Abstract
Nursing staff play a crucial role in maintaining a functional port. Nursing guidelines recommend standard maintenance with 10 ml irrigation without consideration for variations among patients and individual nursing staff. The aim of this study is to identify the efficacy of the current maintenance strategy and analyze the correlation between complications and actual port presentations, based on disassembled intravenous ports after removal from patients. We attempt to organize the information and propose a definite maintenance strategy.
After treatment completion, or due to complications, 434 implanted intravenous ports were removed from patients. All ports were deconstructed to observe their actual presentations and were then analyzed in conjunction with medical records. The correlation between complications and actual presentations was analyzed.
From March 2012 to December 2017, 434 implanted intravenous ports were removed from oncology patients after completion of treatment or catheter related complications. From the view of maintenance related presentations, injection chamber blood clot was highly correlated with chemotherapy completion (P < .001) and malfunction (P = .005), while tip blood clot (P = .043) was related with chemotherapy completion and catheter fibrin (P = .015) was related to malfunction. From the view of structure related presentations, broken catheter integrity was correlated to chemotherapy completion (P = .007), fracture (P < .001), and malfunction (P = .008). Compression groove was related to chemotherapy completion (P = .03) and broken catheter at protruding stud was related to fracture (P = .04), while diaphragm rupture was correlated to chemotherapy completion (P = .048) and malfunction. (P < .001).
Current port maintenance is insufficient for ideal port maintenance, whereby maintenance-related presentations, including tip clot, catheter fibrin, and injection chamber blood clot were identified. We propose a recommended maintenance strategy based on our findings. Structure-related presentations, including broken catheter integrity, broken catheter at protruding stud and diaphragm rupture were seen in patients with longer implantation period. Removal of the implanted port may be considered after 5 years if no disease relapse is noted.
Keywords: catheter infection, fibrin, intravenous port, port maintenance, structural weakness
1. Introduction
A functional intravenous port is crucial for oncology patients. It not only serves to provide secure vascular access but also allows better mobility without external dressing.[1] Three different factors, including structural design, implantation technique, and daily nursing practice may affect functional status of an implanted port. Scientists, clinical practitioners, and manufacturers have sought solutions for decades to keep implanted intravenous ports functional. From the point of view of structural design, there are structural differences between central venous catheter and intravenous port. Central venous catheter is an open system communicating the intravascular space with an extracorporeal connecting portion (Fig. 1A to G, black circle). Intravenous port can be seen as a closed system because the catheter is attached to the injection chamber (Fig. 1G/ H, white circle). This structure means the flow pattern within the intravenous port is quite different from the central venous catheter. The flow within the port is vortex flow in the injection chamber, followed by laminar flow within the attached catheter (Fig. 2A) but the flow within the central venous catheter is entirely laminar flow (Fig. 2B). For this reason the recommendation for central venous catheter irrigation cannot not be applied universally in daily port maintenance.[2] Furthermore, the vortex flow within the injection chamber depends on the orientation of the non-coring needle.[3] Best irrigation can only be achieved when the non-coring needle is inserted into the injection chamber at the middle part of the silicon diaphragm with a 180°-degree orientation.[3] In order to reduce possible residual blood components within an intravenous port, 2 major modifications, including valved catheter tip and realignment of the connecting tube have been proposed (Supplement 1). In the former, a one-way valve is added to prevent blood backflow into the catheter that could be caused by a vacuum effect during non-coring needle withdrawal.[4] In the latter, the connecting tube is realigned to induce a complete vortex within the injection chamber to facilitate effective irrigation.[5] However, further investigations have shown that these structural modifications may not suffice to eliminate catheter occlusion,[6,7] or higher failure rate during function withdrawal test.[8]
Figure 1.
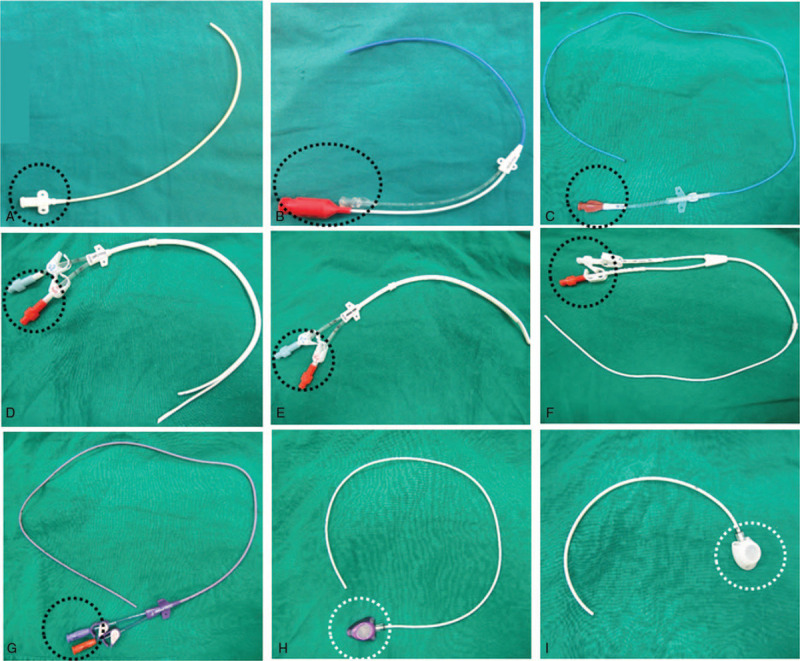
Difference between central venous catheter and intravenous port. A–C: Central venous catheter. D–F: Hickmen catheter for dialysis. G: Peripherally inserted central venous catheter (PICC). H–I: Intravenous ports. The difference from the catheters shown in 5A-G was the injection chamber. (White circle) instead of the connector (Black circle) at one end of the catheter.
