Abstract
BACKGROUND: Individuals with diabetes may develop diabetic foot ulcers due to diabetic peripheral neuropathy. Multiple factors influence the ulcer healing process; oxygen helps in facilitating the different stages of wound healing. OBJECTIVE: The objective of this systematic review was to analyze the different levels of evidence available in the application of topical oxygen therapy, warm oxygen therapy, or other modes of topical oxygen delivery in the healing dynamics of diabetic foot ulcers. METHODS: Databases searched included Pubmed/Medline, Science Direct, Web of Science, Scopus, Cochrane, and CINAHL. The eligibility criteria of studies included participants ≥18 years with chronic non-healing diabetic foot ulcer (duration ≥3 months) receiving warm oxygen or topical oxygen therapy (TOT), and other modes of topical oxygen administration, which were compared with standard care group. Randomized and non-randomized studies were included. The primary outcome measure assessed was the rate of wound healing or wound closure. RESULTS: The review included 5 studies which used different modes of topical oxygen administration. The healing trajectory of the wounds was completely achieved in low-grade ulcers (grade 1), whereas all high-grade ulcers (grades 2, 3, and above) showed either 100% or 50% healing with a reduction in ulcer size and ulcer tissue depth. CONCLUSION: Topical oxygen therapy facilitates wound healing dynamics among individuals with chronic diabetic foot ulcers.
Keywords: diabetic foot ulcer, topical oxygen therapy, topical warm oxygen therapy, wound healing
Abbreviations: DFU - diabetic foot ulcer; HBOT - hyperbaric oxygen therapy; IL - interleukin; MMP - matrix metalloproteinase; TcPO2 - transcutaneous oxygen tension; TCOT - transdermal continuous oxygen therapy; TIMP - tissue inhibitors of metalloproteinase; TNF-α - tumor necrosis factor alpha; TOT - topical oxygen therapy; TWO2 - topical wound oxygen therapy
1. Introduction
Diabetes mellitus is a metabolic disorder associated with a cascade of complications, including the diabetic foot [1] characterized by infection, damage to foot tissue, and development of diabetic foot ulcer (DFU) due to diabetic peripheral neuropathy [2]. The prevalence of DFU in type 2 diabetes is 6.4% globally, with 5.5% in Asia [3], 6.38% in South India [4], and 14.30% in North India [5]. The growing burden of DFU increases the risk for lower extremity amputations [6, 7]. Many surgical and non-surgical strategies aiming to curb this problem include wound debridement, skin grafting, revascularization, frequent dressing of the wound site, and ultimately amputation in very severe cases. Increasing awareness of foot care among people with type 2 diabetes can improve their overall health-related quality of life [8].
Adoption of safe and simple strategies to increase the acceptance of treatment choices for people with DFU brings our focus to the primary factor responsible for enhancing the wound healing process, which is oxygen. The administration of oxygen through oxygen therapy is gaining immense interest and popularity. Evidence on the role of hyperbaric oxygen therapy (HBOT) in wound healing has shown benefits, but it is associated with high treatment costs and the complications of oxygen toxicity and barotrauma [9]. Topical oxygen therapy (TOT) and other modes of topical administration are minimally associated with complications.
The non-invasive application of topical oxygen directly to the wound site bypasses the oxygen transport system, thus creating an oxygen-rich wound bed environment with improved pH levels, which helps to cure infection. TOT may enhance the quality of wound healing, thereby improving the rate of wound closure, which may thus become an adjunct in the management of chronic non-healing diabetic foot ulcers [10]. The objective of this systematic review is to focus on different levels of evidence available in the application of topical oxygen therapy, warm oxygen therapy, and other modes of topical oxygen delivery in the healing dynamics of diabetic foot ulcers.
2. Materials and methods
The procedure for the systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist [11] and registered under PROSPERO with application ID number 150502.
2.1 Search strategy
The systematic electronic search was conducted in the following databases: Pubmed/Medline, Science Direct, Web of Science, Scopus, Cochrane, and CINAHL. The following keywords were used: "wound healing", "oxygen therapy", "foot ulcer" with Boolean operators "AND" and "OR", dating from the inception period until 30th August 2019, with a filter on English language and human studies.
2.2 Inclusion and exclusion criteria
The criteria for studies to be included in this systematic review comprised:
Participants aged ≥18 years.
