Abstract
This case involves a 55-year-old male patient with systolic heart failure and refractory atrial fibrillation due to thyrotoxicosis, who was electrically cardioverted but then developed torsade de pointes and ventricular fibrillation. Rate control was unsuccessful with digoxin, cardizem, labetalol, esmolol and amiodorone. Patient was externally cardioverted after which ECGs showed prolonged QT with frequent premature ventricular contractions. ECGs also showed ‘R-on-T' phenomenon leading to torsades and ventricular fibrillation. Atrial overdrive pacing was used to terminate the dangerous arrhythmia and the patient returned to sinus rhythm. Interestingly, he was found to have new onset thyrotoxicosis and started on methimazole.
Keywords: amiodarone, electrophysiology, R-on-T phenomenon, thyrotoxicosis, torsade de pointes, ventricular fibrillation
Introduction
Thyroid dysfunction produce changes in cardiac contractility, myocardial oxygen consumption, cardiac output, blood pressure, and systemic vascular resistance (SVR) [1]. Although it is well known that thyrotoxicosis can cause sinus tachycardia, atrial fibrillation (Afib) and atrial flutter, ventricular fibrillation (VF) is extremely rare [2]. In thyrotoxicosis, cardiac contractility is enhanced, resting heart rate is increased, and SVR is decreased leading to an increase in cardiac output by 50–300%. Thyroid hormone mediates the expression of both structural and regulatory genes in the cardiac myocyte, in particular, ion channels such as the sodium potassium ATPase, voltage-gated potassium channels and the sodium calcium exchanger that affect the electrochemical responses and contractile function of the myocardium. Hyperthyroidism also resembles a hyperadrenergic state whereby the β1 adrenergic receptors are upregulated [1]. Although supraventricular arrhythmias are more common, one possible mechanism by which VF can occur may be due to coronary vasospasm. Thyroid hormones may increase the hyperreactivity of vascular smooth muscle cells leading to spasms followed by myocardial ischemia and VF. More commonly, ventricular arrhythmias, including VF, are associated with advanced heart failure. Mechanistically, advanced heart failure is associated with structural changes such as fibrosis, regional ventricular hypertrophy, and changes in the mechanical and electrical function that promote the development and maintenance of ventricular arrhythmias [3]. Of interest is the effect of heart failure on acquired QT prolongation. Prolongation of the QT interval is caused by prolongation of the action potential of ventricular myocytes, which can occur as a result of alterations in the cardiac channels, such as potassium channels, secondary to heart failure [4]. New onset thyrotoxicosis in the setting of advanced heart failure and prolonged QT syndrome may increase the risk for a fatal arrhythmia such as torsade de pointes (TdP) or VF.
Case description
Patient is a 55-year-old male with Afib, coronary artery disease, congestive heart failure (ejection fraction 30%) and automatic implantable cardioverter defibrillator (AICD), who presented due to Afib with rapid ventricular response (RVR) and a pulse range of 123–179 bpm. His home cardiac medications consisted of apixaban 5 mg BID, metoprolol succinate XL 25 mg BID, pravastatin 20 mg, furosemide 40 mg and sacubitril/valsartan 24/26 mg BID. In the emergency department, the patient was initially treated with a diltiazem drip, which was unsuccessful in achieving rate control. He was then admitted to inpatient service (day 1) where he was given digoxin 0.25 mg IV once and then started on an esmolol drip but his Afib remained refractory with pulse ranging between 130 and 150 bpm. Cardiology was consulted on day 2, who obtained a new transthoracic echocardiogram, discontinued the esmolol drip and started amiodarone drip over the next 2 days. Despite administration of AV nodal blocking agents and amiodarone, the patient remained in Afib with RVR and pulse of 139 bpm, although he remained largely asymptomatic with blood pressure 130/79. Since he had been compliant with his apixaban, the patient underwent successful DC cardioversion on day 3 whereby he was converted to sinus rhythm with a pulse of 80 bpm after one attempt at 250 Joules. While inpatient, laboratory data showed serum potassium levels ranging from 3.5 to 4.0 mmol/L (reference range 3.5–5.1 mmol/L). Magnesium levels generally stayed within normal range from 1.8 to 2.5 mg/dL, reaching a nadir of 1.7 mg/dL once requiring IV replacement.
