Abstract
We have created a bacterial semi-synthetic organism (SSO) that retains an unnatural base pair (UBP) in its DNA, transcribes it into mRNA and tRNA with cognate unnatural codons and anticodons, and after the tRNA is charged with a non-canonical amino acid, synthesizes proteins containing the non-canonical amino acid. Here, we report the first progress toward the creation of eukaryotic SSOs. After demonstrating proof-of-concept with human HEK293 cells, we show that a variety of different unnatural codon-anticodon pairs can efficiently mediate the synthesis of unnatural proteins in CHO cells. Interestingly, we find that there are both similarities and significant differences between how the prokaryotic and eukaryotic ribosomes recognize the UBP, with the eukaryotic ribosome appearing more tolerant. The results represent the first progress toward eukaryotic SSOs and in fact, suggest that such SSOs might be able to retain more unnatural information than their bacterial counterparts.
Graphical Abstract
INTRODUCTION
Every protein ever produced in a cell has been encoded with a four-letter, two-base-pair genetic alphabet. This generally restricts the composition of proteins to the canonical 20 proteogenic amino acids. While the natural genetic alphabet has allowed for the diversity of life, many potential functionalities are not available, and thus an expansion to include a means to encode non-canonical amino acids (ncAAs), including ones selected to provide a desired activity, might allow for the creation of novel proteins with improved properties, for applications ranging from materials to therapeutics. Efforts to incorporate ncAAs have mostly relied on expansion of the genetic alphabet via stop1 or four-letter codon2–3 suppression, although in these cases incorporation of the ncAA must compete with the codons’ natural functions. To circumvent this limitation, many efforts have focused on the synthesis of genomes with natural stop or rare codons eliminated, thus liberating them for reassignment to ncAAs.4–5 However, rare codons may potentially play important roles in the regulation of translation and protein folding,6 and genome synthesis is impractical as a general strategy, especially with large eukaryotic genomes.
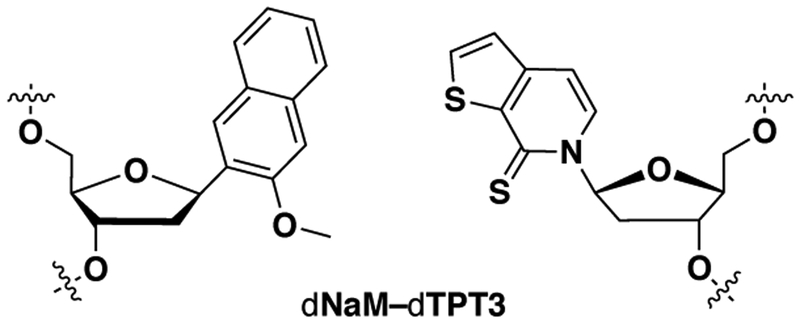
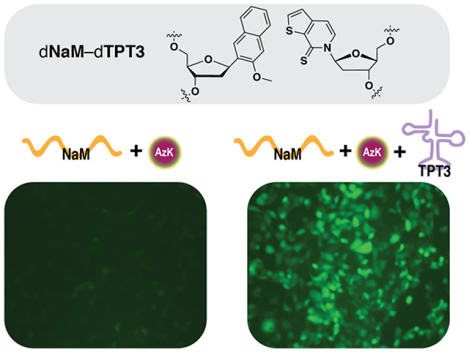
An alternative approach relies on the use of an unnatural base pair (UBP), which would allow for the creation of entirely new codons that are unencumbered by any natural function. By pursuing a medicinal chemistry-like approach,7 we developed a family of UBPs, typified by dNaM-dTPT3 (Figure 1), and used them as the basis of an E. coli semi-synthetic organism (SSO).8–9 The E. coli SSO stores the UBP in its genome or on a plasmid, transcribes it into mRNA and tRNA, and with the tRNA charged with an ncAA by an orthogonal synthetase,10–11 the SSO is able to translate proteins containing the ncAA.12–13 The E. coli SSO has important practical applications as it is currently being used to produce novel therapeutics.14 From a conceptual perspective, the success of the UBP and the SSO suggests that the natural nucleotides are not a unique solution to the challenge of information storage and retrieval; and because the UBPs form via hydrophobic and packing forces without the use of complementary hydrogen bonds, it further suggests that the forces used by nature may not be the only that are suitable. However, testing the generality of the results and harnessing the full potential of their implications requires further work, including exploration of SSOs generated from different organisms. Here, we report our first efforts toward the creation of eukaryotic SSOs.
