Abstract
Gallium-68 prostate-specific membrane antigen positron emission tomography-computed tomography (Ga-68 PSMA PET/CT) is an imaging modality that promises improved sensitivity and specificity of detection of prostate cancer lesions based on their increased uptake of PSMA-based radiotracers. It remains an emerging modality that has not yet been endorsed in the guidelines for the management of prostate cancer pending more established evidence to prove its efficacy. The objective of the study is to assess the value of Ga-68 PSMA PET/CT in the detection and localization of patients diagnosed with intermediate or high risk prostate cancer.
Twenty three patients with intermediate or high risk prostate cancer had undergone Ga-68 PSMA PET/CT imaging prior to robotic assisted radical prostatectomy. Surgical specimens were then submitted for histological examinations. Lesions visualized on PET/CT and histology were independently mapped unto a 36-segment (Prostate Imaging Reporting and Data System version 2 [PI-RADS v.2]) map of the prostate. Concordance of visualization on PET/CT as compared to the histology as gold standard reference was then assessed. Lesions visualized on PET/CT and histology were independently mapped unto a 36-segment (PI-RADS v.2) map of the prostate. Concordance of visualization on PET/CT as compared to the histology as gold standard reference was then assessed.
Sensitivity for all lesions identified on Ga-68 PSMA PET/CT was 42.37%; specificity was 88.61%. Both parameters were higher when considering only index lesions for which sensitivity was 68.42% and specificity was 98.23%. Sensitivity for the index lesions in intermediate risk group was 53.2% and was higher in the high risk group reaching 83.33%.
Ga-68 PSMA PET/CT provides accurate localization of tumor lesions in patients with intermediate and high risk prostate cancer.
Keywords: positron emission tomography, positron emission tomography/computed tomography, prostate cancer, prostate specific membrane antigen, radical prostatectomy
1. Introduction
Globally, prostate cancer is the second most frequently diagnosed malignancy in men. It has a higher incidence in developed countries and has been associated with factors such as lack of physical activity and obesity. Deaths due to prostate cancer in these countries have been on the decline and this has been attributed mainly to earlier detection and/or improved treatment options.[1] Noteworthy is that 5-year survival rate in patients with localized disease is close to 100%; yet, it drops to around 28% in patients with advanced stages.[2] This reflects the importance of early detection in improving prognosis.
The most commonly used imaging modalities for prostate cancer are magnetic resonance imaging (MRI) in patients with primary disease and computed tomography scan (CT) along with technetium-99 m (99mTc)-methylene diphosphonate bone scan (BS) in patients with metastatic disease. PET imaging with Choline-based radiotracers have also been used but have only offered limited sensitivity in patients with prostate-specific antigen (PSA) levels of <1 ng/mL.[3]
Prostate specific membrane antigen (PSMA) is a type II transmembrane protein that is normally expressed in the cytoplasm and on the apical surface of prostatic ductal epithelium. In prostate cancer cells, 100 to 1000 folds increase in expression of PSMA is observed.[4] This increase in uptake gives it potential for use in molecular imaging. Small molecule inhibitors of PSMA have been labeled with different radioactive elements, most commonly Gallium-68, to be used as radiotracers in PET imaging of prostate cancer.
The use of Gallium-68 prostate-specific membrane antigen positron emission tomography-computed tomography (Ga-68 PSMA PET/CT) has not yet been endorsed in the guidelines for primary staging of prostate cancer.[5,6] It remains an emerging imaging modality that promises improved sensitivity and specificity of detection of prostate cancer lesions based on their increased uptake of PSMA-based radiotracers. Published literature on Ga-68 PSMA PET/CT in the primary staging of prostate cancer reports encouraging results and higher accuracy as compared to traditional imaging methods. The reported sensitivities from these studies ranged between 67% and 81.1%, and specificity ranged between 74% and 94.1% for all prostate lesions detected. When considering only index lesions, sensitivity was higher and ranged between 71.4% and 81.1%.[7–9]
The objective of the study is to evaluate the value of Ga-68 PSMA PET/CT in the detection and localization of patients diagnosed with intermediate or high risk prostate cancer and to calculate its sensitivity, specificity, and predictive values using the histopathology obtained from robotic radical prostatectomy as gold standard.
