurolidine-citrate-heparin) has been shown to be as efficacious as an antibiotic-containing lock (containing cefazolin-gentamicin-heparin) for preventing catheter-associated bloodstream infections (70). In this study, a higher rate of resistant strains of methicillin-resistant organisms was reported in the antibiotic lock group. At present, taurolidine containing catheter locks are widely used in Europe, but have not yet been approved for use in the United States. A large, multicenter, randomized trial of a taurolidine-citrate-heparin catheter lock was recently completed and results are available in abstract form, however publication of the manuscript is pending (89).
Citrate 4% locks, when used alone, are not associated with reduction in bloodstream infection rates. The efficacy of catheter-associated bloodstream infection reduction using medium- and high-dose citrate (30%–47%) antimicrobial locks are conflicting: two studies reported a reduction in bloodstream infection, and three studies reported no advantage of citrate over heparin (Table 3) (56,77–80). Trisodium citrate antimicrobial locks, in concentrations of between 30% and 47%, were withdrawn by the US Food and Drug Administration after one patient death, presumably because of overfill of the catheter (88). There are additional reports of symptomatic paresthesia and cardiac and embolic complications due to precipitation of trisodium citrate (30%–47%) in the catheter, and an increase in thrombolytic requirements for catheter dysfunction (90).
Recombinant tissue plasminogen activator used weekly as a catheter locking solution, with heparin twice weekly, has been shown to be associated with a significant reduction in bloodstream infection (approximately 67%) and catheter dysfunction (approximately 50%) (Table 3) (81). Tissue plasminogen activator is not in routine use for bloodstream infection prophylaxis because of excessive immediate costs; however, when counterbalanced by the anticipated reduced cost of bloodstream infection–associated hospitalizations, a decision analysis model calculated that there would be no significant difference in the mean overall cost (91). Again, under the United States health care model, prophylactic tissue plasminogen activator would increase the cost of providing hemodialysis, but the expected savings in reducing catheter-associated bloodstream infection would not be shared with the hemodialysis provider.
A novel, chelator-based, nonantibiotic antimicrobial lock containing citrate 7%-methylene blue-methylparabens-propylparaben was associated with a significant reduction (approximately 70%) in bloodstream infections and in catheter removal for dysfunction (Table 3) (82). This chelator-based antimicrobial locking solution was not approved by the US Food and Drug Administration.
Ethanol antimicrobial lock has also been studied for use in hemodialysis catheters (Table 3) (83–85). The potential advantages of ethanol include its low cost, reduction of biofilm, lack of resistance, and its broad antimicrobial and antifungal properties. In a small study, ethanol lock 70% used once weekly (heparin 1000 U twice weekly) was associated with a reduction in bloodstream infections when compared with heparin lock alone (83). A larger, prospective, randomized study of ethanol 70% plus heparin 2000 U/ml lock instilled three times a week compared with heparin alone reported a significant reduction in catheter-associated bloodstream infections in the ethanol lock group (84). Ethanol lock in high concentrations (70%–100%) has been associated with catheter dysfunction, reports of headaches, nausea, dizziness, fatigue, hepatotoxicity, and structural changes in the catheter integrity, including elution of molecules from the catheter polymers (92). Ethanol lock in a concentration of 30% is not associated with alterations in carbothane catheter integrity (93). In a small study, a lock containing ethanol 30%-citrate 4% was associated with a reduction in the rate of catheters removed for dysfunction compared with heparin (85). The only catheter-associated bloodstream infection episode occurred in the heparin lock group. One silicone catheter was found to have a crack in the ethanol 30% group. Ethanol locks (30%) should only be used with compatible catheters, and the ethanol should be withdrawn before initiation of hemodialysis (92,93). Larger, well powered clinical trials using low concentrations of ethanol 30% locks are needed.
Finally, there was a recently published prospective trial comparing the use of a sodium bicarbonate lock solution (8.4% or 7.5%) with a control group using saline (0.9%) for use in hemodialysis catheters (Table 3) (86). Sodium bicarbonate lock was associated with a significant reduction in catheter-associated bloodstream infections, and was shown to prevent catheter loss caused by bloodstream infections and thrombosis. Bicarbonate exerts antimicrobial activity against various bacteria, including S. aureus, Pseudomonas aeruginosa, and Escherichia coli, by potentiating the antimicrobial properties of mammalian antimicrobial peptides (cathelicidins and defensins), components of innate immunity. The mechanism appears to be bicarbonate’s ability to stimulate global changes in bacterial cell walls, gene expression, and membrane permeability. Alkaline solutions interfere with Staphylococcus adherence and impede biofilm formation (94). Sodium bicarbonate has been shown to have anticoagulant properties in small in vitro studies. By chelating calcium ions, similar to citrate, sodium bicarbonate impairs conversion of fibrin to fibrin clot, and prolongs prothrombin and thrombin clotting times (95). Sodium bicarbonate lock appears to be a safe and cost-effective intervention to reduced catheter-associated bloodstream infections in this single study. The potential advantage of a sodium bicarbonate lock is its anticipated low cost and safety profile. The findings of this study are promising, and larger, well powered studies further evaluating the efficacy of sodium bicarbonate (8.4%) lock in hemodialysis catheters are needed.
