Supplemental Digital Content is available in the text.
Keywords: acute cardiogenic pulmonary edema, acute heart failure, endotracheal intubation, intensive care unit, noninvasive ventilation, prehospital
Objective
The aim of this study was to assess the effect of prehospital noninvasive ventilation for acute cardiogenic pulmonary edema on endotracheal intubation rate and on ICU admission rate.
Methods
We carried out a retrospective study on patients’ prehospital files between 2007 and 2010 (control period), and between 2013 and 2016 (intervention period). Adult patients were included if a diagnosis of acute cardiogenic pulmonary edema was made by the prehospital physician. Exclusion criteria were a Glasgow coma scale score less than 9 or any other respiratory diagnosis. We analyzed the association between noninvasive ventilation implementation and endotracheal intubation or ICU admission with univariable and multivariable regression models. The primary outcome was prehospital endotracheal intubation rate. Secondary outcomes were admission to an ICU, prehospital intervention length, and 30-day mortality.
Results
A total of 1491 patients were included. Noninvasive ventilation availability was associated with a significant decrease in endotracheal intubation rate (2.6% in the control versus 0.7% in the intervention period), with an adjusted odds ratio (OR) of 0.3 [95% confidence interval (CI), 0.1–0.7]. There was a decrease in ICU admissions (18.6% in the control versus 13.0% in the intervention period) with an adjusted OR of 0.6 (95% CI, 0.5–0.9). There was no significant change in 30-day mortality (11.2% in the control versus 11.0% in the intervention period, P = 0.901).
Conclusion
In our physician-staffed prehospital system, use of noninvasive ventilation for acute cardiogenic pulmonary edema decreased both endotracheal intubation and ICU admission rates.
Introduction
Acute cardiogenic pulmonary edema (ACPE) is associated with significant morbidity and mortality. Its treatment, essentially based on expert consensus, can be challenging in the prehospital setting [1,2].
Standard medical therapy includes oxygen administration, vasodilators, and diuretics in the case of hypervolemia [3]. Continuous positive airway pressure (CPAP) or noninvasive ventilation (NIV) should be started without delay in the case of refractory hypoxemia [4,5]. These therapies have been largely validated in the emergency department (ED) and in the ICU [6]. In a review including 32 studies and 2916 subjects, their use was associated with a significant reduction in the endotracheal intubation (ETI) rate [7].
Recently, both CPAP and NIV have been successfully applied in the prehospital setting where tracheal intubation under general anesthesia followed by mechanical ventilation had been standard treatment for decades in cases of persistent hypoxemia [8]. ETI-related complications are, however, more frequent in the prehospital setting, as technical and environmental conditions are often difficult [9]. Crush induction and ETI are therefore procedures requiring well trained practitioners. Failed or traumatic intubations, as well as sedation-related hemodynamic and respiratory instability, are among the most feared immediate complications. Ventilator-associated pneumonia and lung injury are delayed complications that increase both ICU length of stay and mortality [5,10].
The aim of this study was to determine whether prehospital availability of NIV had reduced ETI rates in our prehospital system. We also wanted to assess the effect of this technique on ICU admission rates, on prehospital interventions length, and on mortality.
Methods
Study setting
The emergency prehospital response in Geneva is two tiered. The first tier is composed of an advanced life support ambulance staffed by two paramedics, while the second tier is a medical mobile unit called ‘Service Mobile d’Urgence et de Réanimation’ (SMUR). The last performs more than 5000 missions a year and belongs to the ED of the Geneva University Hospitals, a primary and tertiary care urban teaching hospital admitting 65 000 patients annually. The SMUR takes care of all prehospital life-threatening emergencies in the area. All SMUR vehicles are staffed by an advanced paramedic and by an anesthesia, emergency medicine, or general medicine junior physician. A senior emergency physician is on-call 24/7, and can be dispatched on site for supervision purposes. Emergency calls are handled by professional dispatchers. Both an ambulance and a SMUR are dispatched if an acute respiratory distress is identified.
