Abstract
To explore associated risk factors and their interactions with type 2 diabetes (T2DM) among the elderly with prediabetes in rural areas in China.
A nested case–control study was conducted in a fixed cohort to identify the risk factors for T2DM among the elderly with prediabetes in rural areas of Yiyang City in China. A total of 37 elderly with T2DM were included in the cases group and 111 elderly subjects with prediabetes were matched in the control group. Data related to sociodemographic characteristics, lifestyle behavior, and anthropometric variables were collected by trained staff using standard tools. The risk factors for T2DM were determined using conditional logistic regression analysis, and their additive interactions were also explored.
Multivariable conditional logistic regression analysis results showed that overweight/obesity (odds ratio [OR] = 4.80, 95% confidence interval [CI]: 1.20–12.28), family history of diabetes (OR = 3.63, 95% CI: 1.03–12.81), physically inactive (OR = 3.08, 95% CI: 1.14–8.30), high waist-to-hip ratio (WHR) (OR = 3.15, 95% CI: 1.27–7.80), and inadequate diabetes-specific health literacy (DSHL) (OR = 3.92, 95% CI: 1.14–13.48) increased the risk for T2DM. Additive interactions for T2DM were observed between a family history of diabetes and high WHR with a relative excess risk of interaction (RERI) of 10.02 (95% CI: 4.25, 15.78), and between high WHR and overweight or obesity, with an RERI of 3.90 (95% CI: 0.36, 7.44).
The independent risk factors for T2DM are overweight or obesity, high WHR, family history of diabetes, physically inactive, and inadequate DSHL. High WHR as a risk factor for T2DM has additive interactions with family history of diabetes and overweight or obesity.
Keywords: diabetes, elderly, interaction, prediabetes
1. Introduction
Prediabetes is defined as blood glucose concentrations higher than normal but not high enough to be classified as diabetes, which was categorized into either impaired fasting glucose (IFG) or/and impaired glucose tolerance (IGT).[1] Data from a survey in China indicated that more than 148.2 million adults, and more than 20% of the elderly have prediabetes.[2] Several studies have demonstrated that individuals with prediabetes were at a higher risk status for developing diabetes, especially older adults.[3–5] However, many trials have also shown that people with prediabetes have converted into normal plasma glucose after lifestyle intervention.[6,7] Therefore, investigating the progression rate of prediabetes, identifying influencing factors for diabetes in a timely and accurate manner, and making interventions to reduce the risk for diabetes are necessary among the elderly with prediabetes.
As regards the progression rate from prediabetes to diabetes, a meta-analysis that included 35 articles indicated that the absolute annual incidence of diabetes among individuals with IFG and/or IGT varied from 5% to 10%.[8] Another meta-analysis of prospective observational studies showed that the pooled incidence of diabetes was 47.4, 45.5, and 70.4 per 1000 person–years among subjects with IFG, IGT, and IFG + IGT, respectively, as defined by the World Health Organization (WHO).[9] Moreover, many studies indicated that the incidence of diabetes among the elderly with prediabetes was particularly high.[10–12] A retrospective cohort study among the Chinese elderly with prediabetes showed that the 5-year cumulative incidence of diabetes was 20.1%.[11] Another study showed that, among the elderly people with IFG, an annual progression rate was 6.8%.[12]
Several studies have indicated that there are many factors associated with diabetes among individuals with prediabetes, such as ageing, obesity, elevated glycosylated hemoglobin (HbA1c), fasting insulin, and albuminuria.[13,14] However, there are a few studies that identified the risk factors for diabetes and their interactions among the elderly with prediabetes in rural areas of China. Therefore, the purpose of this study was to identify the associated risk factors and their interactions with type 2 diabetes (T2DM) among the elderly with prediabetes in some rural areas of China. It is hoped that the results of this study could provide a more scientific basis for prediabetes intervention and prevention in rural China.
