Supplemental Digital Content is available in the text
Keywords: drug utilization, epidemiology, Maastricht Study, participation bias, type 2 diabetes
Abstract
Within the southern region of the Netherlands, the Maastricht Study is an on-going observational prospective population-based cohort study that focuses on the etiology of Type 2 diabetes mellitus (T2DM). Representativeness of the participating population is a crucial but often an unknown factor in population-based cohort studies such as the Maastricht Study. We therefore aimed to assess the representativeness of the study population by comparing drug utilization of the participants of the Maastricht Study with the general population of the Netherlands.
Since T2DM patients were oversampled in this study, a sampling method was applied in order to ensure a similar distribution of T2DM over the study population. Drug use in the study population was compared with drug use in the population of the Netherlands, using a Z-test to compare 2 independent proportions.
In general, drug use in the study was similar compared with national data. However, in the age group 65 to 74 years total drug use was lower in the study population (833/1000 persons) versus nationwide data (882/1000 persons). The use of pulmonary medications was lower (104/1000 persons vs 141/1000 persons) and the use of hypnotics/anxiolytics was higher (90/1000 persons vs 36/1000 persons) in the Maastricht Study as compared with national data.
Drug use in the Maastricht Study population is largely comparable to that in the total Dutch population aged 45 to 74. Therefore, data on drug use by participants in the Maastricht Study can be used to perform studies assessing outcomes associated with drug use.
1. Introduction
In 2013 on average 677 out of 1000 inhabitants of the Netherlands received at least one drug prescription. This equals approximately 11.6 million inhabitants, which is ∼70% of the national population. It has been estimated that this has led to 4.3 billion euro nationwide annual costs for drugs in 2013.[1,2] In 2011, 6.5% and 16.1% of the population aged 45 to 64 and 65 to 74 respectively have been diagnosed with type 2 diabetes mellitus (T2DM).[3]
Within the southern region of the Netherlands (Zuid-Limburg) region, the Maastricht Study is an on-going observational prospective population-based cohort study including individuals aged between 40 and 75 years living in the southern region of the Netherlands. Details regarding this study have been reported elsewhere.[4]
Representativeness of the participating population is a crucial but often an unknown factor in population-based cohort studies such as the Maastricht Study. In order to extrapolate findings from this type of studies to the general population it is important to assess the representativeness of the study population and to be aware of deviations. More specifically, in order to compare results of drug outcome studies in the Maastricht Study to outcome studies in the general population drug use should be similar in these populations. Therefore, the objective of this study was to assess the representativeness of drug use of the Maastricht Study by comparing drug utilization of the participants with the general population of the Netherlands.
2. Methods
2.1. Data sources
Data from the Maastricht Study and the Drug Information System of the National Health Care Institute (“Genees- en hulpmiddelen Informatie Project” [GIP]) were used.[2] The Maastricht Study is an observational prospective population-based cohort study. The study focuses on the etiology, pathophysiology, complications, and comorbidities of T2DM and is characterized by an extensive phenotyping approach. Eligible for participation were all individuals aged between 40 and 75 years and living in the southern part of the Netherlands. Participants were recruited through mass media campaigns and from the municipal registries and the regional Diabetes Patient Registry via mailings. Recruitment was stratified according to known T2DM status, with an oversampling of individuals with T2DM, for reasons of efficiency. The present report includes cross-sectional data from the first 3451 participants, who completed the baseline survey between November 2010 and September 2013. The examinations of each participant were performed within a time window of 3 months. The study has been approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sports of the Netherlands (Permit 131088–105234-PG). All participants gave written informed consent. Information regarding drug use was available from electronic dispensing records obtained from community pharmacies. Aggregated national data on drug use per 1000 insured inhabitants of the Netherlands were available from the Drug Information System of the National Health Care Institute (GIP).
2.2. Study population
This study was conducted in Maastricht Study participants who were included between 2011 and 2013. The group of participants who started in 2010 was very small (n = 41) which retained us from studying drug use in 2010. Therefore those participants were excluded from the analyses. Participants younger than 45 and older than 74 years at the date of inclusion (index date) were excluded from the analyses, because aggregated national data were not available on drug use for these age categories. If no informed consent was given for the collection of pharmacy data, participants were excluded. Participants with a diagnosis of type 1 diabetes mellitus (T1DM) were excluded because they were clinically aberrant compared with both T2DM and other non-T2DM participants (Fig. 1).