Patients for whom ACPE is considered the most likely diagnosis are treated with oxygen and standard medical care in accordance with the European Society of Cardiology (ESC) guidelines. Until 2010, ETI was the usual rescue therapy if standard treatment was deemed insufficient, as neither CPAP nor NIV were available. Several techniques and ventilators were tested between 2011 and 2012. NIV, using the Hamilton T1 ventilator (Hamilton Medical, Bonaduz, Switzerland), was finally integrated in our standard of care in 2013. All SMUR physicians and paramedics regularly receive specific training regarding NIV and its accepted indications. Since 2013, the decision to initiate NIV has been made by each physician individually according to this training and to clinical evaluation. If NIV is contraindicated, for example, in the case of coma or of refractory hypotension, an ETI is performed.
A computerized medical file with standardized fields is filled in for each patient and reviewed daily by a senior physician in order to ensure data quality. All data are recorded in a secure database, which dates back to 2007.
Study design and patients
We performed a retrospective before–after study, approved by the institutional ethics committee (Project ID 2016-01373). We proceeded to a computer screening of our database based on diagnostic codes specific to our prehospital unit. We reviewed every SMUR intervention between April 2007 and March 2010 (control period), and between April 2013 and March 2016 (NIV period) and included all patients aged 18 years or older with an acute heart failure (AHF) or ACPE prehospital diagnostic code. All diagnoses were made by prehospital physicians according to a combination of different signs and symptoms and according to the ESC criteria for AHF [3,11].
Patients transported out of a tertiary ED to a definitive care unit were not considered for inclusion, as they had already been treated. We also decided not to include patients treated from April 2010 to March 2013, as this interval was a transition and training period preceding the full NIV treatment implementation.
Patients with a Glasgow Coma Scale (GCS) less than 9, as well as those with any confounding respiratory diagnosis such as chronic obstructive pulmonary disease, were excluded.
Data collection and outcomes
Data from prehospital patient care records were electronically extracted. For the purpose of this study, we defined abnormal values as a heart rate (HR) > 120/minute, a systolic blood pressure (SBP) >140 mmHg (as defined by the 2016 ESC guidelines [12]), an oxygen saturation (SaO2) <90%, a respiratory rate (RR) >30/minute, and a GCS < 15.
Night interventions were defined as those occurring between 7 p.m. and 7 a.m. Supervised interventions described the physical presence of a senior emergency physician on scene.
The primary outcome was prehospital ETI rate. If prehospital rescue intubation had to be performed after an NIV trial, the patient was considered as only having been intubated in order to avoid duplicates.
Secondary outcomes were admission to an ICU during 48 hours following ED arrival (one of our usual quality indicators), intervention length, and 30-day mortality.
Statistical analysis
We used Chi-square test and either Student’s t-test or Wilcoxon-Mann–Whitney test, depending on normality of the variables, for group comparisons.
We performed univariable and multivariable regressions to measure association between NIV availability and ETI rate, and between NIV availability and ICU admission rate.
We chose the variables that would be used in our models based on available evidence as well as on their clinical relevance, before performing any statistical test. To avoid overfitting, we defined that the number of variables included in the multivariable regression models would depend on the number of events (1 variable per 8–10 events). We would include in priority the variables with the strongest significant (P < 0.05) odds ratio (OR) in univariable logistic analysis.
For our primary outcome, we considered that assessment of the individual impact of each vital sign was of particular importance, as prehospital clinicians only have access to few objective parameters that can help them decide whether intubation should be performed.
The relative impact of vital signs is not the same regarding critical care admission as more parameters are then available to guide the decision. We therefore used a composite binary variable in our model for this outcome, ‘abnormal vital signs’, which was considered present when at least half of the available vital signs met the aforementioned criteria.
The association between NIV availability and length of intervention was assessed with a linear regression model. We compared the mortality between the 2 groups by performing a Chi-square test.
