Abstract
Current surgical options for treating genu varum in achondroplasia include tibial and fibular osteotomy and growth modulation using plates and screws. However, a single surgeon consistently treated genu varum using a planned fibular nonunion (PFN). The purpose of this study is to describe his surgical technique and report radiographic and clinical outcomes for the cohort studied.
This is an observational retrospective review. The cohort studied included patients with achondroplasia who had PFN surgery for the treatment of genu varum at a young age (<13 years) and was followed through to skeletal maturity. The surgery included meticulous closure of the periosteum over the remaining fibula. The surgery was considered a success if the patient did not require subsequent surgery and had acceptable or improved clinical alignment. Radiographic measures used to determine change in genu varum included the anatomic tibio-femoral angle (aTFA), tibia varus, and tibia-fibula ratio. Clinically, changes in lower limb alignment were defined using a plumb line and 6 categories of alignment ranging from extreme varus to valgus. Statistics were used to validate the plumb line categorization to available radiographic measures. Other appropriate statistical methods were used with P < .05 considered significant.
Of the 53 PFN cases (27 patients) included in the study, 34 (64%) did not require subsequent surgery and had acceptable or improved alignment. The average age at surgery and follow-up was 6.1 and 17.0 years, respectively. For the 37 limbs (19 patients) with available radiographs, pre- and post-surgery radiographic measures significantly improved including aTFA (3° varus to 2° valgus, P = .003), tibia varus (2° varus to 3° valgus, P = .004), and the tibia-fibula ratio (0.977 to 1.013, P < .001). Clinically, 32 cases (60%) demonstrated significant improvement by translating into an improved alignment and 9 (17%) remained the same (P < .01). Complications were minimal and insignificant. Failures were readily managed by tibia-fibular osteotomies in adolescence and at maturity.
PFN for the treatment of genu varum in young achondroplasia patients significantly improved radiographic and clinical measures of lower limb alignment through skeletal maturity with relatively few complications.
Keywords: achondroplasia, genu varum, planned fibular nonunion
1. Introduction
Achondroplasia represents the largest group of patients with skeletal dysplasias. The incidence is 1 in 15,000 to 40,000 live births.[1–3] Achondroplasia is a bone dysplasia caused by a mutation in fibroblast growth factor receptor 3 gene involved in converting cartilage to bone.[4] Clinical manifestations include shortened stature, proximal shortening of lower and upper extremities, macrocephaly, mid-face hypoplasia, and most, but not all, develop some degree of genu varum, ranging from mild to severe.[5–7] Some studies, particularly Ponseti in 1970, suggest that relative fibular overgrowth in relation to tibial growth contributes to the development of this genu varum, while others found no such relationship.[7–11] Most orthopedic surgeons generally accept that bracing is of no significant value in the treatment of genu varum in this population. Surgical treatment includes tibial osteotomy with or without fibular osteotomy, proximal and distal fibular epiphysiodesis, and growth modulation by plates and screws.[7,9–12] Some have suggested that shortening the fibula effectively tightens the lax lateral collateral ligaments,[7,9,13] but there is little in the literature to suggest that primary surgery on the fibula is likely to be effective.[7]
Frustrated by experiences with failures of tibio-fibular osteotomy performed in children under 8 years of age and after proximal fibular epiphysiodesis (recurrences), Steven E. Kopits, MD (SEK) decided that a procedure that addressed both proximal and distal fibular overgrowth was necessary. He believed the relatively longer fibula was tensioning the tibia into varus. In 1980, he began to perform a planned fibular nonunion (PFN) in these patients by excising a section of the fibula. In 1986, he moved the site of the PFN more proximal in an effort to avoid the possibility of distal ankle valgus. The records and available radiographs were preserved and made available to the senior author Dennis S. Weiner, MD (DSW).
The purpose of this study is to present the clinical and radiographic results of patients with achondroplasia treated with PFN for genu varum operated upon by a single surgeon (SEK).
This report concerns itself solely with whether the PFN was or was not successful in varus correction and in no way concerns itself with the surgeon's indication for treatment.
2. Materials and methods
2.1. Ethical statement
This study was approved with waivers of consent and assent by the institutional review board of the participating institution.
2.2. Study design and patient selection
This is an observational retrospective cohort study. A single surgeon (SEK) performed PFN surgeries from April 1979 to April 2001 and his charts and radiographs were reviewed by the authors to identify patients meeting inclusion/exclusion criteria. Patients were included if they had achondroplasia, underwent PFN surgery for the treatment of genu varum before age 13, and were skeletally mature with at least 2 years follow-up at last visit. Patients were excluded if they had prior or additional tibial or fibular procedures at the time of the PFN or had missing pre- or postoperative clinical data needed for comparison. Skeletal maturity was defined as at least 14 years of age for females and 16 years for males and closure of the distal femoral and proximal tibial growth plates.
2.3. Radiographic analyses
Radiographic measures of pre- and post-surgical lower limb alignment included anatomic tibio-femoral angle (aTFA), tibia-fibula ratio, anatomic lateral distal femoral angle (aLDFA), lateral distal tibial angle (LDTA), medial proximal tibial angle (MPTA), mechanical axis zone, and tibia varus. The technique used for measuring aTFA was outlined by Krackow (K.A. Krackow, MD, unpublished data, 2008, https://www.medschool.lsuhsc.edu/orthopaedics/docs/How%20to%20Measure%20Knee%20Alignment.pdf). Measures of aLDFA, LDTA, and MPTA were as described by Paley.[14] The degree of fibular overgrowth was measured by taking the tibia-fibula ratio as outlined by Matsui, while the mechanical axis zone deviation was measured as described by Stevens.[15,16] Tibia varus and fibular excision site measurements are described in Figure 1.
