Abstract
This study aimed to investigate the effect of comprehensive education and care (CEC) program on anxiety, depression, quality of life, and survival in patients with hepatocellular carcinoma (HCC) who underwent surgical resection.
Totally 136 patients with HCC who underwent hepatectomy were randomly assigned to CEC group and control group as 1:1 ratio. CEC group received health education, psychological nursing, caring activity, and telephone condolence, whereas control group received basic health education and rehabilitation for 12 months. Anxiety and depression were assessed by Hospital Anxiety and Depression Scale (HADS); quality of life was evaluated using European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ-C30).
HADS-Anxiety (HADS-A) score was decreased at 9 month (M9) and M12, and reduction in HADS-A score (M12-M0) was greater in CEC group compared with control group. At M12, percentage of anxiety patients was less, but anxiety severity was similar in CEC group compared with control group. HADS-Depression (HADS-D) score was decreased at M12, and reduction in HADS-D score (M12-M0) was greater in CEC group compared with control group. At M12, percentage of depression patients were less but depression severity was similar in CEC group compared with control group. In addition, QLQ-C30 global health status and functional score was increased at M12, and score improvement (M12-M0) was greater in CEC group compared with control group. In addition, overall survival was longer in CEC group compared with control group.
CEC relieves anxiety and depression, improves quality of life, and prolongs survival in patients with HCC underwent surgical resection.
Keywords: anxiety and depression, comprehensive education and care, hepatocellular carcinoma, quality of life, survival
1. Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death with a continued increase in incidence globally.[1] The survival of patients with HCC has been improved during the past decades largely due to the surveillance of HCC upon high-risk population, such as those with hepatitis B/C viruses infection or nonalcoholic fatty liver disease, and surgery for patients with early-stage HCC.[2] However, problems also co-exist with the increased number of HCC survivors, which are the poor mental health and quality of life.[3] Although to a lesser extent, the psychological distress has long plagued the mental health of HCC survivors and encumbering the recovery after surgery.[4,5] Therefore, countermeasures should be adopted to improve the mental state and quality of life of patients with HCC after surgery.
Educational and psychological interventions are a type of supportive care that works by changing the mental state and behavior patterns of patients with cancer, thereby relieve the psychological distress and improve the quality of life. Several randomized controlled trials (RCTs) have reported the efficacy of educational and psychological interventions on reducing anxiety and depression in patients with cancer.[5–9] A meta-analysis of RCT illustrated that psychological interventions remarkably reduce depression in patients with breast cancer after surgery.[8] And educational interventions, such as a self-care education program in patients with gastric cancer after gastrectomy, are illustrated to improve the quality of life of patients with cancer underwent surgery.[6] However, most of the educational and psychological interventions only focus on a single outcome (depression or quality of life), and the effect of such intervention on both psychological distress and quality of life in patients with HCC after surgery is still rare. Therefore, we designed a comprehensive education and care (CEC) program that included health education, psychological nursing, caring activity, and telephone condolence, and explored its effect on anxiety, depression, quality of life, and survival in patients with HCC after surgery.
2. Materials and methods
2.1. Patients
One hundred thirty-six patients with HCC who underwent hepatectomy at The Central Hospital of Wuhan from January 2014 to December 2015 were consecutively recruited in this study. The screening criteria included the following: diagnosed as primary HCC by clinical and histological examination; age more than 18 years; able to understand the study contents and volunteered to participate in the current study; able to independently complete the assessment questionnaires used in the study; life expectancy above 12 months and able to be regularly followed up, which were assessed by the treating physician according to the clinical experience and patients’ conditions. Following patients were excluded: secondary HCC; complicated with other malignancies; other mental diseases except for anxiety or depression (e.g., schizophrenia); severe cognitive impairment (e.g., Alzheimer disease, dementia); pregnant or lactating women.
2.2. Ethics statement
This study was approved by the Institutional Review Board of The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology. The study was conducted in accordance with the principles expressed in the Declaration of Helsinki and adhered to the standards set by the International Conference on Harmonization and Good Clinical Practice. All patients provided written informed consents.
2.3. Baseline data collection
At entry to the study, the full baseline dataset was obtained through interview, direct assessment, and medical records, and the data included demographic features (such as age, sex, and highest education), medical history (such as history of hepatitis B and history of cirrhosis), chronic comorbidities (hypertension, hyperlipidemia, and diabetes), tumor characteristics (such as tumor nodule number, tumor size, Child-Pugh stage, and Barcelona Clinic Liver Cancer stage), and laboratory indexes (alanine transaminase, aspartate transaminase, alkaline phosphatase, total bilirubin, alpha-fetoprotein, carcino-embryonic antigen, and cancer antigen 199).
2.4. Sample size calculation
The sample size was estimated based on predictions of 15% and 40% patients with anxiety at M12 in CEC group and in the control group, respectively. Under the assumption that at least 15% of the participants would drop out, with a power of 80% and a 2-sided 5% level of significance (α), required a sample size of 68 patients in each group.
