Abstract
Liver resection (LR) is the standard procedure for treating colorectal cancer (CRC) hepatic metastasis; however, LR associated with a high recurrence incidence. This study aimed to determine an optimal post-LR adjuvant chemotherapeutic strategy to improve overall long-term patient outcomes. A retrospective study of 490 patients who had undergone curative LR for CRC hepatic metastasis was performed. Patients who underwent post-LR adjuvant chemotherapy demonstrated high overall survival (OS) rates (hazard ratio [HR] = 0.58, P = .002) but not high recurrence-free survival (RFS) rates (HR = 1.02, P = .885). Moreover, OS was significantly longer in patients who underwent 5-fluorouracil + leucovorin (5-FU/LV; HR = 0.63, P = .039), oxaliplatin-based chemotherapy (HR = 0.45, P < .001), or irinotecan-based chemotherapy with bevacizumab (HR = 0.64, P = .040) than in those who did not. Among patients with carcinoembryonic antigen (CEA) levels of <5 ng/mL at 1 month after LR, significant differences were noted only in those who underwent 5-FU/LV (HR = 0.58, P = .035) and oxaliplatin-based chemotherapy (HR = 0.38, P < .001). In conclusion, perioperative CEA levels are crucial in prognosis and treatment of patients with CRC hepatic metastasis after LR. Additionally, certain regimens of adjuvant chemotherapy alongside post-LR CEA levels may provide beneficial results.
Keywords: adjuvant chemotherapy, CEA, colorectal cancer, liver resection, metastasis
1. Introduction
Liver is the most common organ of distant metastasis in primary colorectal cancer (CRC). Liver resection (LR) is the optimal treatment used for treating CRC with hepatic metastasis.[1,2] Peri-LR carcinoembryonic antigen (CEA) levels are potentially associated with post-LR CRC recurrence and patient survival.[3–6] High pre-LR CEA levels are considered poor prognostic factors and predictors of post-LR CRC recurrence. Moreover, high post-LR CEA levels (≥5 ng/mL) are independent prognostic factors for post-LR CRC recurrence.[7,8] In the era of modern chemotherapy, the incorporation of perioperative CEA monitoring with post-LR adjuvant chemotherapy has been proposed as an effective treatment modality.[9,10] Specifically, perioperative chemotherapy may lead to survival benefit in patients with higher pre-LR CEA levels (>30 ng/mL) but not in those with normal pre-LR CEA levels (<5 ng/mL).
Peri- or postmetastasectomy adjuvant chemotherapy may prevent CRC recurrence after metastasis resection.[11–14] However, the optimal adjuvant chemotherapy after resection of liver metastasis in terms of protocol and regimens remains uncertain.[15–17] Thus, relatively few studies have compared the effects of adjuvant chemotherapy on outcomes of patients after LR on the basis of different chemotherapy regimens. In addition, whether post-LR CEA levels can be/serve as surrogate markers for selecting post-LR adjuvant chemotherapy regimens warrants further research.
Therefore, this retrospective study investigated not only the effects of peri-LR CEA levels on patient outcomes but also the role of post-LR adjuvant chemotherapy and its relation with postoperative CEA levels.
2. Materials and methods
2.1. Patient population
A retrospective analysis of patients who had undergone LR with curative intent for CRC hepatic metastasis between January 2008 and March 2016 at Chang Gung Memorial Hospital, Linkou Medical Centre, Taoyuan, Taiwan was performed. The study was fully reviewed and approved by the Internal Review Board of Chang Gung Memorial Hospital at Linkou (201700231B0), and owing to the retrospective design, the requirement for patients’ written informed consent was waived.
2.2. Evaluation of hepatic metastases
In general, all patients with CRC were thoroughly assessed through computed tomography (CT) scans from the neck to pelvic areas before surgery. Positron emission tomography (PET) or PET/CT was occasionally performed in selected patients as appropriate to confirm occult metastasis. The therapeutic strategy for each patient was selected based on consensus of a multidisciplinary committee of CRC, as previously described.[1] The eligibility of LR for CRC hepatic metastases was mainly considered in relation to the ability for complete removal of all metastatic nodules and preservation of adequate remnant liver volume from LR. No patient received simultaneous radiofrequency tumor ablation and LR in this study.