Figure 1.
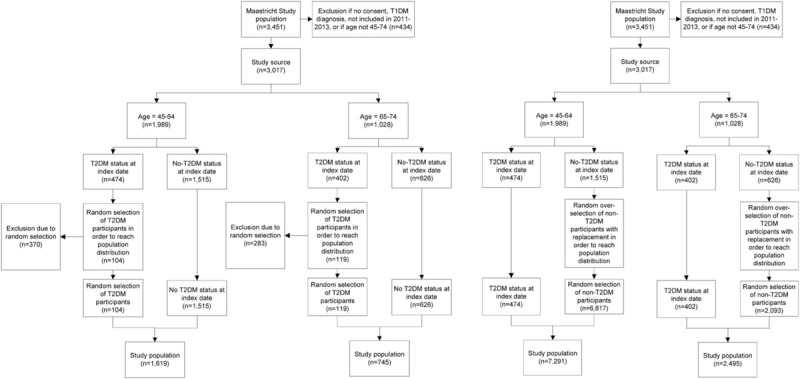
Two methods of selection of study population. In the left panel, the study population is reached by random exclusion of T2DM patients. In the right panel study population is reached by random selection of non-T2DM patients with replacement. The latter results in a study population in which a single subject is included multiple times. T2DM = type 2 diabetes mellitus.
Two methods were applied in order to achieve a study population with 6.5% and 16.1% T2DM patients aged 45 to 64 and 65 to 74 respectively.[3] First, by randomly excluding patients with T2DM. Second, by randomly selecting non-T2DM patients with replacement in the selection pool. The age categories were selected based on the availability of national reference data.
2.3. Random exclusion
For the main analyses, T2DM patients were randomly excluded from the study population in order to achieve a distribution of T2DM representative for the general Dutch population. Consequently, the study population consisted of all participants without T2DM and a random sample of T2DM participants (Fig. 1, left panel). Sensitivity analyses were conducted in order to take into account age distribution within the previously specified categories. In these analyses, overrepresented age categories were reduced in order to achieve a population comparable with the national age distribution.
2.4. Random selection
In additional analyses all T2DM patients were retained and non-T2DM patients were randomly selected, with replacement in the selection pool. Non-T2DM patients may therefore have been included more than once in this population, while no data were lost by excluding T2DM patients (Fig. 1, right panel).
2.5. Diabetes mellitus status and drug exposure
T2DM status was determined by an oral glucose tolerance test (OGTT). Participants with a fasting plasma glucose level of ≥7.0 mmol/L (126 mg/dL) or a 2 hour plasma glucose level ≥11.1 mmol/L (200 mg/dL) were defined as T2DM according to the World Health Organisation (WHO) guidelines. Others were defined as non-T2DM. Drug use in the year prior to index date was determined by at least 1 drug dispensing according to community pharmacy data. We calculated drug use per 1000 participants of the study population. Drug use in the year prior to index date took into account potential variability in seasonal prescription patterns. In order to deal with regulatory or guideline changes throughout the study period, patients contributed to drug exposure in 2 years (Supplementary Figure 1). The proportion contributing to a specific year depended on the index date. If the index date was July 1st, 2012, a prescription in the year prior contributed for 50% to 2012 and for 50% to 2011. Consequently if the index date was April 1st, 2012, a prescription contributed for 25% to 2012 and for 75% to 2011. Drugs were classified according to the WHO's Anatomical Therapeutic Chemical classification (ATC).[5] Drug use was categorized into the following groups (ATC codes): GI-tract (A02), lipid lowering (C10), anti-hyperglycaemic (A10), asthma/COPD (R03, R05CB), antidepressants (N06A), antipsychotics (N05A), and hypnotics/anxiolytics (N05BA, N05CD, N05CF).
