Abstract
Background:
Previous studies investigated the relation of prenatal exposure to bisphenol A (BPA) and birth outcomes, but these results were inconsistent. The aim of this study was to investigate the relation of prenatal exposure to BPA and birth outcomes, provide comprehensive results based on current studies.
Methods:
The PubMed, Cochrane databases, and Web of Science databases were searched systematically by two researchers respectively from their inceptions to Oct. 2018, using the following keywords “bisphenol A, birth weight, birth length, head circumference, gestational age, birth outcomes”. We extracted β coefficient and 95% confidence interval (CI) or β coefficient and standard deviation (SD) from included study. The subgroup analysis was performed to evaluate the potential heterogeneity between studies. We conducted sensitivity analysis by excluding the each individual study to assess the results whether were stable. Finally, the publication bias was performed by accumulative forest plot.
Results:
Seven studies with 3004 participants met the inclusion criteria. BPA had significant positively association with birth weight (β = 21.92, 95%CI: 1.50–42.35, P = .04). No significant associations were found between BPA and birth length, head circumference and gestational age (All of P > .05).
Conclusion:
This meta-analysis demonstrated that the BPA was positively associated with birth weight. Therefore, further studies are needed to investigate the critical sensitive period of influencing fetal development and to investigate the difference on gender.
Keywords: birth outcomes, birth weight, bisphenol A
1. Introduction
Bisphenol A (BPA) is used widely in the manufacture of polycarbonate plastics, epoxy resins which are used to line food cans, food and beverage containers, dental sealants, medical tubing, and thermal receipt papers.[1,2] BPA is ubiquitous in our daily life, people may get exposed to it through many ways. The studies indicate that BPA can release from the polycarbonate drinking bottles, food and beverage containers, dental sealants,[1,3,4] but ingesting food and water in daily life can be a main exposure approach.[1,5] Some studies have demonstrated that BPA can be detected from human plasma, urine, amniotic fluid, follicular fluid, placental tissue, breast milk and umbilical cord blood, adipose tissue.[6–9]
BPA is an endocrine disrupting chemicals (EDCs) that can exert estrogenic and anti-androgenic activities, disturb immune system, influence thyroid and neural function.[5,10] The studies confirm that BPA can pass through the placenta,[11–13] influence fetal growth in the uterus, result in adverse birth outcomes finally.[14] Pregnant People are susceptible to EDCs in gestational period and fetus is sensitive to environmental toxicants.[15] Thus, there are increasing concerns about the influence of BPA on birth outcomes. Many cohort studies have been done to investigate the association between BPA and birth outcomes, but these consequences are inconsistent.[16–22] The latest a published meta-analysis only provides evidence of the association between prenatal exposure to BPA and birth weight, and the results are not widely representative.[23] Hence, the aim of this meta-analysis is to provide summarized evidence on the association between prenatal exposure to BPA and birth outcomes based on current published cohort studies.
2. Materials and methods
2.1. Search strategy
The PRISMA (preferred reporting items for systematic review and meta-analyses) protocol was prospectively conducted.[24] The PubMed, Cochrane databases, and Web of Science databases were searched systematically by 2 researchers respectively from their inceptions to Oct. 2018, using the keywords “bisphenol A”, “birth weight”, “birth length”, “head circumference”, “gestational age”, “birth outcomes” without language restrictions. We also searched the reference lists of all acquired studies to avoid missing. The titles and abstracts were screened firstly. Then the remaining studies were reviewed by full text and identified based on the inclusion criteria. The disagreement between two researchers was solved by discussion. The study began in Oct. 2018. Ethical approval was not necessary, as this study was a meta-analysis based on published studies and did not need handle individual patient data.
2.2. Inclusion criteria
-
(1)
A cohort study.
-
(2)
The time of exposure to BPA for pregnant women was prenatal period.
-
(3)
The exposure way of BPA for pregnant women was in daily life.
