Abstract
In patients with diabetes, where cardiovascular morbidity is highly prevalent, recent cardiovascular outcomes trials have identified therapies in the modern glucagon-like peptide 1 receptor agonist (GLP-1RA) and sodium-glucose cotransporter 2 inhibitor (SGLT2i) classes that significantly reduce cardiovascular events. A number of drugs in both classes have demonstrated reductions in the risk of the composite outcome of major adverse cardiovascular events (myocardial infarction, stroke, and cardiovascular death). In addition, SGLT2i drugs have a substantial impact on hospitalization for heart failure. Because GLP-1RA and SGLT2i are effective in reducing cardiovascular events, independent of their effects on blood glucose, cardiologists should be familiar with how to use them. This review outlines the evidence of cardiovascular benefit for current GLP-1RA and SGLT2i drugs, practical information for prescribing them, and putative mechanisms, so that these therapies can be incorporated along with antihypertensives, statins, and antiplatelet therapies into the routine care of patients.
Keywords: diabetes, atherosclerotic cardiovascular disease, glucagon-like peptide 1 receptor antagonists, sodium-glucose cotransporter 2 inhibitors
Introduction
Diabetes is a well established risk factor for a broad spectrum of cardiovascular diseases, estimated to account for 12–15% attributable risk of myocardial infarction (MI) [1] and stroke [2], as well as nonatherosclerotic cardiovascular disease, including a substantial risk for clinical heart failure [3,4] and atrial fibrillation [5,6]. Cardiovascular disease remains the leading cause of morbidity and mortality in patients with diabetes; approximately 7–10% of global mortality can be linked to diabetes [7,8].
While epidemiologic studies describe an association between glycemia and cardiovascular morbidity and mortality in patients with diabetes [9], large trials of glucose lowering therapies designed to test the effects of tight glucose control on rates of MI, stroke, or cardiovascular death either did not show benefit [10,11] or were stopped early because of evidence of an increased risk of mortality [12]. These data, as well as the results of a meta-analysis that suggested an increased risk of MI and cardiovascular death with rosiglitazone, a thiazolidinedione [13], prompted the FDA to require that all new diabetes drugs and biologics exclude excess cardiovascular risk before approval [upper bound of the 95% confidence interval (CI) <1.8]. Postapproval, new drugs and biologics were required meet a more stringent standard to exclude a 30% increase in risk of atherosclerotic cardiovascular disease (ASCVD) (upper bound of the 95% CI < 1.3) [14].
While these regulatory changes altered the drug development pipeline, clinical cardiologists focused on aggressive lipid and blood pressure reduction, antiplatelet therapies, smoking cessation, weight loss, and physical activity to reduce the burden of cardiovascular disease in patients with diabetes [15,16].
Cardiovascular risk reduction and glucose reduction
Three major classes of medications for the treatment of diabetes were approved after the 2008 U.S. Food and Drug Administration (FDA) guidance and thus had their cardiovascular impact evaluated in large cardiovascular outcome trials. The dipeptidyl peptidase-4 inhibitors (DPP4is) did not show cardiovascular benefit, and though cardiovascular safety has generally been confirmed, saxagliptin showed evidence of an increased risk of hospitalization for heart failure [17]. In contrast, a number of drugs from the glucagon-like peptide 1 receptor agonist class (GLP-1RA) and sodium-glucose cotransporter 2 inhibitor class (SGLT2i) were shown not only to have acceptable cardiovascular safety profiles in primary analyses but subsequently also to reduce major adverse cardiovascular events (MACE) independent of their effects on glycemia. These drugs are leading a paradigm shift in the care for patients with diabetes, providing cardiologists and other clinicians with the opportunity not only to lower blood glucose but in fact to reduce hard cardiovascular outcomes.
We believe that the magnitude of the effects of these medications on major cardiovascular events means that cardiologists need to consider prescribing them in eligible patients, yet in two recent analyses of first-time GLP-1RA and SGLT2i prescriptions across a large tertiary healthcare system, only 4.5% of GLP-1RA prescriptions and 5.1% of SGLT2i prescriptions were written by cardiologists [18,19]. Therefore, the purpose of this review is to provide cardiologists with a practical guide to the use of novel cardioprotective diabetes therapies.
Glucagon-like peptide 1 receptor agonist classes
Mechanism of action
GLP-1 is an incretin peptide hormone released from the distal ileum and colon after enteral intake. GLP-1 receptor activation then stimulates insulin release, inhibits glucagon secretion, slows gastrointestinal transit, and suppresses appetite [20]. Analogs of human GLP-1 have been approved for treatment of type 2 diabetes [21].
Members of the GLP-1RA class that have demonstrated cardiovascular risk reduction in randomized trials include subcutaneous (SC) liraglutide, semaglutide albiglutide, once weekly exenatide, dulaglutide, and oral semaglutide [22–27]. Once daily lixisenatide did not demonstrate a significant reduction in MACE [28]. Prescribing details are outlined in Table 1a and 1b.
Table 1A.