2.6. Statistical analysis
We compared drug use in the study population in 2013 to nationwide drug use in the same year, using a Z-test to compare 2 independent proportions. The Z-test allows an overlap of not >10% of the total population.[6]
3. Results
3.1. Random exclusion
Baseline characteristics of the national and the study populations are depicted in Table 1. On average, when compared with the national population, the study population aged 45 to 64 years were older (56.5 years vs 54.6 years), while age was comparable for participants aged 65 to 74 years (68.5 years vs 68.9 years). More specifically, there was an underrepresentation for the age categories 45 to 49 years, 50 to 54 years, and 70 to 74 years in the study populations, and an overrepresentation among categories 55 to 59 years, 60 to 64 years, and 65 to 69 years, when compared with the national population. Furthermore, among those aged 45 to 64 years, there were more women in the study population (55.8%) compared with the national population (49.8%), yet the opposite was observed for the 65 to 74 years category (study population: 44.0%, national: 51.1%).
Table 1.
Baseline characteristics of the Dutch national population and the study population.
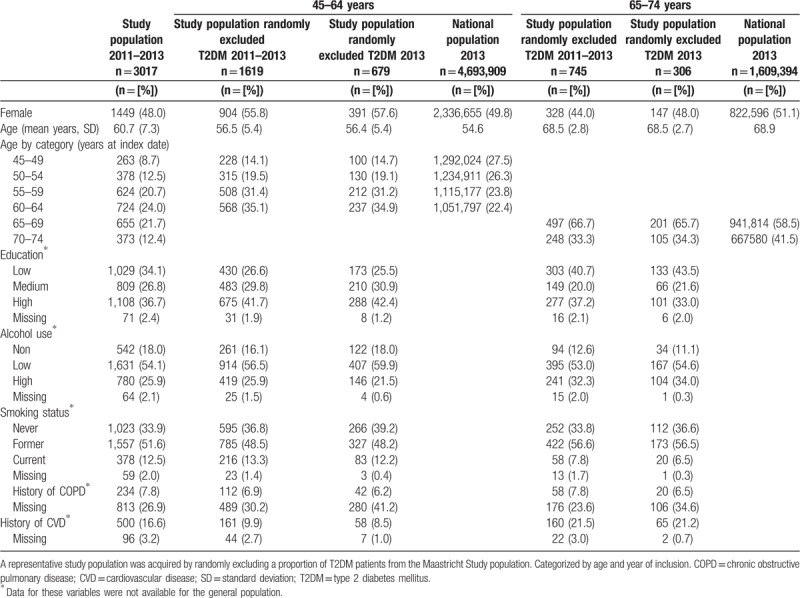
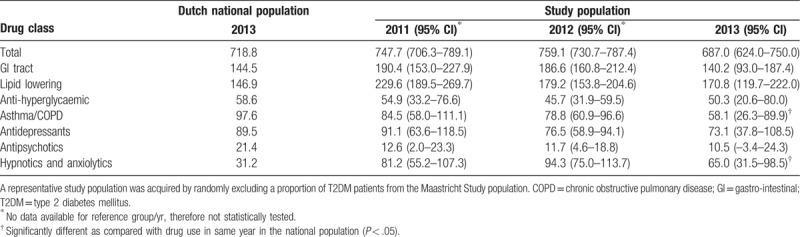
Table 2 presents the total drug use in the study population and the national population among those aged 45 to 64 years of age. For the year 2013, we identified that the total drug use was comparable between the study population (687.0/1000 persons 95% [confidence interval {CI}: 624.0–750.0/1000 persons]) and the national population (718.8/1000 persons). The use of hypnotics/anxiolytics was significantly higher in the study population, as compared with the national population, while use of asthma/chronic obstructive pulmonary disease (COPD) and gastrointestinal (GI)-tract medications tended to be lower in the study population; however, only asthma/COPD use was significantly lower. All other medication use was similar between the 2 groups.
Table 2.
Drug use per 1000 inhabitants/participants aged 45 to 64.