-
(4)
The birth outcomes included birth weight, birth length, head circumference, or gestational age.
2.3. Data extraction
The following information was extracted through predesigned data extraction content by 2 researchers respectively from each included study: publication year, country, sample size, sample, time of sample collection, limits of detection (LOD), time period, eligible criteria of pregnant women, urinary BPA categorization, adjustment in the model, birth outcomes, results expressed as β coefficient (95%CI)or β coefficient (SD). The discrepancy was solved by discussion.
2.4. Assessment of quality
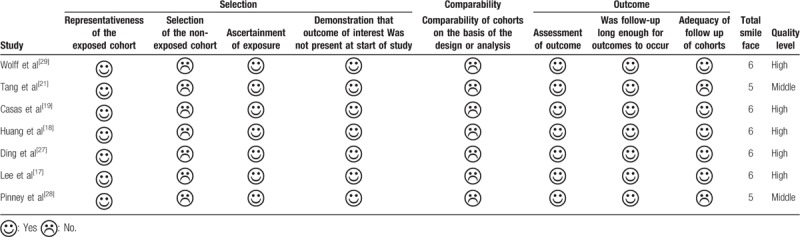
We used the Newcastle-Ottawa Scale (NOS) to assess the methodological quality of included studies.[25] The NOS included 3 categories (Selection, Comparability and Outcome) and 8 items. The NOS ranged from 0 to 9 stars: 4 stars for Selection, 2 stars for Comparability, 3 stars for Outcome. If the total stars was ≥ 6, we regarded the study as high quality, if the total stars was from 3 to 5, we regarded the study as middle quality; if the total stars was <3, we regarded the study as low quality,[26] and we excluded low quality study. The assessment was conducted by 2 researchers respectively, the disagreement was solved by discussion.
2.5. Statistical analysis
The association between prenatal exposure to BPA and birth outcomes was assessed by calculating pooled β coefficient and 95% confidence interval (CI). The heterogeneity of studies was assessed using Chi-squared test and quantified by calculating the I2 statistic. When I2 > 50% or P value <.05 was identified for heterogeneity among studies, we used the random effect model; Otherwise, a fixed effect model was adopted. We conducted subgroup analyses to evaluate the heterogeneity between studies based on country, sample size, LOD, BPA concentration. The sensitivity analysis was performed to assess whether the consequences were influenced by the single study. Finally, we evaluated the publication bias by cumulative forest plot. Meta-analysis was performed using Stata 12.0 version (Stata Corp., College Station, TX). P < .05 was considered statistically significant.
3. Results
3.1. Studies selection and characteristics
The detailed study selection progress was shown in Figure 1. Firstly, 209 studies were identified from PubMed, Web of Science, and Cochrane databases. An additional article was included by scanning the reference lists. Finally, seven studies with 3004 participants were selected into the meta-analysis.[17–19,21,27–29] The data of 2 studies [β (SD)] was acquired by formula transformation.[17,28]
Figure 1.

Flow diagram of studies selection. CI = confidence interval, SD = standard interval.
Table 1 showed the main characteristics of seven studies. Three studies were from USA and Europe,[19,28,29] the remaining studies were from Asia[17,18,21,27]; 6 studies were urine sample,[17–19,21,27,29] 1 study was amniotic fluid sample[28]; 7 studies included birth weight,[17–19,21,27–29] 6 studies included birth length,[17–19,21,27,29] 4 studies included head circumference and gestational age.[18,19,27,29]Table 3 showed the result of quality assessment of included studies. Five studies were high quality,[17–19,27,29] 2 studies were middle quality,[21,28]
Table 1.
Characteristics of included studies.