Statistical analysis was performed using STATA version 15 (Stata Corporation, College Station, Texas, USA).
Results
A total of 32 756 patients’ files were screened. Inclusion criteria were met by 1700 patients, out of which 209 were excluded according to our research protocol. We finally included 1491 patients, 689 in the control period and 802 in the NIV period (see flowchart of inclusion, Supplemental digital content 1, http://links.lww.com/EJEM/A248).
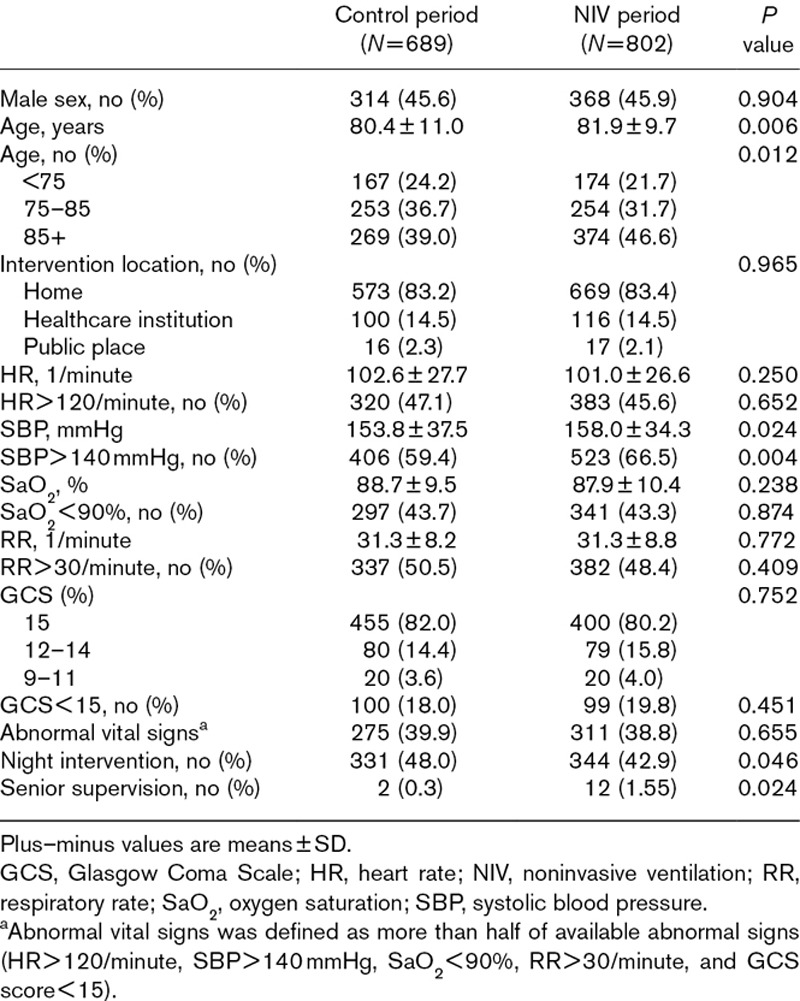
Patients’ characteristics are shown in Table 1. During the NIV period, patients were slightly older, there were less night interventions and more supervised interventions, and 287 patients (35.8%) received NIV.
Table 1.