During this admission, he was also found to have new onset hyperthyroidism with thyroid stimulating hormone (TSH) <0.015 mU/L (reference 0.5–5.0 mU/L) and free thyroxine (T4) level of 4.72 ng/dL (reference 0.9–2.4 ng/dL). Triiodothyronine (free T3) level was 5.7 ng/L (reference 3.6–5.6 ng/L). Send out labs resulted few days later that showed thyroid stimulating immunoglobulin (TSI) level of 0.24. A TSI result <0.10 IU/L is less likely to be positive for thyroid stimulating autoantibodies whereas TSI >0.55 IU/L has a clinical sensitivity and specificity for Graves' disease of 98.6% and 98.5%, respectively. The thyroxine-binding globulin level was 14 μg/mL (reference 13–39 μg/mL). Thyroid ultrasound revealed a 4 mm and a 3 mm hypoechoic nodule in the right lobe of the thyroid and a 7 mm hypoechoic nodule in the left lobe. Endocrinology was consulted who started him on methimazole 20 mg BID PO and tapered dose oral steroids to help reduce thyroid levels [1]. Per chart review, the patient had a normal TSH level of 1.47 mU/L during an admission 3 years prior, indicating that the current thyrotoxicosis is a new change likely related to the nodules found on thyroid ultrasound. Furthermore, this recent change in thyroid function is less likely to be secondary to euthyroid sick syndrome, also known as nonthyroidal illness syndrome, since the hallmark includes a fall in serum T3 levels that may be accompanied by a drop in T4 levels. Serum TSH is usually normal but may be slightly increased or decreased. Nonthyroidal illness syndrome is common in critically ill patients, particularly those in the intensive care units due to imbalance in thyroid hormone production, metabolism and action [5].
After successful cardioversion on day 3, the patient was started on sotalol 80 mg BID PO; however, due to prolonged QTc of 499 ms, the sotalol was stopped the next morning and metoprolol succinate 25 mg BID PO was started. On day 4, the patient remained rate controlled with pulse rate from 69 to 80 bpm and was discharged but unfortunately, he returned the next day after his AICD discharged at home. Device interrogation showed that he was shocked twice. The first was an appropriate ICD shock for VF early in the morning, which terminated the VF but Afib with RVR up to 214 bpm quickly ensued. The second discharge was an inappropriate shock as the defibrillator recognized the heart rate to be in the programmed VF zone threshold for delivering an ICD shock. Although his heart was shocked into sinus rhythm, this left the electrical activity of his heart vulnerable to entry of frequent premature ventricular contractions (PVCs) causing increased chances to develop ‘R-on-T' induced VF [6]. The patient was symptomatic during these episodes as his wife noted syncopal and seizure like activity at home soon after prior discharge. In the triage area, care was taken not to administer any further amiodarone for rhythm control. During this subsequent admission, serum potassium levels ranged from 3.4 to 4.1 mmol/L.
Discussion
Cardiology was reconsulted for cardiac catheterization after the recent echocardiogram showed left ventricular ejection fraction (LVEF) of 10% with diffuse hypokinesis. However, the patient suffered two more ICD shocks for VF/TdP with associated syncope and seizures, once before catheterization and once in the cath recovery unit. Cardiac catheterization did not find significant coronary artery disease but did confirm the severe left ventricular dysfunction. Electrophysiology (EP) was consulted who assisted in the management of the VF/TdP by administering lidocaine IV drip, potassium replacement, magnesium drip and Metoprolol Tartrate IV. Treatment also consisted of changing the AICD device setting to provide atrial overdrive pacing to shorten the QT interval. The ECG rhythm strips in Fig. 1 illustrate the progression of the arrhythmias from initial Afib with RVR (Fig. 1a) to PVC in bigeminy with prolonged QT interval and occurrence of the ‘R-on-T' phenomenon leading to Torsade and VF (Fig. 1b). Persistent TdP and VF is shown in Fig. 1c with conversion to normal sinus rhythm shown in Fig. 1d about 2 h after overdrive atrial pacing. The ‘R-on-T' phenomenon can be dangerous as it can trigger the myocardium into VF when the PVC falls on a T wave from the previous contraction [6]. The etiology of these arrhythmias was clearly related to the new onset thyrotoxicosis as the patient had been well compensated for many years.