2. Material and methods
2.1. Sample size
The number of cases was estimated according to the sample size formula for a 1: r paired case–control study as follows:
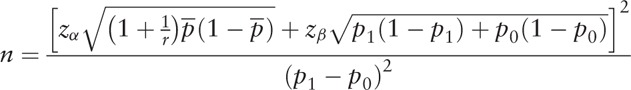 |
where 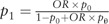 , and
, and 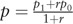 . According to a previous study in China,[15] the exposure rate of low family income in a control group was 13.9% and odds ratio (OR) value was 1.64, respectively. Besides, the estimated theoretical sample was determined as 31, which included an extra 10% to allow for subjects lost to follow-up when α was 0.05, β was 0.20, and r was 3. Furthermore, based on the prevalence of T2DM in rural areas in China, the prevalence was estimated as 20.2% in the elderly,[16] and the theoretical cohort sample size was determined as 154.
. According to a previous study in China,[15] the exposure rate of low family income in a control group was 13.9% and odds ratio (OR) value was 1.64, respectively. Besides, the estimated theoretical sample was determined as 31, which included an extra 10% to allow for subjects lost to follow-up when α was 0.05, β was 0.20, and r was 3. Furthermore, based on the prevalence of T2DM in rural areas in China, the prevalence was estimated as 20.2% in the elderly,[16] and the theoretical cohort sample size was determined as 154.
2.2. Study population
A total of 2144 older adults from 42 villages in Yiyang City of Hunan Province, who were identified using multistage cluster randomized sampling method, were participant in prediabetes screening program between April and July 2015. A detailed description of this study population is provided in a previous study.[17] In brief, a total of 461 older adults were identified with prediabetes using oral glucose tolerance test (OGTT) according to 1999 WHO criteria[1] at baseline as following groups: an IFG group with fasting plasma glucose (FPG) of 6.1 to 7.0 mmol/L (110–126 mg/dL) and a 2-hour postglucose (2-h PG) of <7.8 mmol/L (140 mg/dL); an IGT group with a 2-h PG of 7.8 to 11.1 mmol/L (140–200 mg/dL) and FPG of ≤6.1 mmol/L (110 mg/dL); and an IFG + IGT group. Of the 461 subjects, 189 were randomly selected and followed up to assess the development of T2DM.
2.3. Ethical statement
This study was approved by the Medical Ethics Committee of Central South University (CTXY-150002–7). Written informed consent was obtained from all subjects at the baseline and follow-up stages.
2.4. Case and control definition
Cases were those who developed T2DM during the follow-up period. In this regard, T2DM was determined by self-reporting of the diagnosis made by a physician and diabetes-specific medication used, and it was verified through local health records and hospital admission data. The OGTT was also performed during follow-up period for subjects who were not diagnosed with T2DM. Subjects with FPG ≥7.0 mmol/L or 2-h PG ≥11.1 mmol/L according to the 1999 WHO criteria[1] were considered as potentially having T2DM. The elderly with prediabetes but not diagnosed with T2DM according to the 1999 WHO criteria were selected as controls. The ratio of cases to controls was 1:3 and matched according to gender and age at the time of diagnosis (±3 years) in the control group.
2.5. Data collection
Sociodemographic characteristics (age, gender, marital status, education level, personal annual income, family history of diabetes, and history of chronic disease) and lifestyle behavior (smoking, alcohol drinking, sedentary time, physical activity, dietary pattern, sleep duration, and diabetes-specific health literacy [DSHL]) were collected by trained staff using structured questionnaires through face-to-face interview. Education level was divided into 2 categories: less than 9 years, or 9 years and above. Marital status was classified as married and nonmarried, with nonmarried status including never-married, divorced, and widowed. A family history of diabetes was determined by finding out whether first-degree relatives were diabetic. History of chronic disease was defined as participants self-reporting hypertension, dyslipidemia, coronary heart disease, and others.