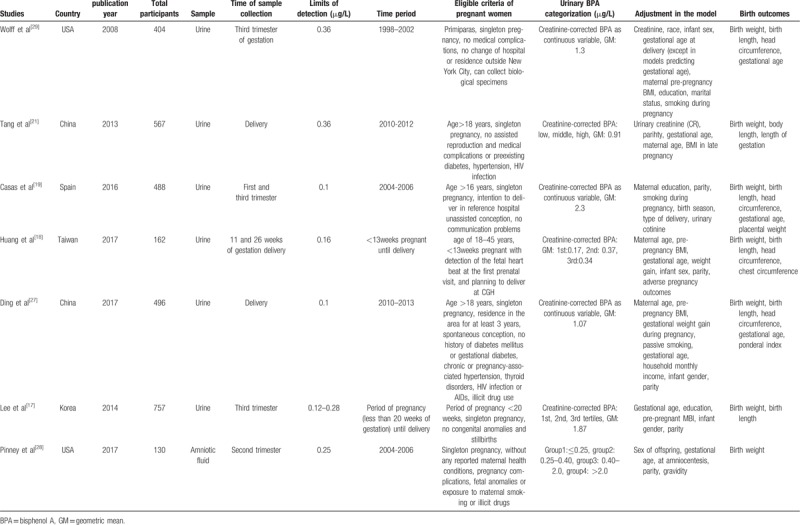
Table 3.
Assessment of methodological quality of included individual studies.
3.2. Main outcomes
3.2.1. Birth weight
The pooled results of 7 studies showed in Figure 2. Heterogeneity was not observed across studies (I2 = 31.8%, P = .137), so fixed effect model was used. There was positively significant association of BPA with birth weight (β = 21.92, 95%CI: 1.50–42.35, P = .04).
Figure 2.
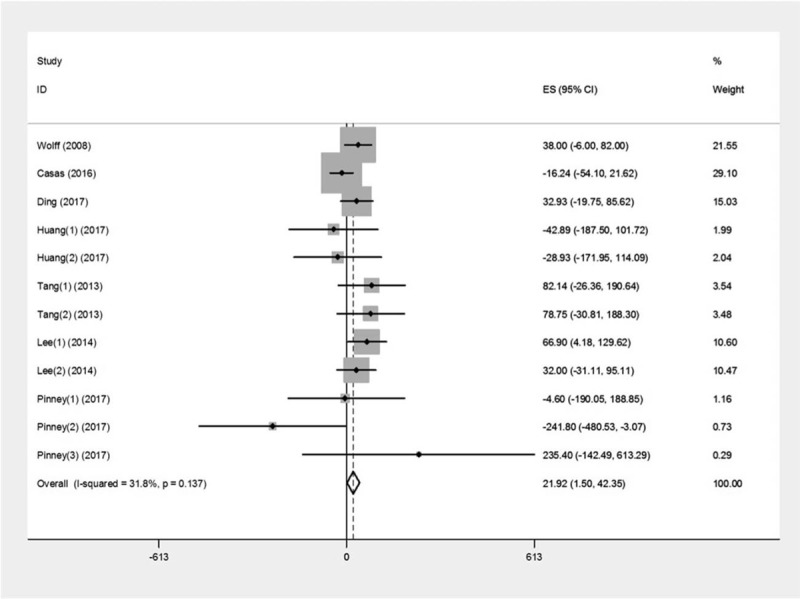
Forest plot of the association between prenatal exposure to BPA and birth weight.
3.2.2. Birth length
The pooled results of 6 studies showed in Figure 3. Heterogeneity was not observed across studies (I2 = 0.0%, P = .996), so fixed effect model was used. There was no significant association of BPA with birth length (β = 0.12, 95%CI: −0.01–0.25, P = .07).
Figure 3.