Fig. 1.
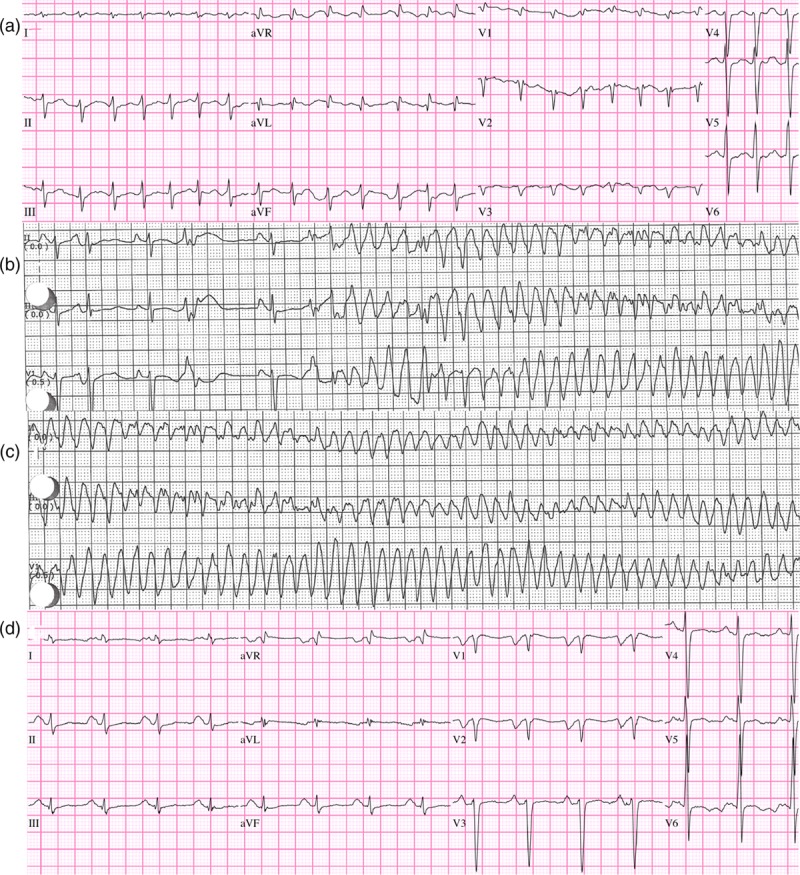
(a) First ED ECG, atrial fibrillation with rapid ventricular response (RVR) 174 bpm; (b) Telemetry after 3 days of amiodarone, sinus rhythm with premature ventricular contractions (PVCs) in bigeminal pattern. Prolonged QT with ‘R on T' PVCs triggering VF/torsades; (c) continued ventricular fibrillation/Torsades; (d) NSR after ICD discharge, dual chamber pacing and sensing, inhibited mode (DDI) to 90 bpm, overdrive atrial pacing to suppress PVCs.
Overdrive pacing has been used for many years to prevent ventricular tachycardia, in particular, arrhythmias with long QT syndromes [7]. Exactly how overdrive works is speculative and not well defined. One theory speculates that ventricular tachycardia termination occurs due to entrance of a critically timed impulse into a reentrant pathway, disrupting the circuit of an established tachycardia [7]. Another theory is that overdrive pacing suppresses tachyarrhythmias by causing more uniform ventricular refractoriness.