In summary, nonantibiotic antimicrobial lock solutions may have an important future role as novel, low-risk approaches to catheter-associated bloodstream infection prevention. Studies combining nonantibiotic antimicrobial locks with chlorhexidine antimicrobial hub devices have not been performed, and so it is unclear if this combination offers additional efficacy.
Interventions Utilizing S. Aureus Decolonization Protocols
Intranasal Mupirocin Ointment
S. aureus nasal carriage is associated with increased risk of hemodialysis catheter–associated bloodstream infections. Decolonization protocols performed in the hemodialysis setting are described in Table 4 (96–100). Intranasal mupirocin prophylaxis was associated with a 94%–100% efficacy for nasal decolonization and a significant reduction in the incidence of S. aureus catheter-associated bloodstream infections. S. aureus decolonization protocols are efficacious and cost-effective (101). Bloom et al. (101) performed a decision analysis evaluating clinical outcomes and cost-effectiveness of three possible management strategies in patients on hemodialysis (1): screen for S. aureus nasal carriage every 3 months and treat those with a positive test result with mupirocin; (2) treat all patients weekly with mupirocin; or (3) no prevention strategy, treat infection only. Eliminating S. aureus nasal carriage with mupirocin markedly reduced the number of infections (approximately 50% reduction) and reduced health care expenditures relative to treating infections when they occur. The potential annual savings to Medicare (in 1996 US dollars) were projected to be $784,000–$1,117,000 per 1000 patients on hemodialysis, depending on which prevention protocol was used. Mupirocin has not been widely adopted in the hemodialysis setting because of concerns of emerging resistance, although reports of mupirocin resistance have largely been conducted in hospitalized patients and with long term mupirocin use. The use of S. aureus decolonization protocols in catheter-dependent patients on hemodialysis should be revisited.
Table 4.
Intranasal mupirocin Staphylococcus decolonization protocols used for the prevention of blood stream infections in the hemodialysis setting
| Study | N | Methods | Standard Definition of Bloodstream Infection Used | Definition of Bloodstream Infection | Protocol | Treatment Group | Outcome | Staphylococcus aureus Infection Rate/Patient yr | P Value | Concerns |
|---|---|---|---|---|---|---|---|---|---|---|
| Kang et al. (95) | 19 | Open, prospective cohort study | No | Not reported | Mupirocin twice per d for 5 d for 6 mo | MRSA nasal carriers | Bloodstream infection | 1 Bloodstream infection | N/A | Potential for resistance |
| Lederer et al. (96) | 16 | Open, prospective cohort study | No | Not reported | Mupirocin three times per d for 5 d for 12 mo | MRSA nasal carriers | Bloodstream infection | 0 | N/A | Potential for resistance |
| Kluytmans et al. (97) | 226 | Open, prospective cohort study compared with historical controls | No | Not reported | Mupirocin twice per d for 5 d, then weekly for 6 mo | Staphylococcus aureus nasal carriers | Bloodstream infection | 0.25 versus 0.04 | <0.001 | No resistance observed |
| Boelaert et al. (98) | 80 | Open, prospective cohort study compared with historical controls | No | Positive blood cultures (two out of six bottles) | Mupirocin three times per d for 5 d, then three times per wk for 6 mo, then weekly for 18 mo | Staphylococcus aureus nasal carriers | Bloodstream infection | 0.097 versus 0.024 | 0.008 | One mupirocin-resistant organism identified |
| Boelaert et al. (99) | 35 | Single-center, randomized, double-blind, placebo-controlled trial | No | Not reported | Mupirocin versus placebo three times per d for 14 d, then three times per wk for 9 mo | All Staphylococcus aureus nasal carriers | Bloodstream infection | 0.489 versus 0.115; 1 bloodstream infection in mupirocin-treated group | <0.05 | No resistance observed |
MRSA, Methicillin resistant Staphylococcus aureus; N/A, not available.
Conclusion
Catheter avoidance is the obvious best strategy for reducing bloodstream infection episodes. Interventions such as early referral of patients to nephrologists, multidisciplinary teams, and vascular access coordinators who provide education to patients; implantation of early-cannulation arteriovenous grafts for hemodialysis; and urgent-start peritoneal dialysis have been shown to be effective at decreasing catheter use. However, in those patients where the use of a catheter is unavoidable, implementation of a multitargeted approach to prevent infections is imperative. Active patient engagement and staff education about appropriate catheter care, and improved adherence to the recommended Centers for Disease Control and Prevention’s core interventions is essential. The development of electronic chair-side checklists/audit tools for catheter care may also improve staff antiseptic technique and patient education in the hemodialysis unit. Novel interventions for catheter-associated bloodstream infection prophylaxis include the following: (1) a chlorhexidine-impregnated transparent exit-site dressing; (2) a chlorhexidine-coated rod hub device; and (3) an antimicrobial lock, preferably one without an antibiotic. There may be an important, underutilized role for S. aureus nasal decolonization protocols using mupirocin for patients on hemodialysis. A combination of these interventions may be optimal. Additional factors to consider when selecting a prophylactic strategy include efficacy, cost, potential for adverse effects such as catheter dysfunction, and local availability/device approval by regulatory agencies. Finally, identifying barriers to safe practices in the hemodialysis setting, using human factors systems engineering, will no doubt be invaluable for reducing infections in the future.