History of chronic non-healing diabetic foot ulcers (i.e. duration of more than 3 months).
Intervention: topical warm therapy, topical oxygen therapy (TOT), or other modes of topical oxygen administration.
Comparison of treatment groups with standard care group who received either no care or basic wound dressing as a treatment.
The primary outcome measure assessed was the rate of wound healing or wound closure, along with the effect of oxygen in the healing dynamics of diabetic foot ulcers. This systematic review included:
2 randomized controlled trials
1 clinical trial
1 case-control study
1 case series, i.e. case report
Preclinical and in-vitro studies with ulcers forming in other areas of the body such as the elbow, sacrum, malleoli, shoulder, and the occiput region or pressure ulcers of unknown etiology were excluded. Data in the form of presentations or conference materials were also excluded from the review.
2.3 Data extraction
The authors MN, GAM, and GK systematically screened studies individually, based on title and abstract in all databases mentioned. Eligibility was assessed independently by MN, GAM, and GK for all studies identified that met the inclusion criteria. Any difference in opinion about the selected studies was mutually resolved between the authors, and finally, 5 studies were selected for this systematic review. We did not find any study that used topical warm oxygen therapy. Therefore, this systematic review discusses studies that included only topical oxygen therapy and its different modes of administration. A customized data extraction form was used which included details of study participant characteristics, study design, level of evidence, ulcer characteristics (like duration, location, and grade), topical oxygen therapy intervention characteristics, and the outcome measure of wound closure, which was extracted from the studies included.
2.4 Assessment of risk of bias
The NIH National Heart, Lung and Blood Institute (U.S. Department of Health and Human studies) checklist for controlled intervention studies, pre-post studies without control group, case-control study, and case series was used to assess the risk of bias of the studies included. The reporting of individual items in the checklists was one of the following:
Yes
No
Cannot be determined or not reported or not applicable
The checklist has been modified, as used previously [12], to provide a quantitative scoring to assess the quality of the included studies. A similar scoring process [12, 13] was used for the purpose of the current review. The quality of a study was rated according to the following scores:
Poor quality if < 4
Fair quality if between 4-5
Good quality if ≥6
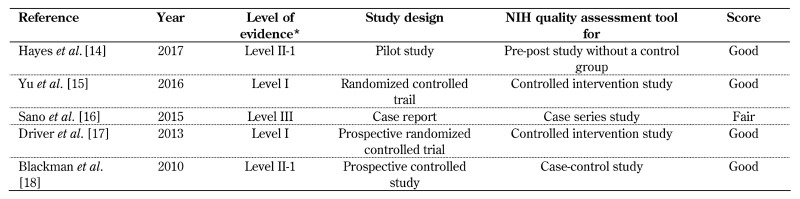
The authors MN, GAM, and GK assessed the studies individually. Any disagreement in rating between them were discussed and agreed upon clarification. The final score per study is shown in Table 1, which represents the mean score from the three authors.
Table 1. Characteristics of the studies included in the review.
Legend: *Reporting of the evidence level of individual studies is based on the evidence-based medicine ratings, as established by the U.S. Preventive Services Task Force (USPSTF).
3. Results
3.1 Retrieval and selection of studies
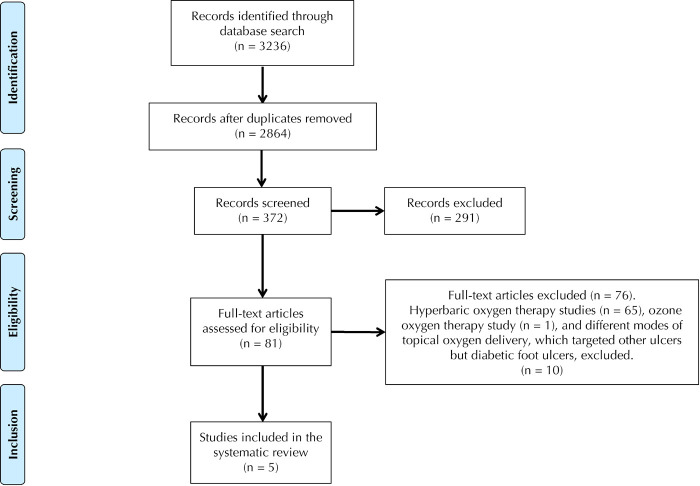
After elimination of duplicates, the initial search yielded 2864 records, which underwent further screening based on title and abstract yielding 372 records, and based on full-text yielding 81 records. Finally, 5 studies were identified that met the inclusion criteria for the systematic review [14-18].