Figure 2.
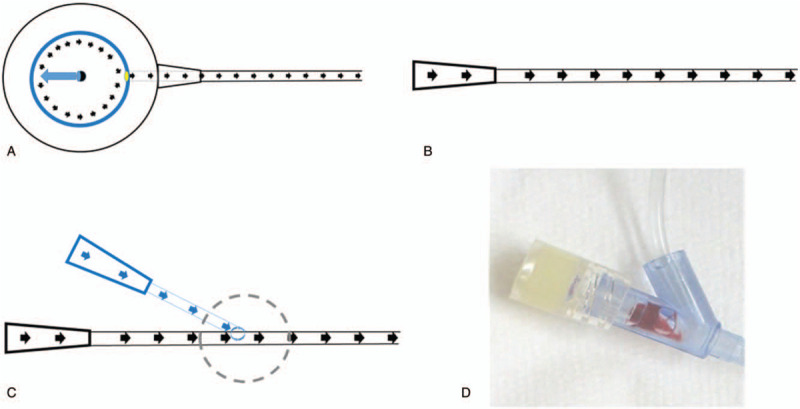
Ideal irrigation flow pattern of an intravenous port and central venous catheter. A: Ideal irrigation flow pattern of an intravenous port: Vortex within the injection chamber followed by laminar flow within the attached catheter. (Black circle: Needle shaft of non-coring needle. Blue ovoid: Opening of non-coring needle. Yellow circle: Opening of injection chamber. Black arrow: Flow direction. B: Ideal irrigation flow pattern of central venous catheter: laminar flow within the whole catheter. C: Flow pattern of connecting tube of a non-coring needle. D: Vortex was noted at the junction site and residual blood clot remaining in the Y-connector.
From the view of implantation technique, vessel cut down technique for intravenous port implantation has been recommended by the guidelines due to the low complication rate.[9–11] The catheter nut angle should be obtuse to avoid catheter impingement[7] and an adequate tip location should be 1 cm below carina, just at the junction site between the superior vena cava (SVC) and the right atrium (RA).[12,13] Subclavian puncture should be avoided in order to prevent iatrogenic pneumo-hemothorax and pinch-off syndrome.[14,15] Late catheter-related complications, such as malfunction, infection, and migration have been reported but were found to be reduced after a standard algorithm was proposed.[16,17] The reported mechanical failure rate was 6.81%.[16] However, catheter complications, including catheter infection and malfunction have been found to persist, despite all implantation being done by standard operation procedure.[17] This observation suggests that current port maintenance practices may be inadequate.
From the view of daily nursing practice, 3 factors may affect the efficacy of port maintenance. First is the orientation of the non-coring needle, which presents a difficulty for nursing staff because the port is invisible during puncture and accurate orientation is impossible to confirm. (Fig. 3A–D) Second is the irrigation volume. According to guidelines[18] and instructions from the manufacturers,[19–21] 10 ml of saline solution is recommended for irrigation of an implanted port, regardless of port size, catheter caliber, and intraluminal volume of the connecting tube. Our simulation has shown that 20 times the intraluminal volume, including port, and connecting tube would be needed as minimal irrigation volume.[22] Third is the flow pattern within the connecting tube (Fig. 2C). Residual blood components within the Y-connector in the ex-vivo simulation[22] have led to microscopic deposit within ports (Fig. 2D). This indirect clinical evidence once again suggests inadequacy of current port maintenance. The aim of this study is to identify the efficacy of the current maintenance strategy and analyze the correlation between complications and actual port presentations, based on analysis of intravenous ports deconstructed after removal from patients.
Figure 3.
Actual puncture of implanted port. A: Actual puncture status of an implanted intravenous port. The puncture holes were dispersed on the whole silicon diaphragm. The puncture hole with some silicon defect that may have been caused by oblique puncture. (White arrow). B. Oblique puncture at peripheral area of silicone diaphragm. C. Oblique puncture at central area of silicone diaphragm. D. Perpendicular puncture at central area of silicone diaphragm.
2. Materials and methods
2.1. Patient selection
From March 2012 to December 2017, 434 implanted intravenous ports were removed from patients after completion of therapy or due to complications. We retrospectively reviewed the medical records for clinical information regarding entry vessel type, intravenous port type, port function, and reason for removal. All patients who underwent port removal were included and collected data were de-identified (anonymized) prior to further analyses. The informed consent requirement was waived since all ports were removed under appropriate clinical scenarios, and they underwent thorough inspection immediately after removal for the purpose of medical records. All study methods were carried out in accordance with approved guidelines.
2.2. Ethics
This study was approved by the Chang Gung Memorial Hospital Institutional Review Board under the number IRB No. 101–4798A3, and was funded by Chang Gung Medical Foundation under the grant numbers CMRPG3F0171 and CMRPG5G0131.
2.3. Intravenous port maintenance
The implanted intravenous ports had been maintained by the following routine maintenance protocol: 10 ml normal saline irrigation once, followed by 10 ml heparin lock (50 U/ml), every time after chemotherapy injection, blood transfusion, medication injection, or blood withdrawal. The irrigation method was pulsatile technique, which is commonly utilized in venous line irrigation and has been proven to provide better irrigation in ex-vivo simulation.[23] Patients undergoing chemotherapy were followed up every 2 to 3 weeks to check port function, and patients not undergoing chemotherapy were followed-up every 3 months.
