Abstract
Background:
This study aimed to clarify the change of the expression of placenta-specific 1 (PLAC1) in the placenta of preeclamptic women and to explore the regulatory effects on thophoblast by PLAC1.
Methods:
Nineteen women with preeclampsia and 19 with normal pregnancies were recruited, and then we determined the expression of PLAC1 by immunohistochemistry (IHC) and Western blotting. To observe the effect of hypoxia on the expression of PLAC1, trophoblasts were cultured at the normoxia or hypoxia condition. Small interference of ribonucleic acid (siRNA) was used to silence PLAC1. The proliferation, migration and invasion of trophoblasts were evaluated with cell counting kit-8 and transwell analysis, and the apoptosis of trophoblast was evaluated by flow cytometry with FITC and PI staining.
Results:
Placental PLAC1 expression was significantly decreased in severe preeclampsia compared with control (P < .001). The expression of PLAC1 in trophoblasts was significantly decreased after treated with low oxygen concentration (P = .018). PLAC1 siRNA significantly inhibited the proliferation (P < .001), the migration (P < .001) and invasion (P < .001) of trophoblasts, but increased the apoptosis (P = .004 for Swan-71; P = .031 for Jar).
Conclusions:
The expression of PLAC1 was declined in preeclampsia and this inhibited the function of trophoblast, suggesting PLAC1 may play a role in the development of preeclampsia.
Keywords: apoptosis, invision, migration, PLAC1, preeclampsia, trophoblast
1. Introduction
Preeclampsia, a disorder characterized by hypertension and proteinuria after 20 weeks of gestation and sometimes accompanied by maternal multi-organ dysfunction, affects 3% to 5% of all pregnancies, and is one of the major causes for maternal and infant mortality and morbidity in the perinatal period.[2] The pathogenesis of preeclampsia is not completely known. However, placenta plays a crucial role in the development of this complication.[2,3]
The placenta, a transient organ that connects fetus to her mother, is critical in maintaining normal pregnancy and fetal growth, and is involved in the pathogenesis of pregnancy-complicated diseases especially preeclampsia. In preeclamptic pregnancies, inappropriate spiral arterial remodeling, shadow placentation, inadequate placental perfusion and hypoxia have been observed.[3,4] Impaired trophoblast invasion and migration are the key step for failure of spiral arteries remodeling. Proliferation of non-invading trophoblasts, excess of proliferative immature intermediate trophoblast,[5,6] and increased trophoblast apoptosis was also noticed in preeclampticplacenta.[7–10] However, the mechanisms leading to dysfunction of trophoblast in preeclampsia remains unknown.
PLAC1, a gene named placenta-specific protein 1, is located at the Xq26 chromosome, which has been thought necessary in placental growth.[11] As a placenta-specific protein, PLAC1 is localized to the membranous compartment in the apical region of the syncytiotrophoblast, expresses throughout pregnancy in human placenta.[11–13] PLAC1 is essential for placental and embryonic development since PlAC1 ablation results in placentomegaly and mild intrauterine growth retardation (IUGR).[13] The knockdown of PLAC1 by RNA interference suppresses the invasion and migration of trophoblast cells, attenuates spontaneous syncytialization of primary human cytotrophoblast cells,[12–14] and shortens the distance of the outgrowth of the induced extravilloustrophoblasts (EVTs) column from the cytotrophoblast column of human placental explants.[3,4] These findings indicate PLAC1 is essential for the invasion, migration and syncytialization of human placental trophoblast and thus play an important role in the placental and embryonic development.
More evidences indicated the link between PLAC1 and preeclampsia. As we described before, PLAC1 gene is on the X chromosome, a region near the HPRT locus.[11] Interestingly, the homologous Plac1 gene of mouse is located to a syntenic site on the X chromosome, which maps to the interspecific hybrid placental dysplasia (Ihpd) locus. Ihpd locus has been considered as one of the major genetic determinants for placental development.[14] Generally speaking, hypertrophy of the placenta improves fetal growth, while placental hypotrophy leads to growth defect or even death of the fetus.[1,15] Besides, it has been showed Plac1-null mice is related to intrauterine growth retardation and has a reduced postnatal viability.[1] Very recently, PLAC1 expression was found to be inversely associated with neonatal birth weight.[16] These findings indicate that PLAC1 plays an important role in the placental development both in human and mouse.