2.5. Randomization
After patients’ eligibility was confirmed and the informed consents were signed, all of 136 enrolled patients were randomly assigned to CEC group and control group in a 1:1 ratio, with 68 patients in each group. Blocked randomization method was applied to ensure a balanced intergroup assignment, and the block size was set as 4. SAS 9.4 (SAS Institute Inc., Cary, NC) was used to create random numbers and to develop random allocation list. Allocation of patients was performed by a nurse who was not involved in other parts of the study.
2.6. Interventions
After surgery, all patients were given conventional care and adjuvant therapy (if necessary) according to the clinical practice. The conventional care included hepatoprotective measures; close monitoring of vital signs; liver function; hemorrhage; blood routines; urine routines and liver function; prevention of infection in the surgical wound; management of pain, diet, activity, and postoperative complications; and so on. Whether patients needed adjuvant therapy was decided by treating physician based on the patients’ status and clinical practice, and we did not intervene it. When patients entered stable status after surgery, interventions of study were performed.
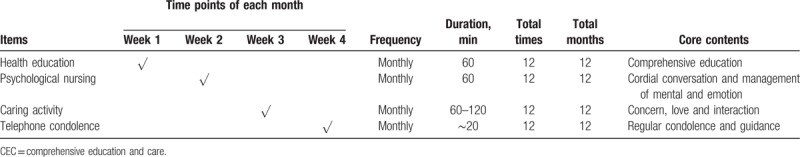
In the CEC group, 12-month CEC program was carried out when stable status of patients was confirmed after surgery (Table 1). The CEC program consisted of 4 items including health education, psychological nursing, caring activity, and telephone condolence, and the details of CEC were as follows:
Table 1.
CEC profiles.
-
1.
Health education: health education materials were given patients at the first week, then monthly education courses (60 minutes each time) were administered to patients by trained nurses at the first week of each month totally for 12 months, and patients were invited to the Rehabilitation Center for receiving education courses. The contents of education included general situation of disease, mature therapeutic techniques, therapy flow, previous successfully treated cases, medication administration, matters needing attention, regular re-examination, management of complications, diet plan, appropriate activity, self-monitoring, emotion, and mental care.
-
2.
Psychological nursing: psychological nursing was carried out by trained nurses though cordial conversation with patients at Rehabilitation Center; it was performed at the second week of each month with 60 minutes each time and lasted for 12 months. On the psychological nursing, trained nurses would comfort and understand patients, encourage patients to pour out distress, help patients eliminate mental tension and emotional excitement and build up confidence to overcome the disease, teach patients self-emotional management and how to maintain a happy mood, and persuade patients to cooperate with treatments and nursing in a positive and optimistic attitude.
-
3.
Caring activity: at the third week of each month, patients and family members were invited to join the monthly caring activity at Rehabilitation Center. It lasted for 60 to 120 minutes each time for a total of 12 months. During the caring activity, patients were encouraged to communicate with each other, and the nurse would organize patients to do appropriate exercises together and participate in some recreational activities, such as playing chess and Mahjong. In addition, the nurse would prepare a small gift for each patient.
-
4.
Telephone condolence: at the fourth week of each month, the nurse would make a phone call to patients to know about their recovery status and mental state, urging them to re-examine regularly, and as soon as possible to give advice for the issues that they met during the recovery. Each telephone support lasted about 20 minutes for a total of 12 months.
In the control group, health education materials were also given to the patients. The instructions about contents of health education materials, care of diet, and mental, as well as other usual guidance were given patients for once (about 60 minutes) on the day of discharge from the hospital. Then the nurse would invite patients to the Rehabilitation Center every 3 months to know about patients’ recovery status and mental state, give patients rehabilitation guidance, help them build up confidence to overcome the disease, and persuade patients to cooperate with treatment, which lasted about 30 minutes each time. As for patients who were unable to attend due to personal reasons, the nurse would call the patients to give rehabilitation guidance (about 30 minutes each time).
2.7. Assessments
At baseline (M0), 3 months after initiation of study (M3), M6, M9, and M12, anxiety and depression of patient were assessed by use of Hospital Anxiety and Depression Scale (HADS), and the quality of life of patients was evaluated using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ-C30 Scale). Before initiation of the study, all patients were given instructions that explained how to fulfill those scales, then they were invited to re-examine and required to independently fulfill those scales at the assessed time points. An independent nurse (unaware of assignment of patients) was responsible for collecting the scales fulfilled by patients at the assessed time points and calculating the HADS-anxiety (HADS-A) score, HADS-depression (HADS-D) score, QLQ-C30 global health status score, QLQ-C30 functional score, and QLQ-C30 symptom score, correspondingly. According to the HADS-A score and HADS-D score, the severity of anxiety and depression was classified as follows: 0 to 7, no anxiety/depression; 8 to 10, mild anxiety/depression; 11 to 14, moderate anxiety/depression; 15 to 21, severe anxiety/depression.[10] In addition, according to QLQ-C30 Scoring Manual, all scales and single-item measures were ranged score of 0 to 100, higher scores in the global health status scale and functional scale indicated better health state and function, whereas higher score in the symptoms scale indicated worse symptoms.[11]
2.8. Follow-up
After 12-month intervention period, all patients were regularly followed up by clinic visit or telephone calls. And the survival data were consecutively collected until December 31, 2018. Median follow-up duration was 35.0 months, and the total follow-up duration was ranging from 4.0 to 59.0 months. Overall survival (OS) was calculated from hepatectomy to patient's death or the last follow-up.
