Supplemental Digital Content is available in the text
Keywords: chronic hepatitis B, interleukin-17, interleukin-26, T helper 17 cells, telbivudine
Abstract
Proinflammatory interleukin-26 (IL-26) is involved in chronic inflammation; however, the role of IL-26 in chronic hepatitis B (CHB) remains unknown.
In this study, serum IL-26 was quantified in a cohort of CHB patients at baseline and during telbivudine (LdT) treatment.
Our results showed that the serum IL-26 level was significantly elevated in CHB patients compared with that in healthy controls and was time-dependently decreased during LdT treatment, accompanying hepatitis B e antigen (HBeAg) seroconversion and reduced serum levels of hepatitis B virus (HBV) DNA, aspartate transaminase, and alanine transaminase across baseline and treatment. In addition, the serum level of IL-26 exhibited a similar declining trend to that of T helper 17 (Th17) cell-secreted IL-17 during LdT treatment in CHB patients. The percentage of IL-26-expressing CD4+ cells was significantly higher than that of IL-26-expressing CD4- cells isolated from the peripheral blood mononuclear cells of CHB patients, suggesting that serum IL-26 might be mainly released from CD4+ T cells. Furthermore, the baseline mRNA levels of IL-26 and orphan nuclear receptor RORγt—an important transcription factor expressed by Th17 cells—were positively correlated and displayed the same declining trend across the baseline and LdT treatment in CHB patients, suggesting that Th17 cells could be a possible cellular source of the increased serum IL-26 in CHB patients.
Taken together, our results suggest that serum IL-26, possibly produced by Th17 CD4+ cells, is a novel and potential biomarker for CHB prognosis and treatment.
1. Introduction
Despite the availability of effective vaccine, chronic hepatitis B virus (HBV) infection remains the leading cause of liver diseases, affecting approximately 240 million people worldwide.[1] Patients with chronic hepatitis B (CHB) are at high risk of developing liver cirrhosis and hepatocellular carcinoma, with 500,000 to 1 million deaths globally each year.[2] During HBV-induced chronic hepatic inflammation, adaptive immune cells, especially T cells, play crucial roles in antiviral defense via releasing cytokines and chemokines.[3,4] Measuring the cytokine and chemokine profiles can help assess disease status and predict clinical outcomes of patients with HBV infection.[5]
Interleukin-26 (IL-26) belongs to the IL-10 family and is mainly produced by activated T helper 17 (TH17) cells, a subset of CD4+ T lymphocytes that typically secrets IL-17.[6] IL-26 induces production of proinflammatory cytokines in some epithelial cell lines and myeloid cells through a receptor complex composed of IL-20R1 and IL-10R2.[7–10] Overexpression of IL-26 has been detected in chronic inflammatory diseases (rheumatoid arthritis, Crohn disease, and hepatitis C), playing a promotive role in inflammatory processes.[7,11,12] Although other members of IL-10 family, like IL-10 and IL-22, have been associated with HBV infection pathogenesis and outcome,[13] little is known about the expression pattern and the role of IL-26 in CHB.
Previous studies have suggested a reciprocal relationship between IL-26 and its cellular source Th17 cells in the pathogenesis of chronic inflammation. For example, upregulated mRNA expression of IL-26 correlates with an increased number of orphan nuclear receptor RORγt+ Th17 cells in the inflamed colonic lesions of patients with Crohn disease.[8] IL-26 promotes the generation of RORγt+ Th17 cells in addition to inducing the production of proinflammatory cytokines in rheumatoid arthritis.[7] In CHB patients, highly enriched circulating and intrahepatic Th17 cells enhance immune activation and liver damage by secreting IL-17A, IL-17F, and IL-22.[14,15] However, the relationship between IL-26 and Th17 cells in CHB remains unknown.
Telbivudine (LdT) is an FDA-approved antiviral agent that inhibits HBV replication and induces hepatitis B e antigen (HBeAg) seroconversion in patients with CHB.[16,17] A recent study showed that the circulating Th17 cell frequency and IL-17 level are significantly increased in HBeAg+ CHB patients compared with those in the healthy controls. Lower ratios of regulatory T/Th17 cells and their secreted cytokines TGF-β1/IL-17 predict a higher HBeAg seroconversion rate in response to LdT treatment.[18] In this study, to elucidate the expression pattern and cellular source of IL-26 in CHB, we analyzed the changes in the serum and cellular levels of IL-26 and discussed their relationship to the changes in HBeAg, HBV DNA, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and IL-17 levels, across baseline and LdT treatment. Our findings may provide serum IL-26 as a potential biomarker for the immune status of the host and as an indicator for treatment success.