For example, mRNA of PLAC1 can be found in maternal plasma, but dispersed rapidly after delivery.[7,17] In addition, PLAC1 mRNA is higher in maternal plasma of preeclamtic women than the normal.[7,18–20] However, the involvement of PLAC1 in preeclampsia has never been investigated. We hypothesized placental expression of PLAC1 may be altered in preeclampsia and this potential change may induce the dysfunction of trophoblast. To verify our hypothesis, we first determined placental levels of PLAC1 protein in women with preeclampsia and normal pregnancy. Moreover, to get insight into the role of PLAC1 in preeclampsia, we also observed the influence of PLAC1 on the functions of trophoblast.
2. Material and methods
2.1. Subjects
Placental tissues were collected after Caesarean section from nineteen women with normal pregnancy and 19 women with severe preeclampsia with the approval from Institutional Ethical Committee of Women's Hospital, School of Medicine, Zhejiang University and informed consents. Preeclampsia was diagnosed and classified according to the criteria recommended by ACOG,[21] and the inclusion and exclusion criteria, which were described in our previous publications.[22]
2.2. Immunohistochemistry staining for PLAC1
For immunohistochemistry, placental tissues were immobilized in 10% formaldehyde and then transferred to paraffin. Paraffin-embedded tissues were cut into 4uM of thickness, then deparaffinized in xylene, dehydrated in alcohol. They were then incubated with Anti-PLAC1 antibody, at dilution of 1:50 at 4°C for 12 hours (cat.no. Ab105395; Abcam, Cambridge, UK) and with horseradish peroxidase (HRP)-conjugated secondary antibodies (cat.no. K5007; Dako, Agilent, Denmark A/S) for 30 minutes at room temperature counterstained. Then stained with 0.5% diaminobenzidine kit with Mayer's haematoxylin (Shanghai genomics, Shanghai, China), The staining of PLAC1 were photographed using a microscope of Olympus CHK, Japan (400×).
2.3. Western blotting
For Western blotting, placental tissues were stored at −80 °C until assay. Proteins extracts (30 μg per lane) were separated on 12% sodium dodeeylsulfate-polyacrylamine gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidenedifluoride (PVDF) membranes (Millipore, Massachusetts, USA), protein concentration was detected through the BCA Protein Kit (Lianke Biotech, China). The membranes were blocked with Tris-buffered saline (TBS) containing 0.1% Tween 20 (TBST) and 5% bovine serum albumin for 1 hour at room temperature, and sequentially incubated with the primary antibodies (anti-PLAC1 antibody: ab105395; Abcam, Cambridge, UK, diluted at 1:500 in TBS/T; anti-actin, Huaan, China, diluted at 1:10000 in TBS/T) overnight at 4oC. The membranes were washed three times with TBS/T and incubated in the TBS/T containing HRP-conjugated secondary antibody at room temperature for 1 hour. The protein bands were detected by enhanced-chemiluminescence (ECL from Fude Biotech, China) and visualized by autoradiography (ImageQuant TL Software, GE healthcare, American). The relative expression of PLAC1 was normalized against internal control β-actin.
2.4. Hypoxia analysis
Trophoblast cells were seeded in 10 cm petri dish at a density of 1×106 cells/dish, and then cultured at the oxygen concentration of 21% (normoxia) or 0.4% (hypoxia) for 48 hours, respectively.
2.5. Cell culture and transfection of siRNA
Cells were cultured as previously describe. Cells were transfected with PLAC1 siRNA (Origene, catalog number: SR307335; concentration: 25 nmol/mL) or scrambled siRNA (Origene; sc37007) using LipofectamineRNAMax (Invitrogen, catalog number: 13778150, America) according to the manufacturer's protocol, and blank group was set without siRNA as control. The transfection medium was replaced with complete medium 24 hours after transfection, and the cells were incubated for further experiment. The expression of PLAC1 was determined with Western blotting as described above.
