Abstract
CKD is a worldwide health problem and the number of patients requiring kidney replacement therapy is rising. In the United States, most patients with ESKD rely on in-center hemodialysis, which is burdensome and does not provide the same long-term benefits as kidney transplantation. Intensive hemodialysis treatments have demonstrated improved clinical outcomes, but its wider adoption is limited by equipment complexity and patient apprehension. Ambulatory devices for hemodialysis offer the potential for self-care treatment outside the clinical setting as well as frequent and prolonged sessions. This article explains the motivation for ambulatory hemodialysis and provides an overview of the necessary features of key technologies that will be the basis for new wearable and implantable devices. Early work by pioneers of hemodialysis is described followed by recent experience using a wearable unit on patients. Finally, ongoing efforts to develop an implantable device for kidney replacement and its potential for implantable hemodialysis are presented.
Keywords: renal replacement therapy; wearable artificial kidney; implantable hemodialysis; implantable artificial kidney; end stage kidney disease; kidney transplantation; self care; global health; Kidney Failure, Chronic; motivation; renal dialysis; Renal Replacement Therapy; Renal Insufficiency, Chronic
Introduction
CKD is a worldwide health problem and the number of patients with CKD is rising. The prevalence is estimated to be 8%–16% worldwide (1). According to the latest US Renal Data System Annual Data Report, over 725,000 Americans have ESKD (2). Of these, 63% are treated with hemodialysis, 7% with peritoneal dialysis, and 30% have a functioning kidney transplant. It is estimated that over 2 million patients worldwide undergo dialysis treatment, and dialysis remains the only long-term organ replacement therapy. Despite the enormous strides in technology and patient care that dialysis has provided, it still does not provide the same long-term survival as with kidney transplantation.
In-center hemodialysis has shortcomings in both the patient's experience and the patient’s physiology. A patient-centered perspective clearly showed that fatigue, poor sleep, inability to travel, and treatment time were among the most bothersome characteristics of the therapy (3). From a physiology perspective, the paroxysmal nature of in-center hemodialysis leads to cyclic volume overload, hypotension, myocardial stunning, arrythmias, and premature cardiac death (4,5). Furthermore, the short treatment times desired by both patients and dialysis centers impair phosphorus removal. Prolonged treatment times of 6–8 hours allow for effective ultrafiltration without hypovolemic shock and allow for intercompartmental equilibration of phosphorus (6,7). This is important because recent evidence suggests that hyperphosphatemia is directly proportional to cardiovascular events and survival in patients on hemodialysis (8). Low dialysis dose is also associated with malnutrition, which is also an important determinant of morbidity and mortality in patients on hemodialysis (9,10).
Home hemodialysis enables patients to have prolonged and more frequent dialysis treatments that confer improved clinical outcomes (reduced BP, left ventricular hypertrophy, pill burden, improved quality of life, and nutritional status) compared with standard thrice-weekly, in-center therapy (11,12). This modality has the potential to empower patients to take care of themselves and provides them with a more personalized hemodialysis schedule. Patients can have reduced dietary and fluid restrictions as well as maintain employment, leading to an overall better quality of life at lower costs for the health care system. However, not all patients are capable of performing home hemodialysis. Conventional equipment is generally bulky, complex, and often needs to be operated by trained clinical staff. Moreover, some patients are apprehensive about dealing with blood and needlesticks and others have space limitations in the home. Consequently, home hemodialysis has been chosen by only 2% of patients on dialysis in the United States (2).
Recent interest in the development of ambulatory (wearable) hemodialysis devices is largely motivated by the desire to implement the benefits of home hemodialysis in a more patient-centric manner. In addition to dialysis adequacy, key patient-centric outcomes include the dialysis-free (untethered-to-machine) time and ability to travel (13). In one study, patients were willing to trade 23 months of life expectancy with home-based dialysis to decrease their travel restrictions (14). Therefore, an ideal ambulatory hemodialysis device would not be bulky and would allow for user-friendly, routine operation by patients with ESKD, as well as facilitating intensive hemodialysis outside the clinical setting.
Key Requirements
Ambulatory procedures are performed when the patient is free to move. More specifically, ambulatory hemodialysis potentially offers a low-impact approach to prolong treatment times without increasing the time patients with ESKD are away from home, family, and work. However, the equipment for ambulatory kidney replacement must be of manageable size and weight for the patient. Ideally, the product should be ergonomic, fit under clothes, or be unobtrusively embedded in the patient’s outfit (15). The device must also be able to deliver sufficient clearance of uremic toxins: prior reports have proposed creatinine clearance and ultrafiltration targets of 30 ml/min (16,17). Finally, as discussed next, the device needs to be safe and meet the various key technical requirements for dialysis membrane characteristics, dialysate regeneration, vascular access, and patient monitoring (16).