2. Methods
2.1. Patients
This study recruited 30 CHB patients admitted to the Division of Infectious Diseases at Southwest Hospital, Third Military Medical University (Chongqing, China) and 28 healthy controls during 2014–2016. The inclusion criteria of CHB patients were as follows:
the presence of hepatitis B surface antigen (HBsAg) and HBeAg in blood samples[19];
no previous systemic antiviral treatment or other medications. Patients with a history of toxic liver damage were excluded.
The demographic and clinical characteristics were collected and summarized in Table 1. Patients were orally given 600 mg LdT once daily. Peripheral blood samples were collected at baseline (week 0) and at weeks 12, 24, 36, and 52 post treatment. Responses to LdT were classified into 2 types: complete response (undetectable serum HBeAg and the serum HBV DNA level < 50 IU/ml) and partial response (only the serum HBV DNA level < 50 IU/ml). This study was approved by the ethics committee of Southwest Hospital. All procedures were in compliance with the Good Clinical Practice Guidelines and the 1975 Declaration of Helsinki. All patients provided written informed consent prior to study initiation.
Table 1.
Clinical characteristics of study participants.
2.2. Enzyme-linked immunosorbent assay (ELISA)
The serum levels of IL-26 and IL-17 were determined using ELISA kits (IL-26: R&D Systems, Minneapolis, MN, USA; IL-17: eBioscience, San Diego, CA, USA) according to the manufacturer's instructions. A standard concentration curve of IL-26 or IL-17 was established. Serum samples were diluted 2-fold in sample diluent. The concentration of IL-26 or IL-17 in each sample was calculated by comparing the experimental values with the standard curve.
2.3. Serological and biochemical analyses of HBV
The serum levels of HBsAg and HBeAg were measured using an Abbott I 2000 automated chemiluminescence immunoassay analyzer (Abbott, IL, USA) as previously described.[20] The amount of serum HBV DNA was determined using a luciferase quantization detection kit (Roche, USA) and quantified using Kaneko method.[21] The serum levels of ALT and AST were measured using an automatic biochemistry analyzer (Hitach, Japan).
2.4. CD4+ T cell isolation and flow cytometry assay
Whole blood samples were collected from each subject at weeks 0 (baseline), 12, 24, 36, and 52 post LdT treatment. Peripheral blood mononuclear cells (PBMCs) were separated from heparinized blood samples using an Optipep Nycoprep Lymphoprep kit (Axis-Shield, Oslo, Norway), followed by CD4+ T cells isolation using a CD4+ T cell isolation kit (Miltenyibiotec, Germany). CD4+ T cells were maintained in RPMI 1640 medium (Hyclone, South Logan, UT, USA) supplemented with 10% foetal bovine serum (GIBCO BRL, Grand Island, NY, USA). After stimulation with 1 μg ml−1 phytohaemagglutinin (PHA; Sigma-Aldrich, St. Louis, MO, USA), 100 U ml−1 IL-2 (eBioscience), and 1 μl ml−1 GolgiStop (BD Biosciences, San Jose, CA, USA) for 6 hours, intracellular staining for cytokines were performed using a Cytofix/Cytoperm kit (eBioscience), FITC-conjugated anti-human CD4 (eBioscience), phycoerythrin-conjugated anti-human IL-26 (R&D), allophycocyanin-conjugated anti-human RORγt (eBioscience), and isotype IgG control. The expression of each cytokine was analysed using a FACSAria cell sorter (BD Biosciences) and FlowJo single cell analysis software (Tree Star, Inc., Ashland, OR, USA).
2.5. Quantitative real-time RT-PCR
After stimulation with 1 μg ml−1 PHA, 100 U ml−1 IL-2, and 1 μl ml−1 GolgiStop for 6 h, total RNA was isolated from 106 CD4+ T cells using TRIzol reagent (TaKaRa, Dalian, China), followed by cDNA synthesis using a PrimeScript RT reagent kit (Takara, Dalian, Chian) according to the manufacturer's instructions. Real-time PCR primers for human RORγt (5’– TGCTGGTTAGGATGTGCCG-3’ and 5’–GAGTGGGAGAAGTCAAAGATGGA-3’), IL-26 (5’- ATACGCTTTGTGGAGGACTTT-3’ and 5’-TTGGCTTTGGTTTACTGACTG-3’), and GAPDH (5’- CGGAGTCAACGGATTTGGTCGTAT-3’ and 5’–AGCCTTCTCCATGGTGGTGAAGAC-3’) were synthesized by Sangon Biotech (Shanghai, China). The cDNAs were amplified (35 cycles of 95°C for 20 s, 62°C for 10 s, and 72°C for 20 s) using corresponding primers and SYBR qPCR SuperMix (Novoprotein, Shanghai, China) on a MxPro-Mx3000P Quantitative Real-Time amplification system (Stratagene, La Jolla, CA, USA). The mRNA level of each gene was calculated as the fold change normalized to that of GAPDH. Data represented the average of 3 independent experiments.