The 5 studies included had different levels of evidence regarding the use of topical oxygen therapy for diabetic foot ulcers. All excluded studies used hyperbaric oxygen therapy, ozone therapy, or other modes of topical oxygen delivery applied to ulcer sites other than the diabetic foot. The study selection process is depicted in Figure 1; the study characteristics are described in Table 1.
Figure 1.
PRISMA flow diagram showing the procedure of study selection.
3.2 Quality assessment tool
A separate quality assessment tool was applied for individual study designs based on NIH quality assessment tool checklists for reporting randomized and non-randomized controlled trials, as shown in Table 1. Almost all studies included in this review were scored as good quality; only one study is of fair quality. The results from the studies included are thus reliable, despite differences in the levels of evidence. The reporting of evidence levels was based on evidence-based medicine rating criteria, as established by the U.S. Preventive Services Task Force (USPSTF) and as shown in Table 1.
3.3 Study participant characteristics
A total of 81 study participants were included in the 5 studies, but one participant in the case report study [16] had a sacral ulcer and thus was excluded from the review. Eventually, the data of 80 participants with chronic non-healing diabetic foot ulcers aged 18 years and above were included. The participants were also suffering from comorbidities other than diabetes mellitus, including peripheral arterial disease (PAD), coronary artery disease (CAD), dyslipidemia, hypertension, smoking history, and chronic renal failure (CRF). In almost all studies, the number of male participants was much higher than that of females (approximately 80-85% males) except in the case report by Sano et al. (2015) [16], which reported an equal ratio of male and female participants.
3.4 Ulcer characteristics
The mean ulcer duration among the topical oxygen therapy intervention group ranged from 24-178 weeks, and the duration of the standard care/control group ranged from 12-64 weeks in 4 studies; the duration was not specified in the case report by Sano et al. [16]. The location of ulcers included areas such as the plantar region of the foot, heel, metatarsal head, toe digits, and midfoot [15, 17, 18]. In two studies, the ulcers were graded based on the University of Texas classification [15, 18]; in one study by the Wagner classification [16]. The other two studies did not specify the grading system used. The ulcer wound areas were mentioned in two out of five studies [17, 18] for both the treatment and control groups, respectively. The study by Driver et al. (2013) [17] reported the ulcer volume (cm3) in the transdermal continuous oxygen therapy (TCOT) group (1.3±0.7) and control group (1.5±0.6), with history of infection and amputation in both groups. The study by Blackman et al. (2010) [18] reported the wound area (cm2) in the topical wound oxygen therapy (TWO2) group (4.1±4.3) and control group (1.4±0.6).
3.5 Outcome measures
The primary outcome measure was wound closure in all five studies. Blackman et al. also assessed the rate of recurrence of ulcer after 24 months following intervention with topical oxygen therapy [18]; a similar follow-up was not assessed in the other studies. Driver et al. also reported levels of various pro-inflammatory cytokines (IL-6, IL-8, IL-10, TNF-α, and IL-1β) and proteases (MMP-1, 2, and 9 and TIMP-1, 2, and 3) in ulcer wound fluid along with the presence of tissue-resistant macrophages, which was not assessed in other studies [17]. In the case report by Sano et al., changes in transcutaneous oxygen tension (TcPO2) values were observed [16].
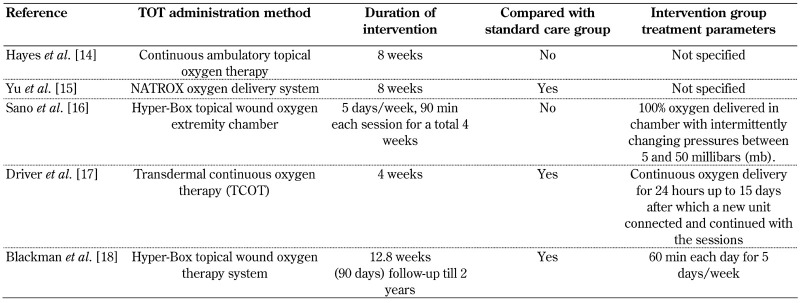
3.6 Duration and application method of topical oxygen therapy
The duration and application methods of topical oxygen therapy for the study participants with chronic non-healing diabetic foot ulcers are shown in Table 2. The negative pressure wound therapy was given along with topical oxygen therapy in the case report by Sano et al. only, not in the other studies. The modified offloading footwear was prescribed to study participants in 3 studies [14, 17, 18], but not in the other 2 studies. The moist wound dressing apart from standard care and topical oxygen therapy intervention were provided to study participants in 3 studies [16-18]. These additional treatment strategies applied by the studies with footwear and dressing care [14, 16-18] could have influenced the results positively by showing a better outcome of wound healing.