Doses, dose adjustments, cautions and contraindications for glucagon-like peptide 1 receptor agonists
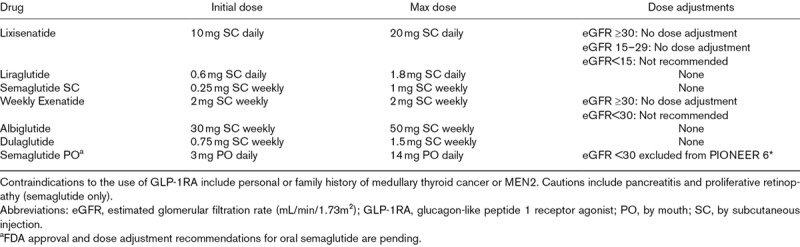
Table 1B.
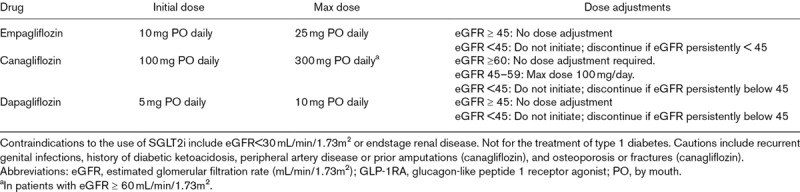
Doses, dose adjustments, cautions and contraindications for sodium-glucose cotransporter 2 inhibitors
Cardiovascular outcomes trials
In trials initially designed to test first cardiovascular safety and then efficacy, GLP-1RAs have demonstrated noninferiority, and in some cases superiority, compared with placebo for the typical atherothrombotic outcome of 3-point MACE, a composite of nonfatal MI, nonfatal stroke, or cardiovascular death [22–27].
The LEADER trial (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) randomized 9340 patients with type 2 diabetes to liraglutide SC or placebo and followed for a median of 3.8 years for 3-point MACE [22]. The study population included both primary and secondary prevention, though the large majority had established cardiovascular disease (72.4% with CVD alone, 15.8% with CVD and at least stage 3 chronic kidney disease). Treatment with liraglutide resulted in a significant reduction in MACE (Table 2) as well as cardiovascular mortality (HR 0.78; 95% CI 0.66–0.93) and overall mortality (HR 0.85; 95% CI 0.74–0.97).
Table 2.
Summary of the published cardiovascular outcomes trials for GLP-1RA and SGLT2i drugs.
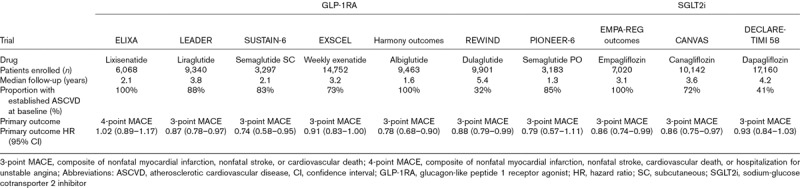
In the SUSTAIN-6 trial (Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes), 3297 patients with type 2 diabetes were randomized to once weekly semaglutide SC or placebo and were followed for a median of 2.1 years. The primary composite outcome was 3-point MACE, and the majority of the patients (83%) had established cardiovascular disease or chronic kidney disease [23]. Semaglutide was noninferior to placebo for reduction of the primary endpoint (HR 0.74, 95% CI 0.58–0.95; Table 2), with a significantly lower risk of nonfatal stroke (HR 0.61; 95% CI 0.38-0.99) and a nonsignificant trend toward fewer nonfatal MIs with semaglutide compared with placebo [23].
The Harmony outcomes trial (A Long-Term, Randomized, Double Blind, Placebo-controlled Study to Determine the Effect of Albiglutide, When Added to Standard Blood Glucose Lowering Therapies, on Major Cardiovascular Events in Patients With Type 2 Diabetes Mellitus) randomized 9463 exclusively secondary prevention patients with type 2 diabetes to once weekly albiglutide SC or placebo [24]. At baseline, all patients had coronary, peripheral, or cerebrovascular disease, and 20% had premorbid heart failure. Treatment with albiglutide resulted in a 22% reduction in 3-point MACE, meeting not only noninferiority for cardiovascular safety but also superiority for efficacy at a median follow-up of 1.6 years (Table 2) [24]. Of note, the addition of SGLT-2 inhibitors was more common in the placebo group, due to lesser glucose control, and may have biased the results toward the null [24].
The EXSCEL trial (Exenatide Study of Cardiovascular Event Lowering) randomized 14 752 patients with type 2 diabetes (73.1% with previous cardiovascular events) to once weekly extended-release exenatide SC or placebo to test noninferiority for safety and superiority for 3-point MACE [25]. Exenatide was noninferior to placebo for safety but not superior for efficacy (Table 2) [25].
The recently published REWIND trial (Researching Cardiovascular Events With a Weekly Incretin in Diabetes) randomized 9901 patients with type 2 diabetes to dulaglutide or placebo, with 31.5% having baseline cardiovascular disease. Dulaglutide SC was superior to placebo for a primary outcome of 3-point MACE at 5.4 years of median follow-up (HR 0.88, 95% CI 0.79–0.99) and significantly reduced nonfatal stroke (HR 0.76, 95% CI 0.61–0.95) [26].