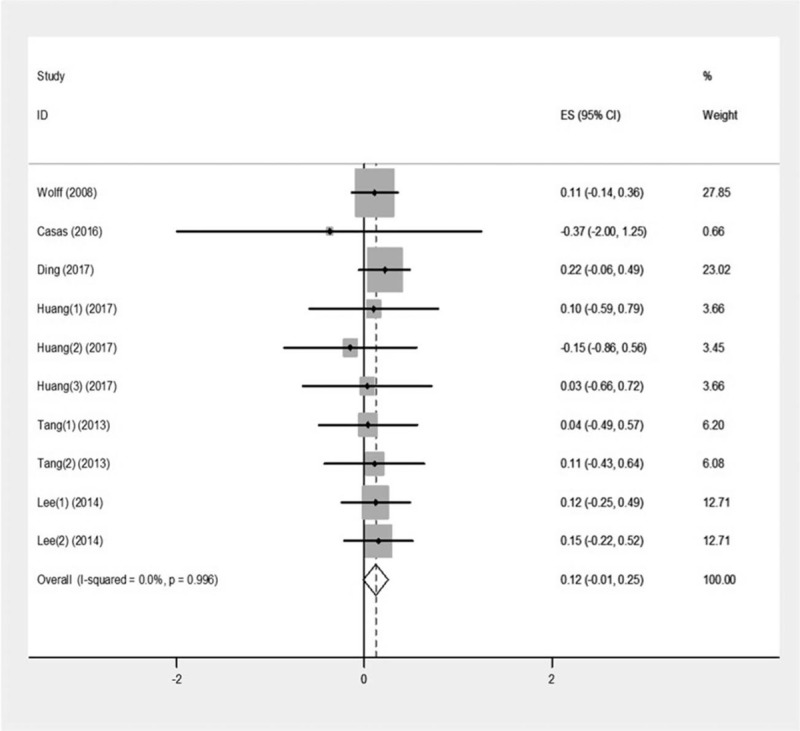
Forest plot of the association between prenatal exposure to BPA and birth length.
3.2.3. Head circumference
Heterogeneity was not observed across studies (I2 = 33.0%, P = .188), so fixed effect model was used. There was no significant association of BPA with head circumference (β = –0.03, 95%CI: –0.14–0.08, P = .60).
3.2.4. Gestational age
Heterogeneity was observed across studies (I2 = 55.4%, P = .062), so random effect model was used. There was no significant association of prenatal exposure to BPA with gestational age (β = –0.07, 95%CI: –0.19–0.06, P = .31).
3.3. Subgroup analysis and sensitivity analysis
The subgroup analysis was conducted based on country, sample size, LOD (Table 2), there was no significant association was found (P > .05). When BPA concentration was ≤ 0.76 μg/L and 0.76–1.3 μg/L, there were positive correlation between BPA and birth weight (β = 70.72, 95%CI: 16.42–125.02; β = 39.63, 95%CI: 7.36–71.91, respectively) (Fig. 4).
Table 2.
Subgroup analysis of the relation of prenatal BPA exposure with birth weight, birth length, head circumference, and gestational age.
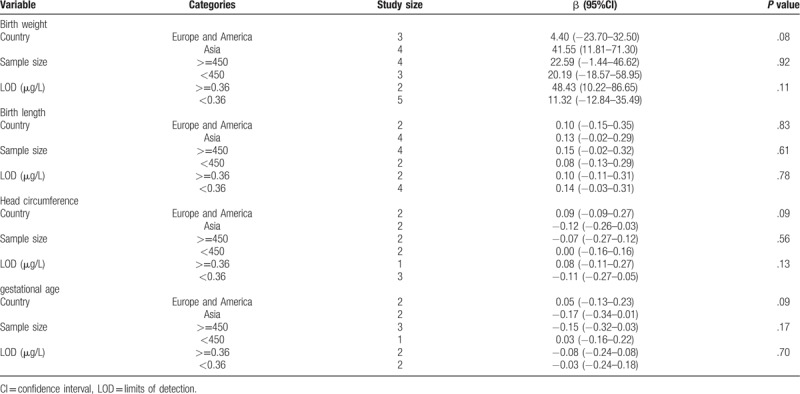
Figure 4.

Forest plot of the association between prenatal BPA exposure levels and birth weight.
We performed sensitivity analysis by excluding the each individual study, these research results did not change evidently.
