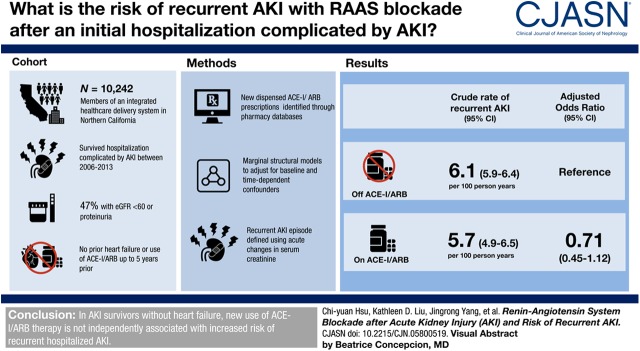
Visual Abstract
Keywords: acute renal failure, renin angiotensin system, humans, angiotensin receptor antagonists, angiotensin-converting enzyme, creatinine, reninangiotensin system, odds ratio, incidence, outpatients, follow-up studies, acute kidney injury, proteinuria, hospitalization, heart failture, survivors, California
Abstract
Background and objectives
How to best medically manage patients who survived hospitalized AKI is unclear. Use of renin-angiotensin system blockers in this setting may increase risk of recurrent AKI.
Design, setting, participants, & measurements
This is a cohort study of 10,242 members of an integrated health care delivery system in Northern California who experienced AKI and survived a hospitalization between January 1, 2006 and December 31, 2013. All study participants did not have prior heart failure or use of angiotensin-converting enzyme inhibitors (ACE-Is) or angiotensin receptor blockers (ARBs) up to 5 years prior. New receipt and time-updated exposure of ACE-Is/ARBs was identified on the basis of dispensed prescriptions found in outpatient health plan pharmacy databases. The main outcome of interest was subsequent episode of hospitalized AKI after discharge from an initial index hospitalization complicated by AKI. Recurrent AKI episode was defined using acute changes in serum creatinine concentrations. Marginal structural models were used to adjust for baseline and potential time-dependent confounders.
Results
Forty-seven percent of the study population had a documented eGFR<60 ml/min per 1.73 m2 or documented proteinuria before hospitalization. With a median of 3 (interquartile range, 1–5) years of follow-up, 1853 (18%) patients initiated use of ACE-Is/ARBs and 2124 (21%) patients experienced recurrent AKI. Crude rate of recurrent AKI was 6.1 (95% confidence interval [95% CI], 5.9 to 6.4) per 100 person-years off ACE-Is/ARBs and 5.7 (95% CI, 4.9 to 6.5) per 100 person-years on ACE-Is/ARBs. In marginal structural causal inference models that adjusted for baseline and potential time-dependent confounders, exposure to ACE-I/ARB use was not associated with higher incidence of recurrent AKI (adjusted odds ratio, 0.71; 95% CI, 0.45 to 1.12).
Conclusions
In this study of AKI survivors without heart failure, new use of ACE-I/ARB therapy was not independently associated with increased risk of recurrent hospitalized AKI.
Introduction
After an episode of AKI, there is a heightened risk of progressive CKD (1,2). Opinion leaders have advocated implementing “reno-protective measures” among those who have survived an episode of AKI, including use of renin-angiotensin system (RAS) blockers (3). Use of angiotensin-converting enzyme inhibitors (ACE-Is) or angiotensin receptor blockers (ARB) may also have beneficial cardiovascular effects (4).
However, use of ACE-Is/ARBs in this setting carries unique potential risks. RAS blockade may increase the risk of developing AKI (5–9), and recurrent AKI is not uncommon among patients who have had one episode of AKI (10–12). Indeed, a few recent studies reported that although ACE-I/ARB therapy after AKI was associated with lower risk of CKD and mortality (13–15), it was also associated with higher risk of recurrent AKI (15).
Prior studies of the risks and benefits of ACE-I/ARB therapy after AKI have been limited by only enrolling patients in an intensive care unit (ICU) (13,14), not considering recurrent AKI (13,14), or capturing recurrent AKI episodes only by diagnostic codes (15), which are known to have suboptimal performance characteristics (16–20). In addition, ACE-I/ARB use was classified on the basis of drug prescription (13–15) rather than dispensation records, key covariates were not time-updated, and potential time-dependent confounders were not accounted for (13–15).
To fill these important knowledge gaps, we ascertained AKI using acute changes in serum creatinine concentrations within a large, integrated health care delivery system; leveraged data from comprehensive health plan pharmacy databases to ascertain exposure to ACE-Is/ARBs; and used causal inference analytic methods (21).
Materials and Methods
Study Sample
The source population included members of Kaiser Permanente Northern California, a large integrated health care delivery system in the San Francisco and greater Bay Area with membership that is highly representative of the local surrounding and statewide population.
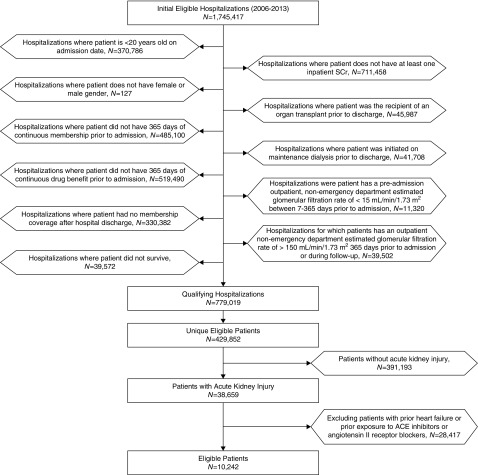
The study sample included all adult (age≥20 years) members who were hospitalized at any of 21 Kaiser Permanente Northern California hospitals between January 1, 2006 and December 31, 2013, and who had at least 12 months of continuous membership and pharmacy benefit before the index admission, who had at least one valid serum creatinine value during the index hospitalization, and who survived to hospital discharge (Figure 1).