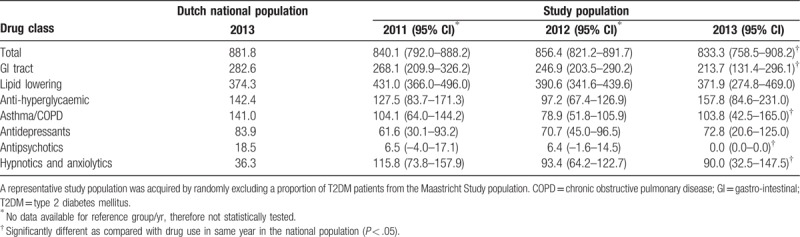
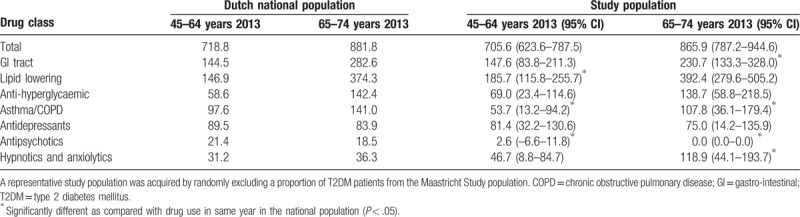
In the study population aged 65 to 74 years, Table 3, total drug use (833.3/1000 persons [95% CI: 758.5–908.2/1000 persons]) was significantly lower compared with the national population (881.8/1000 persons). Similar to the 45 to 64 age group, the use of hypnotics/anxiolytics was significantly higher, while the use of asthma/COPD and GI-tract medication was lower in the study population. Notably, there was no use of antipsychotic drugs in the study population aged 65 to 74.
Table 3.
Drug use per 1000 inhabitants/participants aged 65 to 74.
Sensitivity analyses accounting for age distribution within the pre-specified age categories revealed similar results to our primary analysis, Table 4. In contrast to the primary analysis, among those aged 45 to 64 years, the use of lipid lowering medications was significantly higher, while the use of antipsychotics was significantly lower in the study population, as compared with the national population. In the analyses of the population aged 65 to 74 years, trends by specific drug remained consistent with our primary analysis; however, the total drug use was no longer significantly lower in the study population (865.9 [95% CI: 787.2 to 944.6/1000 persons]) when compared with the national population (881.8/1000 persons).
Table 4.
Drug use per 1000 inhabitants/participants, adjusted for age distribution.
3.2. Random selection
The study population consisting of all participants with T2DM, and a selection with replacement of participants without T2DM, Supplementary Table 1, showed similar baseline characteristics to the primary analysis (Table 1, i.e., study population of all participants without T2DM and a random exclusion of T2DM patients). In the population aged 45 to 64 years, the total drug use in the study population aged 45 to 64 years was comparable with drug use in the national population, Supplementary Table 2. Among the study population, the use of lipid lowering drugs (182.5/1000 persons [95% CI: 152.8–212.1/1000 persons]) and hypnotics/anxiolytics (67.9 95% CI: 48.6–87.1/1000) was significantly higher than among the national population (146.9/1000 persons and 31.2/1000 persons, respectively), while asthma/COPD drug use was significantly lower (61.5/1000 persons [95% CI: 43.1–80/1000 persons] in the study population vs 97.6/1000 persons in the national population).
In the study population aged 65 to 74 years, total drug use was significantly lower (846.5/1000 persons [95% CI: 798.8–894.2/1000 persons]), when compared with the national population (881.8/1000 persons), Supplementary Table 3. Only the use of anti-depressants was comparable between the study population and the national population, while all other drugs showed significant differences. In particular, the study population showed higher use of anti-hyperglycaemic, lipid lowering, and hypnotics/anxiolytics medications, yet had lower use of asthma/COPD and GI-tract medications.
4. Discussion
4.1. Random exclusion
We identified that drug use in the study population was similar to national data. However, differences in drug use were present in some drug classes and across age groups. In all groups, our results suggest that the use of anti-hyperglycaemic drugs and lipid lowering drugs was similar, while there are significant differences for the use of pulmonary medications and the use of hypnotics/anxiolytics between the study population and the national population.
The use of anti-hyperglycaemic and lipid lowering drugs was similar between the study population and the national population. This is reassuring as we randomly sampled the Maastricht Study population in order to create a study population with 6.5% and 16.1% T2DM patients aged 45 to 64 and 65 to 74, respectively. Furthermore, lipid lowering drugs are commonly prescribed to patients with T2DM.[7,8] Therefore, similar use of this co-prescribed drug class is to be expected.
In order to evaluate differences and similarities in drug use between a population-based study, such as the Maastricht Study, and the general population it is important to consider potential determinants for (non-)participation. Several factors that may be involved include the distance to research facility,[9] medical history,[10,11] age,[11–13] sex,[12,14] race,[9,13] and education or income.[9,10,15]
Previous studies have identified an underrepresentation of age extremes in studies that depend on active recruitment. It has been suggested that the time required to participate may be problematic for those at working age or those of advanced age.[11–13] In our study we identified an underrepresentation of participants in the oldest age category (70–74 years). Since older patients are more likely to use a higher number of drugs, this may have caused the lower total drug use identified among the participants aged 65 to 74 years.[16]
Differential participation rates of patients with, and without, an extended medical history have been reported previously.[10,11] It is generally thought that an increased presence of comorbidities is associated with a decline in functional status, which may reduce participation rates.[11] Participation may be especially be problematic for patients with pulmonary morbidities. This is consistent with the results in this study.