2.9. Statistical analysis
Data were displayed as mean and standard deviation, count (percentage), or median and interquartile range. Comparison between groups was determined by independent-sample t test, Chi-square test, or Wilcoxon rank sum test. Survival curve was constructed by Kaplan-Meier method, and comparison of OS between groups was determined by the log-rank test. All statistical analyses were performed in SPSS software version 24.0 (IBM Corporation, Armonk, NY), and all graphs were plotted by GraphPad Prism software version 7.02 (GraphPad Software Inc., San Diego, CA). All tests were 2 sided and P < .05 indicated a significant difference.
3. Results
3.1. Study flow
A total of 201 patients with HCC who underwent surgical resection were initially invited, whereas 27 patients declined to attend prescreening procedure (Fig. 1). And 174 patients were screed for eligibility, among which 38 patients were excluded, including 26 patients who did not meet the inclusion criteria and 12 patients who disagreed to sign the informed consents. The remaining 136 patients who were eligible were randomized into CEC group (N = 68) and control group (N = 68) as 1:1 ratio. During 12-month intervention period, 5 patients in CEC group withdrew due to loss of follow-up, leaving 63 (92.6%) patients who completed the 12-month follow-up; in control group, 6 patients withdrew due to loss of follow-up, leaving 62 (91.2%) patients who completed the 12-month follow-up. After the 12-month intervention, patients in both groups were further followed up without intervention until December 31, 2018. During the extended follow-up, another 5 patients in CEC group and another 6 patients in control group lost follow-up. Based on intention to treat (ITT) principle, all 68 patients in CEC group and all 68 patients in control group were included in final analyses. Per protocol (PP) analysis yielded similar results with that of ITT; therefore, analysis based on PP analysis was not displayed repeatedly in this work.
Figure 1.
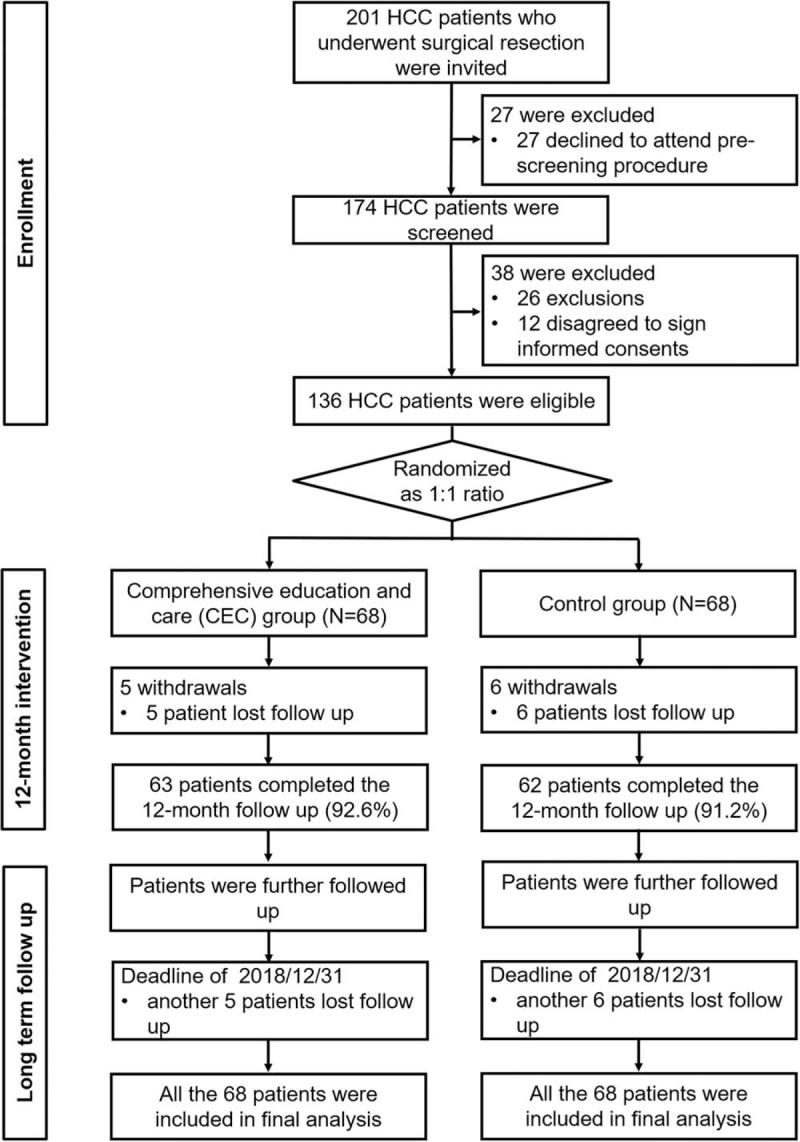
Study flow. CEC = comprehensive education and care, HCC = hepatocellular carcinoma.