Table 2. Characteristics of topical oxygen therapy administered to participants.
3.7 Changes in primary outcome measured, i.e. wound closure
Topical oxygen therapy and its different modes of administration have shown improved healing trajectories in chronic diabetic foot ulcers. Hayes et al. (2017) found complete healing in 56-week old ulcers, along with a 50% reduction in 2-year old ulcers and a minimal 10% reduction in 88-week ulcers [14]. One study that used the Wagner grading for ulcer classification found that wound size reduced significantly (%) in the TCOT group compared to the control group from 21.8±20.0% (week 1) to 49.2±52.3% (week 5), with p < 0.05 [17].
Yu et al. (2016), who used the University of Texas wound classification, observed that all grade 1 ulcers healed completely in both groups, while grade 2 and 3 ulcers in the treatment group healed by 100% and 50%, respectively, compared with control groups (p < 0.001) [15]. Blackman et al. (2010) reported that the TWO2 group had about 82.4% wound closure and complete epithelialization, while the control group had only 45.5% epithelialization [18]. Also, the reported time taken for closure was 56 days (IQR 39-81 days) in the treatment group compared with 93 days (IQR 62-127 days) in the control group. There was no recurrence of ulcers in both groups after 24 months.
3.8 Changes in other outcome measures, i.e. transcutaneous oxygen tension (TcPO2)
Sano et al. found that TcPO2 values significantly improved after treatment with TOT in 4/6 cases and showed complete granulation tissue formation and wound healing with no recurrence [16]. However, no improvement was found in wounds with extremely low TcPO2 values (<10 mmHg) in the 2 cases.
3.9 Changes in other outcome measures, i.e. level of various inflammatory markers
Driver et al. assessed the levels of pro-inflammatory cytokines (IL-6, IL-8, IL-10, and TNF-α, IL-1β) and proteases (MMP-1, 2, and 9 and TIMP-1, 2, and 3) along with the role of macrophages [17]. Significant changes were found in levels of IL-6, which decreased to 82.4±25.2% of baseline. The average levels of IL-8 increased by 260% in the TCOT group compared with baseline levels. Other markers like IL-10, TNF-α, and IL-1β were not detectable in the analysis. Macrophage count decreased in the treatment group from 410±49 at week 1 to 97±52 at week 4 (p < 0.01). MMP-1 increased from 215% to 288% in the treatment group [18]. MMP-2 and MMP-9 levels decreased in the TCOT group compared with the control group after 2 weeks (p < 0.01 and p < 0.05, respectively). The opposite development was seen in the control group during the subsequent 2 weeks. Other proteases like MMP-8, TIMP-2, and TIMP-3 were not detected in the fluid analysis.
4. Discussion
4.1 Oxygen and wound healing
All stages of wound healing are facilitated by oxygen during hemostasis, inflammation, proliferation, maturation, and remodeling through the release of numerous cytokines and mediators that initiate the processes of angiogenesis, thrombosis, granulation tissue formation, and re-epithelialization [19]. Oxygen level under normal skin surface is 45-65 mmHg (Howard et al., 2013 [20]), under chronic wound conditions 5-20 mmHg (Schreml et al., 2010 [21]), and in the absence of vascular supply only 0-5 mmHg in the central area of the wound [21]. Such low oxygen levels below 20 mmHg provoke the wound cells to convert themselves towards an anaerobic metabolic state and thus decelerate the wound healing process.
Interestingly, it is observed that higher oxygen levels of about 160 mmHg can enhance the rate of fibroblast proliferation, formation of proteins, and activity of prolyl hydroxylase at an oxygen level of 250 mmHg [22]. These effects have been highlighted in the consensus expert panel report on the clinical pathway to use topical oxygen therapy in practice [23].