The increased rates of hypnotic/anxiolytic drug use may be caused by a regional effect and by differences in health perception. The use of these drugs is estimated at 27.6/1000 inhabitants in the Zuid-Limburg area compared with 23.4/1000 persons in the national population.[1] However, this does not fully explain the more than doubled rates found in this study. It may, however, be associated with the assumption that a generally healthier, but care seeking population is more likely to participate in a study, such as the Maastricht Study.[15] Individuals from this population are concerned about their health and may therefore visit their general practitioner for unexplained complaints.
Sensitivity analyses that accounted for age distribution showed only minor differences with the primary analyses. In the population aged 45 to 64 this procedure mainly resulted in the exclusion of a proportion of participants between 55 and 64 years. It was expected this would result in a reduction of drug utilization compared with the primary analyses. Surprisingly the opposite was true. This may be explained by the fact that by reducing the proportion of participants, while retaining a population with 6.5% T2DM, predominantly healthier participants were excluded. This effect was less pronounced in the population aged 65 to 74 years. This is possibly due to smaller differences in drug utilization between participants aged 65 to 69 and participants aged 70 to 74 years.
4.2. Random selection
In comparison with the random exclusion population, analyses using the randomly selected population resulted in more significant differences between the study population and the national population, especially in the population aged 65 to 74 years. This is most likely due to a larger sample size, consequently leading to smaller confidence intervals. However, this method may be less valid. By randomly selecting participants with replacement in the selection pool the study population will have less variation than expected when the same sample size is achieved by including new subjects in the study.
4.3. Strengths and limitations
The major strength of this study was the availability of detailed prescription data of the Maastricht Study participants, not only on the index date but also in the year prior to index date. This enabled us to take drug prescriptions that were no longer active at index date into account.
A limitation of this study was the restricted availability of regional drug use data. Drug use in the Zuid-Limburg area is generally higher than the national drug utilization.[1] However, in this study we identified drug use that is largely comparable to national rates. Consequently, there appeared to be an overall participation bias towards a relatively healthy or health-conscious study population. As described previously, this is a known phenomenon in studies such as the Maastricht Study.[10,11] The relatively low drug utilization may also be explained by misclassification of participants with T2DM. In this study they were classified based on an OGTT. However, T2DM is known to be undetected and untreated in a considerable proportion of the population. We may have therefore categorized participants as T2DM patients, while this may have been unknown to their general practitioner. Consequently, these patients did not receive pharmacological treatment as would have been expected for a T2DM patient. Arguably they should therefore have been classified as non-T2DM. However, in the population aged 45 to 64 approximately 0.4% to 1.0% was newly diagnosed based on the OGTT, and in the population aged 65 to 74 this was the case for 1.3% to 1.7%. We therefore expect this misclassification would not have affected the results.
Due to limited availability of national drug use data we were only able to compare drug use in 2013. However, we expect that results would have been comparable in other years, since recruitment methods were similar across the years. Furthermore, we were unable to compare other drug classes, such as drugs used for cardiovascular diseases. This would have been interesting, but not essential for the objective of this study. The statistical procedure used to compare the study populations may also have its limitations. The Z-test is partly affected by the absolute difference between the populations. Therefore, in the case of small numbers a factor 2 difference may result in a non-significant difference, whereas a factor 1.1 may trigger a significant difference in the case of higher numbers.
By excluding the T1DM patients from our study population we have excluded patients who per definition used drugs, which results in an underestimation of the number of patients using anti-hyperglycaemic drugs. However, the prevalence of T1DM is very low (0.65% for the Netherlands in 2016[17]), which limits the impact on our results.