3.2. Baseline characteristics
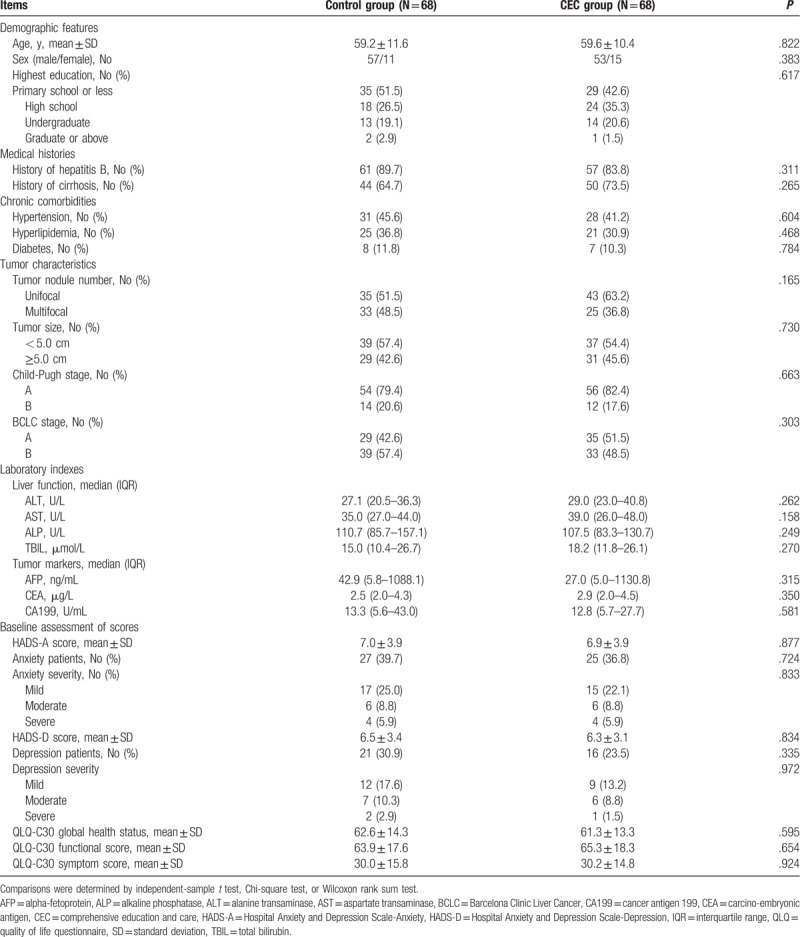
Patients with HCC in CEC group were aged 59.6 ± 10.4 years on average: 53 of them were men and 15 were women (Table 2). In control group, the mean age of HCC patients was 59.2 ± 11.6 years: 57 of them were men and 11 were women. As for the highest education, the numbers of patients with primary school or less, high school, undergraduate, graduate, or above levels were 29 (42.6%), 24 (35.3%), 14 (20.6%), and 1 (1.5%), respectively in CEC group, and 35 (51.5%), 18 (26.5%), 13 (19.1%), and 2 (2.9%), respectively in control group. There was no difference in demographic features, medical histories, chronic comorbidities, tumor characteristics, laboratory indexes, or baseline assessment of scores between CEC group and control group (All P > .05). The detailed baseline characteristics are listed in Table 2.
Table 2.
Baseline characteristics of patients.
3.3. The effect of CEC on anxiety during 12-month intervention
The HADS-A score was similar between CEC group and control group at M0, M3, and M6 (all P > .05), whereas decreased in CEC group compared with control group at M9 (P < .05) and M12 (P < .05) (Fig. 2A), and reduction of HADS-A score (M12-M0) was greater in CEC group compared with control group (P = .003) (Fig. 2B). The number of anxiety patients at M12 was 14 (20.6%) in CEC group, which was lower (P = .024) compared with that in control group [26 (38.2%)] (Fig. 2C). However, the anxiety severity at M12 was similar between the 2 groups (P = .260) (Fig. 2D). The above data indicated that CEC was effective in reducing anxiety in patients with HCC underwent surgical resection.
Figure 2.