2. Materials and methods
A retrospective study was conducted at the American University of Beirut Medical Center which is a tertiary care center located in Beirut, Lebanon. Ethical approval was obtained from the Institutional Review Board (Protocol: BIO-2017-0325).
2.1. Study population
Patients were included if they had performed Ga-68 PSMA PET/CT at our center between March 2016 and December 2017 as part of their preoperative assessment for robotic radical prostatectomy. Only patients whose prostate surgical specimens were available for histologic examination were included. Exclusion criteria included receiving any form of prior treatment. A total of 23 patients were eligible for inclusion in this study.
2.2. Imaging technique and evaluation
Good Manufacturing Practice (GMP) certified 68-Gallium Radiolabelling Kit by Isotope Technologies Garching (Munich, Germany) and single-use sterile cassette by ABX Advanced Biochemical Compounds (ABX) (Radeberg, Germany) were labeled to 10 μg PSMA-11. Each patient received 65 to 178 megabecquerel (MBq) (mean 113.3 ± 21.2 MBq) (1.76–4.81 millicurie [mCi]; mean 3.06 ± 0.57 mCi) of 68Ga-PSMA 11 intravenously. Sixty minutes postinjection of the radiotracer, whole-body images were acquired in supine position using a Philips Gemini TF 16 PET/CT scanner. No adverse reactions to the radiotracer were encountered in any patient.
Images were interpreted with the dedicated commercially available software IntelliSpace Portal 8.0 by Philips Healthcare (Amsterdam, Netherlands), which allows the review of PET, CT and fused imaging data in axial, coronal, and sagittal planes. One board-certified specialist (MH), with 23 years of experience in nuclear medicine and 2 years of experience in reporting Ga-68 PSMA PET/CT scans, reviewed all scans and was blinded to the patients’ clinical information and to the initial transrectal ultrasound guided biopsy. Any focal uptake showing a strong target-to-background ratio compared to surrounding tissue or with maximum standardized uptake value (SUVmax) greater than 2 and did not correspond to physiologic tracer accumulation was considered a lesion positive for malignancy. Additionally, foci with the highest average standardized uptake value were considered index lesions. All positive and index lesions were marked on a 36-segment map of the prostate gland based on the Prostate Imaging Reporting and Data System version 2 (PI-RADS v.2), and their SUVmax values were recorded.
2.3. Surgical technique
All patients underwent robotic assisted radical prostatectomy using the da Vinci Si system. Pelvic lymph node dissection was performed when indicated. The surgeries were performed by a urologist who is fellowship-trained in robotic surgery using a standardized trans-peritoneal 5 port approach with 14 years of experience (AEH). All clinical information including Ga-68 PSMA PET/CT results were reviewed by the primary surgeon and his assistants. No operative or postoperative complications were encountered in any patient.
2.4. Histological technique and evaluation
Surgical specimens were sent to the pathology laboratory at the American University of Beirut Medical Center. The prostate glands were adequately fixed in 10% formalin, then serially sectioned from apex to bladder base into 0.2 cm thick slabs. The total number of slabs varied between the cases depending on the size of the prostate gland. All samples were submitted entirely for histological examination, and all sections were submitted while specifying their location relative to the prostate gland anatomy (ie, apex, middle, or base) and orientation (ie, right or left, anterior or posterior).
One board-certified uropathologist with 14 years of experience (SN) reviewed all pathology slides and was blinded to the patients’ clinical information, initial transrectal ultrasound guided biopsy, and the results of the Ga-68 PSMA PET/CT. For all separate tumor areas, the corresponding Gleason score was determined. Each focus representing tumor was considered a positive lesion. Index lesions were defined as foci with the highest Gleason score. All positive and index lesions were marked on a PI-RADS v.2 map.
2.5. Assessment of concordance
PI-RADS v.2 mapping scheme of the prostate defines 36 regions of the gland. A total of 828 regions for the 23 patients have been examined on both PET/CT and histology. Regions were categorized into positive on both PET/CT and histology, positive on PET/CT and negative on histology, negative on PET/CT and positive on histology, and negative on both PET/CT and histology.
2.6. Statistical analysis
All statistical analysis was performed using SPSS version 25.0 (IBM Corp., Armonk, NY). Categorical variables were described using frequency and percentage. Continuous variables were described using mean and standard deviation. Median and interquartile range was used when appropriate. Measures of diagnostic accuracy were assessed using sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV). Association between continuous variables was assessed using Pearson correlation.