Smoking was defined as smoking 1 or more cigarettes per day in the last 6 months.[18] Alcohol drinking was defined as drinking a glass of wine (approximately 250 mL beer, 100 mL sake, or 20 mL liquor) per day in the last month.[19] Average daily sedentary time in the last week was self-reported and 8 hours per day was the cutoff value dividing participants into 2 groups. Average sleep duration per night in the last week was assessed by asking participants to select from the 3 categories (<7 h per night, 7–8 h per night, and >8 h per night). The International Physical Activity Questionnaire-long version (IPAQ) was used to assess the physical activity levels of the participants in the last week and classifying them into 3 levels: low, moderate, and high. Physically inactive was defined as participant with a low level of physical activity according to the criteria of the IPAQ.[20] Dietary patterns were assessed using the China Health and Nutrition Survey Questionnaire,[21] and the Diet Balance Index (DBI-07) was calculated to evaluate dietary quality. Participants with a DBI score of 0 or lower than 20% of the total scores were defined as having a balanced diet.[22]
DSHL was assessed using the Questionnaire of Health Literacy of Diabetes Mellitus of the Public (HLDMP) in China, which was designed by the Chinese Center for Health Education to assess health literacy about diabetes prevention and control in the general population. This questionnaire has been widely used in epidemiological studies in China, and has high reliability and validity, with a Cronbach's α of 0.866.[23] DSHL can provide a comprehensive evaluation of an individual's diabetes prevention and control knowledge, risk awareness, and ability to manage risk factors. An HLDMP score greater than 32.4 points was defined as having adequate DSHL.[23]
Anthropometric variables including height, weight, waist circumference, and hip circumference were assessed using a set of standard tools. Height was measured to the nearest 0.1 cm using a stadiometer, and weight was measured to the nearest 0.1 kg using a weighing instrument. Waist and hip circumferences were measured to the nearest 1 mm using the recommended measurement methods by training administrator according to the guidelines for the prevention and control of overweight and obesity in Chinese adults.[24] Body mass index (BMI) was calculated according to the formula of kg/m2 and participants were classified into 3 categories of BMI as follows: underweight (<18.5 kg/m2), normal (18.5–24.0 kg/m2), and overweight or obesity (≥24.0 kg/m2).[25] The waist-to-hip ratio (WHR) was calculated by dividing the waist measurement by the hip measurement. A WHR >0.9 in men and >0.8 in women was defined as a high WHR.[26]
2.6. Statistical analysis
Categorical variables were described as counts and percentages, whereas continuous variables were described as means and standard deviations (mean ± SD). The statistical differences in sociodemographic characteristics, lifestyle behaviors, and anthropometric variables between the cases and the controls were analyzed using matched Chi-squared test. The associated risk factors for T2DM were determined using binary conditional logistic regression analysis. Firstly, crude ORs with 95% confidence intervals (95% CIs) were estimated. Secondly, possible confounders were added to the multivariable model and adjusted ORs were estimated. The interaction between risk factors were identified by the Excel table introduced by Andersson et al.[27] Three main indicators were used to determine the existence of additive interactions which are the relative excess risk due to interaction (RERI), the attributable proportion due to interaction (AP), and the synergy index (S). There is no significant additive interaction when the RERI and AP are equal to 0 or S is equal to 1. The statistical analysis was conducted in SAS version 9.2 (SAS Institute Inc, Cary, NC). All statistical tests were two-tailed and P < .05 was considered statistically significant.
3. Results
3.1. Subject characteristics
A total of 37 older adults (16 male, 21 female) developed T2DM after 1 year of follow-up, of whom 13 were IFG, 17 were IGT, and 7 were IFG + IGT. The annual incidence of T2DM among the elderly with prediabetes was 19.7% in the rural areas of Yiyang City in China. Additionally, 111 subjects with prediabetes of same gender and similar age (±3 years) were matched. The average age was 68.8 ± 6.8 years in the cases group and 68.3 ± 8.4 years in the control group. Characteristics of the study population are shown in Table 1.
Table 1.
Characteristics of the study population.