Figure 1.
The dNaM-dTPT3 UBP.
RESULTS AND DISCUSSION
While separate efforts are underway to make the unnatural triphosphates intercellularly available, there is currently no evidence that the UBPs will be functional in eukaryotes. Thus, we started by exploring the translation of unnatural codons. To generate proof-of-concept data, we first sought to characterize protein production after direct, transient, triple transfection with mRNA containing an unnatural codon, tRNA containing a cognate unnatural codon, and DNA encoding an appropriate synthetase to charge the tRNA with a ncAA. The specific components used were an mRNA that encoded EGFP with an unnatural codon at position 151 (EGFP151(NXN); where N refers to one of the natural nucleotides and X refers to NaM), the Methanosarcina mazei tRNAPyl recoded with a cognate unnatural anticodon (tRNAPyl(NYN), where Y refers to TPT3), and the chimeric Methanosacarcina barkeri pyrrolysyl-tRNA synthetase (ChPylRS) which can charge the unnatural tRNAPyl with N6-(2-az-idoethoxy)-carbonyl-l-lysine (AzK)10–11,15
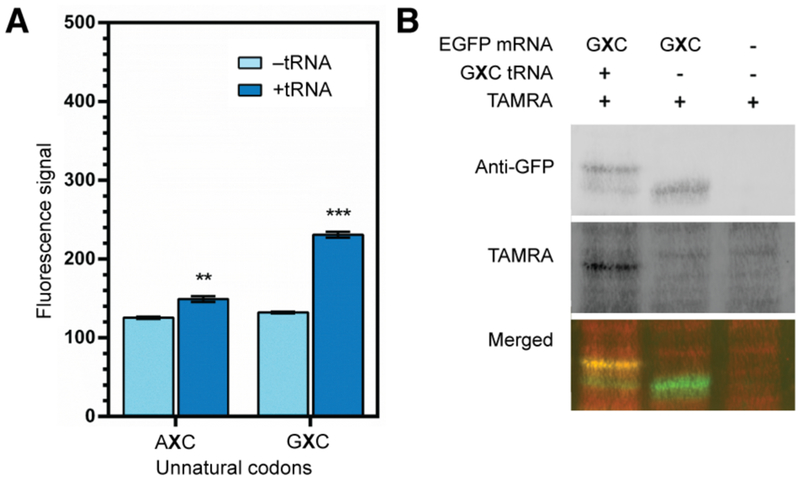
As evaluating translation required successful triple transfection, we performed these proof-of-concept experiments with HEK293T cells due to their high transfection efficiency.16 Plasmids encoding EGFP(AXC)151 and EGFP(GXC)151 were constructed with CS2 3’ and 5’ UTR sequences flanking the coding sequence to enhance mRNA stability.17–18 The codons AXC and GXC were chosen as they have been shown to be decoded well in the E. coli SSO.13,15 The desired mRNAs and cognate tRNAs were produced by in vitro transcription reactions using T7 RNA polymerase. ChPylRS was introduced on a plasmid (pcDNA3.1_C211_IRES_mCher-ry) harboring a bicistronic sequence encoding both ChPylRS and the mCherry marker connected by an internal ribosome binding site. HEK293T cells were transfected with this plasmid when they reached 50% confluence. Cells were grown for 24 h to allow for the expression of the ChPylRS, and then AzK was added to the growth medium and cells were transfected with mRNA only, as a control, or mRNA and the corresponding cognate unnatural tRNA together. Cells were harvested after an additional 24 h and EGFP production in cells expressing the mCherry marker was quantified via flow cytometry. In controls without tRNA, transfection with EGFP(AXC)151 and EGFP(GXC)151 mRNA resulted in detectable levels of EGFP, presumably resulting from readthrough of the unnatural codons when their cognate tRNAs were absent. In contrast, cells transfected with both unnatural mRNA and cognate unnatural tRNA exhibited increased fluorescence. While the increase was modest with EGFP(AXC)151, it was more significant with EGFP(GXC)151 (Figure 2).