Dialysis Membranes
In ambulatory hemodialysis devices, the dialysis membranes may be exposed to a lower blood flow rate for longer treatment duration than conventional in-center dialysis. The dialyzer membrane must exhibit minimal protein adsorption and be thromboresistant to avoid biofouling and clotting, respectively. Conventional hemodialyzers are comprised of polymer hollow-fiber membranes with 30–100 µm thick walls and exhibit nonuniform distribution of pore sizes, which limits their hydraulic permeability and molecular selectivity, respectively (18). Recent advances in membrane technologies are based on semiconductor materials, such as silicon and silicon nitride. For example, silicon nanopore membranes are nominally 0.1–1.0 µm thin, with a highly uniform pore size distribution and parallel slit–shaped pores (19) (Figure 1). The precisely controlled size and shape of the pores makes the silicon nanopore membranes highly selective and permeable compared with polymer membranes. Atomically thin, nanoporous graphene membranes promise to exhibit even greater permeability and tunable selectivity (20). However, there is still limited data regarding biocompatibility and durability of the new materials when exposed to human blood.
Figure 1.
Silicon nanopore membranes are produced using semiconductor manufacturing techniques to achieve superior hydraulic permeability and molecular selectivity. (A) An optical image of a flat-sheet silicon nanopore membrane specimen. (B) A top-view scanning electron microscope image showing parallel slit–shaped pores on membrane surface. (C) A cross-section scanning electron microscope image showing sub-0.5 µm thin membrane with two sub-10 nm wide pores. Modified from reference 19, with permission.
Dialysate Regeneration
For an ambulatory device, the large volumes of dialysate characteristic of in-center hemodialysis are impractical. Consequently, sorbent and enzyme technologies are needed for dialysate regeneration (11,21). The first truly ambulatory hemodialysis device tested in humans used a dialysate circuit volume of 375 ml (22). Existing dialysate regeneration units make use of cation and anion exchangers for the removal of potassium and phosphate, respectively, and activated carbon for the removal of organic waste solutes, such as creatinine and middle molecules (23). Urea removal is generally difficult because of its hydrophilic character and low reactivity. Different techniques for urea removal have been demonstrated with varying levels of success. These include the use of (1) urease, an enzyme that catalyzes hydrolysis of urea into ammonium and carbon dioxide gas, which was implemented in the original REDY (REcirculation of DialYsate) machine; (2) activated carbon; (3) electrochemical degradation of urea to carbon dioxide and nitrogen gas; and (4) removal by adsorbents such as zeolites, resins, silica, and chitosan (23). A potentially attractive technology of urea removal uses a new class of nanomaterials called MXenes (24,25). These are a family of two-dimensional transition-metal carbides and nitrides. MXene two-dimensional nanosheets comprise a few atomic layers of a transition metal interleaved with carbon or nitrogen with surface terminations bonded to the outer metallic layers. Preliminary work demonstrates that titanium carbide-based MXene to be a promising adsorbent for removing urea from dialysate without causing cytotoxicity (24).
Vascular Access
Vascular access is the “Achilles heel” of all hemodialysis therapies, and ambulatory procedures will be no exception. Arteriovenous fistulas, grafts, and catheters all risk infection, accidental blood disconnect, bleeding, clotting, and entrainment of air into the vasculature and air embolism (3,8). For ambulatory devices, vascular access with needles in fistulas and grafts will be especially risky. On the one hand, a small needle dislodgment can result in hematoma or more severe complications such as vessel laceration and bleeding (26). On the other hand, a venous needle dislodgment can lead to catastrophic consequences, including hemorrhagic shock and death (27). Alternatives to standard needles have been examined, albeit to limited extent. Subcutaneously implantable vascular access devices, such as the LifeSite Hemodialysis Access System (Vasca, Tewsbury, MA) and the Dialock Hemodialysis Access System (Biolink, Manfield, MA), were cuffless devices that incorporated internal mechanisms to avoid needle dislodgement (26). A percutaneous device, the Hemaport (Hemapure, Uppsala, Sweden), attempted to provide needle-free arteriovenous access by combining an implanted polytetrafluoroethylene graft with external replaceable lids (28). Although technologically innovative, the wider adoption of these devices was hampered by surgical site complications. Recent efforts to address the consequences of venous needle dislodgement for home hemodialysis have focused around the use of single-needle cannulation strategies. In this implementation, a single needle is used for vascular access, and in the event of a disconnect, the maximum amount of blood loss is limited to the volume in the dialysis circuit (29,30).
Vascular access through catheters minimizes needle dislodgement and locking mechanisms can be implemented to reduce the risk of disconnect. However, the long-term use of tunneled dual-lumen cuffed central venous catheters is associated with high risk of infection and thrombosis (31,32). Catheter thrombosis arises in at least three ways. First, stagnant blood in the catheter either during or between sessions coagulates, obstructing the lumen. Catheter lock solutions of heparin or citrate have been used to chemically interrupt coagulation with some success. Second, adsorption of blood proteins to the catheter material leads to platelet attachment gradual narrowing of the lumen until it is unusable; this is somewhat retarded through the use of low protein binding polymers. Finally, indwelling catheters eventually develop an external fibrin sheath that occludes end and side ports of the lumens. These challenges are partially addressed through the advent of new catheters incorporating biocompatible and antimicrobial coatings, as well design features to enhance blood flow uniformity (33–35).