2.6. Cell proliferation assay
Cells were seeded in 96-well plates with 5000 cells per well. The medium was replaced with the corresponding serum-free medium for 24 hours to synchronize the cells, and then the culture media was replaced with complete medium at the indicated concentrations for 48 hours. Then, 10 ul solution of cell counting kit (CCK) 8 (Dojindo Inc., Kumamoto, Japan) was added, the plates were incubated for 3 hours, and absorbance was measured at 450 nm using a MRX II microplate reader (BioTek Inc., Vermont).
2.7. Cell migration and invasion assay
Cell migration and invasion were analyzed with the transwell analysis. After transfection with PLAC1 siRNA (10 nmol/mL), add 2 × 105 cells in the upper chamber of a Transwell 24-insert plate with medium. For invasion assay, the upper chambers were coated with Matrigel (BD Biosciences, NJ); for migration assay, the coating of Matrigel was omitted. The lower chamber contained 10% FBS medium. After 24 hours, the bottoms of the inserts were fixed in methanol for 10 minutes and stained with hematoxylin and eosin (H&E). The cells that had invaded to the lower surface were photographed and counted using an inverted phase contrast microscope (400 × ).
2.8. Flow cytometric analysis of apoptosis
Swan-71 and Jar were plated in 6-well at a density of 1 × 105 cells/well for 24 hours, and then transfected with siRNA. After 48 hours of transfection, the culture medium was aspirated and the cells were quickly washed twice with ice-cold PBS, and then trypsinized. Cells were collected and re-suspended in 500 ul 1X binding buffer, then added 5 ul Annexin-V-FITC or 10 ul PI solutions (Lianke, China), and incubated for 5 minutes at room temperature in the dark. After that, the rates of apoptotic cells were determined by flow cytometer (BD Biosciences, NJ).
2.9. Statistical analysis
The Kolmogorov-Smirnov test was used to evaluate the distribution of data. Data are presented as the means ± SD of at least three separate experiments and were compared with the analysis of variance (ANOVA) or the Student t test where appropriate. SPSS statistical package (Statistical Analysis System, Chicago, IL, version 20.0) was used for the statistical analysis. Differences were considered statistically significant when P < .05. All experiments were repeated at least 3 times.
3. Results
Table 1 summarized the clinical data of subjects. There were no significant differences in maternal age and fetal gender between normal pregnancy (control) and severe preeclampsia. The significant differences in blood pressure and proteinuria confirmed the diagnosis of preeclampsia, while the differences in gestational age at delivery, and neonatal birth weight were due to preeclampsia.
Table 1.
Clinical characteristics of patients with or without preeclampsia.
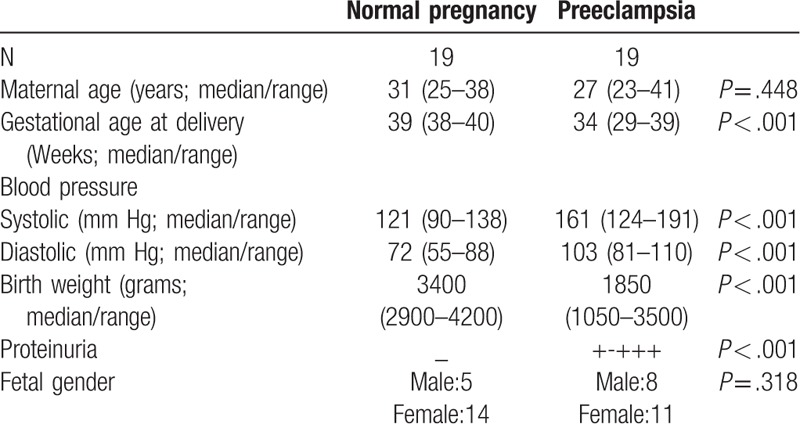
As shown in Figure 1, IHC showed that PLAC1 was mainly expressed in the trophoblasts of placenta (A, B), and placental PLAC1 level was significantly reduced in severe preeclampsia compared with normal pregnancy (Fig. 1: C, D, P < .001).