Patients’ characteristics
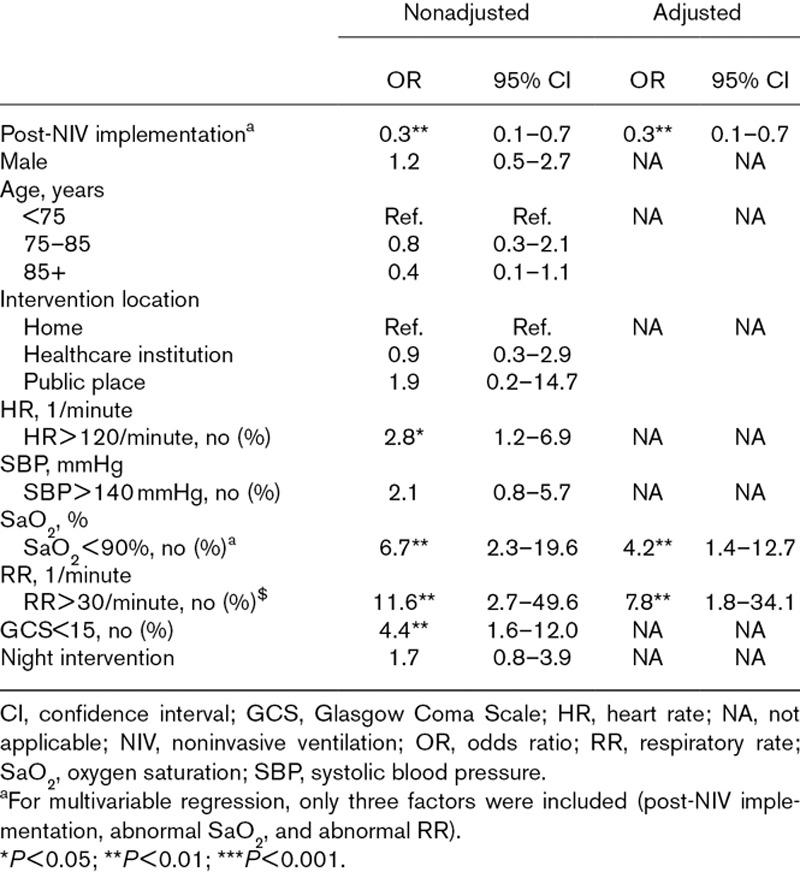
The ETI rate dropped from 2.6% (18/689) during the control period to 0.7% (6/802) during the NIV period [OR, 0.3; 95% confidence interval (CI), 0.1–0.7; P = 0.004]. Significant intubation-associated factors included HR > 120/minute (OR = 2.8; 95% CI, 1.2–6.9), SaO2 < 90% (OR = 6.7; 95% CI, 2.3–19.6), RR > 30/minute (OR = 11.6; 95% CI, 2.7–49.6), and GCS < 15 (OR = 4.4; 95% CI, 1.6–12.0). In a multivariable model, NIV implementation was associated with a decreased ETI rate (OR = 0.3; 95% CI, 0.1–0.7) after adjustment for abnormal SaO2 and abnormal RR (Table 2).
Table 2.
Univariable and multivariable logistic regressions for intubation rate
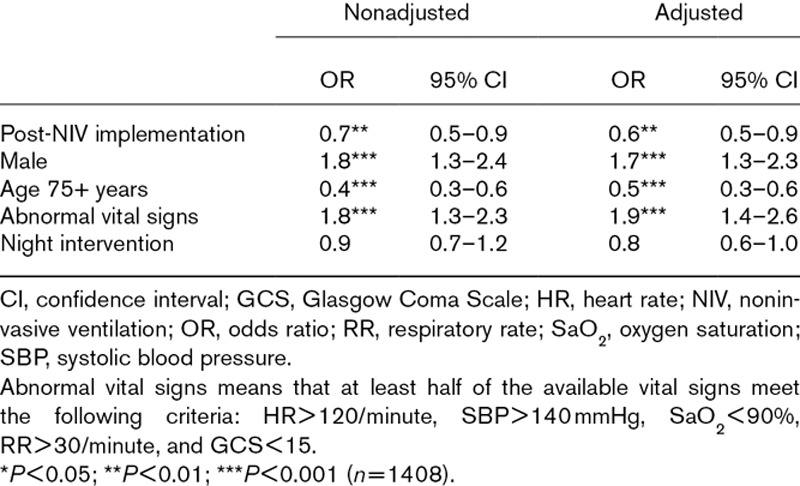
The ICU admission rates were 18.6% (128/689) and 13.0% (104/802) for the control and for the NIV periods, respectively (OR = 0.7; 95% CI, 0.5–0.9; P = 0.011). After adjustment, NIV implementation was associated with a decrease in ICU admissions (OR = 0.6; 95% CI, 0.5–0.9). ICU admissions also significantly depended on abnormal vital signs (OR = 1.9; 95% CI, 1.4–2.6), male gender (OR = 1.7; 95% CI, 1.3–2.3), and age > 75 years (OR = 0.5; 95% CI, 0.3–0.6) (Table 3).