2.3. Perioperative chemotherapy and follow-up
Administration of perioperative chemotherapy was mainly determined according to the consensus of the multidisciplinary committee for CRC, in which the selection of regimens was based on patient's physical condition, the availability and affordability of the chemotherapy drugs, and the criteria of chemotherapeutic drugs covered and reimbursed by National Health Insurance program. In general, neoadjuvant chemotherapy was recommended to patients with hepatic metastases initially considered unresectable or borderline resectable. Subsequently, patients may have been referred for re-evaluation of surgical resection for metastatic lesion after downstaging by neoadjuvant chemotherapy. Post-LR adjuvant chemotherapy was generally recommended for all patients unless it was contraindicated. The options of chemotherapeutic regimens included fluoropyrimidine-based chemotherapy (5-fluorouracil + leucovorin [5-FU/LV] or capecitabine alone), irinotecan-based chemotherapy (leucovorin, 5-fluorouracil, irinotecan [FOLFIRI] or capecitabine plus irinotecan [XELIRI]), oxaliplatin-based chemotherapy (leucovorin, 5-fluorouracil, oxaliplatin [FOLFOX] and capecitabine plus oxaliplatin [XELOX]), and chemotherapy in combination with bioagents such as bevacizumab or cetuximab as appropriate. Usually, the selection of post-LR adjuvant chemotherapeutic regimens was similar to the pre-LR chemotherapeutic regimens or an advanced chemotherapeutic regimens.
All patients who had curative resection of hepatic metastases were regularly followed at our department until death or the end of this study. During follow-up, serum CEA levels and liver ultrasonography were mandatorily assessed at regular intervals. CT or PET/CT scans were performed annually or whenever CRC recurrence was suspected based on the aforementioned clinical assessments.
2.4. Statistical analysis
Recurrence-free survival (RFS) or overall survival (OS) was recorded as the time from the date of LR until the date of disease recurrence or death from any cause. Survival analysis was based on the Kaplan–Meier method and compared using the log-rank test. Categorical and continuous variables were compared using the chi-square and Student t tests, respectively. Hazard ratios (HRs) and 95% confidence intervals (CIs) were assessed using Cox proportional hazards models. All statistical analyses were performed on SPSS for Windows (version 20.0; IBM Inc., Armonk, NY). A P value of <.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
The study recruited 490 patients (332 men [67.8%] and 158 women [32.2%]); median age at the time of LR, 60.3 (range, 28.8–88.0 years). After LR, the median follow-up period was 42.5 (range, 24–266) months. In total, 324 (66.1%) patients had post-LR CRC recurrence, with a median recurrence duration of 13.38 (range, 0.9–81.0) months. First recurrence site could be single or multiple including 62.7% (203 of 324 patients) single site, and 37.3% (121 of 324 patients) multiple sites. Of single site recurrence, liver was the most recurrence site (64.0%; 130 of 203 patients), followed by lung (19.2%; 39 of 203 patients) and intraabdominal (13.3%; 27 of 203 patients), and 4 patients were brain metastases and 2 patients were bone metastases, and 1 patient was noted recurrence at abdominal wall.
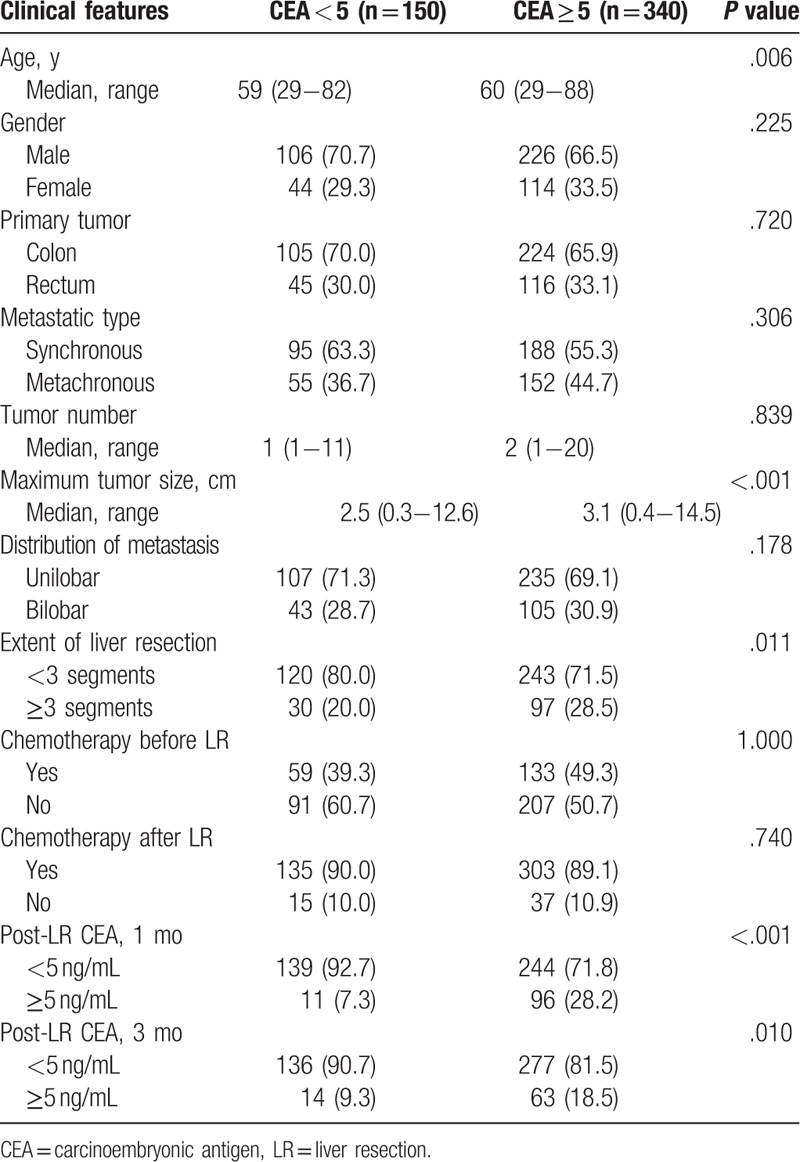
Table 1 summarizes the basic clinical characteristics of the study patients, and clinical features of patients were compared according to pre-LR CEA level (≥5 ng/mL vs <5 ng/mL) as well. The majority of clinical features were similar between the 2 groups. However, patients with pre-LR CEA ≥5 ng/mL had significantly larger tumor size (P < .001) and higher ratio of patients undergone major LR with removal of ≥3 hepatic segments (P = .011) as compared with the other group of pre-LR CEA <5 ng/mL. Additionally, patients in pre-LR CEA <5 ng/mL had a higher percentage of CEA <5 ng/mL at 1 month (P < .001) and 3 months (P = .010) after LR than that of patients in pre-LR CEA ≥5 ng/mL.