The duration of repolarization and refractoriness, also known as the QT interval, is shortened as the heart rate is increased. Increasing the pacing rate narrows the dispersion of refractoriness thereby decreasing the susceptibility to ventricular tachyarrhythmias [7]. In this case, successful application of overdrive pacing is shown in Figs. 2a–d. As stated above, the patient experienced two ICD shocks at home for VF and AFib with RVR. He presented to the Emergency Department on this second admission in sinus rhythm but with frequent PVCs in bigeminal pattern (Fig. 1b). During this second admission, he experienced two more ICD shocks, one on the floor and another after cath recovery (Fig. 2a). Overdrive atrial pacing was successfully applied to suppress PVCs and VF (Fig. 2d). The patient's rhythm eventually converted to normal sinus after 2 h as shown in Fig. 1d.
Fig. 2.
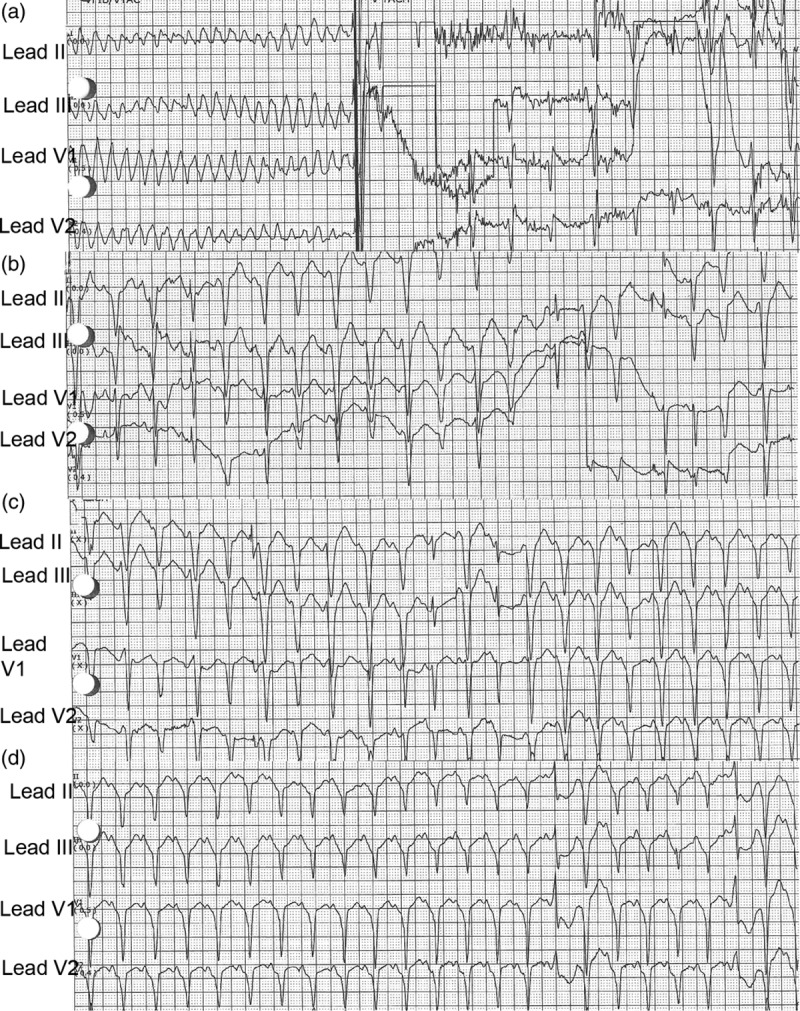
(a–d) This demonstrates successful application of atrial ventricular ‘overdrive pacing'. (a) This shows torsades/ventricular fibrillation and automatic implantable cardioverter defibrillator (AICD) shock delivery. (b) This demonstrates pacing at heart rate (HR) 154 bpm. (c) This shows pacing at HR 124 bpm and (d) pacing at HR 133 bpm. Spikes may be difficult to see in all leads due to settings of the ECG, small amplitude spikes and endocardial ICD leads. (d) This shows regular rate and rhythm with narrow QRS.