Blood Pump
Blood pumps for dialysis have typically been peristaltic roller pumps despite advances in pump technology from the field of mechanical circulatory support. In any (ambulatory) hemodialysis device, the blood propulsion component cannot traumatize the cellular elements of blood to the point of hemolysis. Moreover, the pump motor must be small and quiet, generate tolerable heat, and consume little power (16). The power source must be reliable and safe with a high energy density and power capacity (15). Although the energy density of batteries continues to increase, the resulting heat dissipation, and even fires, have proven a problem in devices ranging from smartphones to laptops to commercial jetliners. Battery packs for blood pumps have been a source of annoyance for patients with ventricular assist devices as they are heavy and must be carried around constantly. However, ambulatory hemodialysis requires lower flow rates than mechanical circulatory support, which should allow for lower-power operation with smaller battery packs.
Patient Monitoring
Conventional hemodialysis machines incorporate a basic monitoring system for tracking blood and dialysate flow and ultrafiltration rate, as well as checking for blood leaks and gas bubbles. The tacit and explicit transfer of responsibility for safety from professional staff in the dialysis center to the patient and family can be a significant barrier to self-care hemodialysis. A device for ambulatory hemodialysis will likely need to incorporate telemetry and automate some safety monitoring and hazard responses to be acceptable to patients and caregivers. Although some safety checks are fairly straightforward yes/no decisions, such as blood leak and bubble detection, others tend to be patient- and prescription-specific, such as access pressures and dialysate conductivity. Determining safe alarm thresholds for vital signs requires individualization as well as clear steps for response to alarms, especially for solitary self-care. Possibly the most challenging safety monitoring problem is detection of venous needle dislodgement. Venous pressures typically exhibit minimal changes with disconnection, and life-threatening blood loss can happen fast, often within a few minutes (27,36). Home and in-center hemodialysis technology approaches have included enuresis pads under the patient or around the access, and optical monitoring for hemoglobin around the access site (36,37). A promising strategy for an integrated monitoring system is found in the architecture of the Tablo platform from Outset Medical (38). It incorporates pressure, flow, temperature, and conductivity sensors to achieve less labor-intensive operation by automating both the treatment management and machine maintenance. Treatment data are stored in the cloud and can be downloaded remotely and/or integrated with the patient’s clinical electronic medical records. With further advances in sensor technology and wireless connectivity, it is feasible to contemplate an “internet of things” approach for communicating key device and physiologic parameters from the patient using an ambulatory hemodialysis to the nephrologist and other clinical staff.
Early Work
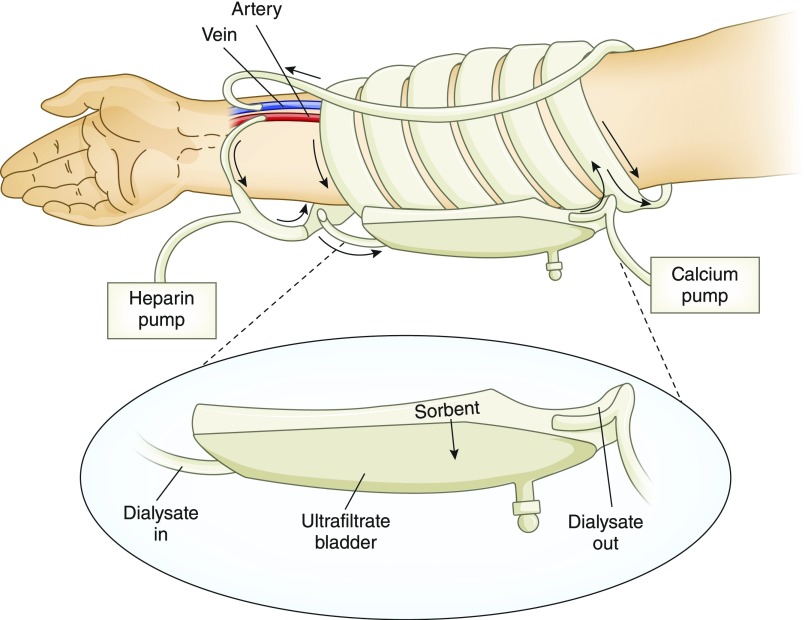
Concepts of wearable devices for ambulatory hemodialysis have been proposed as early as the 1960s (39–42). For example, Newhart proposed a device in which the dialysate would be pumped by patient-generated pulses, such as arterial and breathing pulses, utilizing a check-valve controlled diaphragm (40). Lande et al. (41) proposed a wearable artificial kidney (WAK) located on the forearm near the wrist (Figure 2) (42). In this implementation, blood would be pumped through a miniature dialyzer by periodically blocking the fistula and thereby creating a pressure drop between the arterial and venous side of the fistula.