Table 3.
Univariable and multivariable logistic regressions for intensive care admission
There was no significant difference in intervention length between the two periods (42.2 versus 43.6 minutes, P = 0.055). Finally, the overall 30-day mortality was 11.1%, with no significant difference between the two groups (11.2 versus 11.0%, P = 0.901).
Discussion
This study highlights the association between the availability of prehospital NIV and immediate and short-term outcomes. Once NIV was introduced in our prehospital system, there was a significant reduction in both prehospital ETI and ICU admissions rates regardless of the adjustment model. Length of intervention and mortality remained similar between both periods.
Intrahospital studies have shown that CPAP [13] and NIV [14] decrease the intubation rate in ACPE, and some prehospital studies have shown that both CPAP [1,15] and NIV [16,17] can be used in the field. As far as we know, this is the first study to show a decrease in ETI specifically linked to prehospital NIV.
Over one third of the patients of the intervention group were treated by NIV. This high proportion contrasts with the low number of ETI in the control period and could be explained by the fact that NIV was used not only in the most critically ill, but also in less severe patients to prevent respiratory distress worsening [4]. In addition, in spite of the hypotensive risk associated with positive pressure ventilation, NIV might have been used on some unstable patients provided there was no coma or major hypoperfusion. Indeed, some authors advocate the use of NIV in carefully selected cases of cardiogenic shock associated with respiratory failure [5,18]. Moreover, even though the benefits of positive pressure ventilation on ACPE were already well known in our control period, the advantages of mechanical ventilation were to be weighed against potential ETI-associated complications, inducing a selection of the most unstable patients [19]. Our results therefore also suggest an increase in the proportion of patients who can benefit from positive pressure ventilation.
In fact, respiratory disorders occur according to a continuum of progressive symptoms and signs and the prehospital clinician must decide on the most appropriate treatment on a case-by-case basis. On-site blood gas analysis and left ventricular function assessment by transthoracic portable ultrasound could improve clinical stratification and identification of AHF phenotype in order to target treatments [20]. The ever-widening range of diagnostic and therapeutic tools requires careful evaluation in order to determine the most efficient techniques. Though CPAP and NIV are the only validated techniques used to provide the mandatory intrathoracic positive pressure for ACPE, several authors consider high-flow nasal cannula oxygen for hypoxemia in preselected AHF cases. The efficacy of this technique has yet to be proven in this particular setting, as, conversely to NIV, it neither delivers a reliable positive end-expiratory pressure nor does it add the additional inspiratory aid required in exhausted and hypercapnic patients [5,10].
We found a trend toward a lower ETI rate in older patients. Although aging is not an independent risk factor for difficult intubation in the field [19], mechanical ventilation is associated with an unfavorable prognosis and physicians might try to avoid intubation in elderly patients in order to prevent ICU admissions and complications [21].
Unlike previous ED studies [22], we found a significant decrease in ICU admissions following NIV implementation. It has already been shown that, in comparison to standard medical care, immediate application of positive pressure in prehospital ACPE significantly improves physiological variables and symptoms [23]. More recently, prehospital management has been recognized as a critical component of care in AHF [2] and both CPAP or NIV are now recommended in the case of acute respiratory distress related to this disease in the prehospital setting in order to reverse respiratory failure faster [5]. Early stabilization of our patients thanks to prehospital NIV might have led to easier weaning from ventilation in the ED. Such reduction in the need for further positive pressure ventilation would then lead to a decrease in the ICU admission rate. The continued need for NIV in ED is indeed an indicator of greater severity and of heightened odds of admission to ICU [24].