Table 1.
Clinical features of patients based on pre-liver resection CEA levels.
During the follow-up period, 273 (55.7%) patients died; the remaining 217 (44.3%) remained alive until the end of this study. The cumulative OS and RFS after LR are illustrated in Figure 1, and no significant differences were observed between patients with pre-LR CEA level ≥5 ng/mL and <5 ng/mL. The 1-, 3-, and 5-year RFS rates in patients with pre-LR CEA <5 ng/mL were 57.1%, 29.1%, and 25.6%, respectively, and the 1-, 3-, and 5-year RFS rates in patients with pre-LR ≥5 ng/mL were 56.5%, 28.4%, and 24.7%, respectively (Fig. 1A, P = .803). The 1-, 3-, and 5-year OS rates for patients with pre-LR CEA ≥5 ng/mL were 91.5%, 56.5%, and 41.4%, respectively, and the 1-, 3-, and 5-year OS rates for patients with pre-LR <5 ng/mL were 90.9%, 65.9%, and 49.7%, respectively (Fig. 1B, P = .189).
Figure 1.
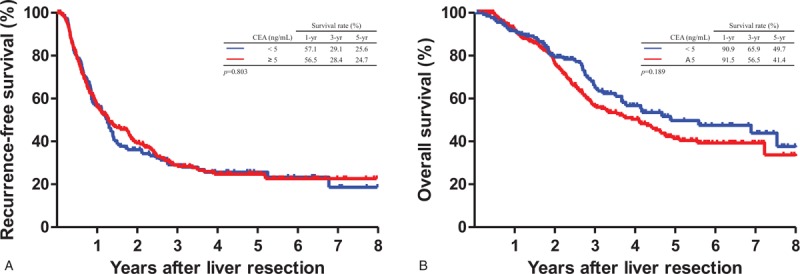
Comparison of cumulative Kaplan−Meier survival curves based on pre-liver resection carcinoembryonic antigen (CEA) levels (≥5 and <5 ng/mL). (A) Recurrence-free survival (P = .803). (B) Overall survival (P = .189). CEA < 5 ng/mL (n = 150) versus CEA ≥ 5 ng/mL (n = 340).
3.2. Relationship between CEA levels and outcomes
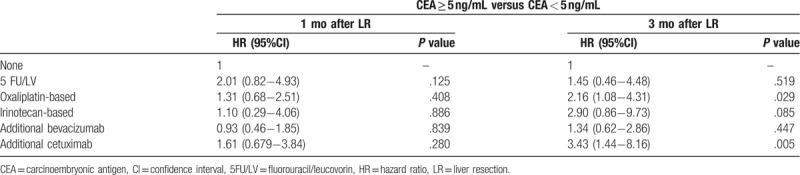
At 1 month post-LR, patients with CEA <5 ng/mL demonstrated significantly favorable RFS (median, 15.6 vs 8.5 months; HR, 1.78; 95% CI, 1.38–2.28; P < .001) and OS (median, 34.4 vs 24.2 months; HR, 2.03; 95% CI, 1.57–2.64; P < .001) as compared with patients with >5 ng/mL CEA (Fig. 2). Table 2 presents the Cox proportional hazard analysis results. At 1, 3, and 5 years, HR comparison for survival demonstrated significant differences; at 3 months post-LR, patients with <5 ng/mL CEA demonstrated similar significant improvements in RFS (median, 15.7 vs 6.12 months; HR, 2.83; 95% CI, 2.16–3.72; P < .001) and OS (median 34.0 vs 23.8 months; HR, 2.50; 95% CI, 1.89–3.31; P < .001) (Fig. 3). Similarly, HR comparisons demonstrated significant differences based on CEA levels 3 months post-LR (Table 2). Additionally, patient outcomes in terms of pre-LR chemotherapy and post-LR CEA levels were analyzed and illustrated in Table 3. There was no significant correlation between pre-LR chemotherapy and post-LR CEA level at 1 month, but oxaliplatin-based chemotherapy and additional cetuximab regimen were significantly associated with post-LR CEA level at 3 months.