Figure 1.
Immunohistochemistry staining for PLAC1 and expression of PLAC1 protein in placenta between normal pregnancy (N) and severe preeclampsia (PE). The positive brown staining in the placental tissues show that PLAC1 expressed in the trophoblast both normal pregnancy (A) and severe preeclampsia (B). PLAC1 protein (30 ug per lane) extracts from placental tissues of normal pregnancy and severe preeclampsia was determined by Western blotting, and there were significant decrease of PLAC1 expression in severe preeclampsia (PE) than control (C, D). Values represent mean ± SD, n = 19 per group, ∗∗∗P < .001.
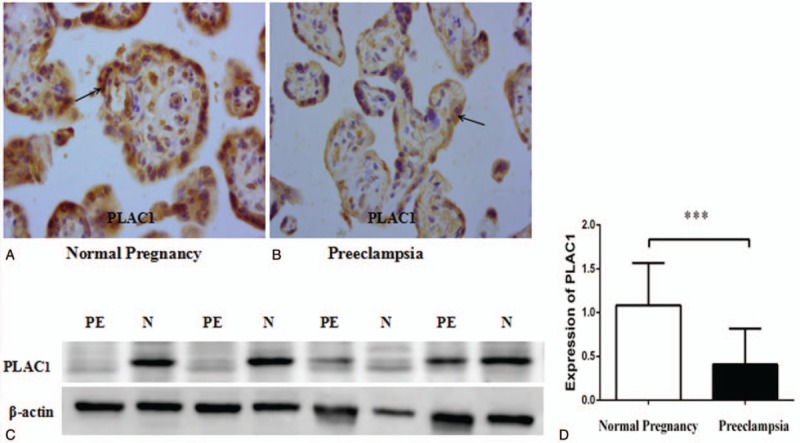
Hypoxia is the characteristic pathology of preeclampsia. To determine if the hypoxia affects PLAC1 expression, trophoblasts (Swan-71 and Jar) were cultured at the oxygen concentration of 21% (normal oxygen concentration) and 0.4% (low oxygen concentration) for 48 hours. We found that PLAC1 expression was significantly reduced when cultured in hypoxia condition compared with the control (Fig. 2: A, B, Swan-71: P = .018).
Figure 2.
The effect on PLAC1 expression by hypoxia andsiRNA in trophoblast cell. Trophoblast cells were incubated in either normoxia (21% O2) or hypoxia (0.4% O2) for 48 h and then we detected the PLAC1 protein of trophoblast by Western blotting (A). Expression of PLAC1 significantly reduced in hypoxia concentration compared with normal oxygen (B). Values represent mean ± SD, n = 3, ∗P < .05. Cells were cultured and transfected with scrambled siRNA, PLAC1 siRNA or blank, PLAC1siRNA significantly reduced PLAC1 expression in trophoblast cell, and there is no significantly difference between blank and scrambled groups (A, B). Values represent mean ± SD, n = 3, ∗P < .05,∗∗P < .01.