Figure 1.
Cohort assembly of adult Kaiser Permanente Northern California members with no prior heart failure or prior exposure to angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers who experienced an episode of AKI while hospitalized between January 1, 2006 and December 31, 2013.
We a priori planned to exclude from consideration patients with known heart failure given that it has its own natural history (e.g., recurrent heart failure exacerbation with AKI), and for which there is a strong clinical indication for ACE-I/ARB therapy, especially if reduced left ventricular systolic function is present. We used previously validated approaches within Kaiser Permanente to identify heart failure (22), which has been shown to have a positive predictive value for clinical heart failure of >92% (23,24). Hospitalizations were also excluded if the patient was receiving maintenance dialysis before admission or had a history of organ transplantation.
To reduce biases due to under-ascertainment of early harm as well as adherence bias related to the therapies of interest, we used a new user design (25) and excluded patients who were receiving ACE-Is/ARBs within 5 years before study entry. For persons who had multiple hospitalizations during the study period, only the first eligible hospitalization was used for this analysis.
The study was approved by the institutional review boards of Kaiser Permanente Northern California and the University of California, San Francisco.
Identification of AKI
We defined an episode of AKI using serum creatinine–based criteria consistent with Kidney Disease: Improving Global Outcomes guidelines (26). For the index hospitalization, AKI was defined as a serum creatinine rise of 0.3 mg/dl or greater within 48 hours during the hospitalization or ≥50% above a baseline, preadmission serum creatinine. Baseline kidney function was defined as the most recent outpatient, non–emergency department serum creatinine concentration between 7 and 365 days before admission (26). All outpatient serum creatinine measurements were performed at the regional health plan laboratory which calibrated their serum creatinine measurement procedures to be traceable to isotope dilution mass spectrometry.
Follow-Up and Recurrence of AKI
Follow-up occurred through December 31, 2015, with censoring due to disenrollment from the health plan, death, onset of end-stage kidney disease (ESKD), and/or administrative end of study follow-up. Death was comprehensively identified from health plan administrative databases, hospitalization and billing claims databases, state death certificate files, and Social Security Administration vital status files.
The outcome of interest was recurrent AKI—a subsequent episode of hospitalized AKI after discharge from an initial index hospitalization complicated by AKI. Recurrent AKI was deemed to have occurred during a subsequent hospitalization in which there was a serum creatinine rise of ≥0.3 mg/dl within 48 hours during that hospitalization or ≥50% above a “baseline” serum creatinine. For the recurrent AKI episode (12), the “baseline” serum creatinine was defined as the more recent value of the following: (1) the most recent inpatient value from the index AKI admission, if it occurred within 365 days before the current admission; or (2) the most recent outpatient serum creatinine 7–365 days before the current admission. This most recent outpatient serum creatinine could not precede the index AKI admission and could not be obtained in an emergency department setting. If there was no “baseline” serum creatinine available for assessment of recurrent AKI, AKI was defined using only the criterion of an acute rise in serum creatinine of ≥0.3 mg/dl over 48 hours during a subsequent hospitalization to avoid potential misclassification (12).
ACE-Is, ARBs, and Other Medication Exposure
The primary exposure, new receipt and time-updated exposure to ACE-I and ARB therapy, was identified on the basis of dispensed prescriptions found in outpatient health plan pharmacy databases at hospital discharge and throughout follow-up. Longitudinal exposure was estimated from drug refill patterns according to the calculated number of days-supply for each dispensed prescription. For any two consecutive dispensed prescriptions, if the second prescription was filled within ≤14 days of the projected end date of the first, the patient was considered continually on the medication. If the second prescription was filled >14 days after the projected end date of the first, the patient was considered not taking the medication from day 15 until the start date of the next prescription. If two prescriptions for the same drug were filled on the same day, we used the longer estimated number of days-supply to determine the end date. If a nonfatal hospitalization occurred during follow-up, the length of stay was added to the estimated days-supply for any prescription crossing that hospitalization because patients were unlikely to take their own medications while hospitalized. Using the same methods, we also ascertained exposure to other relevant prescription medications. Baseline receipt of medications was defined as having an active prescription during the 120 days before the index admission given that chronic medications were typically dispensed in 90- to 100-day prescriptions.
Covariates
We searched for relevant comorbid conditions up to 5 years before each patient’s index AKI hospitalization on the basis of inpatient and ambulatory diagnoses and procedures, laboratory test results, and dispensing records from pharmacy databases. We also collected the most recent outpatient body mass index, BP, and laboratory data measured during the 365 days before the index admission.