3. Results
3.1. Patient characteristics
All 23 patients included in this study were confirmed to have adenocarcinoma of the prostate on histological examination of the surgical specimen. The mean age of the patients was 69 years (±8.74) with mean body mass index of 29.05 kg/m2 (±4.05). Mean presurgery PSA level was 10.84 ng/mL (±7.48) (Table 1). Of the 23 patients, 21 underwent lymphadenectomy during their surgery. Pathological Gleason score of 7 (4 + 3) was the most common and was present in around 47.8% of the surgical specimens. Extra prostatic extension of the disease was present in 39.1% of the patients and the surgical margin was positive for malignancy in 26.1%. Thirteen patients were classified as intermediate risk according to the D’Amico risk grouping criteria and 10 were classified as high risk. Ga-68 PSMA PET/CT was performed at a median of 21 days (Q1 = 12, Q3 = 38) before robotic assisted radical prostatectomy.
Table 1.
Patient characteristics.
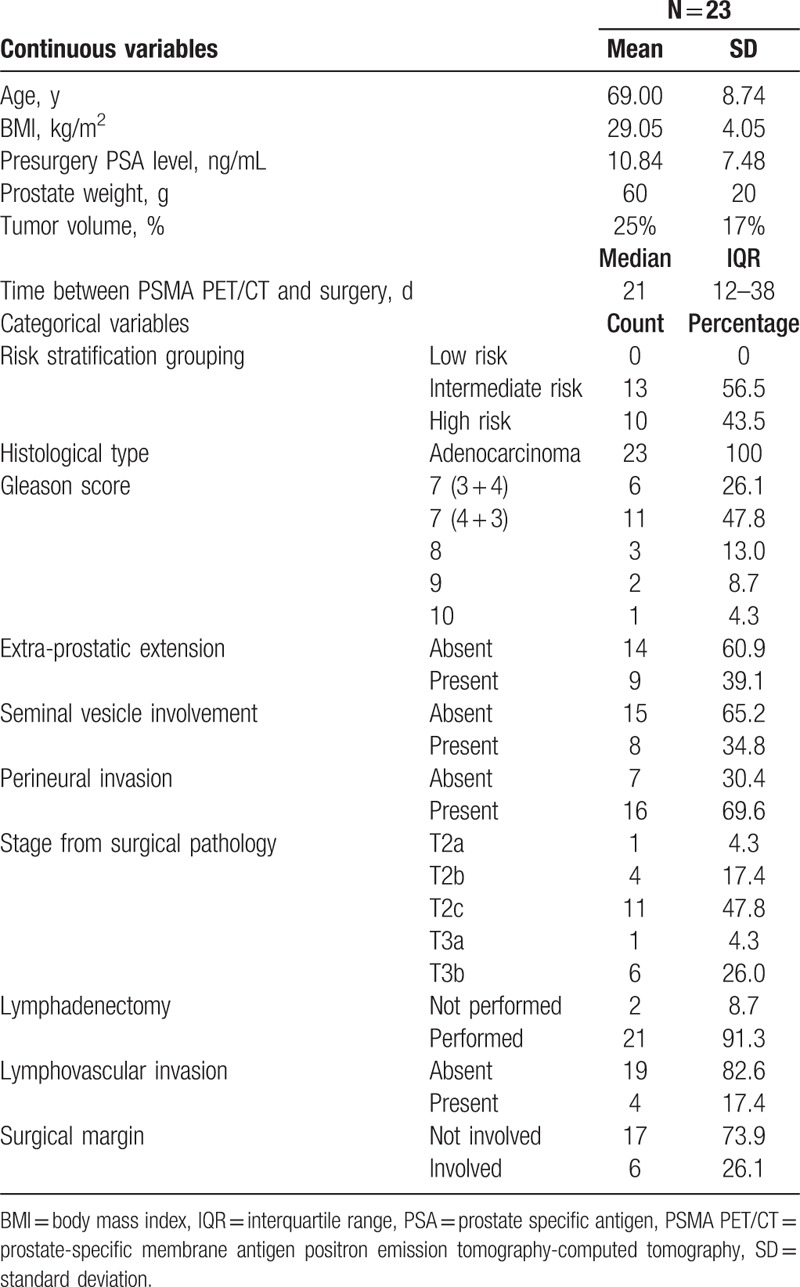
3.2. Ga-68 PSMA PET/CT diagnostic performance for all lesions
Ga-68 PSMA PET/CT identified 204 regions positive for malignancy. A total of 150 of these have been confirmed as true positive by histology and the rest were false positives. Histology further identified 204 regions positive for malignancy that had benign appearance on Ga-68 PSMA PET/CT (false negatives). Sensitivity for all lesions identified on Ga-68 PSMA PET/CT was 42.37% (95% CI, 37.17–47.71); specificity was 88.61% (95% CI, 85.40–91.32). PPV and NPV are respectively 73.53% (95% CI, 67.76–78.59) and 67.31% (95% CI, 65.56–69.36) (Table 2).