3.4. Publication bias
The publication bias was evaluated by accumulative forest plot, we did not observe publication bias.
4. Discussion
This meta-analysis indicated that BPA was positively associated with birth weight, however, not associated with birth length, head circumference and gestational age. The sensitivity analysis showed that the results were consistent after excluding small sample study. The publication bias was not found in the study. The results were almost accordant in the subgroup of country, sample size, publication year and LOD.
This result was not consistent with the latest published meta-analysis,[23] which indicated that prenatal exposure to BPA was not associated with birth weight. That may be because the inclusion criteria of studies and analysis methods were different between the 2 studies. The published meta-analysis included preconception exposure and prenatal exposure, and included case-control studies. Our study only included prenatal exposure, and all of the included studies were cohort studies. In addition, our study included every concentration group of BPA, but the published meta-analysis only included the third trimester or the high concentration group, which can make the result present bias. A European meta-analysis also demonstrated that occupational exposure to BPA was not associated with birth weight.[30] But in this European meta-analysis, the BPA exposure way and countries from which the participants come were different from our study, which can make inconsistent results.
The result suggested that there was positive correlation between prenatal exposure to BPA and birth weight. The animal study also indicated that BPA exposure group had higher birth weight compared to the unexposed group.[31] In the current mechanism researches, BPA may cause adverse health effects by acting on nuclear receptors (NRs). The study showed that BPA can promote Adipogenesis by stimulating the activity of glucocorticoid receptor (GR) in 3T3-L1 preadipocytes.[32] Also, BPA can increase adipocyte number by blinding to estrogen receptor (ER).[33] The subgroup analysis showed that this correlation was more pronounced at relative low concentration of exposure. The animal experiments also showed that BPA can affect birth weight at low concentration,[34,35] but the relevant mechanisms can still need to be further explored. Currently, there were less epidemiological studies to explore the association between the correlation and BPA concentration. Therefore, more prospective studies should be done to investigate the impact of BPA concentration on birth outcomes.
Gender may be a source of heterogeneity, but subgroup analysis was not performed due to data limitation. Relevant studies revealed that there were gender differences on the association between prenatal exposure to BPA and birth outcomes.[17,20,21,27,36,37] Animal experiments also observed gender-specific association.[38,39] Thus, further studies are needed to investigate the association in gender. Gestational period can cause heterogeneity; the subgroup analysis was also not performed due to limited data. The study suggested that late pregnancy can be a sensitive period for exposing to BPA.[40] More researches were needed to explore a sensitive period of BPA exposure in pregnant women.
This study had strict inclusion criteria and exclusion criteria, so the results were reliable. And this study provided summarized evidence about the association between prenatal exposure to BPA and more birth outcomes. But it still had some limitations. First, the sample was not uniform and sample could not represent the authentic exposure level of pregnant women. Second, the definition for study quality cannot be relatively strict. Third, we were unable to analyze the dose-response relationship due to differences in the data description of included studies.
In summary, this meta-analysis reveals that BPA is positively associated with birth weight, but not associated with birth length, head circumference and gestational age. Therefore, further studies are needed to investigate the critical sensitive period of influencing fetal development and to investigate the difference on gender.
Author contributions
Conceptualization: Zhitong Zhou, Xiaofeng Li, Xin Chen.
Data curation: Zhitong Zhou, Yuyang Lei, Wei Wei, Yuxin Zhao, Yizhou Jiang, Ningning Wang.
Formal analysis: Zhitong Zhou, Wei Wei, Yizhou Jiang, Ningning Wang.
Funding acquisition: Xin Chen.
Project administration: Zhitong Zhou, Yuyang Lei, Wei Wei, Yuxin Zhao.
Software: Zhitong Zhou, Yuyang Lei, Wei Wei, Yuxin Zhao, Yizhou Jiang, Ningning Wang.
Supervision: Xiaofeng Li, Xin Chen.