2.4. Definition of complication
Catheter-related complications in this study included infection, migration, fracture, malfunction, and others. Infection was defined as patients presenting with fever or chills and blood culture obtained from peripheral vessels and ports showing positive for bacteria or fungi. Migration was defined as a catheter tip no longer located at the junction site between superior vena cava and right atrium. Fracture was defined as broken catheter integrity, as identified by chest plain film or pocket swelling during irrigation. Malfunction was defined as catheter occlusion during irrigation. Other types of complication included pocket erosion and pocket hematoma.
2.5. Deconstruction of removed intravenous port
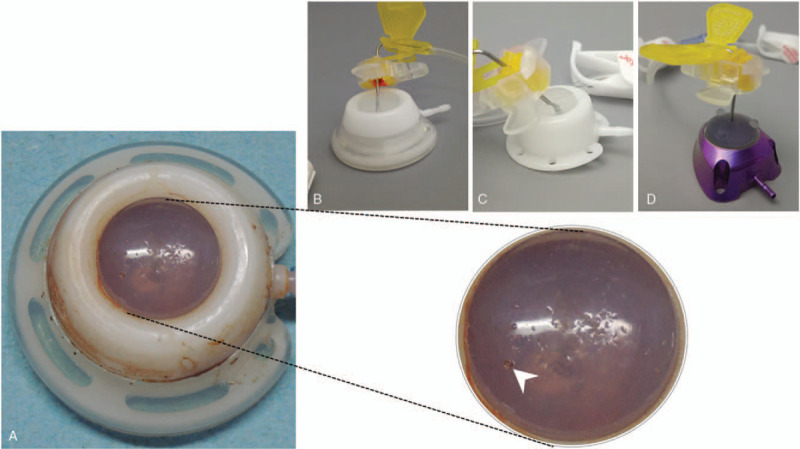
After port removal, port function was checked with a 10 ml normal saline syringe. Ten different brands of ports were collected and analyzed, whereby 4 brands predominated. These intravenous ports shared common design concepts, each 1 being divided into 3 main components, including catheter, locking nut, and port body (Supplement 1). The port body could further be subdivided into connecting tube, injection diaphragm, and injection chamber. We first inspected the structural integrity of the removed intravenous ports and deconstructed them into the 3 main components as aforementioned. Actual presentation (Figs. 4 and 5) of the removed ports after deconstruction was recorded photographically.
Figure 4.
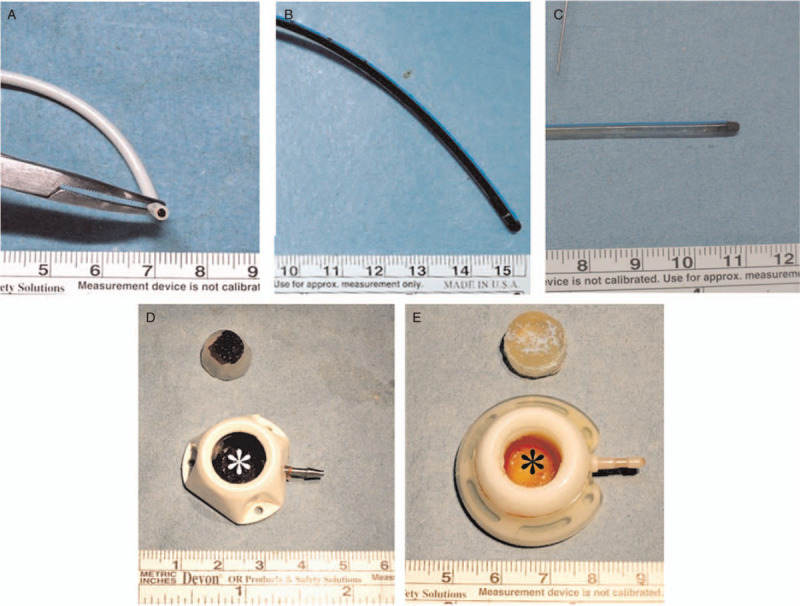
Maintenance-related presentations. A. Tip blood clot (Tyco Fr. 6;Tyco Healthcare Group, CT, USA). B. Catheter blood clot (Bard Fr. 8; Bard Access Systems Inc., Utah, USA). C. Fibrin (Bard Fr. 8; Bard Access Systems Inc., Utah, USA). D. Injection chamber blood clot (White star; Tyco Fr. 6; Tyco Healthcare Group, CT, USA). E. Injection chamber biofilm (Black star; Bard Fr. 8; Bard Access Systems Inc., Utah, USA).
Figure 5.