An astute observation is to challenge the decision to administer the diltiazem drip during the initial ED admission for Afib with RVR. Although non-dihydropyridine calcium channel blockers, such as diltiazem, are commonly used for rate control of Afib, it is worth repeating that diltiazem is best avoided in patients with systolic heart failure due to the negative inotropic and chronotropic effects that can precipitate acute decompensated heart failure [8]. Unfortunately, in this case, the patient was treated with a diltiazem IV drip until the next day when the Cardiology team switched him to an esmolol IV drip. Per chart review, the patient had a documented echocardiogram from 3 years prior, which showed mild to moderate dilated left ventricle with severely reduced LVEF of approximately 25%. In this situation, digoxin may have been a more suitable initial rate control agent.
Admittedly, the exact trigger for this patient's TdP and VF is not clear due to the multitude of confounding factors. As discussed earlier, although very rare, new onset thyrotoxicosis can precipitate VF theoretically by inducing coronary vasospasm leading to myocardial ischemia. Another proposed mechanism is that thyrotoxicosis is associated with early repolarization that can exaggerate the J-point elevation and induce VF [2]. Advanced heart failure marked by maladaptive hypertrophic and fibrotic myocardial remodeling, with or without dyssynchronous contraction, can predispose the heart to the whole spectrum of ventricular arrhythmias [3]. However, in this case, it is also interesting to examine the contribution of acquired long QT syndrome. Briefly, the patient was initially treated with diltiazem drip, digoxin 0.25 mg IV once, esmolol IV drip as well as amiodarone IV drip before DC cardioversion. He was then treated with sotalol 80 mg BID PO for one day before prolonged QTc of 499 ms was noted on telemetry. Given the new onset Graves' disease, administration of amiodarone may have exacerbated ventricular arrhythmias through amiodarone-induced thyrotoxicosis [9]. Of the cardiovascular medications given, sotalol is the most common cause of drug-induced TdP [10], occurring in 2–4% of patients given sotalol [11]. As a Class III, antiarrhythmic drug, sotalol blocks the rapid component of the delayed rectifier potassium channels, which delays repolarization and prolongs the QT interval [12]. Although the patient was only briefly exposed to sotalol, this may have increased the risk of developing TdP, adding to the complexity of this case. Incidentally, the patient had preserved renal function with BUN 13 mg/dL, creatinine 0.80 mg/dL and eGFR > 60 mL/min, thus making renal insufficiency less likely as a cause for sotalol toxicity.
After successful termination of the TdP/VF through AICD atrial overdrive pacing, the patient and his wife, the Cardiology team and the hospitalist agreed to transfer the patient to a heart transplant center for evaluation. In this case, the patient met the indication for transplant based on systolic heart failure with recent LVEF 10% and diffuse hypokinesis, symptoms of NYHA class III or IV despite maximized medical therapy and recurrent life-threatening left ventricular arrhythmias despite an implantable cardiac defibrillator and antiarrhythmic therapy [13]. However, while awaiting evaluation for transplant, additional treatment options exist. First, the patient may benefit from cardiac resynchronization therapy (CRT) to improve quality of life, functional status, and reverse cardiac remodeling to improve LVEF [14]. In this case, an upgrade to a CRT-defibrillator (CRT-D) device may be appropriate [14]. Additionally, the patient may benefit from ablation of the atrioventricular node with subsequent pacing due to unresponsiveness to pharmacotherapy. This ‘ablate and pace' approach also provides more robust control of ventricular rate as well as regularization of the R-R interval [15].