Also recently published was the first cardiovascular outcomes trial of an oral GLP-1RA. The PIONEER 6 trial (Peptide Innovation for Early Diabetes Treatment) randomized 3183 patients to daily oral semaglutide or placebo. The majority (84.7%) had cardiovascular or chronic kidney disease at baseline. Oral semaglutide met noninferiority for the primary outcome of 3-point MACE (HR 0.79, 95% CI 0.57–1.11) [27].
Unlike other cardiovascular outcomes trials of GLP-1RAs, which studied patients with stable cardiovascular disease or elevated risk, the ELIXA trial (Evaluation of Lixisenatide in Acute Coronary Syndrome) examined the impact of lixisenatide on patients with recent acute coronary syndrome. Six thousand sixty-eight patients with type 2 diabetes and an MI or hospitalization for unstable angina in the previous 180 days were randomized to lixisenatide or placebo with a primary outcome of nonfatal MI, nonfatal stroke, cardiovascular death, or urgent revascularization (4-point MACE). The primary outcome showed noninferiority but not superiority of lixisenatide compared with placebo (Table 2) [28].
Prior to the publication of the above Harmony Outcomes, REWIND, and PIONEER 6 trials, a meta-analysis of cardiovascular outcomes trials of GLP-1RAs examined the overall effects of this drug class. In this meta-analysis, which included 33, 457 participants, GLP-1RA treatment significantly reduced 3-point MACE (10% RRR, HR 0.90, 0.82–0.99, P = 0.033), cardiovascular mortality (13% RRR, HR 0.87, 0.79–0.96, P = 0.007), and all-cause mortality (12% RRR, HR 0.88, 0.81–0.95, P = 0.002) when compared with placebo, without any evidence of heterogeneity across trials [29].
Practical considerations
Currently approved GLP-1RAs are self-administered by SC injection, although oral formulations are being studied in ongoing trials [27,30]. Starting and target doses of approved GLP-1RAs are outlined in Table 1a and 1b. Prior to GLP-1RA initiation, baseline antiglycemic drug therapy should be examined for the presence of a DPP4i, which should be discontinued, or for background sulfonylurea or basal insulin, which should be reduced by 50 or 20%, respectively, in patients at elevated risk of hypoglycemia. To improve tolerability, especially of gastrointestinal side effects, slow up-titration from the lowest dose is recommended [31].
Adverse effects
Previous glucose-lowering trials of GLP-1RAs have identified common adverse effects, such as nausea, vomiting, and diarrhea, as well as rare serious events including pancreatitis and pancreatic cancer. The cardiovascular outcomes trials described above similarly identified gastrointestinal side effects associated with GLP-1RA treatment, but there was no significant difference detected in the rates of acute pancreatitis or pancreatic cancer in LEADER, SUSTAIN-6, Harmony Outcomes, EXSCEL, REWIND, or PIONEER 6 trials [22–27].
In addition, animal studies have suggested an increased risk of medullary thyroid cancers in rodent models [32]. For this reason, patients with a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2 were excluded from the LEADER, SUSTAIN-6, Harmony Outcomes, EXSCEL, REWIND, and PIONEER 6 trials. In these trials, GLP-1RA therapy did not increase the risk of medullary thyroid cancer [22–27].
Hypoglycemia, which has been associated with an increased risk of adverse cardiovascular events [11,12], can occur with GLP-1RAs when used in combination with insulin or sulfonylureas. However, in many of the cardiovascular outcomes trials, patients randomized to GLP-1RA had a numerically lower rate of hypoglycemic episodes [22,24]. In LEADER, patients treated with liraglutide were less likely to have the addition of insulin and thus had lower rates of severe hypoglycemia [22]. Patients treated with semaglutide in SUSTAIN-6 did experience higher rates of diabetic retinopathy (HR 1.76; 95% CI 1.11–2.78), perhaps due to marked and rapid reductions in glucose. This has not been observed in other GLP-1RA trials.
Putative mechanisms of cardiovascular benefit
GLP-1RA therapy appears to significantly reduce atherothrombotic cardiovascular events. Hypothesized mechanisms of cardiovascular benefits of GLP-1RAs include both their impact on cardiovascular risk factors, such as blood pressure, as well as direct effects via atherogenesis, inflammatory pathways, and endothelial function [33]. In fact, the primary cardiovascular benefits of GLP-1RAs appear to be largely independent of established atherosclerotic risk factors [29].
GLP-1RA treatment has demonstrated a consistent reduction in weight and blood pressure [34]. In the above cardiovascular outcomes trials, GLP-1RA treatment resulted in a small but significant impact on weight loss, ranging from 0.7 kg with lixisenatide to as much as 4.3 kg with semaglutide SC. Similarly, systolic blood pressure was reduced across the board, ranging from 1 mmHg with abiglutide to 3 mmHg with oral semaglutide [22–28].
In addition to relatively modest but favorable effects on classical cardiovascular risk factors, GLP-1 receptor expression has been identified in cardiovascular tissues, and GLP-1 peptides have been linked to reduction in reactive oxygen species in endothelial cells and cardiomyocytes [33]. Infusion of GLP-1 and GLP-1RA in human subjects has resulted in improvements in left ventricular function, hemodynamics, and reduction in ischemic injury [33].