It is known that the acute wound healing time is approximately 4-6 weeks as compared to chronic wounds, which takes ≥6 weeks of healing. Hypoxia, biofilm formation over the wound area, altered tissue oxygen perfusion levels, changes in cellular response, and defects in collagen synthesis extend the healing time in chronic wounds. Thus, chronic wounds do not improve beyond the inflammatory phase due to impaired oxidative killing [24]. Also, the presence of systemic illnesses like chronic medical conditions, diabetes, obesity, use of medications, age of the individual, presence of infection, alcohol and smoking history, and malnourishment further impair wound healing [25].
4.2 Role of topical oxygen therapy in wound healing
The requirement of oxygen for a person with a healthy clean wound is around 20%, while that for infectious wounds is 50% [10]. The penetration of oxygen across the surface of the skin occurs to a depth of up to 700 µm, validating the physiological basis of topical oxygen delivery [26]. The advantages of topical oxygen therapy as an intervention method are predominantly due to its cost-effectiveness, the availability of a home treatment option, and the minimal risk of developing complications [27] as compared to hyperbaric oxygen therapy or topical hyperbaric oxygen therapy. There is only minimal damage to the vasculature due to oxygen penetration directly from the outer surface of the skin bypassing the oxygen transport system of the body.
4.3 Which ulcer grades benefit most from topical oxygen therapy?
It is crucial to determine the eligible participants who would benefit from topical oxygen therapy. This systematic review included participants with chronic non-healing diabetic foot ulcers of different grades (based on either Wagner classification or the University of Texas classification). It was observed that in participants with higher grades of chronic wounds (grades 2, 3, and above) the wound tissue involved at the wound site was deeper. Thus, complete wound closure through penetration of topical oxygen therapy was not possible in the short duration of treatment. However, some reduction in wound size from baseline was expected, which was discussed in the studies. Although the previous studies showed that the rate of wound healing is dependent on the different grades of ulcer, better outcomes could be achieved with a combination of topical oxygen therapy and comprehensive care including off-loading footwear, proper wound bed dressing, and standard wound care.
Complete healing of such chronic ulcers requires a longer duration of treatment and follow-up. A clinical decision for topical oxygen therapy as a treatment option among participants with chronic ulcers is required by healthcare professionals. A potential aid to making this decision may consist in measuring transcutaneous oxygen tension levels in the wound area, which was addressed by Sano et al. [16]. Also, topical oxygen therapy and its different modes of administration would most likely benefit all low-grade ulcers that have failed to heal following standard wound care by multidisciplinary healthcare teams [27]. Therefore, it can be readily chosen for individuals with low-grade diabetic foot ulcers.
4.4 Strength of this review
This is the first systematic review analyzing the different levels of evidence available regarding the role of topical oxygen therapy and its different modes of administration in the healing dynamics of chronic diabetic foot ulcers among people with diabetes mellitus.
4.5 Limitations of this review
The limitations of this systematic review include the small sample size of studies included in the review and the limited clinical and experimental evidence available from studies assessing the effect of topical oxygen therapy and its effects on the healing of chronic diabetic foot ulcers.
4.6 Recommendations for future directions and clinical significance
Along with topical oxygen therapy, adding warm temperature of oxygen can have local and systemic effects by improving oxygenation levels in the wound bed along with local vasodilatory benefits in the peri-wound areas. Similar effects were observed in a group of healthy volunteers in the study by Joshi et al. [28]. The possible mechanisms that affect wound healing dynamics include upregulation of the wound microenvironment through the expression of modulatory enzymes that may help in the conversion of oxygen to reactive oxygen species, which stimulates vascular endothelial growth factors, resulting in angiogenesis, which may act as a signal to enhance wound healing responses [24]. Another possible mechanism behind cell differentiation during the wound healing process may be wound hyperoxia, facilitating fibroblastic proliferation at the wound site, which may account for the accelerated rate of wound contraction during healing [29]. Molecular oxygen also plays an important role in the collagen synthesis modulated by the enzymes hydroxylase, lysyl hyroxyles, and lysyl oxidase producing procollagen, resulting in remodeling and maturation of the wound [22].
There is a need for future experimental and clinical assessment of the effects of topical warm oxygen therapy. Studies should also investigate the roles of various inflammatory markers which are associated with different stages of wound healing among people with chronic diabetic foot ulcers.