Figure 2.
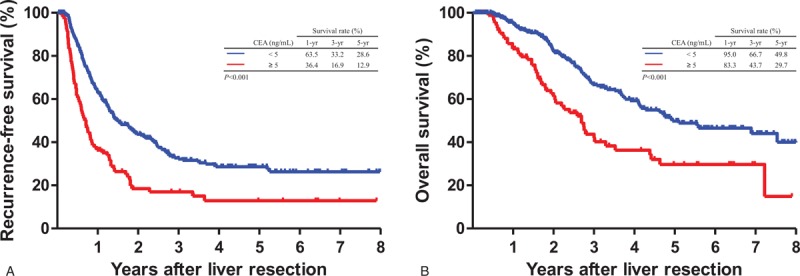
Comparison of Kaplan–Meier recurrence-free survival and overall survival curves based on carcinoembryonic antigen (CEA) levels at 1 month after liver resection. (A) Recurrence-free survival (P < .001). (B) Overall survival (P < .001). CEA < 5 ng/mL (n = 383) versus CEA ≥ 5 ng/mL (n = 107).
Table 2.
Cox proportional hazard analysis of patient outcomes related to post-liver resection carcinoembryonic antigen levels.
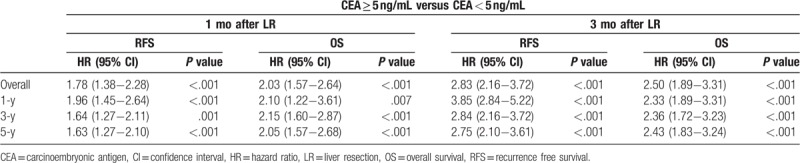
Figure 3.
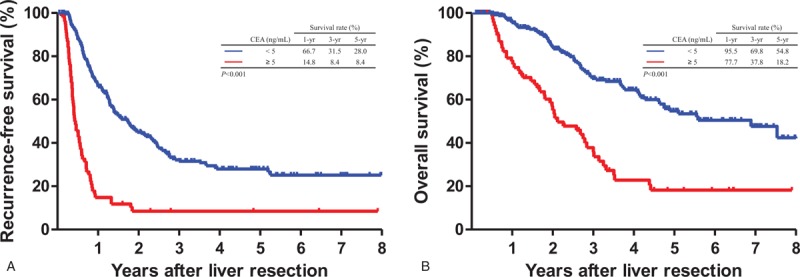
Comparison of Kaplan–Meier recurrence-free survival and overall survival curves according to carcinoembryonic antigen (CEA) levels at 3 months after liver resection. (A) Recurrence-free survival (P < .001). (B) Overall survival (P < .001). CEA < 5 ng/mL (n = 413) versus CEA ≥ 5 ng/mL (n = 77).
Table 3.
Cox proportional hazard analysis of patient outcomes related to pre-liver resection chemotherapy and carcinoembryonic antigen levels.
3.3. Effect of adjuvant chemotherapy on survival outcomes
Of all 490 patients, 438 (89.4%) received post-LR adjuvant chemotherapy. The initial chemotherapeutic regimens comprised fluoropyrimidine (n = 81, 16.5%), irinotecan (n = 37, 7.6%), oxaliplatin (n = 177, 36.0%), bevacizumab (n = 117, 24%), and cetuximab (n = 26, 5.3%). To investigate the impact of post-LR adjuvant chemotherapy on the survival outcomes, RFS and OS were compared between patients with and without post-LR adjuvant chemotherapy. After post-LR adjuvant chemotherapy, median RFS did not differ significantly (9.8 and 9.0 months in patients with and without post-LR adjuvant chemotherapy, respectively; HR, 1.02; 95% CI, 0.69–1.52; P = .885); however, median OS demonstrated a significant difference (25.7 and 22.1 months in patients with and without post-LR adjuvant chemotherapy, respectively; HR = 0.58; 95% CI = 0.40–0.83; P = .002) (Fig. 4).
Figure 4.