Figure 2. Translation of unnatural codons in HEK293T cells.
A. Average (± s.d.; n = 3) EGFP fluorescence signal (total fluorescence signal divided by the number of EGFP-positive cells) of HEK293T cells transfected with unnatural codons with or without cognate tRNAs measured by flow cytometry. *P < 0.05, **P < 0.005, ***P < 0.0005 (two-tailed paired t test). B. The protein shift assay for HEK293T cells transfected with unnatural codon GXC using cell lysate. Representative gels from 3 biological replicates.
Based on the relatively larger tRNA-dependent increase in fluorescence, we examined protein produced with the EGFP(GXC)151 construct. Total cell lysate was subjected to strain-promoted click chemistry to attach a carboxy-tetramethyl-rhodamine (TAMRA) dye (DBCO-TAMRA) to incorporated AzK moieties, which we have shown shifts the electrophoretic mobility of EGFP as analyzed by SDS-PAGE and thus enables an assessment of the fidelity of AzK incorporation by western blotting.13,19 Despite relatively low protein yield and significant background due to non-specific TAMRA labeling, a distinct EGFP signal was apparent, corresponding to approximately 70% of the total EGFP produced, with cells transfected with the synthetase plasmid, EGFP(GXC)151 mRNA, and tRNAPyl(GYC), and grown in medium supplemented with AzK (Figure 2). In contrast, little to no shifted band was observed in lysate prepared from cells transfected without cognate unnatural tRNAs. While the low expression level of EGFP precluded further characterization, these data strongly suggest that AzK is incorporated into EGFP through decoding of the unnatural codons using tRNAs with the cognate unnatural anticodon.
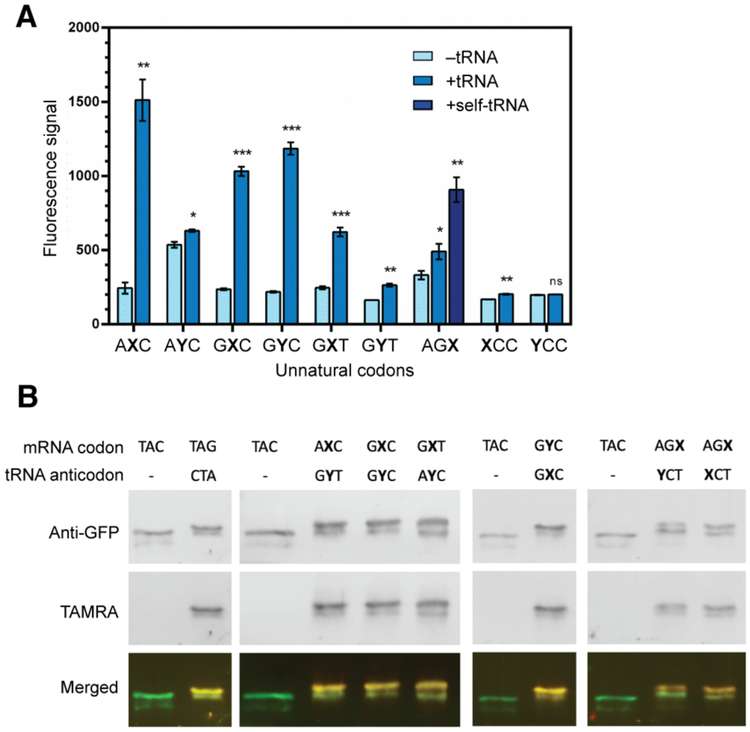
With proof-of-concept unnatural codon-directed ncAA incorporation in HEK293T cells, we turned our attention to CHO cells, which are more commonly used for protein production and typically produce higher yields of recombinant protein than HEK293T cells.20 As CHO cells are less efficiently transfected, we constructed a heterogeneous CHO cell line (CHO-KS3) that stably expresses ChPylRS using the FRT/Flp recombination system, thus reducing transfection to a single RNA co-transfection step. CHO-KS3 cells were transfected with EGFP(AXC)151, EGFP(GXC)151, or EGFP(GXT)151 mRNA, and the cognate tRNA; and AzK was added to the growth medium when cells reached 80% confluence. Cells were harvested after a one-day incubation and then directly subjected to flow cytometry to detect EGFP fluorescence. Control cells not provided with a cognate unnatural tRNA exhibited similar low but detectable levels of EGFP signal, again presumably resulting from readthrough of the unnatural codons by “near cognate” natural tRNAs. In contrast, cells transfected with cognate unnatural tRNAs exhibited significantly increased fluorescence, with EGFP(AXC)151 producing the most fluorescence per cell and EGFP(GXT)151 producing the least. The level of fluorescence observed with both EGFP(AXC)151 and EGFP(GXC)151 was higher than that observed with HEK293T cells (Figure 3).