Figure 2.
Concept of a wearable hemodialyzer situated on the patient’s forearm. Blood is pumped through the long, wide, low-resistance dialyzer by periodically blocking the fistula, which creates a pressure drop between the arterial and venous sides. Dialysate is regenerated by a sorbent cartridge. Modified from reference 41, with permission.
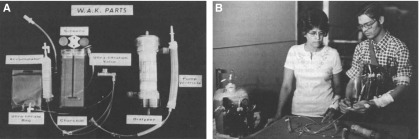
The beginnings of a working ambulatory device for hemodialysis originate in the 1970s, when Willem Kolff developed a “wearable kidney” that could be worn on the chest (43). The wearable unit weighed 3.5 kg (7 lb), comprising a blood and dialysate circuit with pumps, batteries, tubing, and a charcoal regeneration module (Figure 3). It did not have an air bubble or a blood leak detector. Moreover, this device required that the patients also be connected intermittently to a 20 L dialysate batch to allow for urea and potassium removal (11,43). This system was used to treat one patient for 35 consecutive days for 3 hours per day, 6 days per week. Four patients were dialyzed intermittently. Ultrafiltration rates reached 700 ml/h, and mass balance studies demonstrated a daily urea and creatinine removal of 14–20 g and 1.5–2.0 g, respectively. Later on, this type of dialysis system was used more often and patients were sent on “Dialysis in Wonderland” trips (44).
Figure 3.
Wearable artificial kidney developed by Willem Kolff. (A) Components of the wearable artificial kidney developed by Willem Kolff. The wearable module had to be periodically connected to a 20 L dialysate bath to achieve adequate urea and potassium removal. (B) Patient on the left is attached to the 20 L tank of dialysate, whereas the patient on the right is only attached to the wearable unit. Modified from reference 43, with permission.
Current Efforts
Wearable Hemodialysis
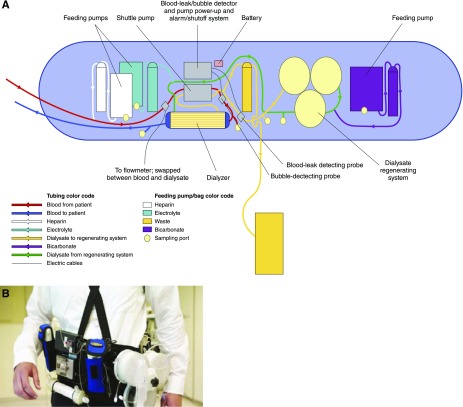
The WAK is an ambulatory hemodialysis device that has recently undergone human trials. The latest version of the WAK (Blood Purification Technologies, Inc.) is a belt-like worn device, that weighs up to 5 kg (11 lb) and connects to vasculature via catheters (Figure 4) (22). It comprises a miniaturized, wearable, sorbent-based hemodialysis system utilizing a commercially available dialyzer (Gambro Polyflux 6H; Baxter) with an effective surface area of 0.6 m2. A pulsatile pump powered by a rechargeable battery with dual-channel separated circuits propels blood and dialysate in a countercurrent fashion through the dialyzer. Dialysate is regenerated using three sorbent-containing cartridges connected in series with a reactor bed of immobilized urease and containing, in order, urease, zirconium phosphate, hydrous zirconium oxide, and activated charcoal. The sorbent system uses urease to convert urea into ammonia and carbonate, which, in the presence of hydrogen ions, forms carbon dioxide. The ammonia is subsequently adsorbed by the zirconium phosphate layer along with other cations, such as calcium, magnesium, and potassium, in exchange for sodium and hydrogen, which are released. The carbon dioxide is vented into the atmosphere as a gas through a semipermeable degassing bubble removal system. The hydrated zirconium oxide layer removes heavy metals such as copper and lead, if present, and also adsorbs anions such as phosphate, in exchange for acetate. Other medium-sized solutes and organic compounds are adsorbed by charcoal in the final sorbent column. The approximate total volume of the blood circuit is 65 ml and that of the dialysate circuit is 375 ml.
Figure 4.
Wearable artificial kidney developed by Victor Gura and colleagues. (A) Schematic of the wearable artificial kidney system. A shuttle pump drives blood through the dialyzer and the spent dialysate goes to a dialysate regenerating system. The system has alarms in place for leaks or bubbles. (B) Patient with the wearable artificial kidney as belt-like worn device that weighs about 5 kg. Figure (A) is modified from reference 45, with permission. Figure (B) is modified from reference 22, with permission.