Table 2.
Number of total lesions found on prostate-specific membrane antigen positron emission tomography-computed tomography and on histology.
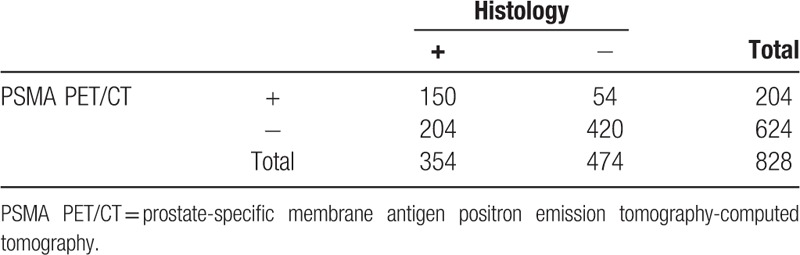
3.3. Ga-68 PSMA PET/CT diagnostic performance for index lesions
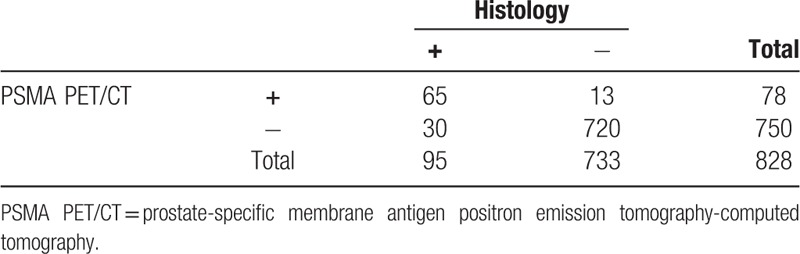
Ga-68 PSMA PET/CT identified 78 regions positive for malignancy that met our criteria of index lesions. A total of 65 of these have been confirmed as true positive by histology and the rest were false positives. Histology further identified 30 regions positive for malignancy and meeting criteria of index lesions that had benign appearance on Ga-68 PSMA PET/CT (false negatives). Examples of true positive, false positive, and false negative index lesions on Ga-68 PSMA PET/CT are correlated to their histological findings in Figure 1. Sensitivity for index lesions identified on Ga-68 PSMA PET/CT was 68.42% (95% CI, 58.08–77.58); specificity was 98.23% (95% CI, 96.99–99.05). PPV and NPV are respectively 83.33% (95% CI, 74.15–89.71) and 96.00% (95% CI, 94.69–96.99) (Table 3).
Figure 1.