2.6. Statistical analysis
Data were expressed as mean ± standard deviation. Statistical analysis was performed using SPSS 18.0 software (IBM, Armonk, NY, USA). The differences between groups were compared using Wilcoxon signed rank test or Mann–Whitney test. The correlation between variables were assessed using the Spearman rank correlation test. A P Value < .05 was considered statistically significant.
3. Results
3.1. Demographic and clinical characteristics of study participants
A total of 30 patients with chronic HBV (21 men and 9 women; 25–53 years old) and 28 healthy controls (16 men and 12 women; 22–49 years old) were recruited at the Department of Infectious Diseases of Southwest Hospital. The demographic and clinical characteristics of participants were summarized in Table 1. CHB patients exhibited increased serum levels of HBV DNA, ALT, and AST, compared with healthy controls at baseline.
3.2. Reduced serum IL-26 level accompanies with decreased HBV infection indicators during LdT treatment in HBV patients
To explore the role of IL-26 in CHB, we monitored the serum levels of IL-26, HBV DNA, ALT, and AST in CHB patients across baseline and LdT treatment. As shown in Figure 1A and 1B, compared with healthy controls, CHB patients had remarkably increased baseline serum levels of both HBV DNA and IL-26 that were significantly and time-dependently reduced in response to LdT treatment. Because HBeAg clearance indicates the therapeutic effect of LdT,[17] we compared the serum IL-26 levels between HBeAg-positive CHB patients at baseline and HBeAg-negative patients at week 24 and 52 post treatment. We found that the serum IL-26 level was dramatically decreased upon HBeAg clearance (Fig. 1C).
Figure 1.
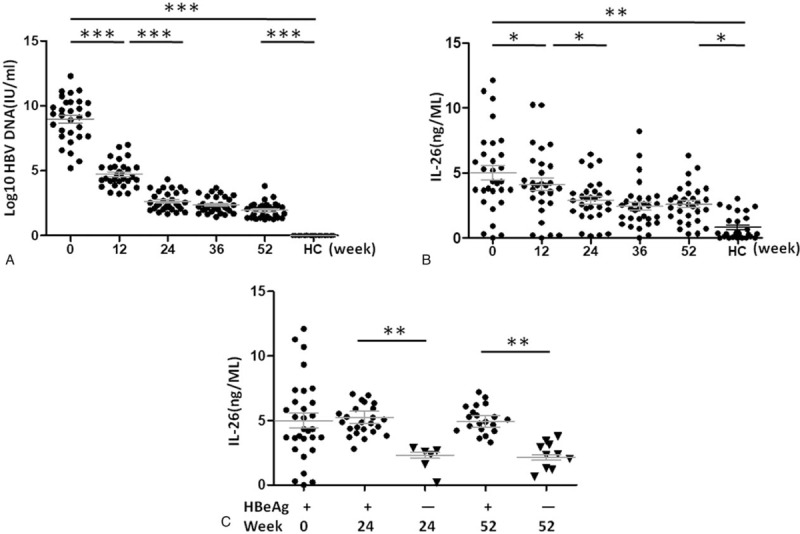
The serum levels of interleukin 26 (IL-26) and hepatitis B virus (HBV) DNA in healthy controls and patients with chronic hepatitis B (CHB). The serum levels of HBV DNA (A) and IL-26 (B) in 28 healthy controls and 30 CHB patients were determined at weeks 0 (baseline), 12, 24, 36, and 52 after telbivudine (LdT) treatment. C. Hepatitis B e antigen (HBeAg)-negative patients (n = 10) were randomly selected at week 24 and 52 post LdT treatment, respectively. The serum IL-26 levels of HBeAg-positive patients at baseline (n = 30) and HBeAg-negative patients (n = 10) at week 24 or 52 were measured. Data are presented as mean ± standard deviation (SD). ∗P < .05, ∗∗P < .01 and ∗∗∗P < .001. HBV = hepatitis B virus, HC = healthy control, IL-26 = interleukin 26, w = week.