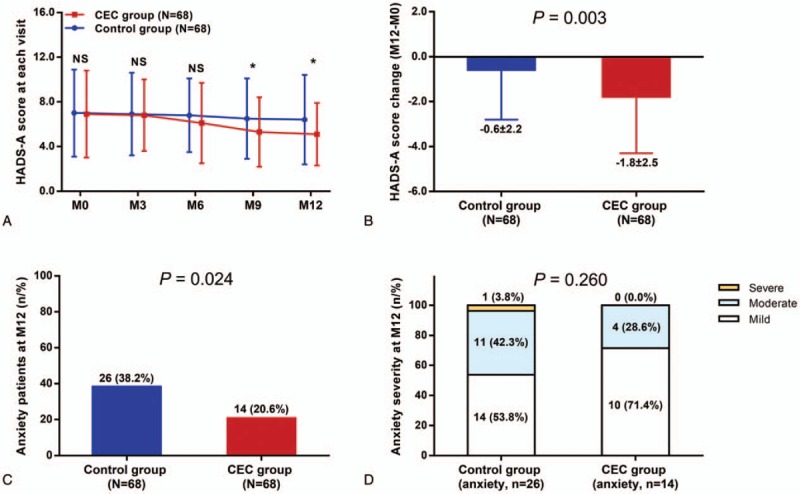
Reduced anxiety by CEC in patients with HCC who underwent surgery. HADS-A score in CEC group was lower compared to control group at M9 and M12 (A) and HADS-A score reduction (M12-M0) was greater in CEC group compared with control group (B). At M12, the percentage of anxiety patients was lower in CEC group compared with control group (C), whereas the anxiety severity was similar between the 2 groups (D). The comparisons between 2 groups were conducted using independent-sample t test, Chi-square test and Wilcoxon rank sum test. P < .05 was considered significant. ∗P < .05. CEC = comprehensive education and care, HADS-A = Hospital Anxiety and Depression Scale-Anxiety, HCC = hepatocellular carcinoma, NS = nonsignificant.
3.4. The effect of CEC on depression during 12-month intervention
The HADS-D score was similar between CEC group and control group at M0, M3, M6, and M9 (all P > .05), whereas decreased in CEC group compared with control group at M12 (P < .05) (Fig. 3A), and reduction of HADS-D score (M12-M0) was larger in CEC group compared with control group (P = .010) (Fig. 3B). There were 11 (16.2%) depression patients in CEC group at M12, which was less compared with that in control group [22 (32.4%)] (P = .028) (Fig. 3C). However, there was no difference in depression severity at M12 between CEC group and control group (P = .255) (Fig. 3D). These suggested that CEC reduced depression in patients with HCC underwent surgical resection.
Figure 3.
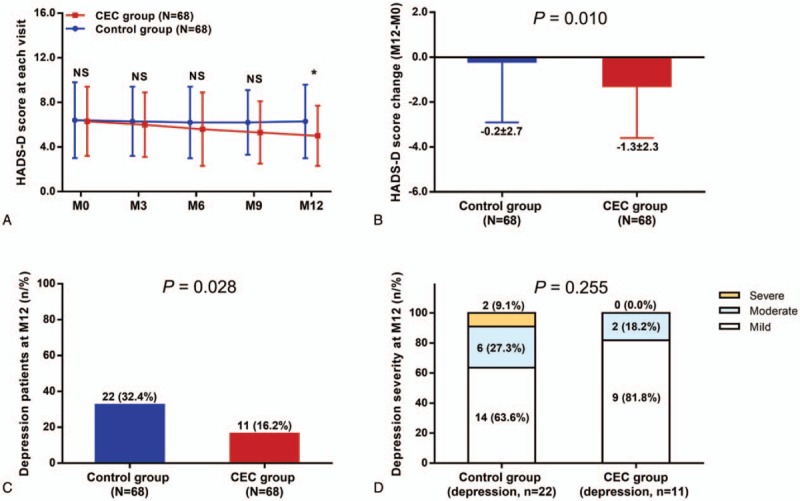
Reduced depression by CEC in patients with HCC who underwent surgery. HADS-D score in CEC group was decreased compared to control group at M12 (A) and the decrease in HADS-D score (M12-M0) was larger in CEC group compared with control group (B). At M12, the percentage of depressive patients was lower in CEC group compared with control group (C), whereas the depression severity was similar between the 2 groups (D). The comparisons between 2 groups were conducted using independent-sample t test, Chi-square test, and Wilcoxon rank sum test. P < .05 was considered significant. ∗P < .05. CEC = comprehensive education and care, HADS-D = Hospital Anxiety and Depression Scale-Depression, HCC = hepatocellular carcinoma, NS = nonsignificant.