Sodium-glucose cotransporter 2 inhibitors
Mechanism of action
The sodium-glucose cotransporter 2 is responsible for proximal renal tubular reabsorption of glucose. Inhibition of SGLT2 prevents glucose resorption, promoting excretion in the urine and thereby reducing circulating blood glucose levels, with a greater effect the higher the plasma glucose concentration and relative protection against hypoglycemia [35].
Members of the SGLT2i class that have demonstrated cardiovascular risk reduction in randomized trials include empagliflozin and canagliflozin, both of which reduced 3-point MACE, and dapagliflozin, which was noninferior for MACE and significantly reduced a composite of cardiovascular death or heart failure hospitalization [36–38]. Prescribing details are outlined in Table 1a and 1b.
Cardiovascular outcomes trials
The first cardiovascular outcomes trial to test an SGLT2i was the EMPA-REG OUTCOME trial (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients). Seven thousand twenty patients with type 2 diabetes and established cardiovascular disease were randomized to empagliflozin or placebo [36]. Treatment with empagliflozin was superior to placebo, resulting in a 14% reduction in 3-point MACE (Table 1a and 1b), driven by a 38% reduction in the risk of cardiovascular death (3.7 vs 5.9%; HR 0.62, 95% CI 0.49–0.77). The rates of heart failure were reduced by 35% in patients randomized to active empagliflozin (HR 0.65, 95% CI 0.50–0.85) [36], as were related endpoints including hospitalization or death from heart failure and investigator reported heart failure [39]. These observations have been replicated in other SGLT2i trials [37,38].
While 10.1% of study population had heart failure at baseline, effects of empagliflozin on heart failure outcomes were consistent across patients with and without baseline heart failure. Similarly, baseline heart failure medication use did not modify the impact of empagliflozin on outcomes [39].
In the CANVAS Program (CANVAS: Canagliflozin Cardiovascular Assessment Study and CANVAS-R: A Study of the Effects of Canagliflozin on Renal Endpoints in Adult Participants With Type 2 Diabetes Mellitus), a total of 10, 142 participants with type 2 diabetes were randomized to canagliflozin or placebo and followed for 3-point MACE for a median of 3.6 years [37]. The study population included both patients with a history of atherosclerotic vascular disease (72.2%) and those at elevated risk (27.8%), and 17.5% of participants had chronic kidney disease at baseline [37].
Compared with placebo, significantly fewer participants on canagliflozin reached the primary outcome of cardiovascular death, nonfatal MI, or nonfatal stroke (Table 1a and 1b). There was also a significant reduction in the risk for hospitalization for heart failure (HR 0.67; 95% CI 0.52–0.87).
In the DECLARE-TIMI 58 (Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58) Trial, 17, 160 patients with type 2 diabetes were randomized to dapagliflozin or placebo and were assessed for primary efficacy outcomes of 3-point MACE and a composite of cardiovascular death or heart failure hospitalization at a median follow-up of 4.2 years [38]. The study population included patients with established, clinically evident atherosclerotic vascular disease (40.6%) and those at elevated risk (59.4%). At baseline, 10% of enrolled patients had a history of heart failure [38].
Dapagliflozin met noninferiority for 3-point MACE (Table 1a and 1b) and reduced the primary efficacy composite endpoint, yielding a 17% decrease in the rate of cardiovascular death or heart failure hospitalization (4.9 vs 5.8%; P = 0.005) driven by a reduction in heart failure hospitalization (HR 0.73, 0.61–0.88) [38]. This benefit for heart failure hospitalization was observed in patients both with (7.8 vs 9.3%, HR 0.83, 0.71–0.98) and without established atherosclerotic cardiovascular disease (2.8 vs 3.4%, HR 0.84, 0.67–1.04; P = 0.99 for interaction).
Practical considerations
SGLT2is are oral daily medications that can be initiated with limited monitoring. Doses of the individual agents described above are outlined in Table 1a and 1b. In patients with well controlled HbA1c or a history of frequent hypoglycemia, recommended medication adjustments include a 50% reduction in sulfonylurea dose and a 20% reduction in basal insulin dose at the time of SGLT2i initiation [31]. If the patient is taking a DPP4i, the prescribing physician may decide to discontinue that medication first before starting the SGLT2i.
Because the mechanism of SGLT2i may induce some osmotic diuresis via glucosuria, consideration should be given to reduce doses of thiazide or loop diuretics at the time of SGLT2i initiation to avoid excessive diuresis and volume depletion [31].
Adverse effects
Due to their effect of increased urinary glucose excretion, SGLT2is are known to increase the risk of genital mycotic infections. In the EMPA-REG trial, genital infections and urosepsis were rare but more frequently reported in the empagliflozin group [36]. Similarly, in DECLARE-TIMI 58, treatment with dapagliflozin was associated with an increased risk of diabetic ketoacidosis (0.3 vs 0.1%, P = 0.02) and of genital infections leading to drug discontinuation (0.9 vs 0.1%, P < 0.001) [38].