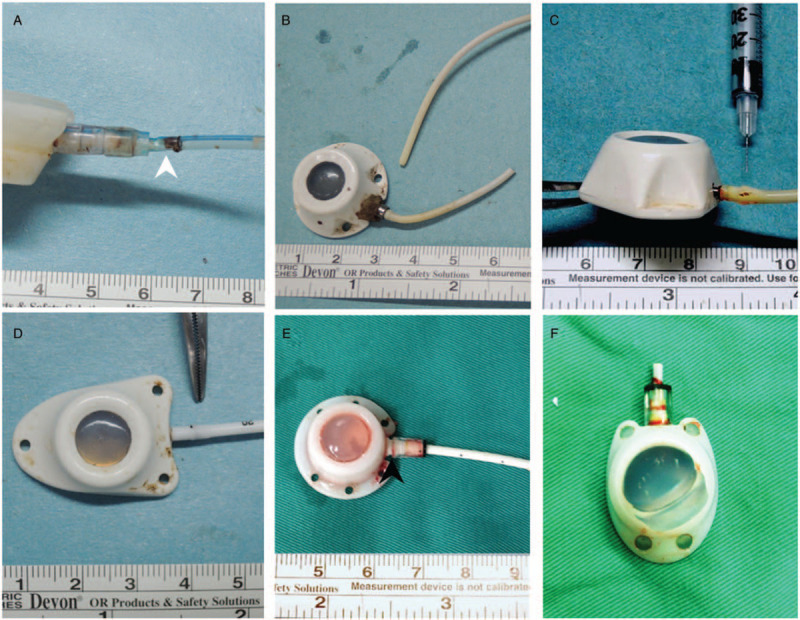
Structure-related presentations. A. Locking nut impingement (white arrow): caused by inadequate pocket creation (Bard Fr. 8; Bard Access Systems Inc., Utah, USA). B. Catheter fracture: Caused by pinch-off syndrome (Medcomp Fr.7; Medcomp Medical Component Inc., PA USA). C. Fracture at protruding stud (Tyco Fr. 6; Tyco Healthcare Group, CT, USA). D. Compression groove: caused by shearing force generated by protruding stud of connecting tube and locking nut (B’Braun Fr 6.5; B’Braun Medical, Boulogne Billancourt, France). E. Locking nut gap: May be caused by mal-assembly or catheter over-mounting to the connecting tube, leading to flaring at the bottom (Bard Fr. 8; Bard Access Systems Inc., Utah, USA). F. Diaphragm rupture (Bard X port Fr. 6; Bard Access Systems Inc., Utah, USA).
2.6. Definition of actual presentations
Tip blood clot was defined as blood clot identified at the catheter tip (Fig. 4A). Catheter blood clot meant blood clot identified within the catheter at any location other than the tip opening (Fig. 4B). Fibrin was thin yellow biofilm deposited over the catheter wall without obvious old blood clot (Fig. 4C). Injection chamber blood clot was defined as blood clot within the injection chamber (Fig. 4D). Injection chamber biofilm referred to yellow biofilm deposit within the injection chamber (Fig. 4E). Locking nut impingement was defined as catheter indentation at the edge of the locking nut (Fig. 5A). The catheter is compressed by the rigid locking nut because the surrounding soft tissue pushes it upward during posture change. Broken catheter integrity was defined as broken catheter integrity (Fig. 5B). Broken catheter at the protruding stud was the situation where the catheter was broken at the mounting over the connecting tube of the injection chamber (Fig. 5C). Compression groove is the indentation caused by the shearing force generated by the protruding stud of the connecting tube and locking nut (Fig. 5D). Locking nut gap is the gap between the bottom of the locking nut and the injection chamber (Fig. 5E). It is caused by mal-assembly, such as catheter over-mounting to the connecting tube, leading to flaring at the bottom. Diaphragm rupture meant the silicone diaphragm was pushed upward and dislodged from the injection chamber (Fig. 5F).
2.7. Statistics
All the collected clinico-pathologic factors were first analyzed by univariate analysis. Categorical variables were compared using Chi-Squares or Fisher exact tests. A P value less than .05 was considered statistically significant. Reported confidence intervals (CI) were assumed to have a coverage probability of 95%. All the analyses were performed using SAS, version 9 (SAS Institute, NC, USA). Multivariate analysis was performed based on the removal reason in order to identify the correlation between actual presentation and the removal reason.
3. Results
From March 2012 to December 2017, 434 implanted intravenous ports were removed from oncology patients after completion of treatment, or due to complications (Table 1). Mean age was 56.9 ± 11.88 years, with females predominant (298/ 434, 68.7%). Of the removed ports, 85.5% had open tip catheter. The mean implantation period was 43.2 ± 25.68 months, and most of the removed ports had been implanted via the superior vena cava. All removed ports underwent a function test prior to deconstruction, whereby the malfunction rate was found to be 16.2%. The main reason for port removal was treatment completion (346/434, 79.7%). Catheter tip clot was identified in 50.4% of the removed ports, while blood clot retained within the catheter was found in 36.8%. Fibrin deposit within the catheter was found in 36.3% of the removed ports. Blood clot and biofilm within the injection chamber were found in 22.1% and 34.8%, respectively. In the subgroup analysis, more ports were implanted via inferior vena cava (IVC) among patients who underwent port removal because of infection (7.7%) and malfunction (4.5%). After implanted ports were removed, ex-vivo functional test was done by non-coring needle. The rates of total occlusion among patients who underwent port removal because of fracture and malfunction were 71.4% and 63.5%, respectively (Supplement 2).
Table 1.
Descriptive statistics (all) N = 434.