Figure 3. Translation of unnatural codons in CHO cells.
A. Average EGFP fluorescence signal (total fluorescence signal divided by the number of EGFP-positive cells) of CHO cells transfected with unnatural codons with or without cognate tRNAs (and self-pairing tRNA for codon AGX) measured by flow cytometry. *P < 0.05, **P < 0.005, ***P < 0.0005 (two-tailed paired t test). B. The protein shift assay for CHO cells transfected with indicated components. Representative gels from 3 biological replicates.
We began our efforts with the NaM codons described above, because they are well translated by the E. coli ribosome; in contrast, among 25 codons tested, no TPT3 codons have been found to be well tolerated by the E. coli ribosome.15 To generate comparative structure-activity relationships between the prokaryotic and eukaryotic ribosomes, CHO-KS3 cells were transfected with EGFP(AYC)151, EGFP(GYC)151 or EGFP(GYT)151, as well as their cognate unnatural tRNAs tRNAPyl(GXT), tRNAPyl(GXC) or tRNAPyl(AXC). In contrast to the E. coli SSO, all three TPT3 codons resulted in increased fluorescence when CHO-KS3 cells were transfected with their cognate tRNAs compared to the controls transfected without tRNAs, and in fact, EGFP(GYC)151 achieved a level of fluorescence similar to that observed with the analogous NaM codon (GXC) (Figure 3).
With higher EGFP expression levels in CHO-KS3 cells, we subjected EGFP(AXC)151, EGFP(GXC)151, EGFP(GXT)151, and EGFP(GYC)151 to additional, quantitative analyses. EGFP was affinity-purified from cell lysates using a tandem C-terminal Strep-tag II and subjected to click chemistry with the DBCO-TAMRA dye, as described above. Purified EGFP was then analyzed by western blotting. From control cells transfected with natural EGFP mRNA, a dominant band was observed with a faster migrating, weaker band (Figure 3). The faster migrating band was attributed to partial Strep tag degradation21 (Figure S1). As expected, neither band showed any TAMRA signal. With transfection of each unnatural mRNA with their cognate tRNA, a similar set of bands was observed, but both were shifted and showed a clear TAMRA signal. These results suggest that in CHO cells, AzK is incorporated into EGFP through decoding either NaM or TPT3 codons with cognate unnatural anticodons.
To confirm the correct encoding of AzK, we used liquid chromatography-tandem mass spectrometry (LC-MS/MS) to analyze protein purified from CHO-KS3 cells transfected with either EGFP(GXC)151 or EGFP(GYC)151 mRNA and their cognate tRNAs. EGFP was purified from transfected cells as described above and then subjected to copper-catalyzed click chemistry to attach a 3-butynylbenzene moiety to AzK, to facilitate MS analysis. The reaction product was purified via SDS-PAGE, and the gel between 25 kDa and 32 kDa was excised, as this region includes both shifted and unshifted EGFP bands. Proteins recovered from the gel slices were digested with trypsin and subjected to nano-LC-MS/MS analysis. Peptide fragments containing the 151st amino acid were detected with masses corresponding to the click reaction product, confirming the specific incorporation of AzK at site 151 (Figure S2). Unmodified peptide was not detected, and while not quantitative, the LC-MS/MS results confirm the incorporation of AzK and suggest that it occurs with high fidelity. While a more thorough sequence context analysis remains to be explored, these data demonstrate that mammalian ribosomes, unlike their E. coli counterparts, are able to decode at least some unnatural codons containing TPT3.