Disclosures
Dr. Allon reports receiving personal fees as a consultant for CorMedix. Dr. Allon is also the Editor-in-Chief of American Society of Nephrology open-access journal Kidney360. Dr. Golestaneh reports grant support from the Cardiorenal Society of America, Horizon Pharmaceuticals, and Montefiore Care Management Organization outside of the submitted work. Dr. Mokrzycki reports stock ownership in Abbott Laboratories. Dr. Golestaneh and Dr. Mokrzycki report positions as Clinical Events Committee Members for Spyral Pivotal Hypertension On-Medications and Spyral Pivotal Hypertension Off-Medications, sponsored by Medtronic. Dr. Abreo and Dr. Fisher have nothing to disclose.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.US Renal Data System: 2018 USRDS Annual Data Report: Volume 2: End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 2.Nguyen DB, Shugart A, Lines C, Shah AB, Edwards J, Pollock D, Sievert D, Patel PR: National healthcare safety network (NHSN) dialysis event surveillance report for 2014. Clin J Am Soc Nephrol 12: 1139–1146, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lok CE, Mokrzycki MH: Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int 79: 587–598, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Mokrzycki MH, Zhang M, Cohen H, Golestaneh L, Laut JM, Rosenberg SO: Tunnelled hemodialysis catheter bacteremia: Risk factors for bacteremia recurrence, infectious complications and mortality. Nephrol Dial Transplant 21: 1024–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Poinen K, Quinn RR, Clarke A, Ravani P, Hiremath S, Miller LM, Blake PG, Oliver MJ: Complications from tunneled hemodialysis catheters: A Canadian observational cohort study. Am J Kidney Dis 73: 467–475, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, Pannu NI, Thomas C, Hemmelgarn BR, Craig JC, Manns B, Tonelli M, Strippoli GF, James MT: Associations between hemodialysis access type and clinical outcomes: A systematic review. J Am Soc Nephrol 24: 465–473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Division of Nosocomial and Occupationl Infectious Diseases, Bureau of Infectious Diseases, Laboratory Centre for Disease Control, Health Canada: Preventing infections associated with indwelling intravascular access devices. Can Commun Dis Rep 23[Suppl 8]: i–iii, 1–32, i–iv, 1–16, 1997 [PubMed] [Google Scholar]
- 8.Vascular Access 2006 Work Group: Clinical practice guidelines for vascular access. Am J Kidney Dis 48[Suppl 1]: S176–S247, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM: CDC definitions for nosocomial infections, 1988. Am J Infect Control 16: 128–140, 1988 [DOI] [PubMed] [Google Scholar]
- 10.O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML, Raad II, Randolph A, Weinstein RA: Guidelines for the prevention of intravascular catheter-related infections. MMWR Recomm Rep 51[RR-10]: 1–29, 2002 [PubMed] [Google Scholar]
- 11.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK: Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of America. Clin Infect Dis 49: 1–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allon M, Brouwer-Maier DJ, Abreo K, Baskin KM, Bregel K, Chand DH, Easom AM, Mermel L, Mokrzycki MH, Patel PR, Roy-Chaudhury P, Shenoy S, Valentini RP, Wasse H: Recommended clinical trial end points for dialysis catheters. Clin J Am Soc Nephrol 13: 495–500, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klevens RM, Edwards JR, Andrus ML, Peterson KD, Dudeck MA, Horan TC; NHSN Participants in Outpatient Dialysis Surveillance: Dialysis surveillance report: National Healthcare Safety Network (NHSN)-data summary for 2006. Semin Dial 21: 24–28, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Wetmore JB, Li S, Molony JT, Guo H, Herzog CA, Gilbertson DT, Peng Y, Collins AJ: Insights from the 2016 peer kidney care initiative report: Still a ways to go to improve care for dialysis patients. Am J Kidney Dis 71: 123–132, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention: Dialysis Safety Core Interventions, 2016. Available at: https://www.cdc.gov/dialysis/prevention-tools/core-interventions.html. Accessed April 11, 2019