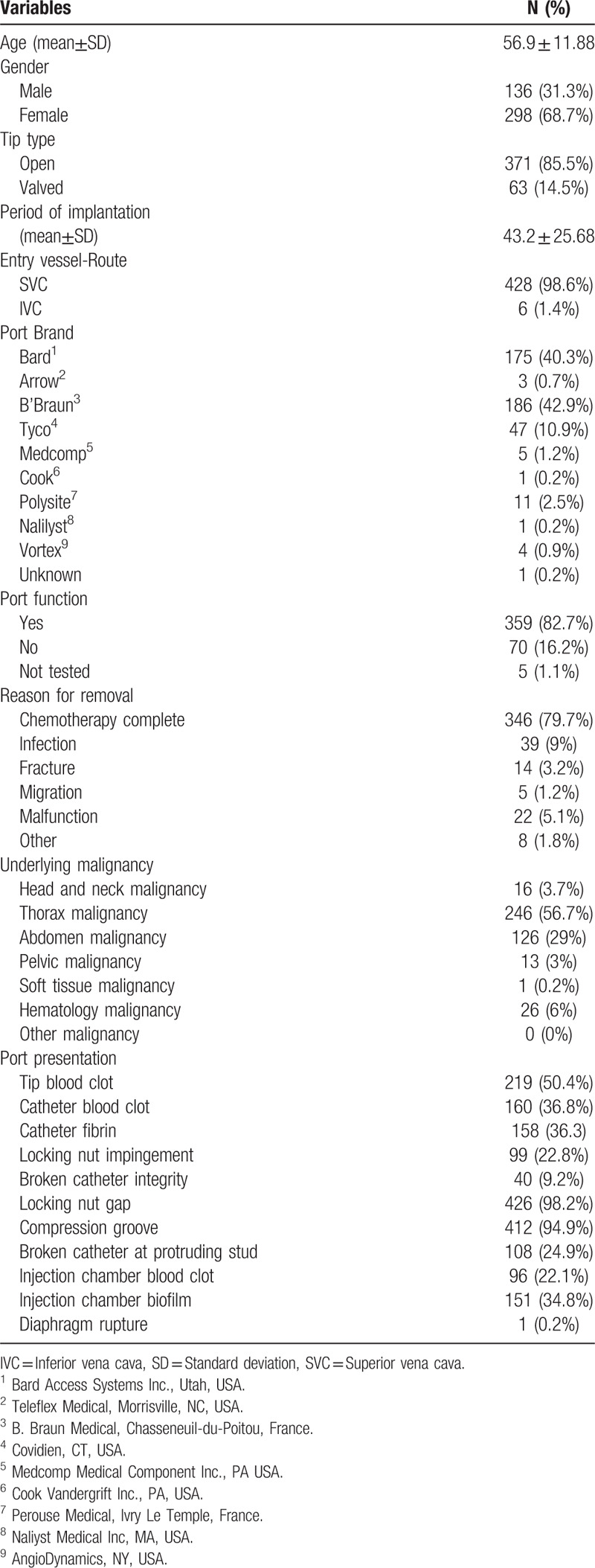
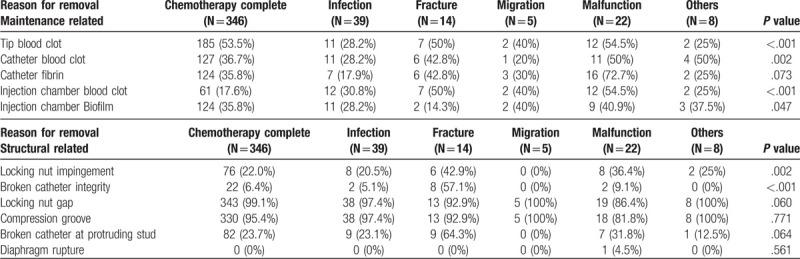
The presentations of removed ports were classified into maintenance- and structure related and are listed in Table 2. A high percentage of tip blood clot presentation was found in ports removed because of treatment completion (53.5%), fracture (50%), or malfunction (54.5%). Similarly, a high rate of catheter blood clot presentation was found in ports removed because of fracture (42.8%) and malfunction (50%). A higher rate of blood retention within the injection chamber was found among patients who presented as fracture (50%), migration (40%), or malfunction (54.5%). A higher percentage of injection chamber biofilm was identified in patients who had completed treatment (35.8%), or presented as fracture (40%) or malfunction (40.9%). With regard to structure related presentations, a higher rate of locking nut impingement was observed after port removal due to catheter fracture (42.9%) and malfunction (36.4%). Broken catheter integrity was found in 57.1% of ports removed because of fracture.
Table 2.
Comparison of actual presentation and reason for port removal.
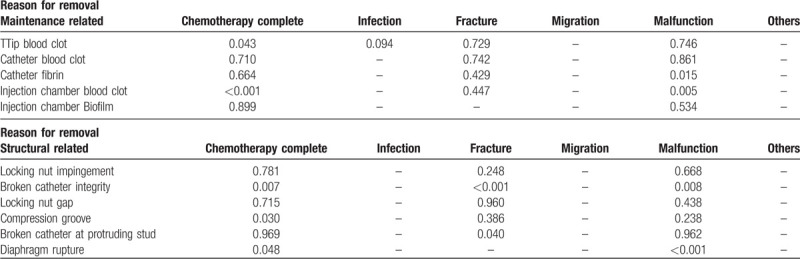
We further analyzed the relationship between port presentation and reason for removal (Supplement 3, Table 3). From the view of maintenance related presentations, injection chamber blood clot was highly correlated to chemotherapy completion (P < .001) and malfunction (P = .005). Tip blood clot (P = .043) was related to chemotherapy completion, while catheter fibrin (P = .015) was related to malfunction. From the view of structure related presentations, broken catheter integrity was correlated to chemotherapy completion (P = .007), fracture (P < .001), and malfunction (P = .008). Compression groove was related to chemotherapy completion (P = .03), while broken catheter at the protruding stud was related to fracture (P = .04). Diaphragm rupture was correlated to chemotherapy completion (P = .048) and malfunction (P < .001). Longer implantation period was identified in patients who underwent port removal because of chemotherapy completion (46.5 ± 22.62 months) and fracture (64.9 ± 41.44 months). Patients who underwent port removal because of infection (13.6 ± 12.6 months) and migration (17.1 ± 15.91 months) had shorter implantation periods (Supplement 4).