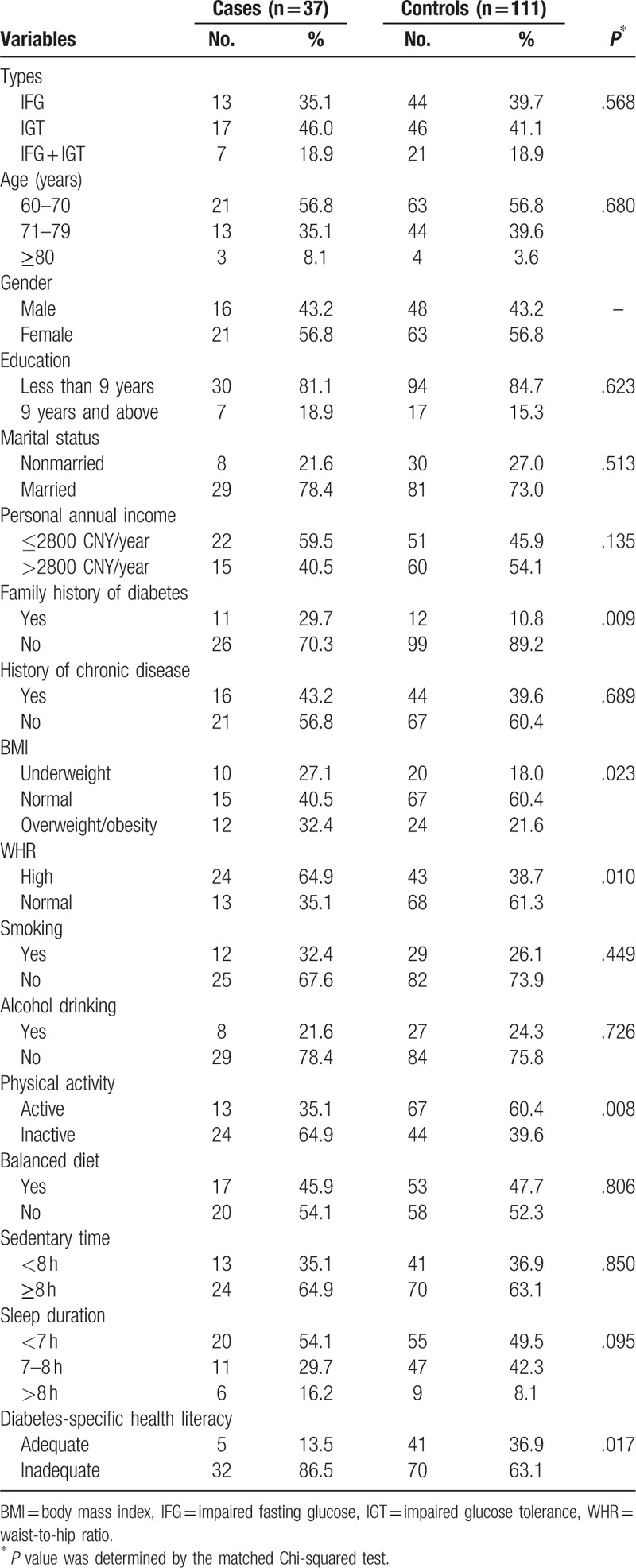
The matched Chi-squared test indicated that there was a significant difference in the distribution of a family history of diabetes, BMI, WHR, physical activity, and DSHL between the 2 groups (All P < .05). The results are shown in Table 1.
3.2. Associated risk factors for T2DM
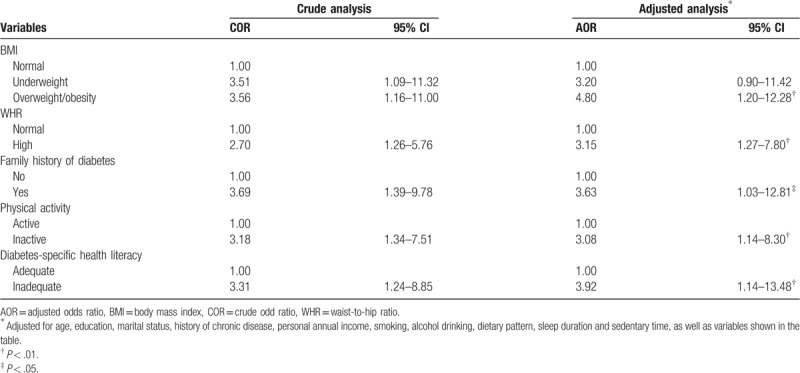
Multivariable binary conditional logistic regression analysis results indicated that the associated risk factors for T2DM among the elderly with prediabetes were overweight or obesity (OR = 4.80, 95% CI: 1.20–12.28), high WHR (OR = 3.15,95% CI: 1.27–7.80), family history of diabetes (OR = 3.63,95% CI: 1.03–12.81), physically inactive (OR = 3.08, 95% CI: 1.14–8.30), and inadequate DSHL (OR = 3.92, 95% CI: 1.14–13.48). The results are shown in Table 2.
Table 2.
Crude and adjusted odds ratios of risk factors for type 2 diabetes among the elderly with prediabetes.