Similarly, the serum IL-26 levels in patients with normal ALT or AST levels (≤40 U/L) were significantly lower than those in patients with elevated ALT or AST levels (> 40U/L) (Fig. 2A and 2B). The serum IL-26 level exhibited a consistent time-dependent downward trend with ALT and AST in HBV patients during LdT treatment (Fig. 2C and 2D). Taken together, these results suggest that the serum IL-26 level may serve as a potential auxiliary indicator for CHB prognosis and LdT treatment success.
Figure 2.
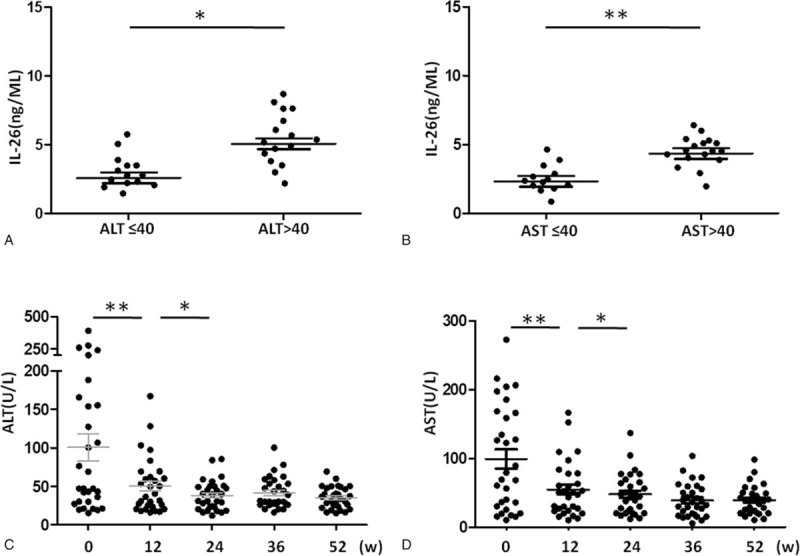
The relationship between the serum levels of IL-26 and alanine aminotransferase (ALT)/aspartate aminotransferase (AST). (A and B) CHB patients were divided into 2 groups based on the serum ALT or AST levels. The serum IL-26 levels were measured and compared. (C and D) The serum ALT and AST levels were measured in CHB patients at weeks 0 (baseline), 12, 24, 36, and 52 post LdT therapy. Data are expressed as mean ± SD. ∗P < .05, ∗∗P < .01; n = 30. ALT, alanine aminotransferase; AST, aspartate aminotransferase; IL-26, interleukin-26.
3.3. The serum IL-26 level positively correlates with the ALT and AST levels in CHB patients at baseline
We further evaluated the correlation of the serum IL-26 level with the ALT, AST, and HBV DNA levels in CHB patients at baseline. As shown in Figure 3A–C, the serum IL-26 level was significantly and positively correlated with the ALT or AST levels, but not with the HBV DNA level, suggesting that the increased serum IL-26 level could indicate liver damage in CHB.
Figure 3.
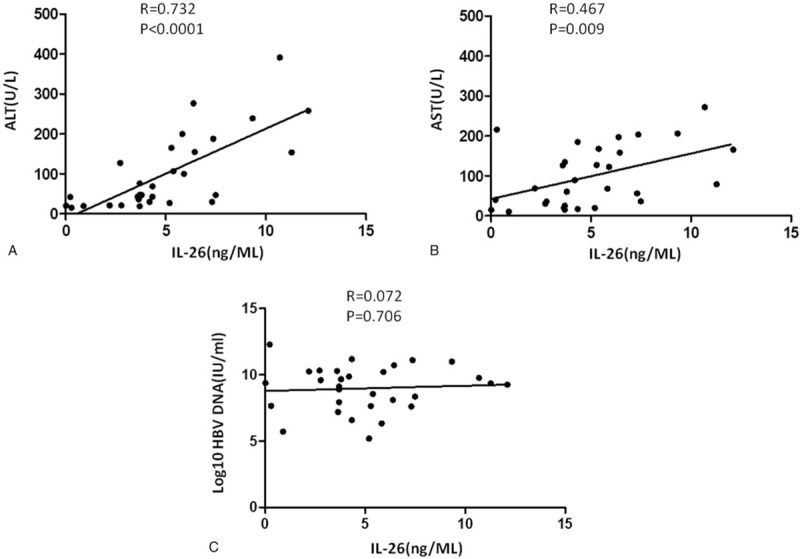
The correlation of the serum IL-26 level with ALT, AST, or HBV DNA level. The correlation analysis was performed between the serum IL-26 level and ALT (A), AST (B), and HBV DNA (C) levels, respectively, in CHB patients at baseline. The P-value of each correlation analysis was indicated. n = 30.