Unique to CANVAS, canagliflozin was associated with an increased risk of amputation (HR 1.97; 95% CI 1.41–2.75), a safety signal not found in other SGLT2i trials to date and the basis of an FDA black box warning [37,40]. Similarly, fractures were more common with canagliflozin in the CANVAS trial; whereas, there was no increase in the rate of fracture with empagliflozin or dapagliflozin [36,38]. However, the CREDENCE trial, a recently published trial of canagliflozin in 4,401 patients with type 2 diabetes and chronic kidney disease did not show an increased risk of amputation or fractures [41].
Putative mechanisms of cardiovascular benefit
In contrast to GLP-1RAs, where the clinical benefits appear to be driven predominantly by a reduction in atherothrombotic endpoints, drugs in the SGLT2i class have demonstrated a prominent impact on heart failure in study populations where the majority of patients did not carry a heart failure diagnosis at baseline.
Several possible mechanisms underlying the benefits of SGLT2is on heart failure have been hypothesized, including direct effect on circulating volume through osmotic diuresis, reduction in typical risk factors including body weight and systolic blood pressure, and via proposed impacts on cardiovascular metabolism. Data from the EMPA-REG trial suggest that markers of plasma volume, specifically hematocrit and hemoglobin, not markers of glycemia, are the most important factors in determining the benefit of empagliflozin [42].
In DECLARE-TIMI 58, dapagliflozin mitigated multiple cardiovascular risk factors in addition to HbA1c, including incremental lowering of weight (1.8 kg), systolic blood pressure (3 mmHg), and diastolic blood pressure (<1 mmHg) [38], findings similar to those demonstrated with empagliflozin and canagliflozin [36,37].
Beyond inducing diuresis and generating modest reductions in weight and blood pressure, several direct mechanisms have been proposed for the reduction in risk of heart failure [43]. SGLT2is may alter myocardial metabolism and improve myocardial efficiency [44].
Special circumstances
Primary vs secondary atherosclerotic cardiovascular disease prevention
The majority of patients included in the existing cardiovascular outcomes trials of GLP-1RA and SGLT2i therapies had existing ASCVD at baseline, so data for patients without established ASCVD, or ‘primary prevention’, are scarce (Table 2). Absolute cardiovascular event rates in that population are lower, further limiting the ability to estimate the effects of GLP-1RAs and SGLT2is in patients without ASCVD. However, a recent meta-analysis that included data from the LEADER, SUSTAIN-6, Harmony Outcomes, EXSCEL, and ELIXA trials, suggested evidence of treatment heterogeneity. GLP-1RA treatment reduced MACE by 13% (HR 0.87, 95% CI 0.82–0.92) in patients with established ASCVD, whereas there was no benefit observed in patients without ASCVD at baseline (HR 1.03, 95% CI 0.87–1.23; P-interaction = 0.028) [45]. Similarly, in a meta-analysis that included data from the EMPA-REG, CANVAS, CANVAS-R, and DECLARE-TIMI 58 trials, SGLT2i reduced MACE by 14% in patients with baseline ASCVD (HR 0.86, 95% CI 0.80–0.93), but no benefit was observed in patients without ASCVD at baseline (HR 1.00, 95% CI 0.87–1.16; P-interaction = 0.0501) [46].
By contrast, SGLT2i reduced the risk of cardiovascular death or heart failure hospitalization by 23% (0.77, 95% CI 0.71–0.84), a benefit that was consistent regardless of the presence or absence of ASCVD or heart failure at baseline [46]. GLP-1RA treatment did not reduce the risk of heart failure hospitalization [45].
Chronic kidney disease
Chronic kidney disease (CKD) is a common complication of diabetes and a prevalent comorbidity in the cardiology clinic. Patients with CKD have elevated rates of MACE, including MI, stroke, heart failure, and cardiovascular death [47]. Patients with significant renal insufficiency, defined as an estimated glomerular filtration rate (eGFR) less than 30 mL/min/1.73 m2 of body surface area, were excluded from all of the above SGLT2i cardiovascular outcomes trials, with a more stringent cutoff of eGFR greater than 60 in the DECLARE-TIMI 58 Trial. By contrast, patients with eGFR less than 30 mg/min/1.73 m2 were eligible for LEADER, SUSTAIN-6, REWIND, and PIONEER 6 [22,23,26,27]. Although a minority of patients enrolled had a reduced eGFR, liraglutide was at least as effective, if not more so, among those with baseline renal dysfunction [22]. Similar observations have been seen with the SGLT2i efficacy for MACE [46].
Beyond their effects on cardiovascular endpoints, GLP-1RA and SGLT2i therapies have been implicated in renal protection. All three major SGLT2i cardiovascular outcomes trials have demonstrated significant reductions in hard renal endpoints. In a meta-analysis, treatment with SGLT2i reduced a composite of reduction in renal function, progression to end-stage kidney disease, or renal death by 45% (HR 0.55, 95% CI 0.48–0.64) [46].