We ascertained diabetes mellitus on the basis of ≥1 primary inpatient discharge diagnosis or ≥2 outpatient diagnoses or receipt of an antidiabetic drug (27). Hypertension was defined as ≥2 ambulatory diagnoses or ≥1 outpatient diagnosis and receipt of an antihypertensive agent (28). Dyslipidemia was defined on the basis of relevant ambulatory diagnoses or receipt of lipid-lowering medications. Cancer was identified on the basis of ≥1 primary discharge diagnosis or ≥2 ambulatory diagnoses of any malignancy other than nonmelanoma skin cancer. Prior coronary heart disease was on the basis of prior hospitalization for acute coronary syndrome, or receipt of percutaneous coronary intervention or coronary artery bypass surgery (29). Prior ischemic stroke was defined as ≥1 primary discharge diagnosis (30). Preadmission GFR (eGFR, ml/min per 1.73 m2) was estimated using baseline serum creatinine information described previously and the Chronic Kidney Disease Epidemiology Collaboration equation (31). Documented proteinuria was defined as an ambulatory urine dipstick result of 1+ or greater (in the absence of a concomitant urinary tract infection) (32).
We handled missing data in several ways in our analyses. For medical conditions and/or procedures, we assumed that the absence of qualifying diagnosis or procedure codes in medical records was evidence of not having had the conditions or procedures. For continuous variables, we created a categoric variable indicating missingness. Patients without a self-reported race of white, black, Asian/Pacific Islander, or Native American were categorized as “other/unknown.” We classified anyone with no evidence of self-reported Hispanic ethnicity as not being Hispanic.
Statistical Methods
Analyses were performed using SAS software version 9.3 (Cary, NC). We compared preadmission patient characteristics for those who did or did not initiate ACE-I/ARB therapy after an index AKI episode. Because of the susceptibility of detecting small differences in large sample sizes by statistical tests, we used Cohen’s D value to characterize the difference in patient characteristics by taking the standardized difference of means or proportions between the two groups and dividing by the pooled estimate (with a value >0.10 considered a potentially meaningful difference) (33). We calculated the crude incidence of recurrent AKI per 100 person-years with associated 95% confidence interval (95% CI) overall and stratified by exposure to ACE-Is/ARBs during follow-up.
We recognize that CKD is a major indication for use of ACE-Is/ARBs, and CKD is also a major risk factor for AKI. Confounders that may influence the receipt and persistent use of ACE-Is/ARBs and the risk of recurrent AKI (e.g., level of eGFR or BP) may vary with time, resulting in time-dependent confounding. Given this situation, to obtain a less biased estimate of the potential causal effect of new receipt and time-updated exposure to ACE-Is/ARBs on the risk of recurrent AKI, we applied marginal structural modeling methods (21). The data were structured to allow the exposure (i.e., ACE-Is/ARBs), outcome (i.e., occurrence of recurrent AKI), right-censoring, and time-dependent covariates to be updated every 30 days during follow-up with appropriate temporal alignment of these factors within each time bin. Within each 30-day bin, we then applied inverse probability weights for ACE-I/ARB exposure, right-censoring due to death, end of membership, and occurrence of ESKD to a weighted pooled logistic regression model where the dependent variable was ACE-I/ARB exposure. We used variables listed in Table 1 as candidate covariates to estimate the separate inverse probability weights for ACE-I/ARB exposure and for right-censoring events, and we specified acute heart failure, eGFR, proteinuria, and systolic BP levels as the relevant potential time-dependent confounders (see Supplemental Figure 1 for directed acyclic graph). Because of the absolute incidence of recurrent AKI being low within each 30-day time bin, the resulting time-discrete adjusted odds ratio and its 95% CI were reported as the approximate relative risk of recurrent AKI associated with new exposure to ACE-I/ARB therapy.
Table 1.
Preadmission characteristics of adult Kaiser Permanente Northern California members between January 1, 2006 and December 31, 2013, who experienced an episode of AKI during the hospital stay and who had no prior heart failure or exposure to ACE-Is/ARBs within 5 yr prior, overall and stratified by exposure of ACE-I/ARB therapy during follow-up
| Characteristic | Overall (n=10,242) | No Use of ACE-I/ARB (n=8389) | Incident Use of ACE-I/ARB (n=1853) | D Value |
|---|---|---|---|---|