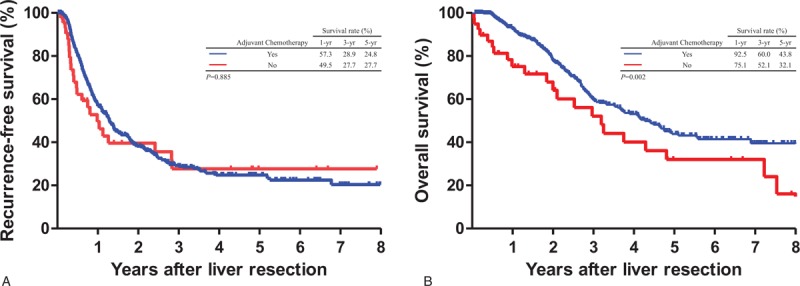
Comparison of Kaplan–Meier survival curves based on post-liver resection adjuvant chemotherapy. Significant differences were not observed for recurrence-free survival (A, P = .885) but were noted for overall survival (B, P = .002). Yes (n = 438) versus No (n = 52).
The clinical features of patients based on post-LR adjuvant chemotherapy are summarized and compared in Table 4. Several significant differences were noted among the 6 treatment subgroups. This cohort composed of heterogeneous clinic-pathological features among different chemotherapy subgroups. However, several significant differences related to chemotherapy subgroups were observed. Subsequently, multivariate regression model was performed to investigate the impact of different regimens on survival outcomes between 5 types of adjuvant chemotherapy (Table 5). The results demonstrated that patients receiving 5-FU/LV (HR, 0.63; 95% CI, 0.41–0.97; P = .039), oxaliplatin-based chemotherapy (HR, 0.45; 95% CI, 0.30–0.67; P < .001), or bevacizumab (HR, 0.64; 95% CI, 0.42–0.98; P = .040) had significantly longer OS than those did not receiving chemotherapy. However, no significant results related to RFS improvement were observed for any of the 5 regimens compared with LR alone.
Table 4.
Clinical features of patients based on post-liver resection adjuvant chemotherapy.
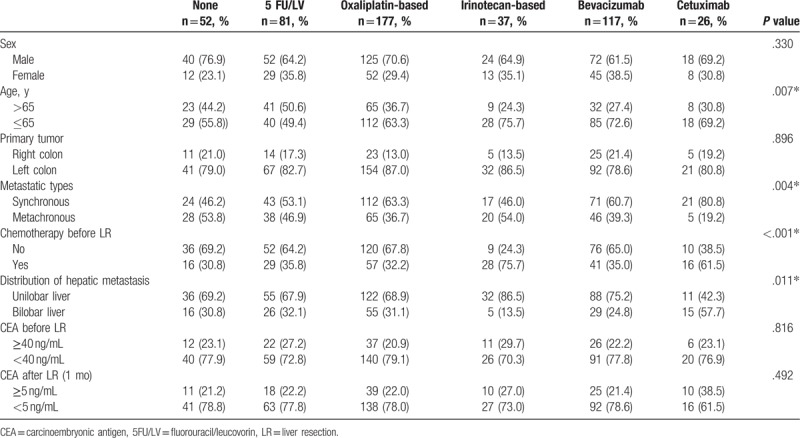
Table 5.
Multiple regression analysis of outcomes related to adjuvant chemotherapy after liver resection.
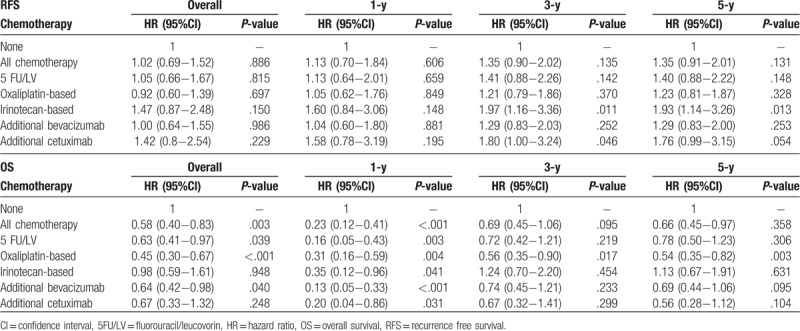
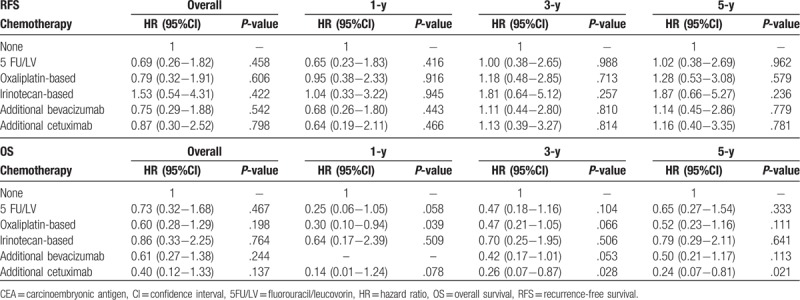
To further clarify outcomes related to posthepatectomy CEA, the subgroup analysis was performed according to CEA levels (<5 and ≥5 ng/mL) 1 month after LR. Patients with <5 ng/mL CEA demonstrated significant differences that persisted for 5-FU/LV (HR, 0.58; 95% CI, 0.35–0.96; P = .035) and oxaliplatin-based chemotherapy (HR, 0.38; 95% CI, 0.24–0.62; P < .001) but not for other chemotherapy types, including irinotecan (HR, 0.93; 95% CI, 0.55–1.7; P = .981), bevacizumab (HR, 0.63; 95% CI, 0.39–1.03; P = .066), and cetuximab (HR, 0.76; 95% CI, 0.32–1.75; P = .521) (Table 6). Significantly, the impact of oxaliplatin-based chemotherapy lasted until post-LR year 5, whereas the effects of 5-FU/LV remained significant only until post-LR year 1. In patients with ≥5 ng/mL CEA, no significant differences related to RFS or OS were noted for any chemotherapy types (Table 7).