5. Conclusion
Drug use in the Maastricht Study population appeared to be largely representative for drug use in the national population aged 45 to 74. However, due to the restricted availability of regional drug use data we were unable to assess representativeness compared with the Zuid-Limburg population. Since drug use in the Zuid-Limburg area is generally higher than the national drug utilization, it is expected that there was an overall participation bias towards a relatively healthy or health-conscious study population. A comparison between Maastricht Study data and regional data should be made to substantiate the findings of the present study. Nonetheless, data from this study could be used to determine the relative risk of specific outcomes associated with drug use. However, care should be taken when examining outcomes associated with the use of pulmonary drugs since functional impairment may have biased participation. In general, when conducting drug use studies using a cohort study, such as the Maastricht Study, it is important to assess representativeness of drug use and to be aware of deviating drug classes.
Acknowledgments
The Maastricht Study was supported by the European Regional Development Fund via OP-Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs (grant 31O.041), Stichting De Weijerhorst (Maastricht, the Netherlands), the Pearl String Initiative Diabetes (Amsterdam, the Netherlands), the Cardiovascular Center (CVC, Maastricht, the Netherlands), CARIM School for Cardiovascular Diseases (Maastricht, the Netherlands), CAPHRI School for Public Health and Primary Care (Maastricht, the Netherlands), NUTRIM School for Nutrition and Translational Research in Metabolism (Maastricht, the Netherlands), Stichting Annadal (Maastricht, the Netherlands), Health Foundation Limburg (Maastricht, the Netherlands), and by unrestricted grants from Janssen-Cilag B.V. (Tilburg, the Netherlands), Novo Nordisk Farma B.V. (Alphen aan den Rijn, the Netherlands), and Sanofi-Aventis Netherlands B.V. (Gouda, the Netherlands). We thank all participants of the Maastricht Study, their community pharmacists, the Apothekers Vereniging Maastricht, and the Verenigde Apotheken Limburg for their cooperation.
Author contributions
Conceptualization: Johannes T.H. Nielen, Johanna H.M. Driessen, Luc Smits, Frank de Vries.
Data curation: Johanna H.M. Driessen, Frank de Vries, Pieter C. Dagnelie, Ronald M.A. Henry, Simone J.S. Sep, Carla J. van der Kallen, Miranda T. Schram, Nicolaas Schaper, Coen D.A. Stehouwer.
Formal analysis: Johannes T.H. Nielen, Johanna H.M. Driessen.
Funding acquisition: Pieter C. Dagnelie, Ronald M.A. Henry, Simone J.S. Sep, Carla J. van der Kallen, Miranda T. Schram, Nicolaas Schaper, Coen D.A. Stehouwer.
Methodology: Johannes T.H. Nielen, Johanna H.M. Driessen, Luc Smits, Frank de Vries.
Supervision: Annelies Boonen, Bart van den Bemt, Hein A.W. van Onzenoort, Cees Neef, Ronald M.A. Henry, Frank de Vries.
Writing – original draft: Johannes T.H. Nielen, Johanna H.M. Driessen, Andrea M. Burden, Frank de Vries.
Writing – review & editing: Johannes T.H. Nielen, Johanna H.M. Driessen, Pieter C. Dagnelie, Annelies Boonen, Bart van den Bemt, Hein A.W. van Onzenoort, Cees Neef, Ronald M.A. Henry, Andrea M. Burden, Simone J.S. Sep, Carla J. van der Kallen, Miranda T. Schram, Nicolaas Schaper, Coen D.A. Stehouwer, Luc Smits, Frank de Vries.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ATC = anatomical therapeutic chemical classification, CI = confidence interval, COPD = chronic obstructive pulmonary disease, GI = gastrointestinal, GIP = Genees- en hulpmiddelen Informatie Project, OGTT = oral glucose tolerance test, T1DM = type 1 diabetes mellitus, T2DM = type 2 diabetes mellitus.
How to cite this article: Nielen JT, Driessen JH, Dagnelie JH, Boonen A, van den Bemt B, van Onzenoort HA, Neef C, Henry RM, Burden AM, Sep SJ, van der Kallen CJ, Schram MT, Schaper N, Stehouwer CD, Smits L, de Vries F. Drug utilization in the Maastricht Study: A comparison with nationwide data. Medicine. 2020;99:1(e18524).
JTHN and JHMD are co-first authors and have equally contributed to the manuscript.
Data sharing: No additional data available.