| Preadmission age, yr | ||||
| Mean (SD) | 62 (19) | 61 (20) | 65 (16) | 0.20 |
| Preadmission age group, yr, n (%) | 0.15 | |||
| 20–49 | 2757 (27) | 2423 (29) | 334 (18) | |
| 50–59 | 1639 (16) | 1322 (16) | 317 (17) | |
| 60–69 | 1902 (19) | 1461 (17) | 441 (24) | |
| 70–79 | 1855 (18) | 1447 (17) | 408 (22) | |
| ≥80 | 2089 (20) | 1736 (21) | 353 (19) | |
| Sex, n (%) | 0.02 | |||
| Women | 5489 (54) | 4479 (53) | 1010 (55) | |
| Men | 4753 (46) | 3910 (47) | 843 (46) | |
| Race, n (%) | 0.02 | |||
| White | 7242 (71) | 5973 (71) | 1269 (69) | |
| Black | 1027 (10) | 817 (10) | 210 (11) | |
| Asian/Pacific Islander | 1347 (13) | 1077 (13) | 270 (15) | |
| Native American | 63 (0.6) | 53 (0.6) | 10 (0.5) | |
| Unknown | 563 (6) | 469 (6) | 94 (5) | |
| Hispanic ethnicity, n (%) | 1460 (14) | 1215 (15) | 245 (13) | 0.04 |
| Preadmission medical history, n (%) | ||||
| Acute coronary syndrome | 234 (2) | 135 (1.6) | 99 (5) | 0.75 |
| Ischemic stroke | 137 (1) | 109 (1) | 28 (2) | 0.09 |
| Transient ischemic attack | 123 (1) | 97 (1) | 26 (1) | 0.12 |
| Peripheral artery disease | 335 (3) | 257 (3) | 78 (4) | 0.20 |
| Mitral and/or aortic valvular disease | 325 (3) | 255 (3) | 70 (4) | 0.14 |
| Atrial fibrillation and/or flutter | 674 (7) | 515 (6) | 159 (9) | 0.22 |
| Coronary artery bypass graft surgery | 51 (0.5) | 35 (0.4) | 16 (0.9) | 0.44 |
| Percutaneous coronary intervention | 121 (1) | 80 (1) | 41 (2) | 0.52 |
| Diabetes mellitus | 1237 (12) | 850 (10) | 387 (21) | 0.52 |
| Hypertension | 3859 (38) | 2870 (34) | 989 (53) | 0.48 |
| Dyslipidemia | 4302 (42) | 3297 (39) | 1005 (54) | 0.37 |
| Chronic liver disease | 562 (6) | 496 (6) | 66 (4) | 0.32 |
| Chronic lung disease | 2184 (21) | 1783 (21) | 401 (22) | 0.01 |
| Hypothyroidism | 1370 (13) | 1109 (13) | 261 (14) | 0.04 |
| Systemic cancer | 2706 (26) | 2287 (27) | 419 (23) | 0.15 |
| Extracranial hemorrhage | 220 (2) | 192 (2) | 28 (2) | 0.26 |
| Preadmission body mass index, kg/m2, n (%) | 0.15 | |||
| <18.5 | 235 (2) | 210 (3) | 25 (1) | |
| 18.5–24.9 | 2739 (27) | 2340 (28) | 399 (22) | |
| 25.0–29.9 | 2939 (29) | 2413 (29) | 526 (28) | |
| 30.0–39.9 | 2687 (26) | 2117 (25) | 570 (31) | |
| ≥40 | 391 (4) | 295 (4) | 96 (5) | |
| Unknown | 1251 (12) | 1014 (12) | 237 (13) | |
| Preadmission systolic BP category, mm Hg, n (%) | 0.22 | |||
| ≤120 | 3534 (35) | 3056 (36) | 478 (26) | |
| 121–129 | 1875 (18) | 1553 (19) | 322 (17) | |
| 130–139 | 2188 (21) | 1762 (21) | 426 (23) | |
| 140–159 | 1539 (15) | 1155 (14) | 384 (20.7) | |
| 160–179 | 304 (3) | 225 (3) | 79 (4) | |
| ≥180 | 88 (0.9) | 64 (0.8) | 24 (1) | |
| Unknown | 714 (7) | 574 (7) | 140 (8) | |
| Preadmission medication use, n (%) | ||||
| α-Blockers | 827 (8) | 649 (8) | 178 (10) | 0.14 |
| Any diuretic | 2249 (22) | 1661 (20) | 588 (32) | 0.38 |
| Any β-blocker | 2196 (21) | 1639 (20) | 557 (30) | 0.35 |
| Calcium channel blocker | 1072 (11) | 809 (10) | 263 (14) | 0.27 |
| Any aldosterone receptor antagonist | 195 (2) | 175 (2) | 20 (1) | 0.41 |
| Nitrate | 164 (2) | 129 (2) | 35 (2) | 0.13 |
| Hydralazine | 78 (0.8) | 68 (0.8) | 10 (0.5) | 0.25 |
| Antiarrhythmic | 60 (0.6) | 46 (0.5) | 14 (0.8) | 0.20 |
| Digoxin | 131 (1) | 109 (1) | 22 (1) | 0.06 |
| Statin | 2204 (22) | 1641 (20) | 563 (30) | 0.35 |
| Other lipid-lowering agent | 221 (2) | 159 (2) | 62 (3) | 0.35 |
| Anti-inflammatory drug | 1552 (15) | 1217 (15) | 335 (18) | 0.16 |
| Antiplatelet agent | 183 (2) | 144 (2) | 39 (2) | 0.13 |
| Diabetic therapy | 511 (5) | 351 (4) | 160 (9) | 0.47 |
| Preadmission ambulatory, non–emergency department laboratory values, n (%) | ||||
| CKD-EPI eGFR, ml/min per 1.73 m2 | 0.07 | |||
| >110 | 1179 (12) | 1061 (13) | 118 (6) | |
| 90–110 | 1721 (17) | 1424 (17) | 297 (16) | |
| 60–89 | 3075 (30) | 2429 (29) | 646 (35) | |
| 45–59 | 1069 (10) | 851 (10) | 218 (12) | |
| 30–44 | 677 (7) | 544 (7) | 133 (7) | |
| 15–29 | 330 (3) | 286 (3) | 44 (2) | |
| Unknown | 2191 (21) | 1794 (21) | 397 (21) | |
| Hemoglobin category, g/dl | 0.01 | |||
| >13.0 | 4058 (40) | 3224 (38) | 834 (45) | |
| 12.0–12.9 | 1605 (16) | 1345 (16) | 260 (14) | |
| 11.0–11.9 | 1208 (12) | 1052 (13) | 156 (8) | |
| 10.0–10.9 | 670 (7) | 602 (7) | 68 (4) | |
| 9.0–9.9 | 332 (3) | 298 (4) | 34 (2) | |
| <9.0 | 284 (3) | 250 (3) | 34 (2) | |
| Unknown | 2085 (20) | 1618 (19) | 467 (25) | |
| Urinary dipstick protein excretion | 0.09 | |||
| Trace | 1481 (15) | 1255 (15) | 226 (12) | |
| 1+ | 2057 (20) | 1705 (20) | 352 (19) | |
| 2+ | 1131 (11) | 920 (11) | 211 (11) | |
| 3+ | 433 (4) | 353 (4) | 80 (4) | |
| Negative or unknown | 5140 (50) | 4156 (50) | 984 (53) | |
| Index hospitalization | ||||
| AKI stage, n (%) | 0.07 | |||