Previously, we showed that an E. coli SSO is also able to translate several codons with the unnatural nucleotide NaM at the third position, including the codon AGX.15 However, in contrast to the second position, decoding occurred with either the “hetero-pairing” tRNAPyl(YCT) or the “self-pairing” tRNAPyl(XCT).NaM-NaM self-pairing at the third position may be facilitated in a fashion similar to wobble-pairing of natural codons. To explore the decoding of unnatural third position codons in mammalian cells, we next tested the AGX codon in the same mRNA context. CHO-KS3 cells were transfected with EGFP(AGX)151 mRNA alone, or co-transfected along with tRNAPyl(YCT) or tRNAPyl(XCT). As in our analysis of the second position unnatural codons, flow cytometry revealed a small amount of readthrough EGFP expression with cells transfected without tRNA. Co-transfecting with tRNAPyl(YCT) resulted in a significant increase in fluorescence, while co-transfecting with tRNAPyl(XCT), the self-pairing tRNA, resulted in an even greater increase in fluorescence (Figure 3). We then used the same protein shift assay described above to further assess the expressed EGFP. Shifted bands were detected in protein purified from cells co-transfected with either tRNAPyl(YCT) or tRNAPyl(XCT) (Figure 3). In both cases, the two shifted bands were again observed, with little to no unshifted band visible. These results demonstrate that with the AGX codon, decoding via either hetero-pairing or self-pairing is at least reasonably efficient.
To further compare the prokaryotic and eukaryotic ribosomes, we explored the translation of codons with an unnatural nucleotide in the first position, which the E. coli ribosome appears unable to decode. EGFP(XCC)151 and EGFP(YCC)151 mRNA were produced in vitro and transfected into CHO-KS3 cells without or with their cognate unnatural tRNA, tRNAPyl(GGY) or tRNAPyl(GGX), respectively. Analysis using flow cytometry indicated a small amount of readthrough when no tRNAs were added in both cases, with EGFP(YCC)151 exhibiting more readthrough than EGFP(XCC)151. When the corresponding tRNAs were added, little to no increase in EGFP signal was observed with either EGFP(XCC)151 or EGFP(YCC)151 (Figure 3). In both cases, EGFP yields were too low for western blot analysis. These data suggest that, as with the E. coli ribosome, first position unnatural codons are not well decoded. This is likely due to the type I A-minor interaction whereby the ribosome strictly selects for a Watson-Crick-like structure at the first position of the codon.22–23
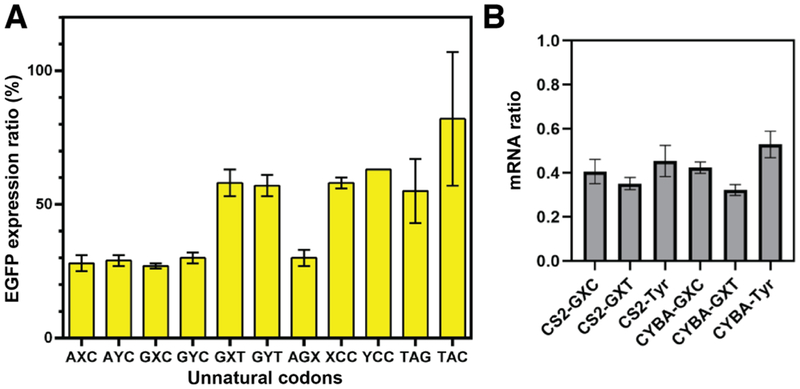
To begin to explore the optimization of unnatural protein production, we next examined the use of alternate 5’ and 3’ UTRs. The combined use of CYBA 5’ and 3’ UTRs have been reported to increase protein production from human cells, while not affecting stability.24 We thus constructed EGFP sequences with all 9 unnatural codons tested above with the CS2 UTRs replaced with CYBA UTRs (CYBA-EGFP(NX/YN)151). CHO-KS3 cells were transfected with these mRNAs without or with a cognate unnatural tRNA. The cells were then analyzed via flow cytometry and the results were compared to their counterparts with CS2 UTRs (Figure 4). The data indicated that in all cases, less protein was produced with the CYBA UTRs than with their CS2 counterparts. For CYBA-EGFP(GXC)151 and CYBA-EGFP(GYC)151 transfected cells, we also assessed unnatural codon decoding fidelity using the gel shift assay as described above. The shifts observed were similar to those observed with the CS2 UTR counterparts (EGFP(GXC)151 and EGFP(GYC)151), respectively (Figure S3), suggesting that the decoding fidelity is not significantly UTR-dependent.