3.3. Interactions of risk factors for T2DM
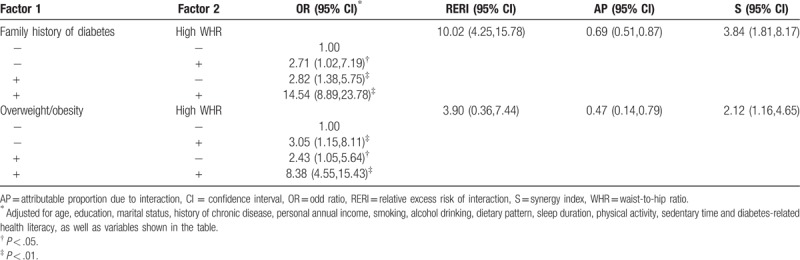
Additive interactions were observed between high WHR and family history of diabetes with RERI of 10.02 (95% CI: 4.25, 15.78), AP of 0.69 (95% CI: 0.51, 0.87), and S of 3.84 (95% CI: 1.81–8.17). Additive interactions were also detected between high WHR and overweight/obesity with RERI of 3.90 (95% CI: 0.36, 7.44), AP of 0.47 (95% CI: 0.14, 0.79), and S of 2.12 (95% CI: 1.16, 4.65) after adjusting for potential confounders. No significant interactions were detected between other risk factors. The results are shown in Table 3.
Table 3.
Interaction of risk factors for type 2 diabetes among the elderly with prediabetes.
4. Discussion
This is the first nested case–control study which examined associated risk factors and their interactions with T2DM among the elderly with prediabetes in the rural areas of Yiyang City in China. It was found that overweight/obesity, high WHR, family history of diabetes, physical inactivity, and inadequate DSHL were the associated risk factors for T2DM after adjusting for potential confounding factors. Furthermore, a high WHR had additive interactions with family history of diabetes and overweight/obesity. Also, this study found that the progression rate of T2DM was 19.7% after 1 year of follow-up among the elderly with prediabetes, which is higher than that reported by other studies conducted among the elderly.[11,12] There are 2 reasons for this phenomenon. On the one hand, our study subjects comprised the elderly with IFG and/or IGT, whereas other studies included the elderly with only IFG. On the other hand, there are differences between this study and other studies in the definitions and assessment criterion for prediabetes and T2DM. Nevertheless, this rate was comparable with that of a study conducted in Tianjin Province, which reported that the incidence of diabetes was 17.1% among the elderly with IFG after 3 years of follow-up.[10]
In addition, this study found that both overweight/obesity and high WHR were increased the risk for T2DM among the elderly with prediabetes. These results are consistence with those in other previous studies.[12,13] For instance, the Strong Heart Study conducted among 1677 American Indians found that obesity significantly increased the risk for T2DM among those with prediabetes by 2.7 times after 7.8 years of follow-up.[13] Moreover, another retrospective cohort study in Singapore among 490 participants with IFG indicated that BMI was positively associated with the risk for T2DM (hazard ratio [HR] = 1.11; 95% CI, 1.06–1.15).[12] Furthermore, this study found that there was additive interaction between high WHR and overweight/obesity, which suggests that there is a reinforcing effect when high BMI and high WHR are present simultaneously. Therefore, there is need to pay more attention to those people who are both generalized and centralized adiposity even though the underlying mechanism between obesity and the risk for T2DM are unclear.
Many studies have demonstrated that family history of diabetes was a major risk factor for both T2DM and prediabetes.[28,29] Similarly, this study showed that family history of diabetes increased the risk for T2DM among the elderly with prediabetes. This outcome may be explained by various genetic factors, such as mitochondrial DNA mutations. Also, this study found that family history of diabetes was not only an independent risk factor for T2DM but also has additive interaction with high WHR. The adjusted odds ratios (aORs) for interaction between family history of diabetes and high WHR were greater than the aORs for only 1 of the 2 factors under discussion, which demonstrates that there may be much stronger association with T2DM when both factors are present. The joint effect of family history of diabetes and waist circumference on diabetes was also found among 1472 Finnish men and 1694 women in a previous study.[30] Additionally, many previous studies demonstrated that a family history of diabetes is independent of proband BMI, and associated with adipocyte hypertrophy and enhanced lipolysis, which suggests that these factors are genetically linked to T2DM.[31,32] Hence these associations may partially explain the rationality of the existence of the interaction between family history of diabetes and high WHR to a certain extent. However, this study found no significant interaction between family history of diabetes and BMI, perhaps because the sample size of this study was relatively small and this might have compromised the effectiveness of the statistical power.