The WAK was evaluated in three separate studies conducted in Vicenza, Italy, London, UK, and Seattle, United States (22,45,46). The device was tested for 24 hours in the most recent study that enrolled ten patients on hemodialysis for 24 hours (22). The trial was stopped after the seventh patient because of device-related technical problems, including excessive carbon dioxide bubbles in the dialysate circuit and variable blood and dialysate flows. During the study, all patients remained hemodynamically stable and there were no serious adverse events. Serum electrolytes and hemoglobin remained stable. Fluid removal was consistent with prescribed ultrafiltration rates. Mean blood flow was 42±24 ml/min, and mean dialysate flow was 43±20 ml/min. Mean urea, creatinine, and β2-microglobulin clearances over 24 hours were 17±10, 16±8, and 5±4 ml/min. Of the five patients who completed the planned 24-hour treatment period, mean 24-hour ultrafiltration volume was 1002±380 ml. Treatment satisfaction with WAK was compared with conventional hemodialysis and patients reported significantly greater overall satisfaction with the ambulatory device. At the 2018 Annual Dialysis Conference, Dr. Victor Gura, the lead investigator for the WAK, revealed that an improved version (WAK 3.0) weighing only 1 kg (2 lb) is under development (47).
Implantable Kidney Replacement Therapy
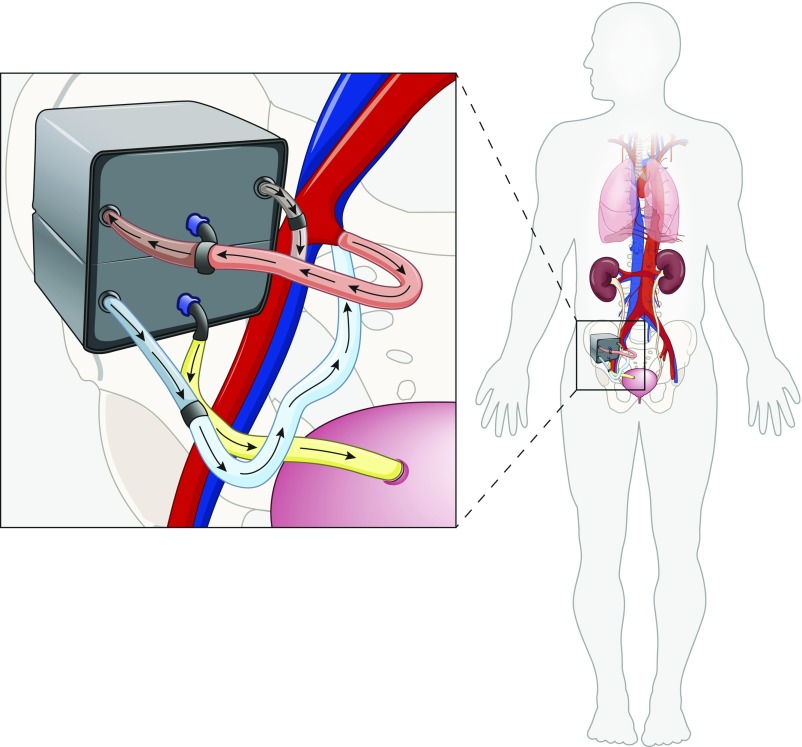
Our team, based at the University of California, San Francisco, and Vanderbilt University Medical Center, has undertaken the development of an implantable artificial kidney that will provide continuous treatment to the patient (Figure 5). The device is a two-stage system that combines a high-efficiency membrane for hemofiltration with a bioreactor of kidney tubule cells for electrolyte balance (48). In its envisioned implementation, the implantable artificial kidney will be implanted in the iliac fossa, similar to a kidney allograft and anastomosed to the iliac vessels for blood flow and to the bladder or ureter. The hemofiltration unit is constructed from biocompatible silicon nanopore membranes, which exhibit high flux and high selectivity such that cardiac perfusion pressure is sufficient enough to drive blood flow without need for battery or electrical power. Various components of the implantable artificial kidney are being evaluated via a series of preclinical studies (18,49–53). Membranes configured in a parallel-plate hemofilter format were implanted in six dogs that received only antithrombotic therapy (acetylsalicylic acid) (18). The devices remained patent and thrombus free despite microscopic fractures in membranes and albumin leakage in ultrafiltrate, which were observed in animals implanted for 5 and 8 days. Intact membranes retained albumin. Currently, the first pilot trial of the hemofiltration unit in patients with ESKD is in the planning stages.
Figure 5.
Concept of the implantable artificial kidney, which is a two-stage device composed of hemofiltration and cell therapy units. Silicon membranes in the hemofiltration unit generate ultrafiltrate using cardiac perfusion pressure, and kidney tubule cells in the cell therapy unit facilitate selective volume reabsorption and other metabolic functions. Excess fluid is directed to the bladder for urine excretion. Modified from reference 53, with permission.