As our study is, by design, both retrospective and without matching, some limitations must be acknowledged. Randomization was of course impossible, and significant bias might remain even after adjustment. Nevertheless, baseline characteristics were similar between both groups, and comparable to those described in other studies [25]. We were unfortunately unable to obtain ED data on left ventricular function, although most patients had not only elevated SBP values but were also more frequently female and older as it is often the case in emergency medicine settings [2]. Though some of the included cases might have been misdiagnosed, such patients are nevertheless treated in the same way as true ACPE cases in clinical practice and must therefore also be taken into account [1].
Some first-line treatment variability might have happened owing to the time elapsed between the two periods and to the changes in the ESC guidelines. Despite the existence of a treatment protocol, both the diagnosis and the ventilator’s use and settings depended on the physician’s evaluation, which is subject to some interindividual difference. Furthermore, the effect of CPAP alone could not be evaluated, as inspiratory aid had to be applied in order to compensate for the breathing circuit resistance.
Finally, even though the OR for ETI was significantly lower in the NIV period, we were not able to adjust our results for more than 2 other variables given the low number of intubations.
Conclusion
Our study suggests that in a physician-staffed prehospital system, use of NIV in patients with ACPE decreases the rate of ETI and the rate of ICU admission. Because of the limitations of the present retrospective study, a multicentric prospective study, encompassing subgroup analysis according to the clinical severity and the etiology of ACPE, should confirm our results.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.euro-emergencymed.com).
References
- 1.Hubble MW, Richards ME, Jarvis R, Millikan T, Young D. Effectiveness of prehospital continuous positive airway pressure in the management of acute pulmonary edema. Prehosp Emerg Care. 2006; 10:430–439 [DOI] [PubMed] [Google Scholar]
- 2.Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, et al. Recommendations on pre-hospital and early hospital management of acute heart failure: a consensus paper from the heart failure association of the european society of cardiology, the european society of emergency medicine and the society of academic emergency medicine–short version. Eur Heart J. 2015; 36:1958–1966 [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ; Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012; 14:803–869 [DOI] [PubMed] [Google Scholar]
- 4.Brochard L, Mercat A, Richard JCM. Ventilation Artificielle: De la Physiologie à la Pratique. 2008, Paris, France: Elsevier Masson [Google Scholar]
- 5.Masip J, Peacock WF, Price S, Cullen L, Martin-Sanchez FJ, Seferovic P, et al. ; Acute Heart Failure Study Group of the Acute Cardiovascular Care Association and the Committee on Acute Heart Failure of the Heart Failure Association of the European Society of Cardiology. Indications and practical approach to non-invasive ventilation in acute heart failure. Eur Heart J. 2018; 39:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess DR. Noninvasive ventilation for acute respiratory failure. Respir Care. 2013; 58:950–972 [DOI] [PubMed] [Google Scholar]
- 7.Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013CD005351. [DOI] [PubMed] [Google Scholar]