| Stage 1 | 7476 (73) | 6079 (73) | 1397 (75) | |
| Stage 2 | 1370 (13) | 1130 (14) | 240 (13) | |
| Stage 3 | 1396 (14) | 1180 (14) | 216 (12) | |
| ICU stay, n (%) | 3173 (31) | 2482 (30) | 691 (37) | |
| ICU length of stay, h | ||||
| Median (interquartile range) | 0 (0–1) | 0 (0–1) | 0 (0–2) | |
| Range | 0–221 | 0–221 | 0–77 | |
| Hospital length of stay, d | 0.02 | |||
| ≤3 d | 2502 (24) | 2015 (24) | 487 (26) | |
| 4.0–7.0 d | 3797 (37) | 3156 (38) | 641 (35) | |
| >7 d | 3943 (39) | 3218 (38) | 725 (39) | |
| Predicted mortality score category, n (%) | 0.18 | |||
| <0.1% | 891 (9) | 743 (9) | 148 (8) | |
| 0.1%–0.4% | 1432 (14) | 1141 (14) | 291 (16) | |
| 0.5%–2.0% | 2626 (26) | 2082 (25) | 544 (29) | |
| 2.0%–5.0% | 1826 (18) | 1464 (18) | 362 (20) | |
| 5.0%–10.0% | 1238 (12) | 1036 (12) | 202 (11) | |
| 10.0%–15.0% | 566 (6) | 458 (6) | 108 (6) | |
| 15.0%–30.0% | 499 (5) | 415 (5) | 84 (5) | |
| ≥30% | 198 (2) | 160 (2) | 38 (2) | |
| Unknown | 966 (9) | 890 (11) | 76 (4) |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; ICU, intensive care unit.
Results
Cohort Assembly and Baseline Characteristics
We identified 10,242 patients who experienced AKI and survived during a hospitalization between January 1, 2006 and December 31, 2013, and who did not have prior heart failure or use of ACE-I/ARB therapy up to 5 years before the index hospitalization (Figure 1). Forty-seven percent of the study population (4814 of 10,242) had a documented eGFR<60 ml/min per 1.73 m2 within the year before hospitalization or documented 1+ or more dipstick proteinuria within 5 years before study entry.
Among eligible patients with AKI, 1853 (18%) initiated the use of ACE-Is/ARBs during a total of 34,966 person-years of follow-up through December 31, 2015. The median time to initiation was 13 months with an interquartile range of 3–34 months. Compared with those who did not initiate ACE-I/ARB therapy during follow-up, patients who initiated ACE-I/ARB therapy were older and had a greater comorbidity burden, including cardiovascular disease and diabetes mellitus (Table 1). In addition, those who initiated ACE-I/ARB therapy during follow-up were more likely to have received cardiac procedures before the index hospitalization and had higher preadmission systolic BP (Table 1). In contrast, incident users of ACE-Is/ARBs during follow-up had a lower prevalence of preexisting liver disease and a lower predicted short-term mortality risk score (Table 1).
ACE-I/ARB Therapy and Recurrent AKI
The mean (SD) observation time was 3.4 (2.8) years for study participants. During follow-up, 2124 (21%) experienced recurrent AKI and 4076 (40%) were censored due to death, kidney replacement therapy, or disenrollment from the health care system. Cases of recurrent AKI occurred a mean (SD) of 16 (22) months and a median (interquartile range) of 6 (1–22) months after index hospitalization. We observed a rate of 5.7 episodes of recurrent AKI per 100 person-years while taking ACE-I/ARB therapy (95% CI, 4.9 to 6.5 episodes per 100 person-years) compared with 6.1 episodes of recurrent AKI per 100 person-years while not receiving ACE-I/ARB therapy (95% CI, 5.9 to 6.4 episodes per 100 person-years) (Table 2). Of the 2124 cases of recurrent AKI, 1241 (58%) were stage 1, 407 (19%) were stage 2, and 420 (20%) were stage 3 in severity (26).
Table 2.
Association of new receipt and time-updated exposure to ACE-Is or ARBs with recurrent AKI
| Variable | Recurrent AKI, N | Follow-Up Person-Yr | Crude Rate of Recurrent AKI per 100 Person-Yr (95% Confidence Interval)a | Adjusted Odds Ratio (95% Confidence Interval)a |
|---|---|---|---|---|
| Off ACE-I/ARB, n=10,242 | 1925 | 31,449 | 6.1 (5.9 to 6.4) | Reference=1 |
| On ACE-I/ARB, n=1853 | 166 | 3517 | 5.7 (4.9 to 6.5) | 0.71 (0.45 to 1.12) |
We used variables listed in Table 1 as candidate covariates to estimate the separate inverse probability weights for ACE-I/ARB exposure and for right-censoring events, and we specified acute heart failure, eGFR, proteinuria, and systolic BP levels as the relevant potential time-dependent confounders (see Supplemental Figure 1 for directed acyclic graph). ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Crude rates and marginal odds ratios of recurrent AKI among eligible adults with no prior heart failure or prior exposure to ACE-Is or ARBs and who experienced an episode of AKI while hospitalized between January 1, 2006 and December 31, 2013. Rates are presented stratified by periods receiving or not receiving ACE-I or ARB therapy during follow-up. Marginal odds ratios weighted by the inverse probability weights for ACE-I/ARB exposure, right-censoring due to death, end of membership, and occurrence of ESKD.