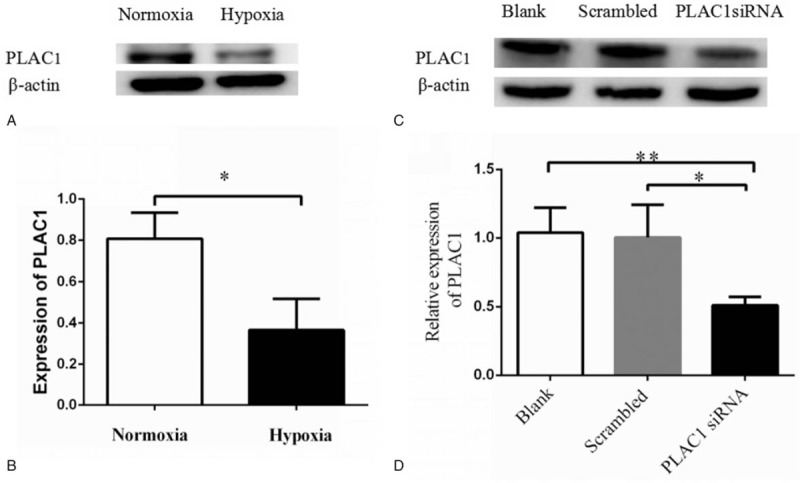
To detect the effect of PLAC1 in trophoblast, cells were transfected by PLAC1 siRNA. Results showed PLAC1 siRNA significantly down-regulate the PLAC1 expression both in Swan-71 and Jar (Fig. 2: C, D, Swan-71: P = .026). PLAC1 siRNA significantly reduced the proliferation of Swan-71 and Jar (Fig. 3: A, Swan-71: P < .001). As shown in Figures 3 and 4, transwell analysis revealed the lower expression of PLAC1 significantly decreased the number of Swan-71 and Jar cells that were found both on the transwell membranes with (Fig. 3: B, C, Swan-71: P < .001) or without Matrigel coating (Fig. 3: D, E, Swan -71, P < .001), and also increased the apoptosis of cells (Fig. 4: A, B, Swan-71: P = .004).
Figure 3.
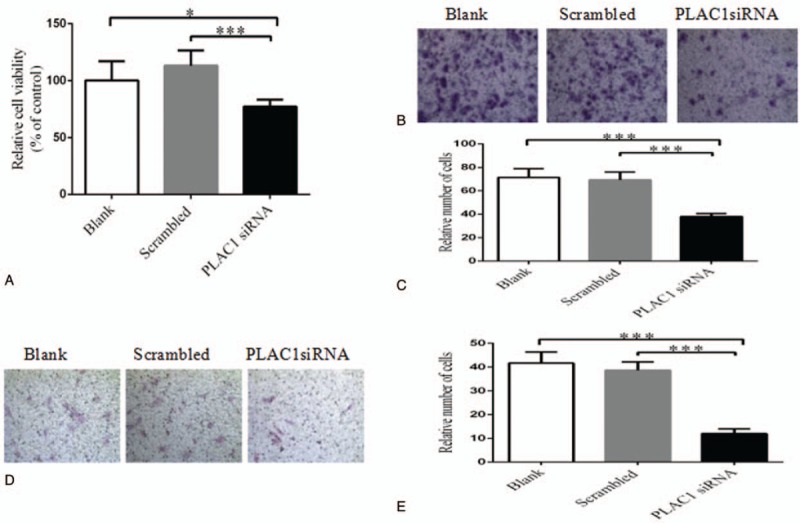
The effect of PLAC1 on trophoblast function. We detected trophoblast cells’ proliferation, migration and invasion by CCK-8 and transwell assay after transfected. PLAC1 siRNA significantly inhibited the cells viability or numbers. Proliferation (A), migration (B, C) and invasion (D, E) of trophoblast cell significantly decreased in PLAC1 siRNA group compared with control, but there is no significantly difference between blank and scrambled groups. Values represent mean ± SD, n = 5 per group, ∗P < .05,∗∗∗P < .001.
Figure 4.