The recently published CREDENCE trial randomized patients with type 2 diabetes and albuminuric CKD on background renin–angiotensin system blockade to canagliflozin or placebo. The trial was stopped early for efficacy of canagliflozin, with a 30% lower risk of the composite primary outcome of end-stage kidney disease, doubling of serum creatinine, or death from renal or cardiovascular cause [41]. Similarly, the risk of end-stage kidney disease, defined as dialysis, transplantation, or a sustained eGFR<15, was reduced by 32%. This trial also recapitulated results from the CANVAS Program, demonstrating a 20% reduction in 3-point MACE and a 39% reduction in heart failure hospitalization [41].
GLP-1RAs have also demonstrated renal protection, though primarily via a reduction in intermediate endpoints such as albuminuria. In a prespecified secondary analysis of the LEADER trial, fewer participants in the liraglutide group met a composite outcome of new onset macroalbuminuria, persistent doubling of serum creatinine, end-stage renal disease, or renal death (HR 0.78; 95% CI 0.67–0.92), driven by new onset persistent macroalbuminuria (HR 0.74; 95% CI 0.60–0.91) [48]. SUSTAIN-6 yielded similar findings [23].
Dose adjustments and contraindications based on eGFR are outlined in Table 1.
Use in combination
At this time, there are no trials examining the impact of combination therapy with both a GLP-1RA and an SGLT2i on cardiovascular outcomes. The DURATION-8 trial randomized 695 patients to exenatide plus dapagliflozin, exenatide plus placebo, or placebo plus dapagliflozin for 28 weeks [49]. Combination therapy with exenatide and dapagliflozin was superior to either alone for the primary endpoint of change in HbA1c as well as several secondary endpoints including weight loss and reduction in systolic blood pressure, with no significant increase in adverse events with combination therapy [49]. These findings suggest that GLP-1RA and SGLT2i therapy may have complementary action not only in glycemic control but potentially in pathways that yield cardiovascular benefit. Combination therapy is endorsed in the current American and European recommendations for approach to glucose management in type 2 diabetes [50].
Use without background metformin therapy
The benefits of empagliflozin and liraglutide were not modified by baseline medication use, although there was a high rate of background metformin therapy in both trials [22,36]. The American Diabetes Association and European Association for the Study of Diabetes consensus recommendations identify metformin as first-line therapy, after which the addition of cardioprotective therapy with a GLP-1RA or SGLT2i can be considered [50]. However, the cardiovascular benefits of GLP-1RA and SGLT2i therapy may be independent of baseline antihyperglycemic medication use.
Use in patients with type I diabetes mellitus
Although these therapies can be used in combination with insulin, neither the GLP-1RA nor SGLT2i classes have been approved for use in patients with Type I diabetes.
Use in patients without diabetes mellitus
Given the notable reductions in heart failure-related outcomes, there is growing interest in the use of SGLT2is in heart failure patients without diabetes, especially as many proposed mechanisms of benefit do not rely on antihyperglycemic effects. To date, SGLT2is are not yet approved for patients without diabetes. However, there are several ongoing trials to evaluate the benefits of SGLT2i drugs in nondiabetic patients with heart failure. These include EMPEROR-PRESERVED and EMPEROR-REDUCED, which evaluate the impact of empagliflozin on a composite of cardiovascular death or heart failure hospitalization in patients with heart failure with a preserved and reduced ejection fraction, respectively, without a requirement for type 2 diabetes (NCT03057951, NCT03057977); and the DAPA-HF trial, which investigates the impact of dapagliflozin on a composite primary outcome of cardiovascular death, heart failure hospitalization, or urgent heart failure visit in patients with reduced ejection fraction, with or without type 2 diabetes (NCT03036124).
Future directions
Ongoing trials
There are multiple ongoing or recently completed trials in these cardioprotective drug classes. In the GLP-1RA class, these include the SELECT trial, which will test the effects of semaglutide on CV outcomes in overweight and obese patients without diabetes (NCT03574597). Multiple SLGT2i trials are also underway, including the VERTIS-CV trial evaluating ertugliflozin in adults with type 2 diabetes and established cardiovascular disease [51]; the SCORED trial evaluating sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, in patients with type 2 diabetes, cardiovascular risk factors, and moderate renal impairment (NCT03315143); and SOLOIST-WHF, which examines sotagliflozin in patients with type 2 diabetes with ejection fraction less than 50% after a heart failure hospitalization (NCT03521934).
Drugs under investigation
There are several drugs under investigation that may further expand the scope of cardioprotective drugs, including recent interest in ‘dual incretin’ therapies of both GLP-1 and glucose-dependent insulinotropic (GIP) hormone. A recently published human phase 2 trial randomized 108 adults with type 2 diabetes to a combined GIP/GLP-1 receptor agonist, open label liraglutide, or placebo for 12 weeks. The dual agonist resulted in significantly better glycemia, more weight loss, and lower total cholesterol [52]. A second phase 2 trial randomized 555 participants with type 2 diabetes to a dual GIP/GLP-1 receptor agonist, dulaglutide, or placebo for 26 weeks. The dual agonist resulted in significantly greater glycemic control and weight loss than dulaglutide [53]. These novel diabetic therapies, which build on existing GLP-1RA therapy, may also have promise for cardioprotection, though this has yet to be studied.