Table 6.
Multiple regression analysis of outcomes related to adjuvant chemotherapy in patients with <5 ng/mL CEA 1 mo post-liver resection.
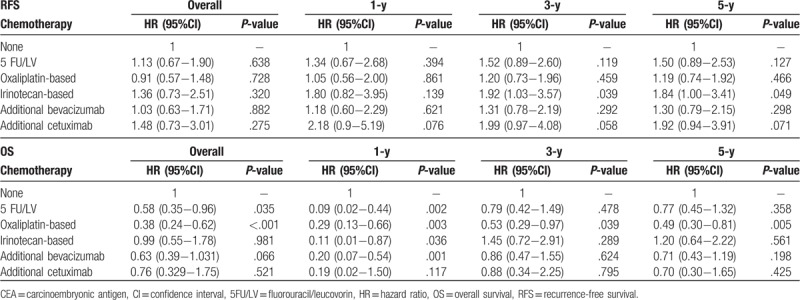
Table 7.
Multiple regression analysis of outcomes related to adjuvant chemotherapy in patients with ≥5 ng/mL CEA 1 mo post-liver resection.
4. Discussion
LR has been the standard treatment for CRC hepatic metastasis and can achieve prolonged survival. However, approximately 75% of patients who had undergone curative-intent LR develop CRC recurrence in post-LR year 1 or 2. Therefore, adjuvant chemotherapy with systemic or intrahepatic artery infusion after LR has been adopted to eradicate micrometastases and prevent CRC recurrence. However, the extent of the benefits of these adjuvant chemotherapies in patients who have undergone curative-intent LR remains unclear. Similarly, the benefit of adding bioagents in this setting for post-LR patients warrants investigation.
Studies have reported the potential benefit of systemic chemotherapy compared with surgery and follow-up alone.[18–20] Moreover, some studies have reported the nonsignificant effects of adjuvant chemotherapy on OS after LR.[21–23] Few recent studies have shown that doublet combination chemotherapy has no significant benefit or even has disadvantages compared with monochemotherapy.[17,24] Moreover, the beneficial effects of additional bioagents with cytotoxic chemotherapy in an adjuvant setting remain inconsistent.[25–27] Thus, recommending effective adjuvant chemotherapy for these patients after LR is difficult.
This retrospective study clarified the role of post-LR adjuvant chemotherapy for CRC hepatic metastasis and its effect on patient outcomes. The results demonstrated that post-LR adjuvant chemotherapy significantly prolonged OS. The adjuvant chemotherapy group was estimated to have a 41.7% increase in OS (HR, 0.58) compared with patients not receiving adjuvant chemotherapy. Moreover, certain chemotherapeutic regimens with 5FU as the backbone with or without oxaliplatin and in combination with bioagents (eg, bevacizumab) had a significant post-LR survival benefit in patients with CRC hepatic metastasis, thereby indicating the importance of chemotherapy in adjuvant settings.
However, adjuvant chemotherapy did not show a significant effect on RFS. In contrast, patients who received certain chemotherapeutic regimens such as irinotecan-based and/or additional cetuximab even had a higher risk of recurrence after LR. These findings are consistent with reports demonstrating a detrimental effect of cetuximab in the adjuvant or neoadjuvant setting of stage III colon cancer.[10] Nonetheless, the current study was not able to well elucidate these results. A possible explanation could be that patients who were subjected to these chemotherapeutic regimens had naturally more severe hepatic metastasis than other patients. As a result, patients who had received irinotecan-based and/or additional cetuximab chemotherapy had a poor outcome in terms of RFS. Additionally, the selection of chemotherapeutic regimens for adjuvant setting might also be affected by the policy of the national health insurance program that only covers and reimburses certain regimens. Hence, it is possible that the policy of national insurance health program would limit oncologist in selection of chemotherapeutic regimens perhaps leading to an existed bias in the current study. Theoretically, adjuvant chemotherapy can eradicate micrometastases. However, identifying subgroup patients that could be considered potential curative resection patients undergoing “true” adjuvant chemotherapy or which subgroup patients as potential noncurative resection cases to be treated as “truly” palliative goal was difficult. Therefore, few studies have used termed “pseudoadjuvant chemotherapy” for aforementioned circumstances. To identify the “true” adjuvant chemotherapy subgroup, this study stratified patients with CRC hepatic metastasis based on serum CEA levels 1 month post-LR (<5 and ≥5 ng/mL). The results indicated the importance of post-LR CEA levels related to adjuvant chemotherapy and chemotherapeutic regimens. In addition, compared with other patients, post-LR adjuvant chemotherapy may benefit more patients with postoperative CEA levels of <5 ng/mL. Consistent with previous studies, the current study supported that CRC recurrence and patient survival are strongly affected by post-LR CEA levels.