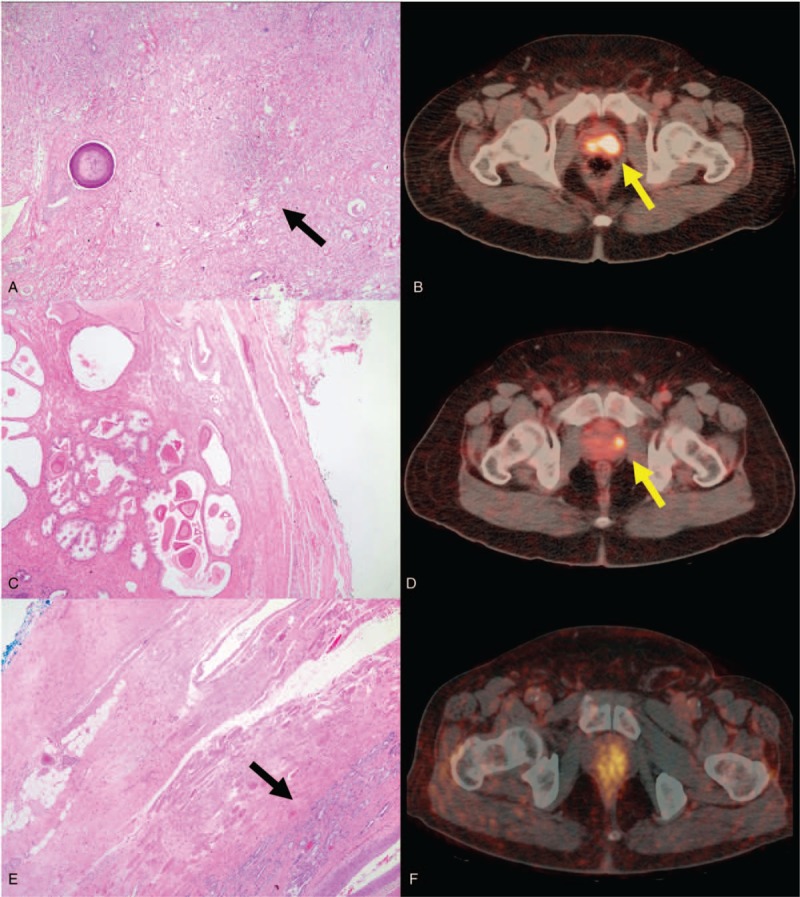
Large focus of carcinoma on histology (A) corresponding to an area with focal uptake on Gallium-68 prostate-specific membrane antigen positron emission tomography-computed tomography (Ga-68 PSMA PET/CT) (B). Absence of carcinoma on histology (C) in an area with focal uptake on Ga-68 PSMA PET/ CT (D). Presence of a small focus of carcinoma (E) in an area without focal uptake on Ga-68 PSMA PET/CT (F).
Table 3.
Number of index lesions found on prostate-specific membrane antigen positron emission tomography-computed tomography and on histology.
3.4. Ga-68 PSMA PET/CT diagnostic performance by risk grouping
In the subgroup analysis by risk group, 13 patients had intermediate risk prostate cancer and 10 patients had high risk prostate cancer. Sensitivity of detection of the index lesion in intermediate risk group was 53.2% (95% CI, 39.2–66.7) and was higher in the high risk group 83.33% (95% 70.4–91.3). Specificity of detection of the index lesions in intermediate risk group was 97.4% (95% CI, 95.4–98.5) and reached 99.4% (95% CI, 97.7–99.8) in the high risk group.
3.5. SUVmax analysis
SUVmax of the index lesion detected on Ga-68 PSMA PET/CT was found to have a moderate linear correlation to Gleason score with a Pearson correlation factor of 0.518 (P = .01). No significant linear correlation was found between SUVmax of the index lesion and the PSA level.
4. Discussion
This study was performed to assess the diagnostic and localization performance of Ga-68 PSMA PET/CT as compared to histologic examination on radical prostatectomy specimen. The findings provide evidence supporting its role in staging of intermediate and high risk prostate cancer patients. It is able to provide very good localization of index lesions with a sensitivity of 68.42% specificity of 98.23%, and as would be expected, both parameters were higher than those for all lesions detected. Sensitivity was higher for high risk compared to intermediate risk patients, specificity was also higher for high risk patients but the difference did not reach statistical significance. Accuracy of localization of lesions is important for patients planned for radiation therapy, as it provides important information that will aid in the planning of their radiation sessions. It is also important for surgical planning and decision on level of nerve sparing to avoid positive surgical margins especially in high risk patients.