Conclusion
In this case, the patient's multiple episodes of VF may have been caused by the new onset thyrotoxicosis (Graves' disease) or more likely, due to a combination of cardiac events. Given his nonischemic cardiomyopathy with LVEF of 10% and diffuse hypokinesis, the patient already had an elevated risk for acquired long QT syndrome [4] and ventricular arrhythmias [3]. Administration of amiodarone in the severe heart failure state may have exacerbated his risk for prolonged QT syndrome. The acquired long QT syndrome along with increased frequency of PVCs, possibly due to thyrotoxicosis [16], may have led to TdP and VF secondary to the ‘R-on-T' phenomenon [6]. Although hypokalemia is a known risk factor for VF, the lowest serum potassium level was 3.4 mmol/L, which was promptly replaced. Given new onset Graves' disease and the long half-life of amiodarone, alternative antiarrhythmics should be used to prevent life threatening arrhythmias [9]. Furthermore, patients with refractory Afib should be screened for thyrotoxicosis before initiation of amiodarone.
Acknowledgements
This study was supported (in whole or in part) by HCA and/or an HCA affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA or any of its affiliated entities.
Consent: Written informed consent was obtained from the patient for the publication of this case report along with the accompanying images.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007; 116:1725–1735 [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi H, Haketa A, Abe M, Tahira K, Hatanaka Y, Tanaka S, et al. Unusual manifestation of graves' disease: ventricular fibrillation. Eur Thyroid J. 2015; 4:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santangeli P, Rame JE, Birati EY, Marchlinski FE. Management of ventricular arrhythmias in patients with advanced heart failure. J Ame Coll Cardiol. 2017; 69:1842–1860. doi: 10.1016/j.jacc.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 4.Kang JY. Cardiac hypertrophy: a risk factor for QT-prolongation and cardiac sudden death. Toxicol Pathol. 2006; 34:58–66. DOI: 10.1080/01926230500419421 [DOI] [PubMed] [Google Scholar]
- 5.Neto AM, Zantut-Wittmann DE. Abnormalities of thyroid hormone metabolism during systemic illness: the low T3 syndrome in different clinical settings. Hindawi publishing corporation. Int J Endocrinol. 2016; 2016:2157583 10.1155/2016/2157583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen AP, Sarmast SA, Kowal RC, Schussler JM. Cardiac arrest due to torsades de pointes in a patient with complete heart block: the “R-on-T” phenomenon. Proc(Bayl Univ Med Cent). 2010; 23:361–362. PMCID: PMC2943449. PMID: 20944757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kowey PR, Engel TR. Overdrive pacing for ventricular tachyarrhythmias: a reassessment. Ann Intern Med. 1983; 99:651–656 [DOI] [PubMed] [Google Scholar]
- 8.Vider E, Zada I. The use of calcium channel blockers in heart failure. J Nurse Pract. 2019; 15:149–150.e1. 10.1016/j.nurpra.2018.07.009 [DOI] [Google Scholar]
- 9.Conen D, Melly L, Kaufmann C, Bilz S, Ammann P, Schaer B, et al. Amiodarone-induced thyrotoxicosis: clinical course and predictors of outcome. J Am Coll Cardiol. 2007; 49:2350–2355 [DOI] [PubMed] [Google Scholar]
- 10.Darpo B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Supplements. 2001; 3Suppl KK70–K80 [Google Scholar]
- 11.Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999; 354:1625–1633 [DOI] [PubMed] [Google Scholar]
- 12.Chong DWS, Ankolekar SJ, McDonald J.2009. [DOI] [PMC free article] [PubMed]
- 13.Alraies MC, Eckman P. Adult heart transplant: indications and outcomes. J Thorac Dis. 2014; 6:1120–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linde C, Ellenbogen K, McAlister FA. Cardiac resynchronization therapy (CRT): clinical trials, guidelines, and target populations. Heart Rhythm. 2012; 9:S3–S13 [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee NA, Upadhyay GA, Ellenbogen KA, McAlister FA, Choudhry NK, Singh JP. Atrioventricular nodal ablation in atrial fibrillation: a meta-analysis and systematic review. Circ Arrhythm Electrophysiol. 2012; 5:68–76 [DOI] [PubMed] [Google Scholar]
- 16.Selmer C, Olesen JB, Hansen ML, Lindhardsen J, Olsen AM, Madsen JC, et al. The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ. 2012; 345:e7895. [DOI] [PMC free article] [PubMed] [Google Scholar]