- 16.Arora P, Obrador GT, Ruthazer R, Kausz AT, Meyer KB, Jenuleson CS, Pereira BJ: Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 10: 1281–1286, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Avorn J, Winkelmayer WC, Bohn RL, Levin R, Glynn RJ, Levy E, Owen W Jr: Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. J Clin Epidemiol 55: 711–716, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Lacson E Jr, Wang W, DeVries C, Leste K, Hakim RM, Lazarus M, Pulliam J: Effects of a nationwide predialysis educational program on modality choice, vascular access, and patient outcomes. Am J Kidney Dis 58: 235–242, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Wilson SM, Robertson JA, Chen G, Goel P, Benner DA, Krishnan M, Mayne TJ, Nissenson AR: The IMPACT (Incident Management of Patients, Actions Centered on Treatment) program: A quality improvement approach for caring for patients initiating long-term hemodialysis. Am J Kidney Dis 60: 435–443, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Gill S, Quinn R, Oliver M, Kamar F, Kabani R, Devoe D, Mysore P, Pannu N, MacRae J, Manns B, Hemmelgarn B, James M, Tonelli M, Lewin A, Liu P, Ravani P: Multi-disciplinary vascular access care and access outcomes in people starting hemodialysis therapy. Clin J Am Soc Nephrol 12: 1991–1999, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polkinghorne KR, Seneviratne M, Kerr PG: Effect of a vascular access nurse coordinator to reduce central venous catheter use in incident hemodialysis patients: A quality improvement report. Am J Kidney Dis 53: 99–106, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Dwyer A, Shelton P, Brier M, Aronoff G: A vascular access coordinator improves the prevalent fistula rate. Semin Dial 25: 239–243, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Mokrzycki MH, Zhang M, Golestaneh L, Laut J, Rosenberg SO: An interventional controlled trial comparing 2 management models for the treatment of tunneled cuffed catheter bacteremia: A collaborative team model versus usual physician-managed care. Am J Kidney Dis 48: 587–595, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Aitken E, Thomson P, Bainbridge L, Kasthuri R, Mohr B, Kingsmore D: A randomized controlled trial and cost-effectiveness analysis of early cannulation arteriovenous grafts versus tunneled central venous catheters in patients requiring urgent vascular access for hemodialysis. J Vasc Surg 65: 766–774, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Shanmuganathan M, Goh BL, Lim CTS: Urgent start intermittent peritoneal dialysis leads to reduction of catheter-related infection and increased peritoneal dialysis penetration. Am J Med Sci 356: 476–480, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Patel PR, Kallen AJ: Bloodstream infection prevention in ESRD: Forging a pathway for success. Am J Kidney Dis 63: 180–182, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Rosenblum A, Wang W, Ball LK, Latham C, Maddux FW, Lacson E Jr: Hemodialysis catheter care strategies: A cluster-randomized quality improvement initiative. Am J Kidney Dis 63: 259–267, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Lee KG, Ng LC, Yeon W, Silva Thore S, Rahman MA, Rofi SNM, Lim WW, Ling ML, Choong HL: Reducing tunneled catheter-related infection in hemodialysis patients with nationwide standardization of catheter care protocol. J Vasc Access 19: 110–111, 2018 [DOI] [PubMed] [Google Scholar]
- 29.O’Keefe C: The authority for certain clinical tasks performed by unlicensed patient care technicians and LPNs/LVNs in the hemodialysis setting: A review. Nephrol Nurs J 41: 247–254, quiz 255, 2014 [PubMed] [Google Scholar]
- 30.Elseviers MM, Van Waeleghem JP; European Dialysis and Transplant Nurses Association/European Renal Care Association: Complications of vascular access: Results of a European multi centre study of the EDTNA/ERCA Research Board. EDTNA ERCA J 29: 163–167, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Brown RS, Brickel K, Davis RB: Two-year observational study of bloodstream infection rates in hemodialysis facility patients with and without catheters. Clin J Am Soc Nephrol 13: 1381–1388, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson S, Wiebe N, Klarenbach S, Pelletier R, Hemmelgarn BR, Gill JS, Manns BJ, Tonelli M; Alberta Kidney Disease Network: Catheter-related blood stream infections in hemodialysis patients: A prospective cohort study. BMC Nephrol 18: 357, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pisoni RL, Zepel L, Port FK, Robinson BM: Trends in US vascular access use, patient preferences, and related practices: An update from the US DOPPS practice monitor with international comparisons. Am J Kidney Dis 65: 905–915, 2015 [DOI] [PubMed] [Google Scholar]
- 34.See I, Shugart A, Lamb C, Kallen AJ, Patel PR, Sinkowitz-Cochran RL: Infection control and bloodstream infection prevention: The perspective of patients receiving hemodialysis. Nephrol Nurs J 41: 37–39, 50, quiz 40, 2014 [PMC free article] [PubMed] [Google Scholar]