At present, topical oxygen therapy has not been tested based on the grading of the ulcer, and there is no detailed information in the available literature regarding the use of topical oxygen therapy based on the wound size or degree of wound infection. However, if oxygen therapy is used independently or in combination with existing clinical interventions, it may be an important adjunct modality in the management of chronic ulcers, especially in the diabetic foot. There is a dearth of literature suggesting mechanisms of underlying problems in healing dynamics of chronic diabetic foot ulcer, which stands as a major challenge in clinical practice. This review intends to shed light on the importance of topical warm oxygen therapy as an adjuvant management for non-healing chronic diabetic foot ulcer, emphasizing the need for more clinical trials in this area.
5. Conclusions
The following conclusions could be drawn from this review:
The application of topical oxygen therapy among people with different grades of chronic diabetic foot ulcers improves wound healing.
Low-grade ulcers (grade 1) heal completely under topical oxygen therapy.
High-grade ulcers (grades 2, 3, and above) show a reduction in wound size by 100% and 50%, respectively, along with a reduction in the depth of tissue involved.
The probability of complete healing of high-grade ulcers needs to be addressed by longer treatment periods and follow-up among participants with chronic diabetic foot ulcers.
The choice of topical oxygen therapy as a treatment modality for eligible participants with varied ulcer grades is the responsibility of the healthcare team delivering the intervention.
Acknowledgments
Author contributions
The authors MN, GAM, and GK contributed to the conceptualization, design, and composition of the review. The literature search was done by MN and GK. The clinical and experimental studies were assessed by MN, GK, and MH. Data acquisition, data analysis, statistical analysis, and preparation of the manuscript were performed by MN, GAM, and GK. Manuscript writing and editing was done by GSR, RS, and SSP. GAM was responsible for verifying the correctness of all statements made in this article.
Acknowledgments
We wish to thank the following institutions for their support of our work: 1. Centre for Diabetic Foot Care and Research (CDFCR), Department of Physiotherapy, Manipal College of Health Professions (MCHP), Manipal Academy of Higher Education (MAHE), Manipal 576104, Karnataka, India. 2. Yostra Labs Pvt. Ltd, Bangalore Bioinnovation Centre (BBC), Bangalore Helix Biotech Park, Electronic City Phase I, Bangalore 560 100, Karnataka, India (neurotouch@yostra.com, www.yostra.com).
Disclosures
The authors report no conflict of interests.
References
- 1.Ibrahim A. IDF clinical practice recommendation on the diabetic foot: a guide for healthcare professionals. Diabetes Res Clin Pract. 2017;127:285–287. doi: 10.1016/j.diabres.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Jakosz N. IWGDF guidelines on the prevention and management of diabetic foot disease (book review). Wound practice and research. Wound Repair Regen. 2019;27(3):144. [Google Scholar]
- 3.Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017;49(2):106–116. doi: 10.1080/07853890.2016.1231932. [DOI] [PubMed] [Google Scholar]
- 4.Maiya AG, Gundmi S, Matpady P, Jadhav R, Lingadakai R, Hande M, Kamath VG, Shivashankar KN, Chinmayee P, Hazari A. et al. Prevalence of foot complications in people with type 2 diabetes mellitus: a community-based survey in rural Udupi. Int J Low Extrem Wounds. 2018;17(3):169–175. doi: 10.1177/1534734618791853. [DOI] [PubMed] [Google Scholar]
- 5.Shahi SK, Kumar A, Kumar S, Singh SK, Gupta SK, Singh TB. Prevalence of diabetic foot ulcer and associated risk factors in diabetic patients from North India. J Diabet Foot Compl. 2012;4(3):83–91. [Google Scholar]
- 6.Richard JL, Schuldiner S. Epidemiology of diabetic foot problems. Rev Med Interne. 2008;29:S222–S230. doi: 10.1016/S0248-8663(08)73949-3. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Geographic disparities in diabetes-related amputations - Texas-Mexico border, 2003. MMWR Recomm Rep. 2006;55(46):1251. [PubMed] [Google Scholar]