This study also found that inactive physical activity was associated with T2DM among the elderly with prediabetes, which is consistence with other studies.[13,33] For example, the Inter99 cohort Study demonstrated that the progression rate to T2DM was lower among physically active individuals with isolated IGT.[33] There are several studies that also showed that moderate to vigorous physical activity may have beneficial effects on insulin and C-peptide metabolism in population at high risk for T2DM.[34,35] In addition, several trials showed that an increase in the level of physical activity may be related to the reduction in the incidence of T2DM among subjects with IGT.[36,37]
Furthermore, this study found that DSHL was associated with T2DM among the elderly with prediabetes, which is comparable with other studies to some degree.[38,39] For instance, a study that included 908 older adults found that lower literacy levels were inversely associated with hemoglobin A1c and blood glucose levels after adjusting for covariates.[38] Also, a meta-analysis of 61 studies with a total of 18,905 patients indicated that health literacy had a small though significant effect on better glycemic control, and had a positive impact on diabetes self-care activities.[39] However, unlike other studies which used general health literacy measures, such as The Rapid Estimate of Adult Literacy in Medicine (REALM) or The Test of Functional Health Literacy in Adults (TOFHLA), this study used a questionnaire which effectively measured the level of health literacy on diabetes prevention and control based on three main domains, namely, diabetes-related knowledge, diabetes-related behavior, and utilization of diabetes information. Previous data from 1318 respondents aged 42 to 96 indicated that there is a direct association between DSHL and patient assessments of their self-care ability, which suggests that DSHL is an important element in the successful management of diabetes.[40] However, a direct relationship between DSHL and the frequency of recommended diabetes self-care behaviors has not been established. Even so, diabetes education is important in the prevention and control of diabetes among individuals with prediabetes because strong evidence suggested that subjects with low health literacy benefited more from diabetes education and showed better improvement in self management behaviors than their peers with adequate health literacy.[41]
The strength of this study is that it is one of few studies to examine the associated risk factors and their interactions with T2DM among the elderly with prediabetes in some rural areas of China. Additionally, the cause and effect is more reliable to infer due to a nested case–control study design based on a cohort. Thus, the findings of this study could provide valuable information on diabetes education and prevention in the aging population of rural areas of China. However, this study has some limitations. Firstly, some unmeasured and unknown potential confounders might have biased the findings. Secondly, the sample size was relatively small and statistical power of findings is limited. Thirdly, participants only from the rural areas of Yiyang City of China, thus generalizing these results to other elderly populations should be considered carefully. Finally, the follow-up period was relatively short and T2DM cases were relatively few, hence further investigations are needed to confirm the findings of this study.
5. Conclusion
The findings of this study suggest that the associated risk factors for T2DM among the elderly with prediabetes are overweight/obesity, high WHR, family history of diabetes, physically inactive, and inadequate DSHL. In addition, there are additive interactions between high WHR and family history of diabetes and overweight/obesity.
Acknowledgments
We thank all the participants very much for their collaboration.
Author contributions
Conceptualization: Huilan Xu.
Investigation: Zhao Hu.
Writing – original draft: Xidi Zhu.
Writing – review & editing: Zhao Hu, Atipatsa Chiwanda Kaminga.
Footnotes
Abbreviations: 2-h PG = 2-hour postglucose, aOR = adjusted odds ratio, AP = attributable proportion due to interaction, BMI = body mass index, CI = confidence interval, DBI = Diet Balance Index, DSHL = diabetes-specific health literacy, FPG = fasting plasma glucose, HLDMP = Health Literacy of Diabetes Mellitus of the Public, IFG = impaired fasting glucose, IGT = impaired glucose tolerance, IPAQ = International Physical Activity Questionnaire, OGTT = oral glucose tolerance test, OR = odd ratio, RERI = relative excess risk of interaction, S = synergy index, T2DM = type 2 diabetes, WHO = World Health Organization, WHR = waist-to-hip ratio.