Conclusion
Approved drugs in the GLP-1RA and SGLT2i classes have demonstrated consistent and significant benefits in reducing adverse cardiovascular outcomes – including MI, stroke, heart failure, and cardiovascular death. Figure 1 outlines a simple clinical algorithm, so that these agents may be more frequently incorporated into usual cardiovascular practice for the benefit of patients. As cardiologists have long focused on attentive management of lipids, blood pressure, antiplatelets, smoking, weight, and physical activity, they now have additional tools to incorporate into the comprehensive care of patients with diabetes at high cardiovascular risk.
Fig. 1.
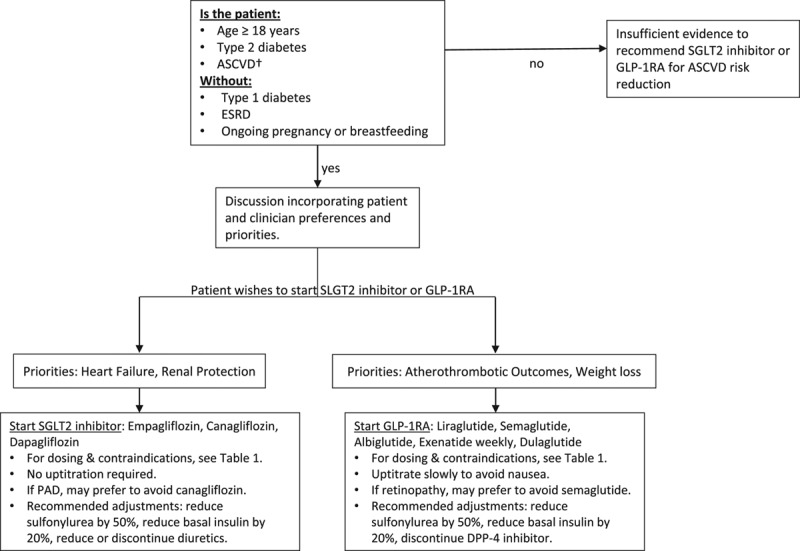
Proposed Clinical Algorithm. American College of Cardiology Expert Clinical Decision Pathway suggested algorithm for the initiation and use of SGLT2i or GLP1RA agents in patients with type 2 diabetes and established ASCVD. There is no agreed upon method for determining high ASCVD risk in the absence of established ASCVD for eligibility for these therapies. †Efficacy has been clearly demonstrated for patients with established ASCVD. However, high-risk primary prevention patients were eligible in the described GLP-1RA and SGLT2i trials if older than 50–60 years of age with multiple typical risk factors including tobacco use, hypertension, and dyslipidemia [26,37,38]. This is one approach to identify patients without established ASCVD for whom these therapies can be considered, although the use of GLP-1RA and SGLT2i therapies can ultimately be left to the discretion of the treating cardiovascular specialist. Adapted with permission [31]. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; DPP4, dipeptidyl peptidase-4; ESRD, end-stage renal disease; GLP-1RA, glucagon-like peptide-1 receptor agonist; PAD, peripheral artery disease; SGLT2 inhibitor, sodium-glucose cotransporter-2 inhibitor.
Acknowledgements
Conflicts of interest
B.M.E. reports grant support from the Novartis and the NHLBI, and consulting income from Roche Diagnostics, Amgen, Novartis, the NIDDK, and the FDA. J.M.B.is supported by a T32 postdoctoral training grant fromthe National Heart, Lung, and Blood Institute (T32HL007604).
References
- 1.Willey JZ, Moon YP, Kahn E, Rodriguez CJ, Rundek T, Cheung K, et al. Population attributable risks of hypertension and diabetes for cardiovascular disease and stroke in the northern Manhattan study. J Am Heart Assoc. 2014; 3:e001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park TH, Ko Y, Lee SJ, Lee KB, Lee J, Han MK, et al. Identifying target risk factors using population attributable risks of ischemic stroke by age and sex. J Stroke. 2015; 17:302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cubbon RM, Adams B, Rajwani A, Mercer BN, Patel PA, Gherardi G, et al. Diabetes mellitus is associated with adverse prognosis in chronic heart failure of ischaemic and non-ischaemic aetiology. Diab Vasc Dis Res. 2013; 10:330–336 [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee NA, Chae CU, Kim E, Moorthy MV, Conen D, Sandhu RK, et al. Modifiable risk factors for incident heart failure in atrial fibrillation. JACC Heart Fail. 2017; 5:552–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez F, Stefanick ML, Greenland P, Soliman EZ, Manson JE, Parikh N, et al. Racial and ethnic differences in atrial fibrillation risk factors and predictors in women: findings from the women’s health initiative. Am Heart J. 2016; 176:70–77 [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The framingham heart study. JAMA. 1994; 271:840–844 [PubMed] [Google Scholar]
- 7.Roglic G, Unwin N. Mortality attributable to diabetes: estimates for the year 2010 Diabetes Res Clin Pract. 2010; 87:15–19 [DOI] [PubMed] [Google Scholar]
- 8.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045 Diabetes Res Clin Pract. 2018; 138:271–281 [DOI] [PubMed] [Google Scholar]
- 9.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. ; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011; 364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. ; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009; 360:129–139 [DOI] [PubMed] [Google Scholar]
- 11.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. ; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 12.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. ; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356:2457–2471 [DOI] [PubMed] [Google Scholar]
- 14.Low Wang CC, Everett BM, Burman KD, Wilson PWF. Cardiovascular safety trials for all new diabetes mellitus drugs? Circulation. 2019; 139:1741–1743 [DOI] [PubMed] [Google Scholar]
- 15.Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, et al. ; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Quality of Care and Outcomes Research, and the American Diabetes Association. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2015; 132:691–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association. 9. Cardiovascular disease and riskManagement: standards of Medical care in Diabetes-2018 Diabetes Care. 2018; 41Suppl 1S86–S104 [DOI] [PubMed] [Google Scholar]