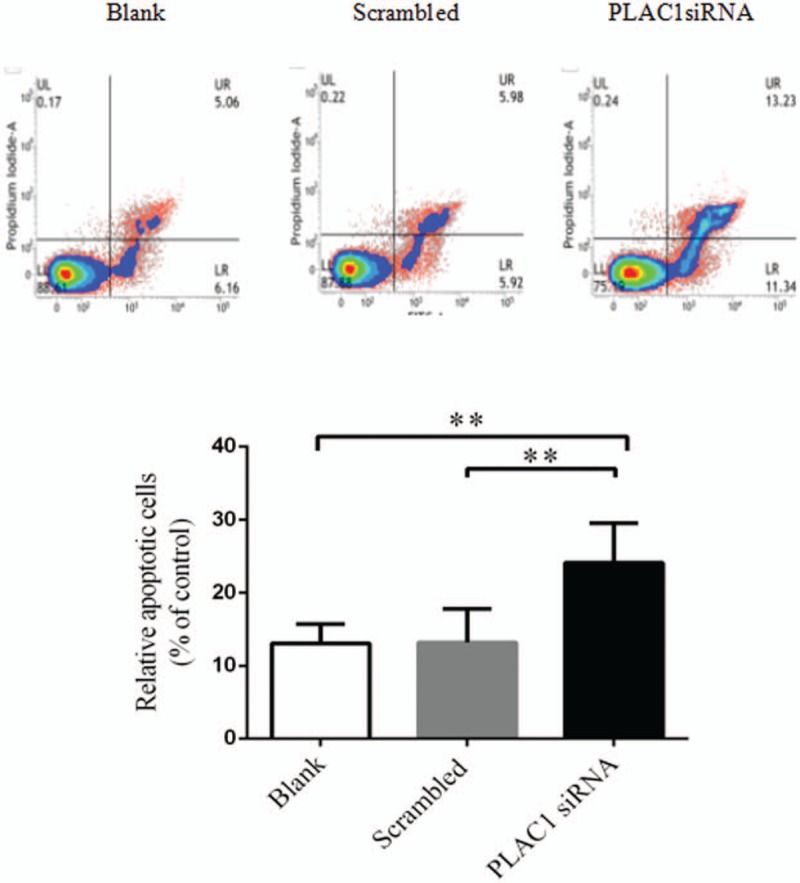
The effect of PLAC1 on trophoblast apoptosis. Flow cytometric analysis of Annexin V-FITC and PI after both staining of Swan-71 and Jar cells, transfected for 48 hours. Alive cell (Annexin V−/PI−) populations were located in the lower left quadrant (LL), early apoptotic cell (Annexin V+/PI−) populations in lower right quadrant (LR), late apoptotic (Annexin V+/PI+) populations in upper right quadrant (UR), and necrotic cells (Annexin V−/PI+) were present in the upper left quadrant (UL). We determined the apoptosis with the data by the sum of early and late apoptotic cells finally. Values represent mean ± SD, n = 6 per group, ∗∗P < .01.
4. Discussion
In the current investigation we confirmed the PLAC1 expression in human placenta and found decreased expression of PLAC1 in the placenta of women with severe preeclampsia compared with women of normal pregnancy for the first time. Hypoxia, a characteristic pathophysiology of preeclampsia, decreased PLAC1 expression by trophoblast. Further, knockdown of PLAC1 by RNA interference suppressed the proliferation, invasion and migration, furthermore, increased the apoptosis of trophoblast cells. These findings imply the decreased placental PLAC1 expression may be involved in the pathogenesis of preeclampsia.
Several studies have reveled higher levels of plasma PLAC1 mRNA concentrations in preeclamptic women than control,[7,18–20] and PLAC1 mRNA levels in maternal plasma dramatically decline at the first day after delivery, and could not be detected one month later.[7,17] These findings suggest the placenta is the origin of PLAC1 mRNA in maternal plasma. However, we observed significantly decreased PLAC1 protein in the placenta of women with severe preeclampsia. There is a discrepancy between decreased PLAC1 protein in placenta and increased mRNA level in maternal plasma. As demonstrated in the current investigation, hypoxia, a characteristic of preeclampsia, inhibited the expression of PLAC1 in placental trophoblast. The damages of placental villi and the apoptosis are increased in preeclampsia; these are consistent with our findings. Hypoxia and oxidative stress, which are present in preeclampsia, could damage the functions of trophoblast cells.[7] However, the reason of the increased mRNA of PLAC1 in plasma is unknown.