In this study, the post-LR CEA level was a significant factor affecting patient RFS and OS. Patients with <5 ng/mL CEA 3 months post-LR had favorable RFS outcomes. Therefore, regular monitoring of CEA levels after LR for metastasis at least within 3 months is strongly recommended. Nevertheless, perioperative FOLFOX may benefit patients with resectable hepatic metastasis, even when preoperative CEA levels are high, although a 5-year OS benefit may not be achieved. Furthermore, the current study observed that postoperative CEA trends were correlated with patient survival and possibly with recurrent patterns after LR for CRC hepatic metastasis. Patients with low post-LR CEA levels were more likely to have a single recurrence site. Therefore, this study concurred with most reports that post-LR CEA levels are important oncological surveillance components, possibly predicting recurrent patterns.
5. Conclusion
In summary, perioperative CEA levels are crucial for defining the prognosis and management of CRC hepatic metastasis after LR. The current study might be limited by its retrospective design; several significant observations may aid in decision-making for therapeutic options to treat CRC hepatic metastasis. The inherent biases might also be associated with retrospective data necessitate caution when interpreting the related results. Nevertheless, in the present study, CEA levels dropping to <5 ng/mL at 1 month post-LR was a favorable factor for both RFS and OS. Regarding post-LR adjuvant chemotherapy use, a beneficial effect may be obtained in terms of use of therapeutic regimens, including monochemotherapy (fluorouracil or leucovorin), oxaliplatin-based chemotherapy, or addition of bioagents (eg, bevacizumab), particularly in patients with <5 ng/mL CEA levels 1 month post-LR. Moreover, in future, post-LR CEA levels might be considered as surrogate markers for therapeutic strategies in terms of selecting post-LR adjuvant chemotherapy. However, to achieve favorable long-term patient outcomes, efforts should be made toward the development of a therapeutic strategy involving surgery and chemotherapeutic regimens.
Acknowledgments
The authors thank grants from the Chang Gung Medical Research Program (CMRPG3I0161) to Kun-Ming Chan.
Author contributions
Conceptualization: Kun-Ming Chan.
Data curation: Hsin-Yuan Hung, Jeng-Fu You, Sum-Fu Chiang, Chen-Fang Lee, Hong-Shiue Chou, Wei-Chen Lee.
Supervision: Wei-Chen Lee.
Writing – original draft: Jy-Ming Chiang.
Writing – review & editing: Kun-Ming Chan.
Footnotes
Abbreviations: CEA = carcinoembryonic antigen, CI = confidence interval, CRC = colorectal cancer, CT = computed tomography, 5-FU/LV = 5-fluorouracil + leucovorin, HR = hazard ratio, LR = liver resection, OS = overall survival, PET = positron emission tomography, RFS = recurrence-free survival.
How to cite this article: Chiang JM, Hung HY, You JF, Chiang SF, Lee CF, Chou HS, Lee WC, Chan KM. Applicability of postoperative carcinoembryonic antigen levels in determining post-liver-resection adjuvant chemotherapy regimens for colorectal cancer hepatic metastasis. Medicine. 2019;98:44(e17696).
Data is available on request through contact with the corresponding author K-MC.
This work was supported by grants from the Chang Gung Medical Research Program (CMRPG3I0161) to K-MC.
The authors have no conflicts of interest to disclose.