Table 3.
Multi-variate analysis of actual presentation vs reason for removal.
4. Discussion
Intravenous ports have been utilized as a secure vascular access for decades.[24] The most important concern is to keep an implanted port functional during the treatment period. From the point of view of implantation technique, a standard algorithm for port implantation [12] has been proposed to avoid catheter-related complications shown in previous studies.[12,14,16,25] No procedure-related complications have been reported since the standardized algorithm was taken into clinical practice but late complications, such as malfunction and infection, are still being reported.[12] Once intravenous ports are implanted, nursing staff play a crucial role in keeping the port functional.[26] Milani et al revealed that occlusion was highly correlated to frequency of irrigation, chemotherapy administering, and blood sampling.[26] All these factors are related to the maintenance protocol after use. However, the only recommendation in the nursing guidelines is 10 ml saline solution for irrigation of implanted port, regardless of port size, catheter caliber, and intraluminal volume of the connecting tube. This study was conducted to clarify the relationship between actual presentations and removal reasons in order to identify possible inadequacies in current maintenance practice.
In this study, we found that female patients had more fracture (85.7%) and malfunction (81.8%) (Supplement 2). This may be connected to soft tissues repetitively pushing the catheter upward during lying, resulting in indentation (Fig. 5A), and may further develop into fracture. More infection was identified in patients who received their port via the inferior vena cava, consistent with results reported in the literature.[27,28] Patients who received port removal due to malfunction (63.6%) and fracture (71.4%) had higher risk of complete occlusion (Supplement 2). The former may be correlated to inadequate irrigation and the latter may be related to broken catheter integrity. From the view of maintenance related presentations, intergroup differences were identified among the different removal reasons, except for fibrin (Table 2). These differences may be related to the irrigation efficacy of maintenance. Higher blood clot presentation rate in the tip, catheter, and injection chamber were identified in fracture and malfunction. This may be due to less irrigation solution reaching the catheter because of leaking to the surrounding soft tissue or difficulty injecting into the port. From the view of structural presentation, more locking nut impingement was identified in patients who underwent port removal because of fracture (42.9%) and malfunction (36.4%). Locking nut impingement is caused by inadequate pocket creation. Initial presentation was lumen narrowing, leading to malfunction and resulting in broken catheter integrity as a result of material fatigue caused by focused stress forces. This finding was similar to our previous studies.[11,14] In addition, more broken catheter integrity was also identified in catheter fracture patients. (57.1%)
In multivariate analysis, tip blood clot (P = .043), and injection chamber blood clot (<0.001) were correlated to ports that were removed because of chemotherapy completion. Theoretically, no maintenance related presentation should be found under good port maintenance. This result implies that blood components are not being completely irrigated under the current maintenance strategy. Furthermore, catheter fibrin (P = .015) and injection blood clot (P = .005) were related to malfunction; a finding which implies that greater retained blood component further progresses to catheter malfunction. In addition, longer implantation period was identified in patients who underwent port removal due to chemotherapy completion (46.5 ± 22.62 months) and fracture (64.9 ± 41.44 months). These findings imply that removal of implanted ports may be considered after 5 years if no disease relapse is noted. Broken catheter integrity was identified in ports that were removed because of chemotherapy completion, fracture and malfunction (Table 3 and Supplement 4). Two common breakage sites, 1 between the locking nut and proximal catheter and 1 on the catheter itself were identified. The former was related to catheter impingement related to inadequate pocket creation or compression by soft tissue, and the latter was related to pinch-off syndrome. Both scenarios are related to stress forces focused on a specific point and are preventable. The former could be avoided by adequate pocket creation and the latter precluded by avoiding subclavian vein puncture.[17] Furthermore, broken catheter at the protruding stud was also identified in patients who underwent port removal because of fracture. This is related to stress generated by the locking nut being focused on the catheter at the mounting point on the protruding stud. This finding was identical in laboratory[29] and clinical observations.[14] Diaphragm rupture was also correlated to chemotherapy completion and malfunction. The former is related to number of puncture times and material fatigue, while the latter is related to functional testing. According to the literature review and following manufacturers’ instructions, a 10 ml syringe is recommended for irrigation because the maximum generated pressure lies below the pressure the ports are designed to withstand.[30] Some nursing staff may utilize a smaller syringe that can generate greater pressure that may then overcome an occluded catheter in order to regain port function, but this may also be the reason that diaphragm rupture was found in malfunctioning ports.
Based on the findings from deconstructed ports, the current maintenance recommendation is insufficient. From the view of ideal maintenance, several aspects should be considered, including the pre-irrigation preparation, irrigation orientation, irrigation method, irrigation volume, catheter-locking method, and maintenance frequency. First, nursing staff should confirm that no blood component is in the connecting tube of the non-coring needle (Fig. 2C, D).[22] Second, the non-coring needle should be inserted as far as possible, perpendicularly to the diaphragm, while keeping the opening opposite the outflow site of the injection chamber. The orientation should not be changed for any reason in order to keep the best irrigation as close as possible to the experimental model.[3] Third, ports should be irrigated by normal saline continuously instead of by pulsatile technique because it invokes intermittent vortex flow that cannot provide effective irrigation and leads to blood clot residue within the injection chamber (Fig. 2D). Fourth, the minimum recommended irrigation volume is the 20 fold of the total intraluminal volume of the implanted ports [22]. While there are variations between different manufacturers, the total intraluminal volume can easily be calculated by the following equation: 20 × (implanted catheter length [cm] × intra luminal volume per length unit [ml/cm] + injection chamber volume). In most clinical scenarios, the intravascular volume of SVC port and IVC port is 0.5 and 0.78 ml, respectively.[22] The recommended irrigation volume is therefore 10 ml for SVC port and 20 ml for IVC port.[22] However, there has been no consensus with regard to the catheter-locking method[31–33] and maintenance frequency[34–37] and further investigation is warranted.