3.4. Reduced serum IL-26 coincides with decreased serum IL-17 during LdT treatment in CHB patients
Th17 cells secret both IL-17 and IL-26.[6] Despite a lack of correlation with the serum IL-26 level (Fig. 4A, P = .969), the serum IL-17 level in CHB patients was significantly higher than those in healthy controls at baseline that was significantly and time-dependently declined during LDT treatment (Fig. 4B), consistent with that observed in the serum IL-26 level (Fig. 1B). These data suggest that IL-17-secreting Th17 cells could be a possible source of circulating IL-26 in CHB patient. The serum levels of TNF-α and IL-23 were also detected in the CHB patients at weeks 0 (baseline), 12, 24, 36, and 52 post LdT therapy. The serum level of the 2 Inflammatory factor in CHB patients didn’t change like IL-17 that was so obvious when treated (Fig. S1).
Figure 4.
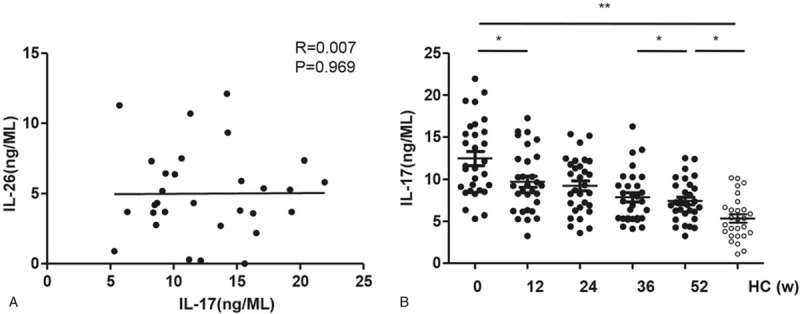
The correlation of the serum IL-26 level with IL-17 level in CHB patients. A. The serum IL-17 level was measured at weeks 0 (baseline), 12, 24, 36, and 52 post LdT therapy in CHB patients. B. The correlation analysis was performed between the serum levels of IL-26 and IL-17 in CHB patients at baseline. Data are expressed as mean ± SD.∗P < .05, ∗∗P < .01; n = 30.
3.5. IL-26 is abundantly expressed in CHB patient-derived CD4+ T cells
To investigate the cellular source of circulating IL-26 in CHB patients, we performed flow cytometric detection of IL-26 protein in CD4– and CD4+ lymphocytes sorted from PBMCs of healthy controls and CHB patients. As shown in Figure 5A, the percentage of IL-26-expressing CD4+ cells was significantly increased in CHB patients compared with that in healthy controls. In CHB patients, the percentage of IL-26-expressing CD4+ cells was considerably higher than that of IL-26-expressing CH4– cells. Consistent results were observed in cellular IL-26 mRNA expression (Fig. 5B). These data suggest that IL-26 might be mainly expressed by CD4+ T cells in CHB patients. In addition, the baseline mRNA levels of both IL-26 and RORγt (a master transcription factor governing Th17 cell differentiation[22] were significantly elevated in CHB patient-derived CD4+ T cells compared with those in healthy control-derived CD4+ T cells. During LdT treatment, the mRNA levels of IL-26 and RORγt showed similar declining trends (Fig. 5C). A significant and positive correlation was observed between baseline IL-26 and RORγt mRNA levels in patient-derived CD4+ T cells (Fig. 5D). Flow cytometric analysis showed that IL-26 and RORγt were co-expressed in 3.4% of CD4+ T cells (Fig. 5E). These results suggest a close relationship between circulating IL-26 and Th17 CD4+ cells.
Figure 5.