3.5. The effect of CEC on quality of life during 12-month intervention
The QLQ-C30 global health status of patients was similar between CEC group and control group at M0, M3, M6, and M9 (all P > .05), whereas increased in CEC group compared with control group at M12 (P < .05) (Fig. 4A), and the rise in QLQ-C30 global health status (M12-M0) was higher in CEC group compared with control group (P = .020) (Fig. 4D). As for QLQ-C30 functional score, it was similar between CEC group and control group at M0, M3, M6, and M9 (all P > .05) but elevated in CEC group compared with control group at M12 (P < .05) (Fig. 4B), and the increase in QLQ-C30 global health status score (M12-M0) was higher in CEC group compared with control group (P = .043) (Fig. 4E). Whereas for QLQ-C30 symptom score, no difference was observed between the 2 groups at M0, M3, M6, M9, or M12 (all P > .05) (Fig. 4C), and the change in QLQ-C30 symptom score was similar between the 2 groups (P = .697) (Fig. 4F). These data revealed that CEC improved QLQ-C30 global health status and QLQ-C30 functional score but not QLQ-C30 symptom score in patients with HCC who underwent surgical resection.
Figure 4.
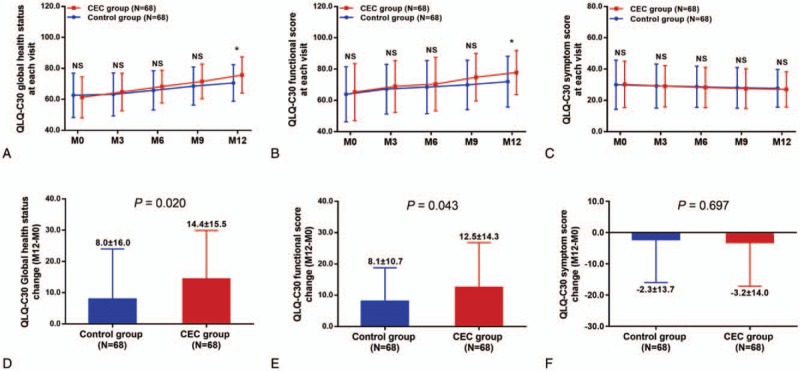
Improved quality of life by CEC in patients with HCC underwent surgery. QLQ-C30 global health status (A) and QLQ-C30 functional score (B) was improved in CEC group compared with control group at M12, whereas QLQ-C30 symptom score was similar between the 2 groups (C). The improvement in QLQ-C30 global health status (M12-M0) (D) and QLQ-C30 functional score (M12-M0) (E) was greater in CEC group compared with control group, whereas change in QLQ-C30 symptom score (M12-M0) was similar between the 2 groups (F). The comparisons between 2 groups were conducted using independent-sample t test. P < .05 was considered significant. ∗P < .05. CEC = comprehensive education and care, HCC = hepatocellular carcinoma, NS = nonsignificant, QLQ-C30 = European Organization for Research and Treatment of Cancer Quality of Life Questionnaire.
3.6. The effect of CEC on survival
The median OS was 37.0 (27.6–46.4 months) in CEC group, which was longer compared with 32.0 (27.0–37.0 months) in control group (P = .026) (Fig. 5).
Figure 5.
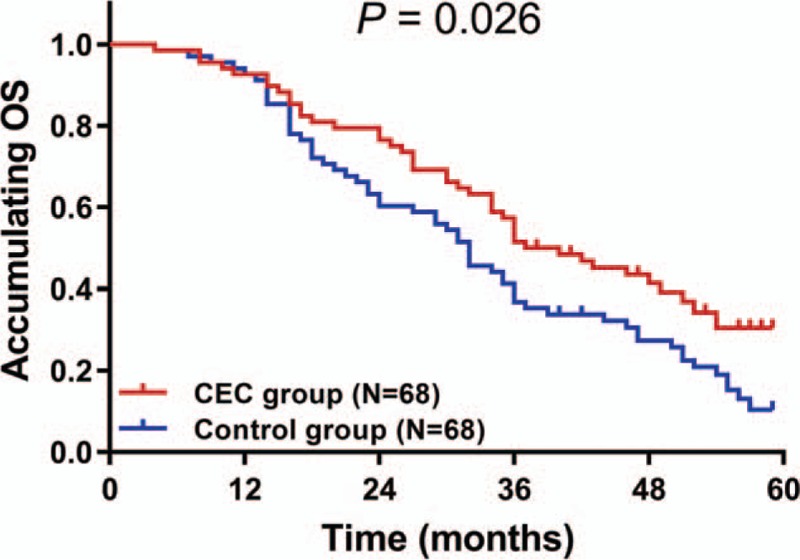
Improved survival by CEC in patients with HCC underwent surgery. The accumulating OS was longer in CEC group compared with control group. Survival curve was constructed by Kaplan-Meier method, and comparison of OS between groups was determined by the log-rank test. P < .05 was considered significant. CEC = comprehensive education and care; HCC = hepatocellular carcinoma; OS = overall survival.
4. Discussion
We compared anxiety, depression, quality of life, and survival between CEC group and control group, and disclosed that CEC effectively reduced anxiety and depression, improved quality of life, and prolonged OS in patients with HCC who underwent surgical resection.