- 8.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Thom SR. Hyperbaric oxygen - its mechanisms and efficacy. Plastic Reconstr Surg. 2011;127(Suppl 1):131S–141S. doi: 10.1097/PRS.0b013e3181fbe2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demling RH. Nutrition, anabolism, and the wound healing process: an overview. Eplasty. 2009;9:e9. [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabeena S, Bhat PV, Kamath V, Bhat SK, Nair S, Chandrabharani K, Arunkumar G. Community-based prevalence of genital human papilloma virus (HPV) infection: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2017;18(1):145–154. doi: 10.22034/APJCP.2017.18.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gundmi S, Maiya AG, Bhat AK, Ravishankar N, Hande MH, Rajagopal KV. Hand dysfunction in type 2 diabetes mellitus: systematic review with meta-analysis. Ann Phys Rehabil Med. 2018;61(2):99–104. doi: 10.1016/j.rehab.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Hayes PD, Alzuhir N, Curran G, Loftus IM. Topical oxygen therapy promotes the healing of chronic diabetic foot ulcers: a pilot study. J Wound Care. 2017;26(11):652–660. doi: 10.12968/jowc.2017.26.11.652. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Lu S, McLaren AM, Perry JA, Cross KM. Topical oxygen therapy results in complete wound healing in diabetic foot ulcers. Wound Repair Regen. 2016;24(6):1066–1072. doi: 10.1111/wrr.12490. [DOI] [PubMed] [Google Scholar]
- 16.Sano H, Ichioka S. Topical wound oxygen therapy for chronic diabetic lower limb ulcers and sacral pressure ulcers in Japan. Wounds Int. 2015;6:20–24. [Google Scholar]
- 17.Driver VR, Yao M, Kantarci A, Gu G, Park N, Hasturk H. A prospective, randomized clinical study evaluating the effect of transdermal continuous oxygen therapy on biological processes and foot ulcer healing in persons with diabetes mellitus. Ostomy Wound Manage. 2013;59(11):19–26. [PubMed] [Google Scholar]
- 18.Blackman E, Moore C, Hyatt J, Railton R, Frye C. Topical wound oxygen therapy in the treatment of severe diabetic foot ulcers: a prospective controlled study. Ostomy Wound Manage. 2010;56(6):24–31. [PubMed] [Google Scholar]
- 19.Kimmel HM, Grant A, Ditata J. The presence of oxygen in wound healing. Wounds. 2016;28(8):264–270. [PubMed] [Google Scholar]
- 20.Howard MA, Asmis R, Evans KK, Mustoe TA. Oxygen and wound care: a review of current therapeutic modalities and future direction. Wound Repair Regen. 2013;21(4):503–511. doi: 10.1111/wrr.12069. [DOI] [PubMed] [Google Scholar]
- 21.Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163(2):257–268. doi: 10.1111/j.1365-2133.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 22.Gordillo GM, Sen CK. Evidence-based recommendations for the use of topical oxygen therapy in the treatment of lower extremity wounds. Int J Low Extrem Wounds. 2009;8(2):105–111. doi: 10.1177/1534734609335149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Consensus round table meeting: clinical pathway for using topical oxygen therapy in practice. https://www.natroxwoundcare.com/site/assets/files/1075/natrox_consensus_-_wounds_uk.S.pdf .
- 24.Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003;186(3):259–263. doi: 10.1016/s0002-9610(03)00211-3. [DOI] [PubMed] [Google Scholar]
- 25.Wallace HA, Bhimji SS. Wound healing phases. StatPearls Publishing; 27. InStatPearls, published online: 2018 Oct 27. [PubMed] [Google Scholar]
- 26.Roe DF, Gibbins BL, Ladizinsky DA. Topical dissolved oxygen penetrates skin: model and method. J Surg Res. 2010;159(1):e29–e36. doi: 10.1016/j.jss.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 27.Mutluoglu M, Cakkalkurt A, Uzun G, Aktas S. Topical oxygen for chronic wounds: a pro/con debate. J Am Coll Clin Wound Spec. 2013;5(3):61–65. doi: 10.1016/j.jccw.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi VS, Sunil Subhash Joshi M. Systemic and local effects of warm oxygen exposure to the lower extremities in healthy volunteers. J Clin Diagn Res. 2017;11(4):CC01. doi: 10.7860/JCDR/2017/23603.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt TK, Pai MP. The effect of varying ambient oxygen tensions on wound metabolism and collagen synthesis. Surg Gynecol Obstet. 1972;135 (4):561–567. [PubMed] [Google Scholar]