When compared to prostate multiparametric MRI (mpMRI), a published study on 75 intermediate and high risk prostate cancer patients described index lesion pooled sensitivity and specificity on T2-weighted, dynamic contrast-enhanced and diffusion-weighted MRI to be 58.5% and 84.3% respectively, while we found the index lesions sensitivity and specificity to be 68.42% and 98.23% respectively. As for the overall all sensitivity and specificity, these were reported as 49.3% and 86.5% respectively compared to our overall sensitivity of 42.37% and specificity of 88.61% on Ga-68 PSMA PET/CT. The PPV and NPV, we found for index lesions, 83.33% and 96.00%, respectively, also surpassed the pooled PPV and NPV on mpMRI which were reported at 72.5% and 74.1%, respectively.[10] Other studies also support these findings that Ga-68 PSMA PET/CT shows better performance in index lesion and overall tumor detection than mpMRI in patients with primary prostate cancer.[7,8]
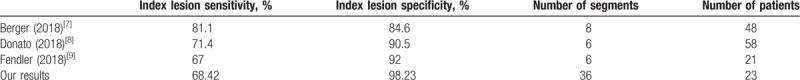
In our study, we opted to use the PI-RADS v.2 sector map which was created to standardize localization and reporting of prostate findings on MRI. This map divides the prostate into 36 segments, 12 in the base, 12 in the midgland, and 12 in the apex.[11] Studies that have looked at the diagnostic performance of Ga-68 PSMA PET/CT have not used a unified segmentation of the prostate. For example, Fendler et al and Donato et al used a 6 segments map and Berger et al used an 8 segments map.[7–9] This created inconsistency and has led to some difficulty in comparing the results obtained. It is evident that following a map with less segments will inflate the sensitivity obtained for localization of lesions. As such, we have decided to follow a well-recognized and validated segmentation of the prostate.
Based on the previously described mapping of the prostate, the reported index lesion sensitivities and specificities were respectively 71.4% and 90.5% by Donato et al, 67% and 92% by Fendler et al, and finally 81.1% and 84.6% by Berger et al. Our index lesion sensitivity was 68.42% and is comparable to what has been described, while our specificity of 98.23% exceeds what they had reported. Variations in these findings between these studies may be somewhat explained by the different methodologies used in each. Nevertheless, they all support the benefit of performing Ga-68 PSMA PET/CT for detection and localization of prostate cancer lesions (Table 4).
Table 4.
Performance of prostate-specific membrane antigen positron emission tomography-computed tomography in detection of index lesions on similar studies.
One study advocated for the use of mpMRI integrated in simultaneous Ga-68 PSMA PET/MRI instead of Ga-68 PSMA PET/CT as lesions in proximity to the bladder were missed on PET/CT but not on PET/MRI.[12] Our center, supported by published literature, uses dual acquisition of PET images at 5 and 60 minutes postinjection of radiotracer. Early imaging at 5 minutes allows for visualization of lesions before radiotracer accumulation in the bladder and provides enough time for adequate uptake of the radiotracer.[13–15] This provides a reasonable solution for centers that lack a PET/MRI facility.
Fendler et al reported that SUVmax of the entire prostate was significantly associated with presurgery PSA level but not Gleason score.[9] In contrast, our results showed that SUVmax of the index lesions, which was the highest in the entire prostate, showed significant correlation with the Gleason score of the surgical specimen. Another report by Bravaccini et al found that PSMA expression on histological examination is significantly correlated with SUVmax, Gleason score, and PSA level.[16] Hence, the lack of significant correlations in our study and that of Fendler et al may be explained the relatively small sample sizes in both studies.
This study was retrospective and nonrandomized which may have introduced selection bias. Also, processing of surgical specimens prior to histological examinations causes shrinkage of the prostate gland, which changes its size from that visualized on PET/CT which may cause differences in anatomical mapping of lesions on the PI-RADS v.2 map.
5. Conclusion
Ga-68 PSMA PET/CT provides accurate localization of index lesions in patients with intermediate and high risk prostate cancer. It has an important role to play in staging of prostate cancer patients and planning for radiation therapy or radical prostatectomy.
Author contributions
Conceptualization: Albert El Hajj, Mohamad B Haidar.
Data curation: Albert El Hajj, Mazen Mansour, Samer Nassif, Mohamad B Haidar.
Formal analysis: Basel Yacoub, Mazen Mansour.
Investigation: Raja Khauli, Mohamad Bulbul, Samer Nassif, Mohamad B Haidar.
Methodology: Albert El Hajj, Mazen Mansour, Raja Khauli, Mohamad Bulbul, Samer Nassif, Mohamad B Haidar.
Project administration: Albert El Hajj, Basel Yacoub, Mohamad B Haidar.
Resources: Albert El Hajj, Mazen Mansour, Samer Nassif.
Supervision: Mohamad B Haidar.