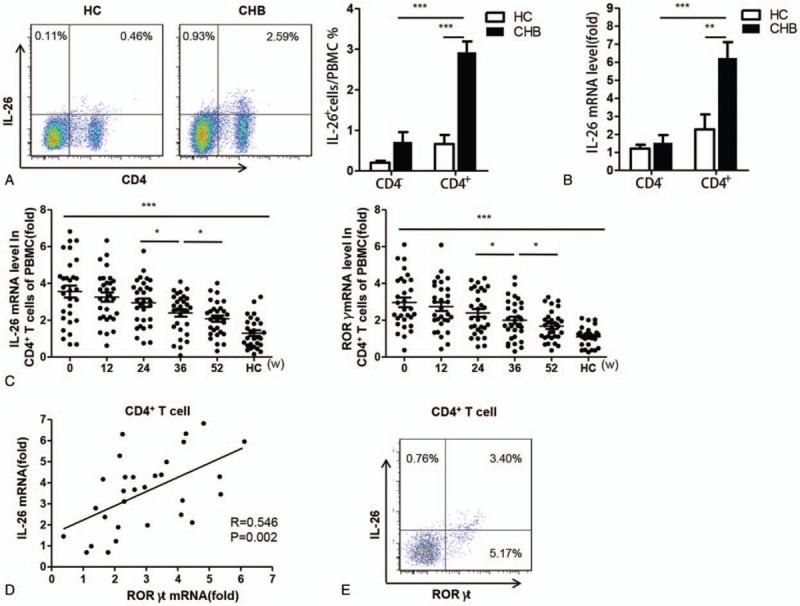
The cellular source of circulating IL-26 in CHB patients. A. CD4– and CD4+ lymphocytes were isolated from peripheral blood mononuclear cells of healthy controls and CHB patients using flow cytometry. The frequencies of IL-26-expressing CD4– and CD4+ lymphocytes were measured. B. The IL-26 mRNA levels were determined in healthy controls- and CHB patients-derived CD4– and CD4+ T cells using quantitative real-time PCR (qRT-PCR). C. The mRNA levels of IL-26 and orphan nuclear receptor RORγt in CD4+ T cells were detected at weeks 0 (baseline), 12, 24, 36, and 52 post LdT treatment using qRT-PCR. D. The IL-26 mRNA level was positively correlated with RORγt mRNA level in CD4+ T cells from CHB patients at baseline. E. The frequencies of IL-26- and RORγt-expressing CD4+ T cells from CHB patients were detected at baseline using flow cytometry. Data are expressed as mean ± SD. ∗P < .05, ∗∗P < .01 and ∗∗∗P < .001; n = 30.
4. Discussion
HBV infection can cause chronic inflammation that involves various immunocytes and proinflammatory cytokines.[23] Proinflammatory cytokine IL-26 contributes to chronic inflammation in multiple autoimmune diseases.[7,11,12] In this study, we demonstrated that the baseline serum IL-26 level was significantly increased in patients with CHB compared with that in healthy controls and that reduced serum IL-26 level coincided with decreased HBV infection indicator levels during LdT therapy, suggesting that circulating IL-26 could indicate the therapeutic effect of LdT in CHB patients. We also found a positive correlation between baseline IL-26 and RORγt mRNA levels in CHB patient-derived CD4+ T cells and observed similar declining trends in circulating IL-26 and IL-17 levels as well as in cellular mRNA expression of IL-26 and RORγt, suggesting that IL-26, IL-17, and RORγt might have the same cellular source (i.e., Th17 cells) in CHB patients.
In this study, the baseline serum IL-26 level was significantly increased in CHB patients compared with that in healthy controls and was gradually decreased during LdT treatment, consistent with that observed in HBV DNA, ALT, and AST, suggesting a role of IL-26 in the inflammatory processes in CHB. Studies have shown that IL-26 plays an antiviral role in primary immune cells, epithelial cells, and keratinocyte cells[9,24,25] by producing proinflammatory cytokines through activating IL-20R1/IL-20R2 complex. Although little is known about the role of IL-26 in chronic HBV infection, a recent study have described that IL-26 can stimulate CD16–CD56bright natural killer (NK) cells to remove HCV-infected hepatocytes by enhancing the expression of tumor necrosis factor-related apoptosis-inducing ligand on NK cell membranes.[12] In addition, IL-26 induces the expression of type I interferon in plasmacytoid dendritic cells by binding to Toll-like receptor 9, further interfering viral replication and reducing damage to hepatocytes.[26] We therefore speculate that IL-26 might have a similar role in chronic HBV infection.
Our results showed that the serum ALT, AST, and HBV DNA levels were consistently declined during LdT treatment in a time-dependent manner, in agreement with previous studies.[14,15,18] In addition, the serum IL-26 level was significantly decreased upon HBeAg clearance after LdT treatment. These data suggest that the circulating IL-26 level may serve as an indicator for treatment success in CHB. Furthermore, the baseline serum IL-26 level positively correlated with ALT and AST levels in CHB patients, suggesting that increased serum IL-26 can indicate liver injury in CHB. Despite a lack of correlation between the serum levels of IL-26 and IL-17 in this study, previous investigations have shown that Th17 cell frequency and the IL-17 level positively correlate with the ALT level in CHB (P < .05),[18] implying a possible relationship between the serum IL-26 and Th17 cells. Because IL-26 is mainly produced by Th17 cells that typically release IL-17 we hypothesized that Th17 cells are possible cellular source of circulating IL-26 in CHB.