There are some limitations to this study. First, this is a retrospective study with a medium sample size. However, this is the first study to demonstrate and investigate the actual presentation of implanted ports and clarify the relationship with the reason for port removal. Second, although ports produced by different manufacturers were included in this study, all ports share common components and similar size and hence can be investigated together. Despite these limitations, we have not only clarified the actual presentation of implanted ports, but also proposed a definite port maintenance strategy.
5. Conclusion
Current port maintenance is insufficient for ideal port maintenance, whereby maintenance-related presentation, including tip clot, catheter fibrin, and injection chamber blood clot were identified. We propose a recommendation for maintenance strategy based on our findings. Structure-related presentation, including broken catheter integrity, broken catheter at protruding stud and diaphragm rupture were seen in patients with longer implantation periods. The implanted ports may be considered for removal after 5 years if no disease relapse has been noted.
Author contributions
Conceptualization: Jui-Ying Fu, Yen Chu, Chia-Hui Cheng, Ching-Feng Wu, Ching Yang Wu.
Data curation: Pin-Li Chou, Jui-Ying Fu, Chia-Hui Cheng, Ching-Feng Wu.
Formal analysis: Ching Yang Wu.
Funding acquisition: Po-Jen Ko, Yun-Hen Liu, Ching Yang Wu.
Investigation: Yen Chu, Chia-Hui Cheng, Yun-Hen Liu.
Methodology: Pin-Li Chou, Jui-Ying Fu, Yen Chu, Ching-Feng Wu, Yun-Hen Liu, Ching Yang Wu.
Project administration: Po-Jen Ko, Yun-Hen Liu, Ching Yang Wu.
Resources: Po-Jen Ko, Ching Yang Wu.
Software: Po-Jen Ko.
Supervision: Yun-Hen Liu, Ching Yang Wu.
Validation: Ching Yang Wu.
Writing – original draft: Pin-Li Chou.
Writing – review & editing: Ching Yang Wu.
Correction
The second grant number in the Ethics section appeared incorrectly as CMRP5G0131 and has been corrected to CMRPG5G0131.
Supplementary Material
Footnotes
Abbreviations: CI = confidence intervals, IVC = inferior vena cava, RA = right atrium, SVC = superior vena cava.
How to cite this article: Chou PL, Fu JY, Cheng CH, Chu Y, Wu CF, Ko PJ, Liu YH, Wu CY. Current port maintenance strategies are insufficient. Medicine. 2019;98:44(e17757).
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Bow EJ, Kilpatrick MG, Clinch JJ. Totally implantable venous access ports systems for patients. receiving chemotherapy for solid tissue malignancies: a randomized controlled clinical trial examining the safety, efficacy, costs, and impact on quality of life. J Clin Oncol 1999;17:1267. [DOI] [PubMed] [Google Scholar]
- [2].Gorski LA, Hadaway L, Hagle M, et al. 2016 Infusion therapy standards of practice. J Infus Nurs 2016;39Suppl 1:S1–59. [DOI] [PubMed] [Google Scholar]
- [3].Guiffant G, Durussel JJ, Flaud P, et al. Flushing ports of totally implantable venous access devices, and impact of the Huber point needle bevel orientation: experimental tests and numerical computation. Med Devices (Auckl) 2012;5:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Carlo JT, Lamont JP, McCarty TM, et al. A prospective randomized trial demonstrating valved implantable ports have fewer complications and lower overall cost than non valved implantable ports. Am J Surg 2004;188:722–7. [DOI] [PubMed] [Google Scholar]
- [5].Stevens B, Moenter S, Barton SE, et al. A randomized, prospective trial of conventional ports vs. the vortex“ clear-flow” reservior port in adult oncology patient. J Vasc Access Device 2000;5:37–40. [Google Scholar]
- [6].Biffi R, De Braud F, Orsi F, et al. A randomized, prospective trial of central venous port connect to standard open ended or Groshong catheter in adult oncology patients. Cancer 2001;92:1204–12. [DOI] [PubMed] [Google Scholar]
- [7].Goossens GA, Verbeeck G, Moons P, et al. Functional evaluation of conventional “ Celsite” venous port versus “ Vortex’ ports with a tangential outlet: a prospective randomized pilot study. Support Care Cancer 2008;16:1367–74. [DOI] [PubMed] [Google Scholar]
- [8].Biffi R, Pittiruti M, Gillet JP, et al. Valved central venous catheter connected to subcutaneous port: a multicenter phase IV study based on a cohort 50 oncology patients. J Vasc Access 2002;3:147–53. [DOI] [PubMed] [Google Scholar]
- [9].B. Sousa J, Furlanetto M, Hutka P, et al. Central venous access in oncology: ESMO Clinical Practice Guidelines. Ann Oncol 2015;26Suppl 5:v152–68. [DOI] [PubMed] [Google Scholar]
- [10].Di Carlo I, Pulvirenti E, Mannino M, et al. Increased use of percutaneous technique for totally implantable venous access devices. is it real progress? a27-year comprehensive review on early complications. Ann Surg Oncol 2010;17:1649–56. [DOI] [PubMed] [Google Scholar]