As revealed in this study and others, PLAC1 has a critical role in promoting biological features at least including viability, migration and invasion of trophoblast cells.[23] Placentation is a highly migrate and invasive process in normal pregnancy, which the EVTs derived from the placenta migrate into the uterine wall and deeper in myometrium.[1,15] This invasion is very important for the remodeling of spiral arteries, which makes spiral artery dilated, non-vascular activity and low resistance vessel to provide adequate nutrients and oxygen for the fetus.[24] Relatively small placenta, shadow placentation and inadequate remodeling of spiral artery are central pathologies of preeclampsia. Inhibited proliferation, migration and invasiveness and increased apoptosis are among the causes for above-mentioned preeclamptic pathophysiology. Our in vitro investigation revealed the down-regulation of RNA interference inhibited PLAC1 expression by trophoblast, while placental PLAC1 expression was decreased in preeclampsia. This points to the importance of low PLAC1 expression in the pathology of preeclampsia.
The pathophysiology of preeclampsia is much more complex than what was mentioned above. Whether aberrant placental PLAC1 level in preeclampsia has impact on other biological feature of trophoblast cells, or is associated with other pathologies of preeclampsia still need further investigation. In addition, whether the aberrant PLAC1 expression is the intrinsic feature of trophoblast cells in the setting of preeclampsia, or is induced by hypoxia as demonstrated in this study also need investigation.
The limitations of this research include relative small sample size and having no mild preeclampsia women. It is unknown whether PLAC1 is also decreased in other kinds of preeclampsia cases. Further, a larger sample size is needed to investigate the relation between PLAC1 and preeclampsia including mild and severe cases in the future.
In summary, the placental expression of PLAC1 is markedly decreased in preeclampsia, which could be induced by hypoxia, and down-regulation of PLAC1 inhibits the proliferation, migration, invasion of trophoblast, and increased the apoptosis, suggesting aberrant placental PLAC1 expression may be involved in the pathogenesis of preeclampsia.
Author contributions
Data curation: Dandan Sun, Jiamin Xie, Peng Wang, Miaomiao Wang.
Formal analysis: Liuxia Wan, Mengkai Du, Yu Lei, Minyue Dong.
Investigation: Liuxia Wan, Dandan Sun, Jiamin Xie, Mengkai Du, Huihua Wang, Minyue Dong.
Methodology: Peng Wang, Yu Lei, Hanzhi Wang, Minyue Dong.
Project administration: Minyue Dong.
Supervision: Hanzhi Wang, Minyue Dong.
Writing – original draft: Liuxia Wan, Minyue Dong.
Writing – review & editing: Jiamin Xie, Huihua Wang, Hanzhi Wang, Minyue Dong.
Footnotes
How to cite this article: Wan L, Sun D, Xie J, Du M, Wang P, Wang M, Lei Y, Wang H, Wang H, Dong M. Declined placental PLAC1 expression is involved in preeclampsia. Medicine. 2019;98:44(e17676).
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Natural Scientific Foundation of China (grant number 81370726); the Jiaxing Science and Technology bureau (grant number 2017AY33050); Zhejiang Medical and Health Science and Technology Project (2015KYB-397).
No conflicts of interest exits in the submission of this manuscript, and all authors for publication approve manuscript.
References
- [1].Burton GJ, Jauniaux E. The cytotrophoblastic shell and complications of pregnancy. Placenta 2017;60:134–9. [DOI] [PubMed] [Google Scholar]
- [2].Mol BWJ, Roberts CT, Thangaratinam S, et al. Pre-eclampsia. Lancet (London, England) 2016;387:999–1011. [DOI] [PubMed] [Google Scholar]
- [3].James JL, Stone PR, Chamley LW. Cytotrophoblast differentiation in the first trimester of pregnancy: evidence for separate progenitors of extravillous trophoblasts and syncytiotrophoblast. Reproduction (Cambridge, England) 2005;130:95–103. [DOI] [PubMed] [Google Scholar]