At the 2018 American Society of Nephrology Kidney Week meeting, preliminary work demonstrating the feasibility of an implantable silicon nanopore membrane-based hemodialyzer was presented. A compact cartridge (approximately 120 ml) constructed from polycarbonate and stainless steel, mounted with a subtherapeutic level of silicon nanopore membrane area in a parallel-plate hemofilter format, was implanted in the abdomen of a healthy Yucatan minipig. Catheters to supply dialysate were tunneled subcutaneously and attached to the silicon nanopore membrane-based hemodialyzer. Three-hour hemodialysis sessions using an external dialysate pump were performed for three consecutive days after surgery. The animal was treated with daily acetylsalicylic acid and clopidogrel beginning 3 days before the implantation and continuing throughout the postoperative period. The animal tolerated surgical implantation and subsequent dialysis sessions without complication. Over the course of the study, creatinine clearance ranged from 11 to 42 ml/min per m2 and urea clearance ranged from 26 to 74 ml/min per m2. With further refinement of the external dialysate recirculation unit, which may include portable pumps and sorbent regeneration technologies, the silicon nanopore membrane-based hemodialyzer could be implemented for ambulatory hemodialysis without the risks of accidental blood disconnect associated with needle-based vascular access.
Conclusions
In conclusion, ambulatory hemodialysis is attractive for its potential to allow intensive self-care treatment by the patient with ESKD outside the clinical setting. Recent results from the clinical evaluation of a wearable unit suggest the need for additional advances in hemofiltration membranes, dialysate regeneration, vascular access, and patient monitoring systems. Preclinical testing of an implantable dialyzer demonstrates the feasibility of eliminating repeated vascular access and blood pumps. However, questions around implantation technique, device lifetime, ultrafiltration control, and periodic maintenance will require further consideration. For both wearable and implantable devices, the potential need for administration of continuous or intermittent anticoagulation therapy without compromising safety will need to be addressed. Future work will need to continue to focus on the technology improvements to facilitate ambulatory implementation and clinical evaluation.
Disclosures
Dr. Fissell and Dr. Roy are founders of Silicon Kidney LLC. Dr. Fissell reports receiving other fees from Silicon Kidney LLC. In addition, Dr. Fissell has a patent (US 7,540,963) and other patents issued through his employer(s). Dr. Roy has multiple patents issued (US Patents 7,048,856, 7,540,963, 9,403,126, 9,737,653, and 9,802,158). Dr. Hojs reports grants from Fulbright Grant during the conduct of the study. Dr. Roy reports grants from NIH/NIBIB during the conduct of the study.
Acknowledgments
The authors would like to thank Drs. Jarrett Moyer and Jimmy Ly of University of California, San Francisco, for the information on the implantable silicon nanopore membrane-based hemodialyzer.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System: 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 3.Urquhart-Secord R, Craig JC, Hemmelgarn B, Tam-Tham H, Manns B, Howell M, Polkinghorne KR, Kerr PG, Harris DC, Thompson S, Schick-Makaroff K, Wheeler DC, van Biesen W, Winkelmayer WC, Johnson DW, Howard K, Evangelidis N, Tong A: Patient and caregiver priorities for outcomes in hemodialysis: An International Nominal Group Technique Study. Am J Kidney Dis 68: 444–454, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 4: 1925–1931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton JO, Korsheed S, Grundy BJ, McIntyre CW: Hemodialysis-induced left ventricular dysfunction is associated with an increase in ventricular arrhythmias. Ren Fail 30: 701–709, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Lindsay RM, Alhejaili F, Nesrallah G, Leitch R, Clement L, Heidenheim AP, Kortas C: Calcium and phosphate balance with quotidian hemodialysis. Am J Kidney Dis 42[1 Suppl]: 24–29, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Bergman A, Fenton SS, Richardson RM, Chan CT: Reduction in cardiovascular related hospitalization with nocturnal home hemodialysis. Clin Nephrol 69: 33–39, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Cozzolino M, Ciceri P, Galassi A: Hyperphosphatemia: A novel risk factor for mortality in chronic kidney disease. Ann Transl Med 7: 55, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zha Y, Qian Q: Protein nutrition and malnutrition in CKD and ESRD. Nutrients 9, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pupim LB, Caglar K, Hakim RM, Shyr Y, Ikizler TA: Uremic malnutrition is a predictor of death independent of inflammatory status. Kidney Int 66: 2054–2060, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Kooman JP, Joles JA, Gerritsen KG: Creating