In marginal structural causal inference models with inverse probability weights that adjusted for baseline and potential time-dependent confounders, exposure to ACE-I/ARB use was not significantly associated with higher incidence of recurrent AKI (adjusted odds ratio, 0.71; 95% CI, 0.45 to 1.12) (Table 2).
Discussion
Better evaluation and management of patients who experience AKI has been recognized as an important clinical and public health priority (3,34–37). Yet, there are currently scant data to guide evidence-based management in this large and vulnerable population (38).
Interest has turned recently to the role of ACE-I/ARB therapy after an episode of AKI. Although RAS blockade may slow the loss of kidney function and reduce rates of cardiovascular disease events and mortality (4,39–41), it may also increase risk of experiencing additional episodes of AKI for which this population is at uniquely high risk. Concern by physicians about the risk for recurrent AKI may explain, in part, observations such as those from a recent Canadian study showing that after coronary angiography, compared with patients without AKI, those with AKI had lower odds of subsequent receipt of ACE-I/ARB therapy (42).
Certain recent human studies suggest that ACE-I/ARB therapy after AKI is associated with numerous clinical benefits, consistent with results from some animal models (43). For example, Chou et al. (13) reported that among 587 Taiwanese patients who had cardiac surgery–associated AKI, use of ACE-Is/ARBs was associated with a lower risk of ensuing CKD; of note, death and recurrent AKI were not examined as outcomes (13). Gayat et al. (14) reported among 611 patients with AKI discharged from 21 European ICUs that ACE-I/ARB prescription at discharge was associated with lower 1-year all-cause mortality, but this protective association was not observed among ICU survivors who did not have AKI (14). This study also did not examine occurrence of recurrent AKI (14). Finally, Brar et al. (15) recently reported that in a large Canadian community-based population of 46,253 adults who experienced an episode of AKI during a hospitalization, receipt of ACE-I/ARB therapy within 6 months after discharge was associated with reduced mortality (adjusted hazard ratio [aHR], 0.85; 95% CI, 0.81 to 0.89) but also a significant risk of subsequent hospitalization involving AKI (aHR, 1.25; 95% CI, 1.08 to 1.46) and no difference in ESKD (aHR, 0.96; 95% CI, 0.86 to 1.06).
We believe that our study provides unique insights and reassuring data regarding the safety of ACE-I/ARB therapy among patients after AKI using a more rigorous study design and analytic approach. In contrast to Brar et al. (15), we did not find that ACE-I/ARB use after AKI led to differences in the risk of experiencing recurrent AKI in our marginal structural model causal inference analyses that accounted for baseline, time-updated, and potential time-dependent confounders. The robustness of our conclusions is additionally bolstered by several study design strengths. First, we excluded patients with preexisting, clinically recognized heart failure from the study population which we believe reduces potential important confounding by indication, (relatively) healthy user, and other types of biases (44). In contrast, in the study by Brar et al. receipt of ACE-Is/ARBs within 6 months after AKI was associated with an increase in subsequent risk of hospitalization for heart failure (aHR, 1.69; 95% CI, 1.18 to 2.41), a finding that is challenging to interpret given randomized evidence of ACE-I and ARB therapy for the prevention of heart failure complications (45). Second, we time-updated key covariates relevant to the initiation and persistence of ACE-I/ARB therapy during each 30-day window during follow-up, including eGFR and systolic BP, and modeled them as potential time-dependent confounders using a marginal structural modeling approach. Prior studies, however, did not have follow-up information on kidney function or BP after the initial index AKI hospitalization and did not address potential time-dependent confounding (13–15). Third, new users versus never users of ACE-Is/ARBs over time were classified on the basis of records on longitudinal receipt of dispensed medications throughout follow-up and not using only a physician prescription or medical record descriptions (13–15); in contrast, the study by Brar et al. (15) only required one prescription within 6 months of discharge to classify a patient as being an ACE-I/ARB user. Fourth, we defined the outcome of recurrent AKI using acute changes in serum creatinine values identified from inpatient laboratory databases, which is currently the “gold standard” in AKI epidemiology studies and superior to relying on administrative billing codes. Finally, our study also has good external validity. The Kaiser Permanente Northern California membership is sociodemographically diverse and highly representative of the local surrounding and statewide population (46,47). Kaiser Permanente is a major regional provider for Medical/Medicaid patients as well, especially after the implementation of the Affordable Care Act in California, which further enhances the generalizability of our findings to patients receiving medical care.
Our study also has certain limitations. We excluded patients with known heart failure so our results are not generalizable to that subset of patients. However, we believe that the dilemma facing clinicians as to whether to start ACE-Is/ARBs after AKI is less of an issue among patients who have a nonrenal indication for ACE-Is/ARBs, such as heart failure. We did not examine the associations of ACE-I/ARB therapy post-AKI with outcomes such as death or development or progression of CKD. A relatively small proportion of patients in our sample had dialysis-requiring AKI (AKI-D), and these patients may be the ones who are at highest risk for progressive CKD and may benefit the most from renoprotective measures such as RAS blockade (48). We did not have information on the specific indication(s) for starting ACE-Is/ARBs. This is a limitation shared by other observational studies on this topic (13–15). We did not explore the issue of resumption of RAS blockade in patients who were taking ACE-Is/ARBs before the index AKI admission. Although our study included proteinuria as a time-updated covariate in the marginal structural model, proteinuria was determined on the basis of dipstick urinalysis results and not quantified by urine protein or urine albumin concentration. Because we used a new user design in this study to reduce biases associated with analyzing prevalent users of ACE-Is/ARBs (25), our results may not apply to patients who had been on ACE-Is/ARBs before AKI. Finally, our study population was limited to patients with health insurance in Northern California, so results may not be fully generalizable to uninsured patients or to all geographic or practice settings.