- [11].Hsu CC, Kwan GN, Evans-Barns H, et al. Venous cutdown versus the Seldinger technique for placement of totally implantable venous access ports. Cochrane Database Syst Rev 2016;8:CD008942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wu CY, Fu JY, Feng PH, et al. Risk factors and possible mechanisms of intravenous port catheter migration. Eur J Vasc Endovasc Surg 2012;44:82–7. [DOI] [PubMed] [Google Scholar]
- [13].Fu JY, Wu CF, Ko PJ, et al. Analysis of chest X-ray plain film images of intravenous ports inserted via the superior vena cava. Surg Today 2014;44:1513–21. [DOI] [PubMed] [Google Scholar]
- [14].Wu CY, Fu JY, Feng PH, et al. Catheter fracture of intravenous ports and its management. World J Surg 2011;35:2403–10. [DOI] [PubMed] [Google Scholar]
- [15].Aitken DR, Minton JP. The “pinch-off sign”: a warning of impending problems with permanent subclavian catheters. Am J Surg 1984;148:633–6. [DOI] [PubMed] [Google Scholar]
- [16].Wu CF, Ko PJ, Wu CY, et al. A single-center study of vascular access sites for intravenous ports. Surg Today 2014;44:723–31. [DOI] [PubMed] [Google Scholar]
- [17].Wei WC, Wu CY, Wu CF, et al. The treatment results of a standard algorithm for choosing the Best entry vessel for intravenous port implantation. Medicine (Baltimore) 2015;94:e1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goossens GA. Flushing and locking of venous catheters: available evidence and evidence deficit. Nurs Res Pract 2015;2015:985686.doi: 10.1155/2015/985686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Polysite®: Instruction and Indication p5. [Google Scholar]
- [20].Nursing guide for Power port (implantable port)/Power lock (safety infusion set) Bard Access System p9. [Google Scholar]
- [21].Celsite® Implantable Access Port System: Instruction for use p7. [Google Scholar]
- [22].Wu CY, Cheng CH, Fu JY, et al. Recommended irrigation volume for an intravenous port: Ex vivo simulation study. PLoS One 2018;13:e0201785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ferroni A, Gaudin F, Guiffant G, et al. Pulsative flushing as a strategy to prevent bacterial colonization of vascular access devices. Med Devices (Auckl) 2014;7:379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Niederhuber JE, Ensminger W, Gyves JW, et al. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery 1982;92:706–12. [PubMed] [Google Scholar]
- [25].Wu CY, Hu HC, Ko PJ, et al. Risk factors and possible mechanisms of superior vena cava intravenous port malfunction. Ann Surg 2012;255:971–5. [DOI] [PubMed] [Google Scholar]
- [26].Milani A, Mazzocco K, Gandini S, et al. Incidence and determinants of port occlusions in cancer outpatients: a prospective cohort study. Cancer Nurs 2017;40:102–7. [DOI] [PubMed] [Google Scholar]
- [27].Ribeiro RC, Abib SC, Aguiar AS, et al. Long-term complications in totally implantable venous access devices: randomized study comparing subclavian and internal vein puncture. Pediatr Blood Cancer 2012;58:274–7. [DOI] [PubMed] [Google Scholar]
- [28].Hartkamp A, van Boxtel AJ, Zonnenberg BA, et al. Totally implantable venous access devices: evaluation of complications and a prospective comparative study of two different port systems. Neth J Med 2000;57:215–23. [DOI] [PubMed] [Google Scholar]
- [29].Wu CY, Fu JY, Wu CF, et al. Initial experiences with a new design for a preattached intravenous port device. J Biomed Mater Res B Appl Biomater 2018;106:1017–27. [DOI] [PubMed] [Google Scholar]
- [30].Teichgräber UK, Gebauer B, Benter T, et al. Central venous access catheters: radiological management of complications. Cardiovasc Intervent Radiol 2003;26:321–33. Review. [DOI] [PubMed] [Google Scholar]
- [31].Rosenbluth G, Tsang L, Vittinghoff E, et al. Impact of decreased heparin dose for flush-lock of implanted venous access ports in pediatric oncology patients. Pediatr Blood Cancer 2014;61:855–8. [DOI] [PubMed] [Google Scholar]
- [32].Bradford NK, Edwards RM, Chan RJ. Heparin versus 0.9% sodium chloride intermittent flushing for the prevention of occlusion in long term central venous catheters in infants and children: a systematic review. Int J Nurs Stud 2016;59:51–9. [DOI] [PubMed] [Google Scholar]
- [33].Solinas G, Platini F, Trivellato M, et al. Port in oncology practice: 3-monthly locking with normal saline for catheter maintenance, a preliminary report. J Vasc Access 2017;18:325–7. [DOI] [PubMed] [Google Scholar]
- [34].Hopkins M. Infusion port maintains excellent blood return after 77 weeks without flushing. Oncol Nurs Forum 1993;20:731. [PubMed] [Google Scholar]
- [35].Kuo YS, Schwartz B, Santiago J, et al. How often should a port-A-cath be flushed? Cancer Invest 2005;23:582–5. [DOI] [PubMed] [Google Scholar]
- [36].Kefeli U, Dane F, Yumuk PF, et al. Prolonged interval in prophylactic heparin flushing for maintenance of subcutaneous implanted port care in patients with cancer. Eur J Cancer Care (Engl) 2009;18:191–4. [DOI] [PubMed] [Google Scholar]
- [37].Ignatov A, Ignatov T, Taran A, et al. Interval between port catheter flushing can be extended to four months. Gynecol Obstet Invest 2010;70:91–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