Stress-related psychological factors have profound effects on health in cancer, which arouse people's attention toward psychological therapy for patients with cancer.[12] And the benefits of educational and psychological interventions have been evidenced in several cancers. In Malaysia, a psychoeducational intervention focusing on medical information about cancer, problem-solving skills, and communication is shown to reduce depression and improve the well-being of patients with breast cancer undergoing allopathic medication.[13] Another postoperative educational intervention for patients with laryngeal cancer decreases the self-rating anxiety score and self-rating depression score, as well as improved quality of life.[14] In non–small-cell lung cancer, patients involved in a Web-based health education program present with better mental state, less symptom distress, and improved quality of life compared with controls.[15] In addition, a systemic review about the effectiveness of nursing interventions illustrated that psychological nursing intervention has a significant effect on spiritual well-being, including reducing anxiety and distress, sleep, and fatigue meliorating in various cancer patients.[16] These evidences emphasize the efficiency of educational and psychological interventions in reducing mental distress and improve quality of life in patients with cancer. However, the intervention in most of the study only focuses on a single aspect and the intervention was not comprehensive. Moreover, for patients with HCC who underwent surgical resection, such intervention program is rarely reported. We conducted the CEC program in patients with HCC underwent surgical resection, and used HADS-A/D scores to assess anxiety and depression, which were previously shown to have internal consistency and invariant regarding to sex.[17] Our study disclosed that the anxiety and depression were reduced, and quality of life was improved in CEC group compared with control group within 12-month intervention period, which was consistent with the previously reported interventions. This might be attributable to that the health education sessions in CEC informed patients with HCC about the disease, which alleviated the anxiety and fear caused by the blind to the disease, thereby reduced the psychological distress of patients with HCC. In addition, psychological nursing in CEC directly intervened patients’ mental state from a psychological point of view, thus, ameliorated anxiety and depression of patients with HCC, and improved their quality of life. In addition, the caring activities and frequent phone calls allowed interaction between nurses and patients with HCC to deliver more concern and love, which to some extend soothed patients’ emotion and eliminated the feeling of isolation, thereby relieving mental distress and improved quality of life. In addition, previous evidence suggest that patients’ education can positively or negatively affect anxiety and depression outcomes; however, in our study, there was no difference in level of education between control group and CEC group at baseline (Table 2).[18] Therefore, the effect of education level on patients’ anxiety and depression outcomes was greatly diminished. In addition, age and sex were strong determinants for depression and anxiety in patients with cancer. However, because our study was an RCT, the demographic characteristics including age and sex were matched between CEC group and control group, which minimized the influence of age or sex on study outcomes.
It has been demonstrated that psychological factors are associated with survival in patients with cancer.[19] For instance, depression, hopelessness, and emotional repression are predictive factors for shorter survival in patients with cancer.[20] Based on that, the effects of educational and psychological interventions on survival in patients with cancer have been investigated. One educational and psychological intervention conducted in patients who are surgically treated for regional breast cancer results in reduced risk of breast cancer recurrence and lower risk of death.[21] Another psychological therapy conducted in varying kinds of medically incurable metastatic cancer is also shown to have a life-prolonging effect.[22] These studies display that educational and psychological interventions are associated with favorable survival in patients with cancer. And considering that CEC effectively reduced psychological distress and improved quality of life in patients with HCC, it was also interesting to know its influence on patients’ survival. We observed that the accumulating OS was longer in CEC group compared with control group, suggesting that CEC prolonged the survival of patients with HCC underwent surgical resection. This could be explained by: patients in CEC group were better informed about the disease, so they would perform more actively in disease management and self-care, which might increase their survival. Patients with reduced anxiety and depression might experience less psychological distress and improved quality of life, which reduced suicidal risk. In addition, they might adhere to the postoperative treatments more actively, which enhanced treatment efficacy and thereby improved survival. Therefore, patients in CEC group, whose anxiety and depression were reduced would have longer survival compared to controls.
There were several limitations to our study. Patients’ anxiety, depression, or quality of life assessment was based on single measurement scale, whereas the assessment based on more scales might further verify the effect of CEC. Considering the financial and labor cost, the intervention period in this study was only 12 months. Therefore, longer intervention was needed to better assess the effect of CEC on anxiety, depression, and quality of life in patients with HCC in future study. Although the patients in this study were recruited according to the calculated sample size, a large-scale study was still needed to validate our results.
In conclusion, CEC relieves anxiety and depression, improves quality of life, and prolongs survival in patients with HCC underwent surgical resection.
Author contributions
Conceptualization: Adan Fu.
Data curation: Jingjing Wang, Chenli Yan.
Formal analysis: Jingjing Wang, Chenli Yan.
Methodology: Jingjing Wang, Chenli Yan.
Resources: Adan Fu.
Supervision: Adan Fu.