- 35.Kosa SD, Gafni A, House AA, Lawrence J, Moist L, Nathoo B, Tam P, Sarabia A, Thabane L, Wu G, Lok CE: Hemodialysis Infection Prevention Protocols Ontario-Shower Technique (HIPPO-ST): A pilot randomized trial. Kidney Int Rep 2: 228–238, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans EC, Hain D, Kear TM, Dork LA, Schrauf C: Hemodialysis catheter outcomes pilot study: No dressing coverage with prescribed showering. Nephrol Nurs J 41: 53–64, 72, quiz 65, 2014 [PubMed] [Google Scholar]
- 37.Levin A, Mason AJ, Jindal KK, Fong IW, Goldstein MB: Prevention of hemodialysis subclavian vein catheter infections by topical povidone-iodine. Kidney Int 40: 934–938, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Sesso R, Barbosa D, Leme IL, Sader H, Canziani ME, Manfredi S, Draibe S, Pignatari AC: Staphylococcus aureus prophylaxis in hemodialysis patients using central venous catheter: Effect of mupirocin ointment. J Am Soc Nephrol 9: 1085–1092, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Johnson DW, MacGinley R, Kay TD, Hawley CM, Campbell SB, Isbel NM, Hollett P: A randomized controlled trial of topical exit site mupirocin application in patients with tunnelled, cuffed haemodialysis catheters. Nephrol Dial Transplant 17: 1802–1807, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Lok CE, Stanely KE, Hux JE, Richardson R, Tobe SW, Conly J: Hemodialysis infection prevention with polysporin ointment. J Am Soc Nephrol 14: 169–179, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Battistella M, Bhola C, Lok CE: Long-term follow-up of the Hemodialysis Infection Prevention with Polysporin Ointment (HIPPO) study: A quality improvement report. Am J Kidney Dis 57: 432–441, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Johnson DW, van Eps C, Mudge DW, Wiggins KJ, Armstrong K, Hawley CM, Campbell SB, Isbel NM, Nimmo GR, Gibbs H: Randomized, controlled trial of topical exit-site application of honey (Medihoney) versus mupirocin for the prevention of catheter-associated infections in hemodialysis patients. J Am Soc Nephrol 16: 1456–1462, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention: Appendix 1: Search Strategy and Evidence Summary Supporting the 2017 Updated Recommendations on the Use of Chlorhexidine-Impregnated Dressings for Prevention of Intravascular Catheter-Related Infections. Available at: https://www.cdc.gov/infectioncontrol/guidelines/bsi/c-i-dressings/appendix/index.html. Accessed April 11, 2019
- 44.Timsit JF, Mimoz O, Mourvillier B, Souweine B, Garrouste-Orgeas M, Alfandari S, Plantefeve G, Bronchard R, Troche G, Gauzit R, Antona M, Canet E, Bohe J, Lepape A, Vesin A, Arrault X, Schwebel C, Adrie C, Zahar JR, Ruckly S, Tournegros C, Lucet JC: Randomized controlled trial of chlorhexidine dressing and highly adhesive dressing for preventing catheter-related infections in critically ill adults. Am J Respir Crit Care Med 186: 1272–1278, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Safdar N, O’Horo JC, Ghufran A, Bearden A, Didier ME, Chateau D, Maki DG: Chlorhexidine-impregnated dressing for prevention of catheter-related bloodstream infection: A meta-analysis*. Crit Care Med 42: 1703–1713, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camins BC, Richmond AM, Dyer KL, Zimmerman HN, Coyne DW, Rothstein M, Fraser VJ: A crossover intervention trial evaluating the efficacy of a chlorhexidine-impregnated sponge in reducing catheter-related bloodstream infections among patients undergoing hemodialysis. Infect Control Hosp Epidemiol 31: 1118–1123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Apata IW, Hanfelt J, Bailey JL, Niyyar VD: Chlorhexidine-impregnated transparent dressings decrease catheter-related infections in hemodialysis patients: A quality improvement project. J Vasc Access 18: 103–108, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Bonkain F, Racapé J, Goncalvez I, Moerman M, Denis O, Gammar N, Gastaldello K, Nortier JL: Prevention of tunneled cuffed hemodialysis catheter-related dysfunction and bacteremia by a neutral-valve closed-system connector: A single-center randomized controlled trial. Am J Kidney Dis 61: 459–465, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Brunelli SM, Njord L, Hunt AE, Sibbel SP: Use of the TEGO needlefree connector is associated with reduced incidence of catheter-related bloodstream infections in hemodialysis patients. Int J Nephrol Renovasc Dis 7: 131–139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hymes JL, Mooney A, Van Zandt C, Lynch L, Ziebol R, Killion D: Dialysis catheter-related bloodstream infections: A cluster-randomized trial of the ClearGuard HD Antimicrobial Barrier Cap. Am J Kidney Dis 69: 220–227, 2017 [DOI] [PubMed] [Google Scholar]