Writing – original draft: Zhitong Zhou, Yuyang Lei, Xin Chen.
Footnotes
Abbreviations: BPA = bisphenol A, CI = confidence interval, EDCs = endocrine disrupting chemicals, GM = geometric mean, LOD = limits of detection, SD = standard deviation.
How to cite this article: Zhou Z, Lei Y, Wei W, Zhao Y, Jiang Y, Wang N, Li X, Chen X. Association between prenatal exposure to bisphenol a and birth outcomes. Medicine. 2019;98:44(e17672).
This work was supported by the National Natural Science Foundation of China under Grant (number 81602871); Liaoning Provincial Doctor Initial Funding of China under Grant (number 20170520127); Liaoning Provincial education department of Scientific and technological research of China under Grant (number L2016032).
The authors have no conflict of interest.
References
- [1].Vandenberg LN, Hauser R, Marcus M, et al. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007;24:139–77. [DOI] [PubMed] [Google Scholar]
- [2].Calafat AM, Kuklenyik Z, Reidy JA, et al. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 2005;113:391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Le HH, Carlson EM, Chua JP, et al. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett 2008;176:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Calafat AM, Ye X, Wong LY, et al. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 2008;116:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mikolajewska K, Stragierowicz J, Gromadzinska J. Bisphenol A - application, sources of exposure and potential risks in infants, children and pregnant women. Int J Occup Med Environ Health 2015;28:209–41. [DOI] [PubMed] [Google Scholar]
- [6].Chen M, Zhu P, Xu B, et al. Determination of nine environmental phenols in urine by ultra-high-performanceliquid chromatography-tandem mass spectrometry. J Anal Toxicol 2012;36:608–15. [DOI] [PubMed] [Google Scholar]
- [7].Vandenberg LN, Chahoud I, Heindel JJ, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cien Saude Colet 2012;17:407–34. [DOI] [PubMed] [Google Scholar]
- [8].Ikezuki Y, Tsutsumi O, Takai Y, et al. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod 2002;17:2839–41. [DOI] [PubMed] [Google Scholar]
- [9].Geens T, Neels H, Covaci A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 2012;87:796–802. [DOI] [PubMed] [Google Scholar]
- [10].Richter CA, Birnbaum LS, Farabollini F, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 2007;24:199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Takahashi O, Oishi S. Disposition of orally administered 2,2-Bis(4-hydroxyphenyl)propane (Bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ Health Perspect 2000;108:931–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Balakrishnan B, Henare K, Thorstensen EB, et al. Transfer of bisphenol A across the human placenta. Am J Obstet Gynecol 2010;202:393.e1-7. [DOI] [PubMed] [Google Scholar]
- [13].Schonfelder G, Wittfoht W, Hopp H, et al. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect 2002;110:A703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Troisi J, Mikelson C, Richards S, et al. Placental concentrations of bisphenol A and birth weight from births in the Southeastern U.S. Placenta 2014;35:947–52. [DOI] [PubMed] [Google Scholar]
- [15].Wigle DT, Arbuckle TE, Turner MC, et al. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev 2008;11:373–517. [DOI] [PubMed] [Google Scholar]
- [16].Miao M, Yuan W, Zhu G, et al. In utero exposure to bisphenol-A and its effect on birth weight of offspring. Reprod Toxicol 2011;32:64–8. [DOI] [PubMed] [Google Scholar]
- [17].Lee BE, Park H, Hong YC, et al. Prenatal bisphenol A and birth outcomes: MOCEH (Mothers and Children's Environmental Health) study. Int J Hyg Environ Health 2014;217:328–34. [DOI] [PubMed] [Google Scholar]
- [18].Huang YF, Pan WC, Tsai YA, et al. Concurrent exposures to nonylphenol, bisphenol A, phthalates, and organophosphate pesticides on birth outcomes: a cohort study in Taipei, Taiwan. Sci Total Environ 2017;607-608:1126–35. [DOI] [PubMed] [Google Scholar]