Writing – original draft: Jingjing Wang, Chenli Yan.
Writing – review and editing: Adan Fu.
Footnotes
Abbreviations: CEC = comprehensive education and care, HADS = Hospital Anxiety and Depression Scale, HADS-A = HADS-Anxiety, HADS-D = HADS-Depression, HCC = hepatocellular carcinoma, ITT = intention to treat, PP = per protocol, QLQ = quality of life questionnaire, RCT = randomized controlled trial.
How to cite this article: Wang J, Yan C, Fu A. A randomized clinical trial of comprehensive education and care program compared to basic care for reducing anxiety and depression, improving quality of life and survival in hepatocellular carcinoma patients underwent surgery. Medicine. 2019;98:44(e17552).
JW and CY contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301–14. [DOI] [PubMed] [Google Scholar]
- [2].Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin 2012;62:394–9. [DOI] [PubMed] [Google Scholar]
- [3].Tang F, Wang J, Tang Z, et al. Quality of life and its association with physical activity among different types of cancer survivors. PLoS One 2016;11:e0164971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chiu CC, Lee KT, Wang JJ, et al. Health-related quality of life before and after surgical resection of hepatocellular carcinoma: a prospective study. Asian Pac J Cancer Prev 2018;19:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim YS, Do H, Lee JW, et al. Patient reporting pain intensity immediately after surgery can be associated with underlying depression in women with breast cancer. Psychooncology 2016;25:308–15. [DOI] [PubMed] [Google Scholar]
- [6].Pool MK, Nadrian H, Pasha N. Effects of a self-care education program on quality of life after surgery in patients with esophageal cancer. Gastroenterol Nurs 2012;35:332–40. [DOI] [PubMed] [Google Scholar]
- [7].Thomas ML, Elliott JE, Rao SM, et al. A randomized, clinical trial of education or motivational-interviewing-based coaching compared to usual care to improve cancer pain management. Oncol Nurs Forum 2012;39:39–49. [DOI] [PubMed] [Google Scholar]
- [8].Xiao F, Song X, Chen Q, et al. Effectiveness of psychological interventions on depression in patients after breast cancer surgery: a meta-analysis of randomized controlled trials. Clin Breast Cancer 2017;17:171–9. [DOI] [PubMed] [Google Scholar]
- [9].Li Z, Geng W, Yin J, et al. Effect of one comprehensive education course to lower anxiety and depression among Chinese breast cancer patients during the postoperative radiotherapy period—one randomized clinical trial. Radiat Oncol 2018;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- [11].Tung HY, Chao TB, Lin YH, et al. Depression, fatigue, and QoL in colorectal cancer patients during and after treatment. West J Nurs Res 2016;38:893–908. [DOI] [PubMed] [Google Scholar]
- [12].Lehto US, Ojanen M, Vakeva A, et al. Early quality-of-life and psychological predictors of disease-free time and survival in localized prostate cancer. Qual Life Res 2019;28:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ram S, Narayanasamy R, Barua A. Effectiveness of group psycho-education on well-being and depression among breast cancer survivors of Melaka, Malaysia. Indian J Palliat Care 2013;19:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Han J, Nian H, Zheng ZY, et al. Effects of health education intervention on negative emotion and quality of life of patients with laryngeal cancer after postoperative radiotherapy. Cancer Radiother 2018;22:1–8. [DOI] [PubMed] [Google Scholar]
- [15].Huang CC, Kuo HP, Lin YE, et al. Effects of a web-based health education program on quality of life and symptom distress of initially diagnosed advanced non-small cell lung cancer patients: a randomized controlled trial. J Cancer Educ 2019;34:41–9. [DOI] [PubMed] [Google Scholar]
- [16].Tuominen L, Stolt M, Meretoja R, et al. Effectiveness of nursing interventions among patients with cancer: an overview of systematic reviews. J Clin Nurs 2019;28:2401–19. [DOI] [PubMed] [Google Scholar]
- [17].Djukanovic I, Carlsson J, Arestedt K. Is the Hospital Anxiety and Depression Scale (HADS) a valid measure in a general population 65-80 years old? A psychometric evaluation study. Health Qual Life Outcomes 2017;15:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sharma Dhital P, Sharma K, Poudel P, et al. Anxiety and depression among patients with coronary artery disease attending at a cardiac center, Kathmandu, Nepal. Nurs Res Pract 2018;2018:4181952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Watson M, Haviland JS, Greer S, et al. Influence of psychological response on survival in breast cancer: a population-based cohort study. Lancet 1999;354:1331–6. [DOI] [PubMed] [Google Scholar]
- [20].Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med 2010;40:1797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer 2008;113:3450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cunningham AJ, Phillips C, Lockwood GA, et al. Association of involvement in psychological self-regulation with longer survival in patients with metastatic cancer: an exploratory study. Adv Mind Body Med 2000;16:276–87. [DOI] [PubMed] [Google Scholar]