How to cite this article: Hu Z, Zhu X, Kaminga AC, Xu H. Associated risk factors and their interactions with type 2 diabetes among the elderly with prediabetes in rural areas of Yiyang City. Medicine. 2019;98:44(e17736).
ZH and XZ contributed equally to the article.
The data analyzed during this study are included in the article. The numerical data used to support the findings of this study are available from the corresponding author upon reasonable request.
This work was supported by the Teachers Research Fund of Central South University (2013JSJJ034).
The authors have no conflicts of interest to disclose.
References
- [1].Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- [2].Yang W, Lu J, Weng J, et al. China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–101. [DOI] [PubMed] [Google Scholar]
- [3].Forouhi NG, Luan J, Hennings S, Wareham NJ. Incidence of type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990–2000. Diabet Med 2007;24:200–7. [DOI] [PubMed] [Google Scholar]
- [4].Nathan DM, Davidson MB, DeFronzo RA, et al. American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753–9. [DOI] [PubMed] [Google Scholar]
- [5].Perreault L, Pan Q, Mather KJ, et al. Diabetes Prevention Program Research Group. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012;379:2243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–9. [DOI] [PubMed] [Google Scholar]
- [7].Penn L, White M, Oldroyd J, et al. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health 2009;9:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 2007;78:305–12. [DOI] [PubMed] [Google Scholar]
- [9].Morris DH, Khunti K, Achana F, et al. Progression rates from HbA1c 6.0-6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia 2013;56:1489–93. [DOI] [PubMed] [Google Scholar]
- [10].Zhang W, Yang X, Han P, et al. Risk factors for developing diabetes after 3 years among community-dwelling elderly with impaired fasting glucose. J Diabetes 2019;11:107–14. [DOI] [PubMed] [Google Scholar]
- [11].Fu SN, Luk W, Wong CK, Cheung KL. Progression from impaired fasting glucose to type 2 diabetes mellitus among Chinese subjects with and without hypertension in a primary care setting. J Diabetes 2014;6:438–46. [DOI] [PubMed] [Google Scholar]
- [12].Ang YG, Wu CX, Toh MP, et al. Progression rate of newly diagnosed impaired fasting glycemia to type 2 diabetes mellitus: a study using the National Healthcare Group Diabetes Registry in Singapore. J Diabetes 2012;4:159–63. [DOI] [PubMed] [Google Scholar]
- [13].Wang H, Shara NM, Calhoun D, et al. Incidence rates and predictors of diabetes in those with prediabetes: the Strong Heart Study. Diabetes Metab Res Rev 2010;26:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dong J, Yang ZK, Chen Y. Older age, higher body mass index and inflammation increase the risk for new-onset diabetes and impaired glucose tolerance in patients on peritoneal dialysis. Perit Dial Int 2016;36:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Luo G, Yang J, Wang Z, et al. Analysis on the risk factors of type 2 diabetes mellitus among the elderly at Bazhong city in Sichuan province. Chin J Diabetes Mellitus 2011;03:42–5. In Chinese. [Google Scholar]
- [16].Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017;317:2515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Qin L, Xu H. A cross-sectional study of the effect of health literacy on diabetes prevention and control among elderly individuals with prediabetes in rural China. BMJ Open 2016;6:e011077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Silventoinen K, Sans S, Tolonen H, et al. WHO MONICA Project. Trends in obesity and energy supply in the WHO MONICA Project. Int J Obes Relat Metab Disord 2004;28:710–8. [DOI] [PubMed] [Google Scholar]
- [19].Fogli-Cawley JJ, Dwyer JT, Saltzman E, et al. The 2005 Dietary Guidelines for Americans Adherence Index: development and application. J Nutr 2006;136:2908–15. [DOI] [PubMed] [Google Scholar]
- [20].Fan M, Lyu J, He P. Chinese guidelines for data processing and analysis concerning the International Physical Activity Questionnaire. Zhonghua Liu Xing Bing Xue Za Zhi 2014;35:961–4. [PubMed] [Google Scholar]