- 8.Bakke SA, Botker MT, Riddervold IS, Kirkegaard H, Christensen EF. Continuous positive airway pressure and noninvasive ventilation in prehospital treatment of patients with acute respiratory failure: a systematic review of controlled studies. Scand J Trauma Resusc Emerg Med. 2014; 22:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pepe PE, Roppolo LP, Fowler RL. Prehospital endotracheal intubation: elemental or detrimental?. Crit Care. 2015; 19:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beng Leong L, Wei Ming N, Wei Feng L. High flow nasal cannula oxygen versus noninvasive ventilation in adult acute respiratory failure: a systematic review of randomized-controlled trials. Eur J Emerg Med. 2019; 26:9–18 [DOI] [PubMed] [Google Scholar]
- 11.Renier W, Winckelmann KH, Verbakel JY, Aertgeerts B, Buntinx F. Signs and symptoms in adult patients with acute dyspnea: a systematic review and meta-analysis. Eur J Emerg Med. 2018; 25:3–11 [DOI] [PubMed] [Google Scholar]
- 12.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016; 252:207–274 [DOI] [PubMed] [Google Scholar]
- 13.Pang D, Keenan SP, Cook DJ, Sibbald WJ. The effect of positive pressure airway support on mortality and the need for intubation in cardiogenic pulmonary edema: a systematic review. Chest. 1998; 114:1185–1192 [DOI] [PubMed] [Google Scholar]
- 14.Masip J, Betbesé AJ, Páez J, Vecilla F, Cañizares R, Padró J, et al. Non-invasive pressure support ventilation versus conventional oxygen therapy in acute cardiogenic pulmonary oedema: a randomised trial. Lancet. 2000; 356:2126–2132 [DOI] [PubMed] [Google Scholar]
- 15.Templier F, Dolveck F, Baer M, Chauvin M, Fletcher D. ‘Boussignac’ continuous positive airway pressure system: practical use in a prehospital medical care unit. Eur J Emerg Med. 2003; 10:87–93 [DOI] [PubMed] [Google Scholar]
- 16.Craven RA, Singletary N, Bosken L, Sewell E, Payne M, Lipsey R. Use of bilevel positive airway pressure in out-of-hospital patients. Acad Emerg Med. 2000; 7:1065–1068 [DOI] [PubMed] [Google Scholar]
- 17.Weitz G, Struck J, Zonak A, Balnus S, Perras B, Dodt C. Prehospital noninvasive pressure support ventilation for acute cardiogenic pulmonary edema. Eur J Emerg Med. 2007; 14:276–279 [DOI] [PubMed] [Google Scholar]
- 18.Hongisto M, Lassus J, Tarvasmaki T, Sionis A, Tolppanen H, Lindholm MG, et al. Use of noninvasive and invasive mechanical ventilation in cardiogenic shock: a prospective multicenter study. Int J Cardiol. 2017; 230:191–197 [DOI] [PubMed] [Google Scholar]
- 19.Freund Y, Duchateau FX, Devaud ML, Ricard-Hibon A, Juvin P, Mantz J. Factors associated with difficult intubation in prehospital emergency medicine. Eur J Emerg Med. 2012; 19:304–308 [DOI] [PubMed] [Google Scholar]
- 20.Strnad M, Prosen G, Borovnik Lesjak V. Bedside lung ultrasound for monitoring the effectiveness of prehospital treatment with continuous positive airway pressure in acute decompensated heart failure. Eur J Emerg Med. 2016; 23:50–55 [DOI] [PubMed] [Google Scholar]
- 21.Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, et al. ; Mechanical Ventilation International Study Group. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002; 287:345–355 [DOI] [PubMed] [Google Scholar]
- 22.Hensel M, Strunden MS, Tank S, Gagelmann N, Wirtz S, Kerner T. Prehospital non-invasive ventilation in acute respiratory failure is justified even if the distance to hospital is short. Am J Emerg Med. 2019; 37:651–656 [DOI] [PubMed] [Google Scholar]
- 23.Plaisance P, Pirracchio R, Berton C, Vicaut E, Payen D. A randomized study of out-of-hospital continuous positive airway pressure for acute cardiogenic pulmonary oedema: physiological and clinical effects. Eur Heart J. 2007; 28:2895–2901 [DOI] [PubMed] [Google Scholar]
- 24.Goldraich L, Austin PC, Zhou L, Tu JV, Schull MJ, Mak S, et al. Care setting intensity and outcomes after emergency department presentation among patients with acute heart failure. J Am Heart Assoc. 2016; 5:e003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013; 128:1810–1852 [DOI] [PubMed] [Google Scholar]