- 51.Brunelli SM, Van Wyck DB, Njord L, Ziebol RJ, Lynch LE, Killon DP: Cluster-randomized trial of devices to prevent catheter-related bloodstream infection. J Am Soc Nephrol 29: 1336–1343, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pervez A, Ahmed M, Ram S, Torres C, Work J, Zaman F, Abreo K: Antibiotic lock technique for prevention of cuffed tunnel catheter associated bacteremia. J Vasc Access 3: 108–113, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Dogra GK, Herson H, Hutchison B, Irish AB, Heath CH, Golledge C, Luxton G, Moody H: Prevention of tunneled hemodialysis catheter-related infections using catheter-restricted filling with gentamicin and citrate: A randomized controlled study. J Am Soc Nephrol 13: 2133–2139, 2002 [DOI] [PubMed] [Google Scholar]
- 54.McIntyre CW, Hulme LJ, Taal M, Fluck RJ: Locking of tunneled hemodialysis catheters with gentamicin and heparin. Kidney Int 66: 801–805, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Nori US, Manoharan A, Yee J, Besarab A: Comparison of low-dose gentamicin with minocycline as catheter lock solutions in the prevention of catheter-related bacteremia. Am J Kidney Dis 48: 596–605, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Venditto M, du Montcel ST, Robert J, Trystam D, Dighiero J, Hue D, Bessette C, Deray G, Mercadal L: Effect of catheter-lock solutions on catheter-related infection and inflammatory syndrome in hemodialysis patients: Heparin versus citrate 46% versus heparin/gentamicin. Blood Purif 29: 268–273, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Landry DL, Braden GL, Gobeille SL, Haessler SD, Vaidya CK, Sweet SJ: Emergence of gentamicin-resistant bacteremia in hemodialysis patients receiving gentamicin lock catheter prophylaxis. Clin J Am Soc Nephrol 5: 1799–1804, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernández-Gallego J, Martín M, Gutiérrez E, Cobelo C, Frías P, Jironda C, Hidalgo P, Jiménez T: Prophylaxis with gentamicin locking of chronic tunnelled central venous catheters does not cause bacterial resistance. Nefrologia 31: 308–312, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Moran J, Sun S, Khababa I, Pedan A, Doss S, Schiller B: A randomized trial comparing gentamicin/citrate and heparin locks for central venous catheters in maintenance hemodialysis patients. Am J Kidney Dis 59: 102–107, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Moore CL, Besarab A, Ajluni M, Soi V, Peterson EL, Johnson LE, Zervos MJ, Adams E, Yee J: Comparative effectiveness of two catheter locking solutions to reduce catheter-related bloodstream infection in hemodialysis patients. Clin J Am Soc Nephrol 9: 1232–1239, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goh TL, Wei J, Semple D, Collins J: The incidence and costs of bacteremia due to lack of gentamicin lock solutions for dialysis catheters. Nephrology (Carlton) 22: 485–489, 2017 [DOI] [PubMed] [Google Scholar]
- 62.Onder AM, Chandar J, Billings A, Simon N, Gonzalez J, Francoeur D, Abitbol C, Zilleruelo G: Prophylaxis of catheter-related bacteremia using tissue plasminogen activator-tobramycin locks. Pediatr Nephrol 24: 2233–2243, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Bleyer AJ, Mason L, Russell G, Raad II, Sherertz RJ: A randomized, controlled trial of a new vascular catheter flush solution (minocycline-EDTA) in temporary hemodialysis access. Infect Control Hosp Epidemiol 26: 520–524, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Campos RP, do Nascimento MM, Chula DC, Riella MC: Minocycline-EDTA lock solution prevents catheter-related bacteremia in hemodialysis. J Am Soc Nephrol 22: 1939–1945, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saxena AK, Panhotra BR: The impact of catheter-restricted filling with cefotaxime and heparin on the lifespan of temporary hemodialysis catheters: A case controlled study. J Nephrol 18: 755–763, 2005 [PubMed] [Google Scholar]
- 66.Saxena AK, Panhotra BR, Sundaram DS, Morsy MN, Al-Ghamdi AM: Enhancing the survival of tunneled haemodialysis catheters using an antibiotic lock in the elderly: A randomised, double-blind clinical trial. Nephrology (Carlton) 11: 299–305, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Al-Hwiesh AK, Abdul-Rahman IS: Successful prevention of tunneled, central catheter infection by antibiotic lock therapy using vancomycin and gentamycin. Saudi J Kidney Dis Transpl 18: 239–247, 2007 [PubMed] [Google Scholar]