To further investigate the cellular source of serum IL-26 in CHB, we tested the protein and mRNA expression of IL-26 in CD4– and CD4+ lymphocytes derived from healthy controls and CHB patients. Our results suggest that serum IL-26 might be mainly released from CD4+ T cells, evidenced by the significantly increased percentage of IL-26-expressing CD4+ cells compared with that of IL-26-expressing CD4– cells isolated from the PBMC of CHB patients. Furthermore, the mRNA levels of IL-26 and RORγt were positively correlated and displayed the same declining trend across the baseline and LdT treatment. Although RORγt expression is not only confined in Th17 cells,[27] RORγt, as a transcription factor, can control the expression of key genes in Th17 cells. Knockdown of RORγt reduces the expression of a number of genes, including IL-17A, IL-17F, IL-22, and IL-26, during Th17 differentiation,[28] consistent with the same expression patterns of IL-17, IL-26, and RORγt observed in CHB patients across the baseline and LdT treatment. We therefore speculate that the Th17 subset of CD4+ T cells might be one of the cellular sources of circulating IL-26 in CHB patients.
Previous research shows that the viral infectivity modulation capability of IL-26 is dependent on the expression of IL-26 receptor (IL-26R). It is known that the IL-26R consists of the IL-20R1 and IL-10R2 receptor chains that are expressed differently in different tissues. The heterodimeric structure IL-10R2/IL-20R1 has been initially described as an IL-26 receptor on epithelial cells, which signals via STAT1 and STAT3. However, converging evidences support the existence of a distinct IL-26 receptor on immune cells unlike that on epithelial cells.[28] We detected respectively the mRNA and protein levels of IL-10R2 and IL-20R1 in liver specimens of normal people and hepatitis B patients, and found that there was no statistical difference between them. IL-10R2 and IL-20R1 can be expressed in liver cells of CHB, and are not affected by HBV infection (Fig. S2). Unfortunately, we did not do dimeric IL-26 receptor research in liver tissue. The nature of the IL-26 receptor expressed by human immune cells is undergoing further research.
In summary, compared with healthy controls, CHB patients have significantly increased serum IL-26 levels at baseline that is time-dependently decreased during LdT treatment, consistent with HBV infection indicators like the serum HBV DNA, ALT, and AST levels. In addition, the expression patterns of serum and cellular IL-26 are similar to those of IL-17 and RORγt, respectively, suggesting that IL-17- and RORγt-expressing Th17 cells are possible cellular source of serum IL-26 elevation in CHB patients. Our results suggest IL-26 as a novel potential indicator for inflammation in chronic HBV infection and provide some new information about the mechanism underlying the serum IL-26 elevation in CHB patients.
Acknowledgments
The authors would like to thank all participants in this study.
Author contributions
Conceptualization: Liwen Luo, Li Jiang.
Data curation: Liwen Luo, Li Jiang, Zhiqiang Tian.
Formal analysis: Xinqi Zhang.
Writing – original draft: Zhiqiang Tian, Xinqi Zhang.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, CHB = chronic hepatitis B, HBeAg = hepatitis B e antigen, HBV = hepatitis B virus, IL-26 = interleukin-26, LdT = telbivudine, PBMCs = peripheral blood mononuclear cells, Th17 = T helper 17.
How to cite this article: Luo L, Jiang L, Tian Z, Zhang X. The serum interleukin-26 level is a potential biomarker for chronical hepatitis B. Medicine. 2020;99:1(e18462).
LL and LJ contributed equally to this work.
This work was supported by the Research Program of Foundation of Science and Applicate Technology of Chongqing (Nos.cstc2015jcyjA10119, cstc2018jcyjAX0598), the President Foundation of General Hospital of Jinan Military Region (2015MS05) and Third Military Medical University (TMMU) key project for clinical research (Grant No. 2012 XLC05).
The authors declare no conflict of interests.
Supplemental Digital Content is available for this article.