Figure 4. Protein expression ratio between mRNA with CYBA UTRs and mRNA with CS2 UTRs.
A. Ratio of average (± s.d.; n = 3) EGFP fluorescence signal observed with CYBA and CS2 UTRs. B. Ratio of mRNA abundance at 4 and 8 h post-transfection as measured by RT-qPCR.
While the reduced level of expression observed with the CYBA UTRs may be due to the use of hamster cells instead of human cells, the magnitude of the effect, quite unexpectedly, was significantly different among the different unnatural codons. When decoding with their cognate unnatural tRNAs (the self-pairing tRNA was used with the AGX codon), the XCC, YCC, GXT, and GYT codons with CYBA UTRs exhibited expression levels that were ~60% of their CS2 counterparts, while expression levels with the AXC, AYC, GXC, GYC, and AGX codons with CYBA UTRs were only ~30% of their CS2 counterparts (Figures 4 and S3). Comparing the performance of the unnatural codons to the amber construct CYBA-EGFP(TAG)151 and natural construct CYBA-EGFP(TAC)151 used as controls, CYBA-EGFP(TAG)151 and CYBA-EGFP(TAC)151 exhibited expression levels that were ~60% and ~80% of their CS2 UTR counterparts.
To test whether the observed unnatural codon-dependent UTR effect may have originated from differences in mRNA stability, we compared the level of mRNA at 8 h post-transfection to that at 4 h post-transfection for EGFP(TAC)151, EGFP(GXC)151, EGFP(GXT)151, CYBA-EGFP(TAC)151, CYBA-EGFP(GXC)151 and CYBA-EGFP(GXT)151 using reverse transcription coupled with quantitative PCR (see Supporting Information for details). The differences observed in degradation among these different constructs do not account for the differences in protein expression, and thus other factors must be responsible. One way that UTRs are thought to affect translation is by regulating ribosome recruitment efficiency.17–18 However, it is difficult to rationalize how this could affect the translation of a codon that is far removed from either the 5’ or 3’ UTR (in this case by at least 350 nt). While little is known about the CS2 and CYBA UTRs, neither is thought to adopt a structured fold known to recruit ribosomes.25 Nonetheless, it is interesting to speculate that the UTRs may help recruit different subpopulations of ribosomes, as multiple ribosome subpopulations are known to exist in a single cell,26 and may, for example, be differentiated by variable translation elongation efficiencies.27 Unlike with the translation of natural codons, this could in principle have a more significant effect on how the ribosome handles different unnatural codons, perhaps similar to our observation that ribosomes from prokaryotes and eukaryotes decode different unnatural codons differently. Further experiments are required to test this fascinating possibility.