- 68.Kim SH, Song KI, Chang JW, Kim SB, Sung SA, Jo SK, Cho WY, Kim HK: Prevention of uncuffed hemodialysis catheter-related bacteremia using an antibiotic lock technique: A prospective, randomized clinical trial. Kidney Int 69: 161–164, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Rijnders B, DiSciullo GJ, Csiky B, Rutkowski B, Appelt K, Cheronis J, Aitchison R, Gordon G, Jadoul M, Fluck R: Locking hemodialysis catheters with trimethoprim-ethanol-Ca-EDTA to prevent bloodstream infections: A randomized, evaluator-blinded clinical trial. Clin Infect Dis 69: 130–136, 2019 [DOI] [PubMed] [Google Scholar]
- 70.Bueloni TNV, Marchi D, Caetano C, de Souza Cavalcante R, Mendes Amaral ML, Ponce D: Cefazolin-gentamicin versus taurolidine-citrate for the prevention of infection in tunneled central catheters in hemodialysis patients: A quasi-experimental trial. Int J Infect Dis 85: 16–21, 2019 [DOI] [PubMed] [Google Scholar]
- 71.Allon M: Prophylaxis against dialysis catheter-related bacteremia with a novel antimicrobial lock solution. Clin Infect Dis 36: 1539–1544, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Betjes MG, van Agteren M: Prevention of dialysis catheter-related sepsis with a citrate-taurolidine-containing lock solution. Nephrol Dial Transplant 19: 1546–1551, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Solomon LR, Cheesbrough JS, Ebah L, Al-Sayed T, Heap M, Millband N, Waterhouse D, Mitra S, Curry A, Saxena R, Bhat R, Schulz M, Diggle P: A randomized double-blind controlled trial of taurolidine-citrate catheter locks for the prevention of bacteremia in patients treated with hemodialysis. Am J Kidney Dis 55: 1060–1068, 2010 [DOI] [PubMed] [Google Scholar]
- 74.Solomon LR, Cheesbrough JS, Bhargava R, Mitsides N, Heap M, Green G, Diggle P: Observational study of need for thrombolytic therapy and incidence of bacteremia using taurolidine-citrate-heparin, taurolidine-citrate and heparin catheter locks in patients treated with hemodialysis. Semin Dial 25: 233–238, 2012 [DOI] [PubMed] [Google Scholar]
- 75.Winnicki W, Herkner H, Lorenz M, Handisurya A, Kikić Ž, Bielesz B, Schairer B, Reiter T, Eskandary F, Sunder-Plassmann G, Sengoelge G: Taurolidine-based catheter lock regimen significantly reduces overall costs, infection, and dysfunction rates of tunneled hemodialysis catheters. Kidney Int 93: 753–760, 2018 [DOI] [PubMed] [Google Scholar]
- 76.Al-Ali F, Hamdy AF, Hamad A, Elsayed M, Zafar Iqbal Z, Elsayed A, Ibrahim R, Tolba H, Buanan H, Fawzy A: Safety and efficacy of taurolidine/urokinase versus taurolidine/heparin as a tunneled catheter lock solution in hemodialysis patients: A prospective, randomized, controlled study. Nephrol Dial Transplant 33: 619–626, 2018 [DOI] [PubMed] [Google Scholar]
- 77.Weijmer MC, van den Dorpel MA, Van de Ven PJ, ter Wee PM, van Geelen JA, Groeneveld JO, van Jaarsveld BC, Koopmans MG, le Poole CY, Schrander-Van der Meer AM, Siegert CE, Stas KJ; CITRATE Study Group: Randomized, clinical trial comparison of trisodium citrate 30% and heparin as catheter-locking solution in hemodialysis patients. J Am Soc Nephrol 16: 2769–2777, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Winnett G, Nolan J, Miller M, Ashman N: Trisodium citrate 46.7% selectively and safely reduces staphylococcal catheter-related bacteraemia. Nephrol Dial Transplant 23: 3592–3598, 2008 [DOI] [PubMed] [Google Scholar]
- 79.Power A, Duncan N, Singh SK, Brown W, Dalby E, Edwards C, Lynch K, Prout V, Cairns T, Griffith M, McLean A, Palmer A, Taube D: Sodium citrate versus heparin catheter locks for cuffed central venous catheters: A single-center randomized controlled trial. Am J Kidney Dis 53: 1034–1041, 2009 [DOI] [PubMed] [Google Scholar]
- 80.Correa Barcellos F, Pereira Nunes B, Jorge Valle L, Lopes T, Orlando B, Scherer C, Nunes M, Araújo Duarte G, Böhlke M: Comparative effectiveness of 30 % trisodium citrate and heparin lock solution in preventing infection and dysfunction of hemodialysis catheters: A randomized controlled trial (CITRIM trial). Infection 45: 139–145, 2017 [DOI] [PubMed] [Google Scholar]
- 81.Hemmelgarn BR, Moist LM, Lok CE, Tonelli M, Manns BJ, Holden RM, LeBlanc M, Faris P, Barre P, Zhang J, Scott-Douglas N; Prevention of Dialysis Catheter Lumen Occlusion with rt-PA versus Heparin Study Group: Prevention of dialysis catheter malfunction with recombinant tissue plasminogen activator. N Engl J Med 364: 303–312, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Maki DG, Ash SR, Winger RK, Lavin P; AZEPTIC Trial Investigators: A novel antimicrobial and antithrombotic lock solution for hemodialysis catheters: A multi-center, controlled, randomized trial. Crit Care Med 39: 613–620, 2011 [DOI] [PubMed] [Google Scholar]