- [21].Zhang B, Zhai FY, Du SF, et al. The China Health and Nutrition Survey, 1989–2011. Obes Rev 2014;15Suppl 1:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu R, Zhao Y, Yan H, et al. Using the revised Chinese diet balance index Quality of Diet to evaluate the quality of diet among rural residents in Hanzhong, Shaanxi province and relative influencing factors. Zhonghua Liu Xing Bing Xue Za Zhi 2014;35:1087–90. [PubMed] [Google Scholar]
- [23].Li L, Li Y, Nie X, et al. An analysis of health literacy about diabetes prevention and control and its influencing factors among the residents in six provinces in China. Zhonghua Yu Fang Yi Xue Za Zhi 2014;48:561–5. [PubMed] [Google Scholar]
- [24].Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci 2004;17Suppl:1–36. [PubMed] [Google Scholar]
- [25].Wu Y. Overweight and obesity in China. BMJ 2006;333:362–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Obesity in Asia Collaboration. Is central obesity a better discriminator of the risk of hypertension than body mass index in ethnically diverse populations? J Hypertens 2008;26:169–77. [DOI] [PubMed] [Google Scholar]
- [27].Andersson T, Alfredsson L, Källberg H, et al. Calculating measures of biological interaction. Eur J Epidemiol 2005;20:575–9. [DOI] [PubMed] [Google Scholar]
- [28].Wagner R, Thorand B, Osterhoff MA, et al. Family history of diabetes is associated with higher risk for prediabetes: a multicentre analysis from the German Center for Diabetes Research. Diabetologia 2013;56:2176–80. [DOI] [PubMed] [Google Scholar]
- [29].Moonesinghe R, Beckles GL, Liu T, Khoury MJ. The contribution of family history to the burden of diagnosed diabetes, undiagnosed diabetes, and prediabetes in the United States: analysis of the National Health and Nutrition Examination Survey, 2009–2014. Genet Med 2018;20:1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ning F, Pang Z, Laatikainen T, et al. Qingdao 2006 Diabetes Survey and FINRISK 2002 Study. Joint effect of family history of diabetes with obesity on prevalence of type 2 diabetes mellitus among Chinese and Finnish men and women. Can J Diabetes 2013;37:65–71. [DOI] [PubMed] [Google Scholar]
- [31].Dahlman I, Ryden M, Arner P. Family history of diabetes is associated with enhanced adipose lipolysis: evidence for the implication of epigenetic factors. Diabetes Metab 2018;44:155–9. [DOI] [PubMed] [Google Scholar]
- [32].Lai Y, Qi J, Tao X, et al. Associations of grandparental diabetes mellitus with grandchild BMI status. BMC Public Health 2019;19:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Engberg S, Glumer C, Witte DR, et al. Differential relationship between physical activity and progression to diabetes by glucose tolerance status: the Inter99 Study. Diabetologia 2010;53:70–8. [DOI] [PubMed] [Google Scholar]
- [34].Ungethum K, Jolink M, Hippich M, et al. Physical activity is associated with lower insulin and C-peptide during glucose challenge in children and adolescents with family background of diabetes. Diabet Med 2019;36:366–75. [DOI] [PubMed] [Google Scholar]
- [35].Kriska AM, Pereira MA, Hanson RL, et al. Association of physical activity and serum insulin concentrations in two populations at high risk for type 2 diabetes but differing by BMI. Diabetes Care 2001;24:1175–80. [DOI] [PubMed] [Google Scholar]
- [36].Tuomilehto J, Lindstrom J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50. [DOI] [PubMed] [Google Scholar]
- [37].Horton ES. Effects of lifestyle changes to reduce risks of diabetes and associated cardiovascular risks: results from large scale efficacy trials. Obesity 2009;17Suppl 3:S43–8. [DOI] [PubMed] [Google Scholar]
- [38].Lamar M, Wilson RS, Yu L, et al. Associations of literacy with diabetes indicators in older adults. J Epidemiol Community Health 2019;73:250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marciano L, Camerini AL, Schulz PJ. The role of health literacy in diabetes knowledge, self-care, and glycemic control: a meta-analysis. J Gen Intern Med 2019;34:1007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yamashita T, Kart CS. Is diabetes-specific health literacy associated with diabetes-related outcomes in older adults? J Diabetes 2011;3:138–46. [DOI] [PubMed] [Google Scholar]
- [41].Kim S, Love F, Quistberg DA, Shea JA. Association of health literacy with self-management behavior in patients with diabetes. Diabetes Care 2004;27:2980–2. [DOI] [PubMed] [Google Scholar]