CONCLUSION
We have demonstrated that unnatural codons may be decoded with at least reasonable efficiency and fidelity in both HEK293T and CHO cells. Interestingly, recognition by the eukaryotic ribosomes shows both similarities and differences with recognition mediated by the E. coli ribosome. First position codons XCC and YCC cannot be efficiently decoded in either E. coli or CHO cells. While second position NaM codons appear to be efficiently decoded in both organisms, second position TPT3 codons can only be decoded in CHO cells. Finally, the third position codons appear to be decoded in both E. coli and CHO cells by both their cognate hetero-pairing tRNA as well as their non-cognate self-pairing tRNA. Thus, while recognition at the first and third positions of the codon appears to be similar, the prokaryotic and eukaryotic ribosomes show distinctly different recognition at the second position. In addition to illuminating interesting differences between the ribosomes, the data suggest that eukaryotic SSOs may be inherently able to store more information than their bacterial counterparts. Future studies will be directed at further elucidation of the different structure-activity relationships governing UBP recognition by prokaryotic and eukaryotic ribosomes, including the potential role of UTRs. In addition to elucidating fundamental aspects of biology, along with ongoing efforts to make unnatural triphosphates available within eukaryotic cells and to explore their use in replication and transcription, these results represent the first progress towards eukaryotic SSOs with important practical and conceptual implications.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health (GM118178 to F.E.R.). A.W.F. was supported by a National Science Foundation Graduate Research Fellowship (NSF/DGE-1346837). The authors thank Dr. James Paulson for gifting Flip-in CHO-K1 cells, Dr. Joel Gottesfeld for use of cell culture resources, the Scripps Center for Metabolomics and Linh Hoang for facilitating Nano-LC-MS/MS analyses, and the Scripps Automated Synthesis Facilities and Dr. Jason Chen for facilitating quantitative high-resolution MS analyses.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Methods, Sequence information (Tables 1–2) and plasmid maps, Schemes S1–S2, Figures S1–S5 (PDF).
The authors declare the following competing financial interests: a patent application has been filed based on the use of UBPs in SSOs and F.E.R. has a financial interest (shares) in, and a consulting agreement with, Synthorx, Inc., a company that has commercial interests in the UBP.
REFERENCES
- (1).Xiao H; Schultz PG, At the Interface of Chemical and Biological Synthesis: An Expanded Genetic Code. Cold Spring Harb Perspect. Biol 2016, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Anderson JC; Wu N; Santoro SW; Lakshman V; King DS; Schultz PG, An Expanded Genetic Code with a Functional Quadruplet Codon. Proc. Natl. Acad. Sci. USA 2004, 101, 7566–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Neumann H; Wang K; Davis L; Garcia-Alai M; Chin JW, Encoding Multiple Unnatural Amino Acids via Evolution of a Quadruplet-Decoding Ribosome. Nature 2010, 464, 441–444. [DOI] [PubMed] [Google Scholar]
- (4).Fredens J; Wang K; de la Torre D; Funke LFH; Robertson WE; Christova Y; Chia T; Schmied WH; Dunkelmann DL; Beranek V;Uttamapinant C; Llamazares AG; Elliott TS; Chin JW, Total Synthesis of Escherichia coli with a Recoded Genome. Nature 2019, 569, 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Italia JS; Addy PS; Wrobel CJ; Crawford LA; Lajoie MJ; Zheng Y; Chatterjee A, An Orthogonalized Platform for Genetic Code Expansion in both Bacteria and Eukaryotes. Nat. Chem. Biol 2017, 13, 446–450. [DOI] [PubMed] [Google Scholar]
- (6).Chaney JL; Clark PL, Roles for Synonymous Codon Usage in Protein Biogenesis. Annu. Rev. Biophys 2015, 44, 143–166. [DOI] [PubMed] [Google Scholar]
- (7).Feldman AW; Dien VT; Romesberg FE, Chemical Stabilization of Unnatural Nucleotide Triphosphates for the in Vivo Expansion of the Genetic Alphabet. J. Am. Chem. Soc 2017, 139, 2464–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Malyshev DA; Dhami K; Lavergne T; Chen T; Dai N; Foster JM; Correa IR Jr.; Romesberg FE, A Semi-Synthetic Organism with an Expanded Genetic Alphabet. Nature 2014, 509, 385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Zhang Y; Lamb BM; Feldman AW; Zhou AX; Lavergne T; Li L; Romesberg FE, A Semisynthetic Organism Engineered for the Stable Expansion of the Genetic Alphabet. Proc. Natl. Acad. Sci. USA 2017, 114, 1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Nguyen DP; Lusic H; Neumann H; Kapadnis PB; Deiters A; Chin JW, Genetic Encoding and Labeling of Aliphatic Azides and Alkynes in Recombinant Proteins via a Pyrrolysyl-tRNA Synthetase/tRNA(CUA) Pair and Click Chemistry. J. Am. Chem. Soc 2009, 131, 8720–8721. [DOI] [PubMed] [Google Scholar]
- (11).Chatterjee A; Sun SB; Furman JL; Xiao H; Schultz PG, A Versatile Platform for Single- and Multiple-Unnatural Amino acid Mutagenesis in Escherichia coli. Biochemistry 2013, 52, 1828–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ledbetter MP; Karadeema RJ; Romesberg FE, Reprograming the Replisome of a Semisynthetic Organism for the Expansion of the Genetic Alphabet. J. Am. Chem. Soc 2018, 140, 758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Zhang Y; Ptacin JL; Fischer EC; Aerni HR; Caffaro CE; San Jose K; Feldman AW; Turner CR; Romesberg FE, A Semi-synthetic Organism that Stores and Retrieves Increased Genetic Information. Nature 2017, 551, 644–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Synthorx Inc. www.synthorx.com.