BB receives research grants to his department from Pfizer and Roche and occasionally speakers honoraria form Pfizer, Roche, Abbvie, and MSD. AB receives research grants to her department from Amgen Abbvie, Pfizer, and Merck and occasionally speakers honoraria from Pfizer, UCB, Janssen, and Sandoz. PE receives research grants to his department from Stryker, Active implants, Carbylan Biosurgery, DSM Biomedical, Regentis, and occasionally speakers honoraria from Biomet and Push braces. PD has received unrestricted grants from NWO, EU, and nutritional industry for research unrelated to this topic. AMB is supported by a Canadian Institutes of Health Research (CIHR) Post-Doctoral Fellowship. All other authors have no conflicts of interest to declare.
Supplemental Digital Content is available for this article.
References
- [1].The Drug Information System of National Health Care Institute, Netherlands (GIP) accessible through: http://www.zorgatlas.nl/zorg/genees-en-hulpmiddelen/geneesmiddelengebruik/gebruikers-geneesmiddelen-per-zorgkantoorregio/#breadcrumb (June 17th, 2016). [Google Scholar]
- [2].Genees- en hulpmiddelen Informatie Project. GIPeiling 2013 2014;35:46–52. [Google Scholar]
- [3].NIVEL. Available at: http://www.nivel.nl/nl/incidentie-en-prevalentiecijfers-in-de-huisartsenpraktijk (accessed: July 3rd, 2016). [Google Scholar]
- [4].Schram MT, Sep SJS, van der Kallen CJ, et al. The Maastricht Study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol 2014;29:439–51. [DOI] [PubMed] [Google Scholar]
- [5].WHO's ATC/DDD index 2016. Available at: http://www.whocc.no/atc_ddd_index/ (April 5th, 2016). [Google Scholar]
- [6].Hayes LJ, Berry G. Comparing the part with the whole: should overlap be ignored in public health measures? J Public Health (Oxf) 2006;28:278–82. [DOI] [PubMed] [Google Scholar]
- [7].Wijnands JM, van Durme CM, Driessen JH, et al. Individuals with Type 2 diabetes mellitus are at an increased risk of gout but this is not due to diabetes: a population-based Cohort Study. Medicine (Baltimore) 2015;94:e1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Knapen LM, van Dalem J, Keulemans YC, et al. Use of incretin agents and risk of pancreatic cancer: a population-based cohort study. Diabetes Obes Metab 2016;18:258–65. [DOI] [PubMed] [Google Scholar]
- [9].Linne A, Leander K, Lindström D, et al. Reasons for non-participation in population-based abdominal aortic aneurysm screening. Br J Surg 2014;101:481–7. [DOI] [PubMed] [Google Scholar]
- [10].Jackson R, Chambless LE, Yang K, et al. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. J Clin Epidemiol 1996;49:1441–6. [DOI] [PubMed] [Google Scholar]
- [11].Jacobsen SJ, Mahoney DW, Redfield MM, et al. Participation bias in a population-based echocardiography study. Ann Epidemiol 2004;14:579–84. [DOI] [PubMed] [Google Scholar]
- [12].Slattery ML, Edwards SL, Caan BJ, et al. Response rates among control subjects in case-control studies. Ann Epidemiol 1995;5:245–9. [DOI] [PubMed] [Google Scholar]
- [13].Moorman PG, Newman B, Millikan RC, et al. Participation rates in a case-control study: the impact of age, race, and race of interviewer. Ann Epidemiol 1999;9:188–95. [DOI] [PubMed] [Google Scholar]
- [14].Olson SH. Reported participation in case-control studies: changes over time. Am J Epidemiol 2001;154:574–81. [DOI] [PubMed] [Google Scholar]
- [15].Lorant V, Demarest S, Miermans PJ, et al. Survey error in measuring socio-economic risk factors of health status: a comparison of a survey and a census. Int J Epidemiol 2007;36:1292–9. [DOI] [PubMed] [Google Scholar]
- [16].Statline, Statistics Netherlands (CBS) accessible through: http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=81071NED&D1=1&D2=0&D3=0-8,18-23&D4=0-1,60,79,128,161,182,200,227,241,258,285,297,317,340,l&D5=l&HDR=T,G1,G4,G2&STB=G3&VW=T (18th March 2016). [Google Scholar]
- [17].Peters ML, Huisman EL, Schoonen M, et al. The current total economic burden of diabetes mellitus in the Netherlands. Neth J Med 2017;75:281–97. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