- 83.Broom JK, Krishnasamy R, Hawley CM, Playford EG, Johnson DW: A randomised controlled trial of Heparin versus EthAnol Lock THerapY for the prevention of Catheter Associated infecTion in Haemodialysis patients--the HEALTHY-CATH trial. BMC Nephrol 13: 146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sofroniadou S, Revela I, Kouloubinis A, Makriniotou I, Zerbala S, Smirloglou D, Kalocheretis P, Drouzas A, Samonis G, Iatrou C: Ethanol combined with heparin as a locking solution for the prevention of catheter related blood stream infections in hemodialysis patients: A prospective randomized study. Hemodial Int 21: 498–506, 2017 [DOI] [PubMed] [Google Scholar]
- 85.Vercaigne LM, Allan DR, Armstrong SW, Zacharias JM, Miller LM: An ethanol/sodium citrate locking solution compared to heparin to prevent hemodialysis catheter-related infections: A randomized pilot study. J Vasc Access 17: 55–62, 2016 [DOI] [PubMed] [Google Scholar]
- 86.El-Hennawy AS, Frolova E, Romney WA: Sodium bicarbonate catheter lock solution reduces hemodialysis catheter loss due to catheter-related thrombosis and blood stream infection: An open-label clinical trial [published online ahead of print January 21, 2019]. Nephrol Dial Transplant 10.1093/ndt/gfy388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raad II, Fang X, Keutgen XM, Jiang Y, Sherertz R, Hachem R: The role of chelators in preventing biofilm formation and catheter-related bloodstream infections. Curr Opin Infect Dis 21: 385–392, 2008 [DOI] [PubMed] [Google Scholar]
- 88.US Food and Drug Administration: FDA Issues Warning on TriCitrasol Dialysis Catheter Anticoagulant, 2000. Available at: http://www.m2.com/m2/web/story.php/2000A7C52BD5BF4A5240802568C4003B0B87. Accessed April 11, 2019 [Google Scholar]
- 89.Agarwal A, et al.: Poster 441. Presented at the National Kidney Foundation Spring Clinical Meetings, Boston, MA, May 8–12, 2019
- 90.Willicombe MK, Vernon K, Davenport A: Embolic complications from central venous hemodialysis catheters used with hypertonic citrate locking solution. Am J Kidney Dis 55: 348–351, 2010 [DOI] [PubMed] [Google Scholar]
- 91.Manns BJ, Scott-Douglas N, Tonelli M, Ravani P, LeBlanc M, Dorval M, Holden R, Moist L, Lok C, Zimmerman D, Au F, Hemmelgarn BR: An economic evaluation of rt-PA locking solution in dialysis catheters. J Am Soc Nephrol 25: 2887–2895, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mermel LA, Alang N: Adverse effects associated with ethanol catheter lock solutions: A systematic review. J Antimicrob Chemother 69: 2611–2619, 2014 [DOI] [PubMed] [Google Scholar]
- 93.Bell AL, Jayaraman R, Vercaigne LM: Effect of ethanol/trisodium citrate lock on the mechanical properties of carbothane hemodialysis catheters. Clin Nephrol 65: 342–348, 2006 [DOI] [PubMed] [Google Scholar]
- 94.Farha MA, French S, Stokes JM, Brown ED: Bicarbonate alters bacterial susceptibility to antibiotics by targeting the proton motive force. ACS Infect Dis 4: 382–390, 2018 [DOI] [PubMed] [Google Scholar]
- 95.Wong DW, Mishkin FS, Tanaka TT: The effects of bicarbonate on blood coagulation. JAMA 244: 61–62, 1980 [PubMed] [Google Scholar]
- 96.Kang YC, Tai WC, Yu CC, Kang JH, Huang YC: Methicillin-resistant Staphylococcus aureus nasal carriage among patients receiving hemodialysis in Taiwan: Prevalence rate, molecular characterization and de-colonization. BMC Infect Dis 12: 284, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lederer SR, Riedelsdorf G, Schiffl H: Nasal carriage of meticillin resistant Staphylococcus aureus: The prevalence, patients at risk and the effect of elimination on outcomes among outclinic haemodialysis patients. Eur J Med Res 12: 284–288, 2007 [PubMed] [Google Scholar]
- 98.Kluytmans JA, Manders MJ, van Bommel E, Verbrugh H: Elimination of nasal carriage of Staphylococcus aureus in hemodialysis patients. Infect Control Hosp Epidemiol 17: 793–797, 1996 [DOI] [PubMed] [Google Scholar]
- 99.Boelaert JR, Van Landuyt HW, Godard CA, Daneels RF, Schurgers ML, Matthys EG, De Baere YA, Gheyle DW, Gordts BZ, Herwaldt LA: Nasal mupirocin ointment decreases the incidence of Staphylococcus aureus bacteraemias in haemodialysis patients. Nephrol Dial Transplant 8: 235–239, 1993 [PubMed] [Google Scholar]
- 100.Boelaert JR, De Smedt RA, De Baere YA, Godard CA, Matthys EG, Schurgers ML, Daneels RF, Gordts BZ, Van Landuyt HW: The influence of calcium mupirocin nasal ointment on the incidence of Staphylococcus aureus infections in haemodialysis patients. Nephrol Dial Transplant 4: 278–281, 1989 [DOI] [PubMed] [Google Scholar]
- 101.Bloom BS, Fendrick AM, Chernew ME, Patel P: Clinical and economic effects of mupirocin calcium on preventing Staphylococcus aureus infection in hemodialysis patients: A decision analysis. Am J Kidney Dis 27: 687–694, 1996 [DOI] [PubMed] [Google Scholar]