- 17.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. ; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013; 369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 18.Vaduganathan M, Patel RB, Singh A, McCarthy CP, Qamar A, Januzzi JL, Jr, et al. Prescription of glucagon-like peptide-1 receptor agonists by cardiologists. J Am Coll Cardiol. 2019; 73:1596–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaduganathan M, Sathiyakumar V, Singh A, McCarthy CP, Qamar A, Januzzi JL, Jr, et al. Prescriber patterns of SGLT2I after expansions of U.S. Food and Drug Administration labeling. J Am Coll Cardiol. 2018; 72:3370–3372 [DOI] [PubMed] [Google Scholar]
- 20.Riedel MJ, Wheeler MB, Salapatek AM, MacDonald PE, El-Kholy W, Light PE. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2007; 51:S434–S442 [DOI] [PubMed] [Google Scholar]
- 21.Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015; 6:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. ; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016; 375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. ; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016; 375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 24.Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Sr, Granger CB, Jones NP, et al. ; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018; 392:1519–1529 [DOI] [PubMed] [Google Scholar]
- 25.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. ; EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017; 377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. ; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019; 394:121–130 [DOI] [PubMed] [Google Scholar]
- 27.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019. 10.1056/NEJMoa1901118 [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. ; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015; 373:2247–2257 [DOI] [PubMed] [Google Scholar]
- 29.Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, et al. ; EXSCEL Study Group. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018; 6:105–113 [DOI] [PubMed] [Google Scholar]
- 30.Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, et al. ; PIONEER 4 Investigators. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019; 394:39–50 [DOI] [PubMed] [Google Scholar]
- 31.Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL, Jr, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the american college of cardiology task force on expert consensus decision pathways. J Am Coll Cardiol. 2018; 72:3200–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010; 151:1473–1486 [DOI] [PubMed] [Google Scholar]
- 33.Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017; 136:849–870 [DOI] [PubMed] [Google Scholar]
- 34.Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016; 18:317–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez V, Weiss MC, Weintraub H, Goldberg IJ, Schwartzbard A. Cardiovascular disease leads to a new algorithm for diabetes treatment. J Clin Lipidol. 2017; 11:1126–1133 [DOI] [PubMed] [Google Scholar]
- 36.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 37.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. ; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377:644–657 [DOI] [PubMed] [Google Scholar]
- 38.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. ; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–357 [DOI] [PubMed] [Google Scholar]
- 39.Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, et al. ; EMPA-REG OUTCOME® trial investigators. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016; 37:1526–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fadini GP, Avogaro A. SGLT2 inhibitors and amputations in the US FDA adverse event reporting system. Lancet Diabetes Endocrinol. 2017; 5:680–681 [DOI] [PubMed] [Google Scholar]
- 41.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380:2295–2306. NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 42.Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018; 41:356–363 [DOI] [PubMed] [Google Scholar]
- 43.Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 inhibition and cardiovascular events: why did EMPA-REG outcomes surprise and what were the likely mechanisms? Diabetologia. 2016; 59:1333–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018; 61:2108–2117 [DOI] [PubMed] [Google Scholar]
- 45.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019; 139:2022–2031 [DOI] [PubMed] [Google Scholar]
- 46.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393:31–39 [DOI] [PubMed] [Google Scholar]
- 47.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004; 351:1296–1305 [DOI] [PubMed] [Google Scholar]
- 48.Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. ; LEADER Steering Committee and Investigators. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017; 377:839–848 [DOI] [PubMed] [Google Scholar]
- 49.Frías JP, Guja C, Hardy E, Ahmed A, Dong F, Öhman P, Jabbour SA. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016; 4:1004–1016 [DOI] [PubMed] [Google Scholar]
- 50.Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018; 61:2461–2498 [DOI] [PubMed] [Google Scholar]
- 51.Cannon CP, McGuire DK, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, et al. ; VERTIS-CV Investigators. Design and baseline characteristics of the evaluation of ertugliflozin efficacy and safety cardiovascular outcomes trial (VERTIS-CV). Am Heart J. 2018; 206:11–23 [DOI] [PubMed] [Google Scholar]
- 52.Frias JP, Bastyr EJ, III, Vignati L, Tschöp MH, Schmitt C, Owen K, et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 2017; 26:343–352.e2 [DOI] [PubMed] [Google Scholar]
- 53.Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018; 392:2180–2193 [DOI] [PubMed] [Google Scholar]