a wearable artificial kidney: Where are we now? Expert Rev Med Devices 12: 373–376, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Walsh M, Culleton B, Tonelli M, Manns B: A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int 67: 1500–1508, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Evangelidis N, Tong A, Manns B, Hemmelgarn B, Wheeler DC, Tugwell P, Crowe S, Harris T, Van Biesen W, Winkelmayer WC, Sautenet B, O’Donoghue D, Tam-Tham H, Youssouf S, Mandayam S, Ju A, Hawley C, Pollock C, Harris DC, Johnson DW, Rifkin DE, Tentori F, Agar J, Polkinghorne KR, Gallagher M, Kerr PG, McDonald SP, Howard K, Howell M, Craig JC; Standardized Outcomes in Nephrology–Hemodialysis (SONG-HD) Initiative: Developing a set of core outcomes for trials in hemodialysis: An International Delphi Survey. Am J Kidney Dis 70: 464–475, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Morton RL, Snelling P, Webster AC, Rose J, Masterson R, Johnson DW, Howard K: Dialysis modality preference of patients with CKD and family caregivers: A discrete-choice study. Am J Kidney Dis 60: 102–111, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Kim JC, Garzotto F, Nalesso F, Cruz D, Kim JH, Kang E, Kim HC, Ronco C: A wearable artificial kidney: Technical requirements and potential solutions. Expert Rev Med Devices 8: 567–579, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Armignacco P, Lorenzin A, Neri M, Nalesso F, Garzotto F, Ronco C: Wearable devices for blood purification: Principles, miniaturization, and technical challenges. Semin Dial 28: 125–130, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Fissell WH, Roy S: The implantable artificial kidney. Semin Dial 22: 665–670, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Kensinger C, Karp S, Kant R, Chui BW, Goldman K, Yeager T, Gould ER, Buck A, Laneve DC, Groszek JJ, Roy S, Fissell WH: First implantation of silicon nanopore membrane hemofilters. ASAIO J 62: 491–495, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song S, Blaha C, Moses W, Park J, Wright N, Groszek J, Fissell W, Vartanian S, Posselt AM, Roy S: An intravascular bioartificial pancreas device (iBAP) with silicon nanopore membranes (SNM) for islet encapsulation under convective mass transport. Lab Chip 17: 1778–1792, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidambi PR, Jang D, Idrobo JC, Boutilier MSH, Wang L, Kong J, Karnik R: Nanoporous atomically thin graphene membranes for desalting and dialysis applications. Adv Mater 29, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Murisasco A, Baz M, Boobes Y, Bertocchio P, el Mehdi M, Durand C, Reynier JP, Ragon A: A continuous hemofiltration system using sorbents for hemofiltrate regeneration. Clin Nephrol 26[Suppl 1]: S53–S57, 1986 [PubMed] [Google Scholar]
- 22.Gura V, Rivara MB, Bieber S, Munshi R, Smith NC, Linke L, Kundzins J, Beizai M, Ezon C, Kessler L, Himmelfarb J: A wearable artificial kidney for patients with end-stage renal disease. JCI Insight 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Gelder MK, Mihaila SM, Jansen J, Wester M, Verhaar MC, Joles JA, Stamatialis D, Masereeuw R, Gerritsen KGF: From portable dialysis to a bioengineered kidney. Expert Rev Med Devices 15: 323–336, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Meng F, Seredych M, Chen C, Gura V, Mikhalovsky S, Sandeman S, Ingavle G, Ozulumba T, Miao L, Anasori B, Gogotsi Y: MXene sorbents for removal of urea from dialysate: A step toward the wearable artificial kidney. ACS Nano 12: 10518–10528, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Overbury SH, Kolesnikov AI, Brown GM, Zhang Z, Nair GS, Sacci RL, Lotfi R, van Duin ACT, Naguib M: Complexity of intercalation in MXenes: Destabilization of urea by two-dimensional titanium carbide. J Am Chem Soc 140: 10305–10314, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Castro AC, Lorenzin A, Neri M, Nayak Karopadi A, Ronco C, Marchionna N: Wearable artificial kidney and wearable ultrafiltration device vascular access-future directions. Clin Kidney J 12: 300–307, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Waeleghem JP, Chamney M, Lindley EJ, Pancírová J: Venous needle dislodgement: How to minimise the risks. J Ren Care 34: 163–168, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Ahlmén J, Goch J, Wrege U, Larsson R, Honkanen E, Althoff P, Danielson BG: Preliminary Results from the Use of New Vascular Access (Hemaport) for Hemodialysis. Hemodialysis International 7: 73–104, 2003. 19379346 [Google Scholar]