References
- [1].MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med 2015;5:a021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Morikawa K, Shimazaki T, Takeda R, et al. Hepatitis B: progress in understanding chronicity, the innate immune response, and cccDNA protection. Ann Transl Med 2016;4:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005;5:215–29. [DOI] [PubMed] [Google Scholar]
- [4].Vinader V, Afarinkia K. A beginner's guide to chemokines. Future Med Chem 2012;4:845–52. [DOI] [PubMed] [Google Scholar]
- [5].Estevez J, Chen VL, Podlaha O, et al. Differential serum cytokine profiles in patients with chronic hepatitis B, C, and hepatocellular carcinoma. Sci Rep 2017;7:11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Donnelly RP, Sheikh F, Dickensheets H, et al. Interleukin-26: an IL-10-related cytokine produced by Th17 cells. Cytokine Growth Factor Rev 2010;21:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Corvaisier M, Delneste Y, Jeanvoine H, et al. IL-26 is overexpressed in rheumatoid arthritis and induces proinflammatory cytokine production and Th17 cell generation. PLoS Biol 2012;10:e1001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dambacher J, Beigel F, Zitzmann K, et al. The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut 2009;58:1207–17. [DOI] [PubMed] [Google Scholar]
- [9].Hor S, Pirzer H, Dumoutier L, et al. The T-cell lymphokine interleukin-26 targets epithelial cells through the interleukin-20 receptor 1 and interleukin-10 receptor 2 chains. J Biol Chem 2004;279:33343–51. [DOI] [PubMed] [Google Scholar]
- [10].Sheikh F, Baurin VV, Lewis-Antes A, et al. Cutting edge: IL-26 signals through a novel receptor complex composed of IL-20 receptor 1 and IL-10 receptor 2. J Immunol 2004;172:2006–10. [DOI] [PubMed] [Google Scholar]
- [11].Fujii M, Nishida A, Imaeda H, et al. Expression of Interleukin-26 is upregulated in inflammatory bowel disease. World J Gastroenterol 2017;23:5519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Miot C, Beaumont E, Duluc D, et al. IL-26 is overexpressed in chronically HCV-infected patients and enhances TRAIL-mediated cytotoxicity and interferon production by human NK cells. Gut 2015;64:1466–75. [DOI] [PubMed] [Google Scholar]
- [13].Truelove AL, Oleksyk TK, Shrestha S, et al. Evaluation of IL10, IL19 and IL20 gene polymorphisms and chronic hepatitis B infection outcome. Int J Immunogenet 2008;35:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ge J, Wang K, Meng QH, et al. Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J Clin Immunol 2010;30:60–7. [DOI] [PubMed] [Google Scholar]
- [15].Zhang JY, Zhang Z, Lin F, et al. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology 2010;51:81–91. [DOI] [PubMed] [Google Scholar]
- [16].Jiang H, Wang J, Zhao W. Lamivudine versus telbivudine in the treatment of chronic hepatitis B: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 2013;32:11–8. [DOI] [PubMed] [Google Scholar]
- [17].Lai CL, Gane E, Liaw YF, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 2007;357:2576–88. [DOI] [PubMed] [Google Scholar]
- [18].Yang X, Li J, Liu J, et al. Relationship of Treg/Th17 balance with HBeAg change in HBeAg-positive chronic hepatitis B patients receiving telbivudine antiviral treatment: a longitudinal observational study. Medicine (Baltimore) 2017;96:e7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Andrews JA, Lewinsohn PM. Suicidal attempts among older adolescents: prevalence and co-occurrence with psychiatric disorders. J Am Acad Child Adolesc Psychiatry 1992;31:655–62. [DOI] [PubMed] [Google Scholar]
- [20].Li A, Yuan Q, Huang Z, et al. Novel double-antigen sandwich immunoassay for human hepatitis B core antibody. Clin Vaccine Immunol 2010;17:464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang J, Cai Y, Ji H, et al. Serum IL-33 levels are associated with liver damage in patients with chronic hepatitis B. J Interferon Cytokine Res 2012;32:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guendisch U, Weiss J, Ecoeur F, et al. Pharmacological inhibition of RORgammat suppresses the Th17 pathway and alleviates arthritis in vivo. PLoS One 2017;12:e0188391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang J, Liu Y, Xie L, et al. Association of IL-17A and IL-17F gene polymorphisms with chronic hepatitis B and hepatitis B virus-related liver cirrhosis in a Chinese population: a case-control study. Clin Res Hepatol Gastroenterol 2016;40:288–96. [DOI] [PubMed] [Google Scholar]
- [24].Braum O, Klages M, Fickenscher H. The cationic cytokine IL-26 differentially modulates virus infection in culture. PLoS One 2013;8:e70281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Che KF, Tengvall S, Levanen B, et al. Interleukin-26 in antibacterial host defense of human lungs. Effects on neutrophil mobilization. Am J Respir Crit Care Med 2014;190:1022–31. [DOI] [PubMed] [Google Scholar]
- [26].Meller S, Di Domizio J, Voo KS, et al. T(H)17 cells promote microbial killing and innate immune sensing of DNA via interleukin 26. Nat Immunol 2015;16:970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Steinmetz OM, Summers SA, Gan PY, et al. The Th17-defining transcription factor RORgammat promotes glomerulonephritis. J Am Soc Nephrol 2011;22:472–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Castro G, Liu X, Ngo K, et al. RORgammat and RORalpha signature genes in human Th17 cells. PLoS One 2017;12:e0181868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