- [19].Casas M, Valvi D, Ballesteros-Gomez A, et al. Exposure to bisphenol A and phthalates during pregnancy and ultrasound measures of fetal growth in the INMA-Sabadell Cohort. Environ Health Perspect 2016;124:521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chou WC, Chen JL, Lin CF, et al. Biomonitoring of bisphenol A concentrations in maternal and umbilical cord blood in regard to birth outcomes and adipokine expression: a birth cohort study in Taiwan. Environ Health 2011;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tang R, Chen MJ, Ding GD, et al. Associations of prenatal exposure to phenols with birth outcomes. Environ Pollut 2013;178:115–20. [DOI] [PubMed] [Google Scholar]
- [22].Ferguson KK, Meeker JD, Cantonwine DE, et al. Urinary phthalate metabolite and bisphenol A associations with ultrasound and delivery indices of fetal growth. Environ Int 2016;94:531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hu CY, Li FL, Hua XG, et al. The association between prenatal bisphenol A exposure and birth weight: a meta-analysis. Reprod Toxicol 2018;79:21–31. [DOI] [PubMed] [Google Scholar]
- [24].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [25].Wells GA, Shea B, Conell DO, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 2011. [Google Scholar]
- [26].Yang Y, Zhang D, Feng N, et al. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology 2014;147:1031–42. [DOI] [PubMed] [Google Scholar]
- [27].Ding G, Wang C, Vinturache A, et al. Prenatal low-level phenol exposures and birth outcomes in China. Sci Total Environ 2017;607-608:1400–7. [DOI] [PubMed] [Google Scholar]
- [28].Pinney SE, Mesaros CA, Snyder NW, et al. Second trimester amniotic fluid bisphenol A concentration is associated with decreased birth weight in term infants. Reprod Toxicol 2017;67:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wolff MS, Engel SM, Berkowitz GS, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect 2008;116:1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Birks L, Casas M, Garcia AM, et al. Occupational exposure to endocrine-disrupting chemicals and birth weight and length of gestation: a European meta-analysis. Environ Health Perspect 2016;124:1785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang L, Zhang HY, Ma CC, et al. Effect of bisphenol A exposure during early development on glucose metabolism and adipokine expression in adolescent female rats. Mol Cell Toxicol 2013;9:385–91. [Google Scholar]
- [32].Sargis RM, Johnson DN, Choudhury RA, et al. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 2010;18:1283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol 2011;73:135–62. [DOI] [PubMed] [Google Scholar]
- [34].Rubin BS, Murray MK, Damassa DA, et al. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect 2001;109:675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Somm E, Schwitzgebel VM, Toulotte A, et al. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect 2009;117:1549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Huo W, Xia W, Wan Y, et al. Maternal urinary bisphenol A levels and infant low birth weight: a nested case-control study of the Health Baby Cohort in China. Environ Int 2015;85:96–103. [DOI] [PubMed] [Google Scholar]
- [37].Lee YM, Hong YC, Ha M, et al. Prenatal Bisphenol-A exposure affects fetal length growth by maternal glutathione transferase polymorphisms, and neonatal exposure affects child volume growth by sex: from multiregional prospective birth cohort MOCEH study. Sci Total Environ 2018;612:1433–41. [DOI] [PubMed] [Google Scholar]
- [38].Johnson SA, Painter MS, Javurek AB, et al. Sex-dependent effects of developmental exposure to bisphenol A and ethinyl estradiol on metabolic parameters and voluntary physical activity. J Dev Orig Health Dis 2015;6:539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Howdeshell KL, Hotchkiss AK, Thayer KA, et al. Exposure to bisphenol A advances puberty. Nature 1999;401:763–4. [DOI] [PubMed] [Google Scholar]
- [40].Ohtani N, Suda K, Tsuji E, et al. Late pregnancy is vulnerable period for exposure to BPA. J Vet Med Sci 2018;80:536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]