- 29.Huang SH, Shah S, Thomson BK, Laporte S, Filler G, Lindsay RM: What is single needle cannulation hemodialysis: Is it adequate? Blood Purif 38: 13–17, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Haroon S, Davenport A: Haemodialysis at home: Review of current dialysis machines. Expert Rev Med Devices 15: 337–347, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Miller LM, Clark E, Dipchand C, Hiremath S, Kappel J, Kiaii M, Lok C, Luscombe R, Moist L, Oliver M, MacRae J; Canadian Society of Nephrology Vascular Access Work Group: Hemodialysis tunneled catheter-related infections. Can J Kidney Health Dis 3: 2054358116669129, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poinen K, Quinn RR, Clarke A, Ravani P, Hiremath S, Miller LM, Blake PG, Oliver MJ: Complications from tunneled hemodialysis catheters: A Canadian observational cohort study. Am J Kidney Dis 73: 467–475, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Mojibian H, Spector M, Ni N, Eliseo D, Pollak J, Tal M: Initial clinical experience with a new heparin-coated chronic hemodialysis catheter. Hemodial Int 13: 329–334, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Clark TW, Isu G, Gallo D, Verdonck P, Morbiducci U: Comparison of symmetric hemodialysis catheters using computational fluid dynamics. J Vasc Interv Radiol 26: 252–259.e2, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Hockenhull JC, Dwan KM, Smith GW, Gamble CL, Boland A, Walley TJ, Dickson RC: The clinical effectiveness of central venous catheters treated with anti-infective agents in preventing catheter-related bloodstream infections: A systematic review. Crit Care Med 37: 702–712, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Axley B, Speranza-Reid J, Williams H: Venous needle dislodgement in patients on hemodialysis. Nephrol Nurs J 39: 435–445, quiz 446, 2012 [PubMed] [Google Scholar]
- 37.Ahlmén J, Gydell KH, Hadimeri H, Hernandez I, Rogland B, Strömbom U: A new safety device for hemodialysis. Hemodial Int 12: 264–267, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Wilcox SB, Carver M, Yau M, Sneeringer P, Prichard S, Alvarez L, Chertow GM: Results of human factors testing in a novel Hemodialysis system designed for ease of patient use. Hemodial Int 20: 643–649, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Scott RD: Biological Sciences Laboratory, Inc., assignee. Wearable Dialysis Apparatus, US patent 3,388,803. June 18, 1968 [Google Scholar]
- 40.Newhart EE: Newhart-Jones Ltd., assignee. Ambulatory Hemodialysis Apparatus, US Patent 3,864,259. February 4, 1975 [Google Scholar]
- 41.Lande AJ, Roberts M, Pecker EA: In search of a 24 hours per day artificial kidney. J Dial 1: 805–823, 1977 [DOI] [PubMed] [Google Scholar]
- 42.Roberts M, Lee DB: Wearable Artificial Kidneys: A Historical Perspective. Presented at the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, September 7–12, 2009 [Google Scholar]
- 43.Stephens RL, Jacobsen SC, Atkin-thor E, Kolff W: Portable/wearable artificial kidney (WAK) - initial evaluation. Proc Eur Dial Transplant Assoc 12: 511–518, 1976 [PubMed] [Google Scholar]
- 44.Kolff WJ: Lasker Clinical Medical Research Award. The artificial kidney and its effect on the development of other artificial organs. Nat Med 8: 1063–1065, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Davenport A, Gura V, Ronco C, Beizai M, Ezon C, Rambod E: A wearable haemodialysis device for patients with end-stage renal failure: A pilot study. Lancet 370: 2005–2010, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Gura V, Ronco C, Nalesso F, Brendolan A, Beizai M, Ezon C, Davenport A, Rambod E: A wearable hemofilter for continuous ambulatory ultrafiltration. Kidney Int 73: 497–502, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Gura V: The wearable kidney: The latest developments. Annual Dialysis Conference, Orlando, FL, March 3–6, 2018 [Google Scholar]
- 48.Salani M, Roy S, Fissell WH 4th: Innovations in wearable and implantable artificial kidneys. Am J Kidney Dis 72: 745–751, 2018 [DOI] [PubMed] [Google Scholar]
- 49.Brakeman P, Miao S, Cheng J, Lee CZ, Roy S, Fissell WH, Ferrell N: A modular microfluidic bioreactor with improved throughput for evaluation of polarized renal epithelial cells. Biomicrofluidics 10: 064106, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S, Heller J, Iqbal Z, Kant R, Kim EJ, Durack J, Saeed M, Do L, Hetts S, Wilson M, Brakeman P, Fissell WH, Roy S: Preliminary diffusive clearance of silicon nanopore membranes in a parallel plate configuration for renal replacement therapy. ASAIO J 62: 169–175, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buck AKW, Goebel SG, Goodin MS, Wright NJ, Groszek JJ, Moyer J, Singh S, Bluestein D, Fissell WH, Roy S: Original article submission: Platelet stress accumulation analysis to predict thrombogenicity of an artificial kidney. J Biomech 69: 26–33, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim S, Feinberg B, Kant R, Chui B, Goldman K, Park J, Moses W, Blaha C, Iqbal Z, Chow C, Wright N, Fissell WH, Zydney A, Roy S: Diffusive silicon nanopore membranes for hemodialysis applications. PLoS One 11: e0159526, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fissell WH, Roy S, Davenport A: Achieving more frequent and longer dialysis for the majority: Wearable dialysis and implantable artificial kidney devices. Kidney Int 84: 256–264, 2013 [DOI] [PubMed] [Google Scholar]