References
- [1].Chan KM, Wu TH, Wang YC, et al. Clinical relevance of oncologic prognostic factors in the decision-making of pre-hepatectomy chemotherapy for colorectal cancer hepatic metastasis: the priority of hepatectomy. World J Surg Oncol 2018;16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nordlinger B, Van Cutsem E, Gruenberger T, et al. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol 2009;20:985–92. [DOI] [PubMed] [Google Scholar]
- [3].Dexiang Z, Li R, Ye W, et al. Outcome of patients with colorectal liver metastasis: analysis of 1,613 consecutive cases. Ann Surg Oncol 2012;19:2860–8. [DOI] [PubMed] [Google Scholar]
- [4].Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309–18. discussion 318−321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 1996;77:1254–62. [PubMed] [Google Scholar]
- [6].Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125–35. [DOI] [PubMed] [Google Scholar]
- [7].Araujo RL, Gonen M, Allen P, et al. Positive postoperative CEA is a strong predictor of recurrence for patients after resection for colorectal liver metastases. Ann Surg Oncol 2015;22:3087–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sasaki K, Margonis GA, Andreatos N, et al. Pre-hepatectomy carcinoembryonic antigen (CEA) levels among patients undergoing resection of colorectal liver metastases: do CEA levels still have prognostic implications? HPB (Oxford) 2016;18:1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Polastro L, El Hachem G, Hendlisz A. Pseudoadjuvant chemotherapy in resectable metastatic colorectal cancer. Curr Opin Oncol 2018;30:269–75. [DOI] [PubMed] [Google Scholar]
- [10].Sorbye H, Mauer M, Gruenberger T, et al. Predictive factors for the benefit of perioperative FOLFOX for resectable liver metastasis in colorectal cancer patients (EORTC Intergroup Trial 40983). Ann Surg 2012;255:534–9. [DOI] [PubMed] [Google Scholar]
- [11].Kanemitsu Y, Kato T, Shimizu Y, et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone as treatment for liver metastasis from colorectal cancer: Japan Clinical Oncology Group Study JCOG0603. Jpn J Clin Oncol 2009;39:406–9. [DOI] [PubMed] [Google Scholar]
- [12].Kim HR, Min BS, Kim JS, et al. Efficacy of oxaliplatin-based chemotherapy in curatively resected colorectal cancer with liver metastasis. Oncology 2011;81:175–83. [DOI] [PubMed] [Google Scholar]
- [13].Kim SY, Kim HJ, Hong YS, et al. Resected colorectal liver metastases: does the survival differ according to postoperative chemotherapy regimen? J Surg Oncol 2009;100:713–8. [DOI] [PubMed] [Google Scholar]
- [14].Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208–15. [DOI] [PubMed] [Google Scholar]
- [15].Brandi G, Derenzini E, Falcone A, et al. Adjuvant systemic chemotherapy after putative curative resection of colorectal liver and lung metastases. Clin Colorectal Cancer 2013;12:188–94. [DOI] [PubMed] [Google Scholar]
- [16].Liu JH, Hsieh YY, Chen WS, et al. Adjuvant oxaliplatin- or irinotecan-containing chemotherapy improves overall survival following resection of metachronous colorectal liver metastases. Int J Colorectal Dis 2010;25:1243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ychou M, Hohenberger W, Thezenas S, et al. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol 2009;20:1964–70. [DOI] [PubMed] [Google Scholar]
- [18].Hasegawa K, Saiura A, Takayama T, et al. Adjuvant oral uracil-tegafur with leucovorin for colorectal cancer liver metastases: a randomized controlled trial. PLoS One 2016;11:e0162400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Parks R, Gonen M, Kemeny N, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg 2007;204:753–61. discussion 761−753. [DOI] [PubMed] [Google Scholar]
- [20].Saiura A, Yamamoto J, Hasegawa K, et al. A combination of oral uracil-tegafur plus leucovorin (UFT + LV) is a safe regimen for adjuvant chemotherapy after hepatectomy in patients with colorectal cancer: safety report of the UFT/LV study. Drug Discov Ther 2014;8:48–56. [DOI] [PubMed] [Google Scholar]
- [21].Mauri D, Zarkavelis G, Filis P, et al. Postoperative chemotherapy with single-agent fluoropyrimidines after resection of colorectal cancer liver metastases: a meta-analysis of randomised trials. ESMO Open 2018;3:e000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol 2008;26:4906–11. [DOI] [PubMed] [Google Scholar]
- [23].Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 2006;24:4976–82. [DOI] [PubMed] [Google Scholar]
- [24].Nishioka Y, Moriyama J, Matoba S, et al. Prognostic impact of adjuvant chemotherapy after hepatic resection for synchronous and early metachronous colorectal liver metastases. Dig Surg 2018;35:187–95. [DOI] [PubMed] [Google Scholar]
- [25].Primrose J, Falk S, Finch-Jones M, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol 2014;15:601–11. [DOI] [PubMed] [Google Scholar]
- [26].Rong Z, Martel G, Vandenbroucke-Menu F, et al. Impact of peri-operative bevacizumab on survival in patients with resected colorectal liver metastases: an analysis of the LiverMetSurvey. HPB (Oxford) 2014;16:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Turan N, Benekli M, Koca D, et al. Adjuvant systemic chemotherapy with or without bevacizumab in patients with resected liver metastases from colorectal cancer. Oncology 2013;84:14–21. [DOI] [PubMed] [Google Scholar]