We believe that our study makes an important contribution to the literature. On the basis of pathophysiologic considerations and extrapolation from clinical trials in other settings, many have hypothesized that RAS blockade after AKI would be associated with favorable outcomes in terms of slowing future loss of kidney function and reducing risk of cardiovascular disease events and all-cause death. However, many physicians withhold ACE-Is/ARBs in the setting of AKI, so safety signals regarding an increased risk of recurrent AKI may make providers hesitate before prescribing ACE-Is/ARBs after AKI. We believe that our data are therefore reassuring and contribute toward painting a picture of a favorable risk-benefit profile for RAS blockade after AKI that can inform clinical practice until there is more definitive evidence from randomized, controlled trials.
In conclusion, although there is much interest in improving care after AKI, there is scant evidence to guide physicians about what is the most appropriate care for these patients. Our results support that RAS blockade using ACE-I/ARB therapy after an episode of hospitalized AKI does not appear to increase the risk of recurrent hospitalized AKI, and contribute important information to help guide clinicians until more definitive evidence from randomized, controlled trials is available.
Disclosures
Dr. Hsu reports a grant from Satellite Healthcare outside of the submitted work. Dr. Liu reports stock ownership in Amgen and personal fees from Durect, Potrero Med, Quark, and Theravance, all outside of the submitted work. Dr. Zheng reports a position as partner at The Permanente Medical Group. Dr. Glidden, Dr. Pravoverov, Ms. Tan, and Dr. Yang have nothing to disclose.
Funding
Dr. Glidden is supported by a grant from the National Institutes of Health (R01 DK101507). Dr. Go is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01 DK101507 and R01 DK114014). Dr. Hsu is supported by a grant from the NIDDK (R01 DK101507 and R01 DK114014). Dr. Liu is supported by grants from the National Heart, Lung, and Blood Institute and the NIDDK (R01 DK101507, R01 DK114014, and U01HL123004). The funder had no role in study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Renin-Angiotensin System Blockade after Acute Kidney Injury: The Plot Thickens,” on pages 2–4.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05800519/-/DCSupplemental.
Supplemental Figure 1. Directed acyclic graph describing conceptual underpinning of the marginal structural models used.
References
- 1.Hsu CY: Yes, AKI truly leads to CKD. J Am Soc Nephrol 23: 967–969, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein SL, Jaber BL, Faubel S, Chawla LS; Acute Kidney Injury Advisory Group of American Society of Nephrology : AKI transition of care: A potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol 8: 476–483, 2013 [DOI] [PubMed] [Google Scholar]
- 4.van Vark LC, Bertrand M, Akkerhuis KM, Brugts JJ, Fox K, Mourad JJ, Boersma E: Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: A meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone system inhibitors involving 158,998 patients. Eur Heart J 33: 2088–2097, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarro I, Poveda R, Torras J, Castelao AM, Grinyó JM: Acute renal failure associated to renin angiotensin system (RAS) inhibitors--its burden in a nephrology department. Nephrol Dial Transplant 23: 413–414, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Arora P, Rajagopalam S, Ranjan R, Kolli H, Singh M, Venuto R, Lohr J: Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 3: 1266–1273, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cirit M, Toprak O, Yesil M, Bayata S, Postaci N, Pupim L, Esi E: Angiotensin-converting enzyme inhibitors as a risk factor for contrast-induced nephropathy. Nephron Clin Pract 104: c20–c27, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Dreischulte T, Morales DR, Bell S, Guthrie B: Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin-angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int 88: 396–403, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Evans M, Carrero JJ, Szummer K, Åkerblom A, Edfors R, Spaak J, Jacobson SH, Andell P, Lindhagen L, Jernberg T: Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in myocardial infarction patients with renal dysfunction. J Am Coll Cardiol 67: 1687–1697, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Siew ED, Parr SK, Abdel-Kader K, Eden SK, Peterson JF, Bansal N, Hung AM, Fly J, Speroff T, Ikizler TA, Matheny ME: Predictors of recurrent AKI. J AM Soc Nephro 27: 1190–1200, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koulouridis I, Price LL, Madias NE, Jaber BL: Hospital-acquired acute kidney injury and hospital readmission: A cohort study. Am J Kidney Dis 65: 275–282, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu KD, Yang J, Tan TC, Glidden DV, Zheng S, Pravoverov L, Hsu CY, Go AS: Risk factors for recurrent acute kidney injury in a large population-based cohort. Am J Kidney Dis 73: 163–173, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou YH, Huang TM, Pan SY, Chang CH, Lai CF, Wu VC, Wu MS, Wu KD, Chu TS, Lin SL: Renin-angiotensin system inhibitor is associated with lower risk of ensuing chronic kidney disease after functional recovery from acute kidney injury. Sci Rep 7: 46518, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gayat E, Hollinger A, Cariou A, Deye N, Vieillard-Baron A, Jaber S, Chousterman BG, Lu Q, Laterre PF, Monnet X, Darmon M, Leone M, Guidet B, Sonneville R, Lefrant JY, Fournier MC, Resche-Rigon M, Mebazaa A, Legrand M; FROG-ICU investigators : Impact of angiotensin-converting enzyme inhibitors or receptor blockers on post-ICU discharge outcome in patients with acute kidney injury. Intensive Care Med 44: 598–605, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Brar S, Ye F, James MT, Hemmelgarn B, Klarenbach S, Pannu N; Interdisciplinary Chronic Disease Collaboration : Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with outcomes after acute kidney injury. JAMA Intern Med 178: 