Validation: Basel Yacoub, Raja Khauli, Mohamad Bulbul.
Visualization: Basel Yacoub.
Writing – original draft: Basel Yacoub, Mazen Mansour.
Writing – review & editing: Albert El Hajj, Basel Yacoub, Raja Khauli, Mohamad Bulbul, Mohamad B Haidar.
Footnotes
Abbreviations: Ga-68 PSMA PET/CT = Gallium-68 prostate-specific membrane antigen positron emission tomography-computed tomography, mpMRI = multiparametric MRI, NPV = negative predictive value, PI-RADS v.2 = Prostate Imaging Reporting and Data System version 2, PPV = positive predictive value, PSA = prostate-specific antigen, SUVmax = maximum standardized uptake value.
How to cite this article: El Hajj A, Yacoub B, Mansour M, Khauli R, Bulbul M, Nassif S, Haidar MB. Diagnostic performance of Gallium-68 prostate-specific membrane antigen positron emission tomography-computed tomography in intermediate and high risk prostate cancer. Medicine. 2019;98:44(e17491).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- [3].Albisinni S, Artigas C, Aoun F, et al. Clinical impact of 68 Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: preliminary analysis of a multidisciplinary approach. BJU Int 2017;120:197–203. [DOI] [PubMed] [Google Scholar]
- [4].Maurer T, Eiber M, Schwaiger M, et al. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol 2016;13:226. [DOI] [PubMed] [Google Scholar]
- [5].Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol 2018;199:683–90. [DOI] [PubMed] [Google Scholar]
- [6].Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part II: recommended approaches and details of specific care options. J Urol 2018;199:990–7. [DOI] [PubMed] [Google Scholar]
- [7].Berger I, Annabattula C, Lewis J, et al. 68 Ga-PSMA PET/CT vs. mpMRI for locoregional prostate cancer staging: correlation with final histopathology. Prostate Cancer Prostatic Dis 2018;21:204. [DOI] [PubMed] [Google Scholar]
- [8].Donato P, Roberts MJ, Morton A, et al. Improved specificity with 68 Ga PSMA PET/CT to detect clinically significant lesions “invisible” on multiparametric MRI of the prostate: a single institution comparative analysis with radical prostatectomy histology. Eur J Nucl Med Mol Imaging 2018;46:20–30. [DOI] [PubMed] [Google Scholar]
- [9].Fendler WP, Schmidt DF, Wenter V, et al. 68Ga-PSMA PET/CT detects the location and extent of primary prostate cancer. J Nucl Med 2016;57:1720–5. [DOI] [PubMed] [Google Scholar]
- [10].Isebaert S, Van den Bergh L, Haustermans K, et al. Multiparametric MRI for prostate cancer localization in correlation to whole-mount histopathology. J Magn Reson Imaging 2013;37:1392–401. [DOI] [PubMed] [Google Scholar]
- [11].Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS prostate imaging–reporting and data system: 2015, version 2. Eur Urol 2016;69:16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Freitag MT, Radtke JP, Afshar-Oromieh A, et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in 68 Ga-PSMA-11-PET of PET/CT and PET/MRI: comparison with mpMRI integrated in simultaneous PET/MRI. Eur J Nucl Med Mol Imaging 2017;44:776–87. [DOI] [PubMed] [Google Scholar]
- [13].Kabasakal L, Demirci E, Ocak M, et al. Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl Med Commun 2015;36:582–7. [DOI] [PubMed] [Google Scholar]
- [14].Uprimny C, Kroiss AS, Decristoforo C, et al. Early dynamic imaging in 68Ga- PSMA-11 PET/CT allows discrimination of urinary bladder activity and prostate cancer lesions. Eur J Nucl Med Mol Imaging 2017;44:765–75. [DOI] [PubMed] [Google Scholar]
- [15].Perveen G, Arora G, Damle NA, et al. Role of early dynamic positron emission tomography/computed tomography with 68Ga-prostate-specific membrane antigen-HBED-CC in patients with adenocarcinoma prostate: initial results. Indian J Nucl Med 2018;33:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bravaccini S, Puccetti M, Bocchini M, et al. PSMA expression: a potential ally for the pathologist in prostate cancer diagnosis. Sci Rep 2018;8:4254. [DOI] [PMC free article] [PubMed] [Google Scholar]