- [4].Osol G, Moore LG. Maternal uterine vascular remodeling during pregnancy. Microcirculation (New York, NY: 1994) 2014;21:38–47. [DOI] [PubMed] [Google Scholar]
- [5].Redline RW, Patterson P. Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Human Pathol 1995;26:594–600. [DOI] [PubMed] [Google Scholar]
- [6].Kaya B, Nayki U, Nayki C, et al. Proliferation of trophoblasts and Ki67 expression in preeclampsia. Arch Gynecol Obstet 2015;291:1041–6. [DOI] [PubMed] [Google Scholar]
- [7].Kodama M, Miyoshi H, Fujito N, et al. Plasma mRNA concentrations of placenta-specific 1 (PLAC1) and pregnancy associated plasma protein A (PAPP-A) are higher in early-onset than late-onset pre-eclampsia. J Obstetr Gynaecol Res 2011;37:313–8. [DOI] [PubMed] [Google Scholar]
- [8].Cartwright JE, Whitley GS. Strategies for investigating the maternal-fetal interface in the first trimester of pregnancy: What can we learn about pathology? Placenta 2017;60:145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Walker JJ. Pre-eclampsia. Lancet (London, England) 2000;356:1260–5. [DOI] [PubMed] [Google Scholar]
- [10].Meekins JW, Pijnenborg R, Hanssens M, et al. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol V 101 1994;669–74. [DOI] [PubMed] [Google Scholar]
- [11].Cocchia M, Huber R, Pantano S, et al. PLAC1, an Xq26 gene with placenta-specific expression. Genomics 2000;68:305–12. [DOI] [PubMed] [Google Scholar]
- [12].Chang WL, Wang H, Cui L, et al. PLAC1 is involved in human trophoblast syncytialization. Reproduct Biol 2016;16:218–24. [DOI] [PubMed] [Google Scholar]
- [13].Jackman SM, Kong X, Fant ME. Plac1 (placenta-specific 1) is essential for normal placental and embryonic development. Mol Reproduct Dev 2012;79:564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chang WL, Yang Q, Zhang H, et al. Role of placenta-specific protein 1 in trophoblast invasion and migration. Reproduction (Cambridge, England) 2014;148:343–52. [DOI] [PubMed] [Google Scholar]
- [15].Zechner U, Reule M, Orth A, et al. An X-chromosome linked locus contributes to abnormal placental development in mouse interspecific hybrid. Nat Genet 1996;12:398–403. [DOI] [PubMed] [Google Scholar]
- [16].Deyssenroth MA, Li Q, Lacasana M, et al. Expression of placental regulatory genes is associated with fetal growth. J Perinat Med 2017;45:887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Concu M, Banzola I, Farina A, et al. Rapid clearance of mRNA for PLAC1 gene in maternal blood after delivery. Fetal Diagn Ther 2005;20:27–30. [DOI] [PubMed] [Google Scholar]
- [18].Fujito N, Samura O, Miharu N, et al. Increased plasma mRNAs of placenta-specific 1 (PLAC1) and glial cells-missing 1 (GCM1) in mothers with pre-eclampsia. Hiroshima J Med Sci 2006;55:9–15. [PubMed] [Google Scholar]
- [19].Purwosunu Y, Sekizawa A, Farina A, et al. Cell-free mRNA concentrations of CRH, PLAC1, and selectin-P are increased in the plasma of pregnant women with preeclampsia. Prenat Diagn 2007;27:772–7. [DOI] [PubMed] [Google Scholar]
- [20].Zanello M, Sekizawa A, Purwosunu Y, et al. Circulating mRNA for the PLAC1 gene as a second trimester marker (14-18 weeks’ gestation) in the screening for late preeclampsia. Fetal Diagn Ther 2014;36:196–201. [DOI] [PubMed] [Google Scholar]
- [21].Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol 2009;200:481.e481-487. [DOI] [PubMed] [Google Scholar]
- [22].Yan Y, Peng H, Wang P, et al. Increased expression of fatty acid binding protein 4 in preeclamptic Placenta and its relevance to preeclampsia. Placenta 2016;39:94–100. [DOI] [PubMed] [Google Scholar]
- [23].Silva WA, Jr, Gnjatic S, Ritter E, et al. PLAC1, a trophoblast-specific cell surface protein, is expressed in a range of human tumors and elicits spontaneous antibody responses. Cancer Immun 2007;7:18. [PMC free article] [PubMed] [Google Scholar]
- [24].Brosens I, Pijnenborg R, Vercruysse L, et al. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 2011;204:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]