1681–1690, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grams ME, Plantinga LC, Hedgeman E, Saran R, Myers GL, Williams DE, Powe NR; CDC CKD Surveillance Team : Validation of CKD and related conditions in existing data sets: A systematic review. Am J Kidney Dis 57: 44–54, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlasschaert ME, Bejaimal SA, Hackam DG, Quinn R, Cuerden MS, Oliver MJ, Iansavichus A, Sultan N, Mills A, Garg AX: Validity of administrative database coding for kidney disease: A systematic review. Am J Kidney Dis 57: 29–43, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL: Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol 17: 1688–1694, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM: Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J: Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 9: 682–689, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie D, Yang W, Jepson C, Roy J, Hsu JY, Shou H, Anderson AH, Landis JR, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Statistical methods for modeling time-updated exposures in cohort studies of Chronic Kidney Disease. Clin J Am Soc Nephrol 12: 1892–1899, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH: Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA 296: 2105–2111, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Gurwitz JH, Magid DJ, Smith DH, Goldberg RJ, McManus DD, Allen LA, Saczynski JS, Thorp ML, Hsu G, Sung SH, Go AS: Contemporary prevalence and correlates of incident heart failure with preserved ejection fraction. Am J Med 126: 393–400, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McManus DD, Hsu G, Sung SH, Saczynski JS, Smith DH, Magid DJ, Gurwitz JH, Goldberg RJ, Go AS; Cardiovascular Research Network PRESERVE Study : Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. J Am Heart Assoc 2: e005694, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray WA: Evaluating medication effects outside of clinical trials: New-user designs. Am J Epidemiol 158: 915–920, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for Acute Kidney Injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 27.Selby JV, Ray GT, Zhang D, Colby CJ: Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care 20: 1396–1402, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS: Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 362: 2155–2165, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, Jensvold NG, Selby JV, Singer DE: Anticoagulation therapy for stroke prevention in atrial fibrillation: How well do randomized trials translate into clinical practice? JAMA 290: 2685–2692, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd., Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS: The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74: 101–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Austin PC: Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38: 1228–1234, 2009 [Google Scholar]
- 34.Healthy people 2020: Chronic Kidney Disease. Available at: http://www.healthypeople.gov/2020/topics-objectives/topic/chronic-kidney-disease/objectives. Accessed July 3, 2017
- 35. National Institute of Diabetes and Digestive and Kidney Diseases: Improving care for patients after hospitalization with AKI. 2019. Available at: https://www.niddk.nih.gov/news/meetings-workshops/2019/improving-care-patients-hospitalization-aki. Accessed February 25, 2019.
- 36.Silver SA, Goldstein SL, Harel Z, Harvey A, Rompies EJ, Adhikari NK, Acedillo R, Jain AK, Richardson R, Chan CT, Chertow GM, Bell CM, Wald R: Ambulatory care after acute kidney injury: An opportunity to improve patient outcomes. Can J Kidney Health Dis 2: 36, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silver SA, Harel Z, Harvey A, Adhikari NK, Slack A, Acedillo R, Jain AK, Richardson RM, Chan CT, Chertow GM, Bell CM, Wald R: Improving care after acute kidney injury: A prospective time series study. Nephron 131: 43–50, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Vanmassenhove J, Vanholder R, Lameire N: Points of concern in post acute kidney injury management. Nephron 138: 92–103, 2018 [DOI] [PubMed] [Google Scholar]
- 39.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD; The Collaborative Study Group : The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 40.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) : Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 41.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G; Heart Outcomes Prevention Evaluation Study Investigators : Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 342: 145–153, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Leung KC, Pannu N, Tan Z, Ghali WA, Knudtson ML, Hemmelgarn BR, Tonelli M, James MT; APPROACH and AKDN Investigators : Contrast-associated AKI and use of cardiovascular medications after acute coronary syndrome. Clin J Am Soc Nephrol 9: 1840–1848, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng SY, Chou YH, Liao FL, Lin CC, Chang FC, Liu CH, Huang TM, Lai CF, Lin YF, Wu VC, Chu TS, Wu MS, Lin SL: Losartan reduces ensuing chronic kidney disease and mortality after acute kidney injury. Sci Rep 6: 34265, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN; SOLVD Investigators : Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325: 293–302, 1991 [DOI] [PubMed] [Google Scholar]
- 45.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C: 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation 136: e137–e161, 2017 [DOI] [PubMed] [Google Scholar]
- 46.Krieger N: Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health 82: 703–710, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon NP: Characteristics of Adult Health Plan Members in the Northern California Region Membership, as Estimated from the 2011 Member Health Survey, Oakland, CA, Division of Research, Kaiser Permanente Medical Care Program, 2013 [Google Scholar]
- 48.Cerdá J, Liu KD, Cruz DN, Jaber BL, Koyner JL, Heung M, Okusa MD, Faubel S; AKI Advisory Group of the American Society of Nephrology : Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol 10: 1859–1867, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.