- (15).Fischer EC; Hashimoto K; Zhang Y; Feldman AW; Dien VT; Karadeema RJ; Adhikary R; Ledbetter MP; Krishnamurthy R; Romesberg FE, New Codons for the Efficient Production of Unnatural Proteins in a SemiSynthetic Organism. Nat. Chem. Biol. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Pear WS; Nolan GP; Scott ML; Baltimore D, Production of High-Titer Helper-Free Retroviruses by Transient Transfection. Proc. Natl. Acad. Sci. USA 1993, 90, 8392–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Mayr C, Regulation by 3’-Untranslated Regions. Annu. Rev. Genet 2017, 51, 171–194. [DOI] [PubMed] [Google Scholar]
- (18).Leppek K; Das R; Barna M, Functional 5’ UTR mRNA Structures in Eukaryotic Translation Regulation and How to Find Them. Nat. Rev. Mol. Cell. Biol 2018, 19, 158–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Jewett JC; Sletten EM; Bertozzi CR, Rapid Cu-free Click Chemistry with Readily Synthesized Biarylazacyclooctynones. J. Am. Chem. Soc 2010, 132, 3688–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wurm FM, Production of Recombinant Protein Therapeutics in Cultivated Mammalian Cells. Nat. Biotechnol 2004, 22, 1393–1398. [DOI] [PubMed] [Google Scholar]
- (21).Kittur FS; Lalgondar M; Hung CY; Sane DC; Xie J, C-Terminally Fused Affinity Strep-tag II is Removed by Proteolysis from Recombinant Human Erythropoietin Expressed in Transgenic Tobacco Plants. Plant Cell Rep 2015, 34, 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Rozov A; Westhof E; Yusupov M; Yusupova G, The Ribosome Prohibits the G*U wobble Geometry at the First Position of the Codon-Anticodon Helix. Nucleic Acids Res 2016, 44, 6434–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ogle JM; Brodersen DE; Clemons WM Jr.; Tarry MJ; Carter AP; Ramakrishnan V, Recognition of Cognate Transfer RNA by the 30S Ribosomal Subunit. Science 2001, 292, 897–902. [DOI] [PubMed] [Google Scholar]
- (24).Ferizi M; Aneja MK; Balmayor ER; Badieyan ZS; Mykhaylyk O; Rudolph C; Plank C, Human Cellular CYBA UTR Sequences Increase mRNA Translation without Affecting the Half-life of Recombinant RNA Transcripts. Sci. Rep 2016, 6, 39149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ferizi M; Leonhardt C; Meggle C; Aneja MK; Rudolph C; Plank C; Radler JO, Stability Analysis of Chemically Modified mRNA Using Micropattern-Based Single-Cell Arrays. Lab Chip 2015, 15, 3561–3571. [DOI] [PubMed] [Google Scholar]
- (26).Xue S; Barna M, Specialized Ribosomes: a New Frontier in Gene Regulation and Organismal Biology. Nat. Rev. Mol. Cell. Biol 2012, 13, 355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Kurylo CM; Parks MM; Juette MF; Zinshteyn B; Altman RB; Thibado JK; Vincent CT; Blanchard SC, Endogenous rRNA Sequence Variation Can Regulate Stress Response Gene Expression and Phenotype. Cell Rep 2018, 25, 236–248 e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.