Figure 1.
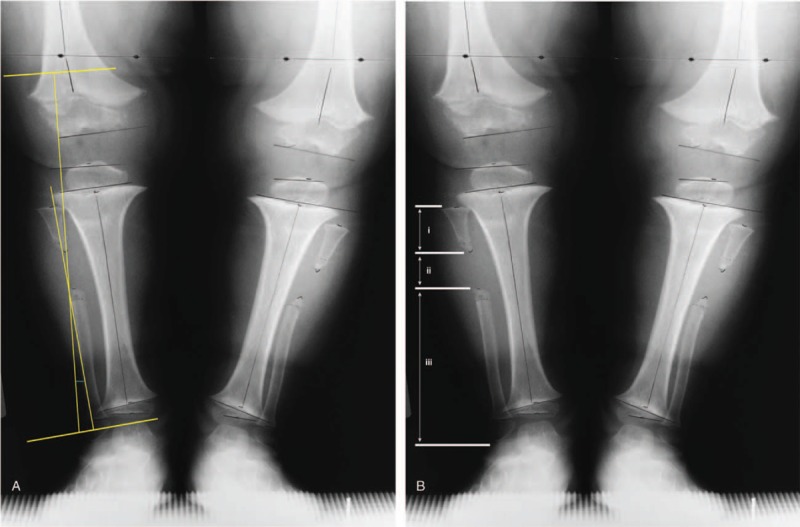
Description of the following radiographic measurements taken: (A) tibia varus and (B) location of the fibular excision. Tibia varus was measured similar to the Cobb angle, where a line was drawn parallel to the tibial plafond and a second line drawn parallel to the proximal tibial condyles and the angle formed by the intersection of the lines perpendicular to these parallel lines representing tibia varus or valgus. The fibular excision site was calculated using the following measures: (i) length between the head of the fibula and first cut; (ii) the gap created by the excision, and (iii) length from the distal cut to the lateral malleolus. A ratio (i/[i + ii + iii]) with a value less than 0.30 was considered a proximal excision and a value greater than or equal to 0.30 was considered a mid-shaft incision.
2.4. Clinical assessment of lower limb alignment
Chart data included a clinical assessment of lower extremity alignment. As part of a separate study, the primary surgeon (SEK) measured and documented lower limb alignment in a standardized manner where both the pelvis and the knees were brought parallel with the ground and any leg length discrepancy was corrected with an appropriate lift to level the pelvis. Once the pelvis and knees were level to the ground, lower limb alignment was defined by a plumb line dropped from the anterior superior iliac spine.[7] Six categories were used to describe this alignment: (A) normal alignment where the plumb line bisected the knee and ankle joints; (B) moderate varus where the plumb line transected the knee and ankle joints medial or lateral, but was still within the condyle; (C) severe varus where the plumb line failed to transect either the knee or ankle; (D) extreme varus where the plumb line did not transect the knee and ankle; (AK) normal alignment with slight valgus, and; (BK) moderate valgus alignment where the plumb line transected the knee and ankle joints medial or lateral, but was still within the condyle. We compared the recorded clinical plumb line alignment pre- and post-surgery and determined it to be improved, the same, or failed. Improvement was defined as transitioning from a more varus category into a more valgus aligned or neutral clinical category. The clinical plumb line assessments were also compared to radiographic measures to verify sub-group distinctiveness. The PFN surgery was considered a success if the patient did not require subsequent tibial or fibular surgery and had acceptable or improved clinical alignment.
2.5. Surgical indications
All available operative reports, manuscripts describing the surgical procedure, and videos were reviewed by the current authors. Indications for PFN included disproportionate growth between the fibula and tibia, ideally identified before the child was 7 years of age. Additional indications were pain and lateral weight-bearing instability of the knee “thrust” and lateral radiographic gaping at midstance. In achondroplasia, 1 leg commonly bows somewhat more than the other but both were operated upon simultaneously once a decision was made for surgery. Contraindications were a valgus bow at the proximal middle junction of the proximal fibula or at midshaft or a valgus tilt at the ankle. We reiterate and emphasize that we did not evaluate the surgeon's decision-making process in regards to surgical indications but confined our evaluation to the success or failure of the operative procedure.
2.6. Statistical analysis
Data for statistical analyses were imported into SPSSv25.0 (IBM Corp, New York, NY) software. Statistics used to compare pre- and post-surgical radiographic measures included paired samples t tests. McNemar test was used to determine improvement in post-surgical clinical plumb-line grades. Statistics used to compare clinical plumb-line categories to radiographic measures included single factor analysis of variance with Tukey honest significant difference post-hoc tests. Statistical significance was defined as P < .05 via 2-sided testing.
2.7. Surgical procedure and hospital course
Below is a brief description of the surgical procedure of the more complex proximal PFN with representative images from the surgery presented in Figure 2. Note that special precautions taken to protect the branches of the peroneal nerve.
Figure 2.
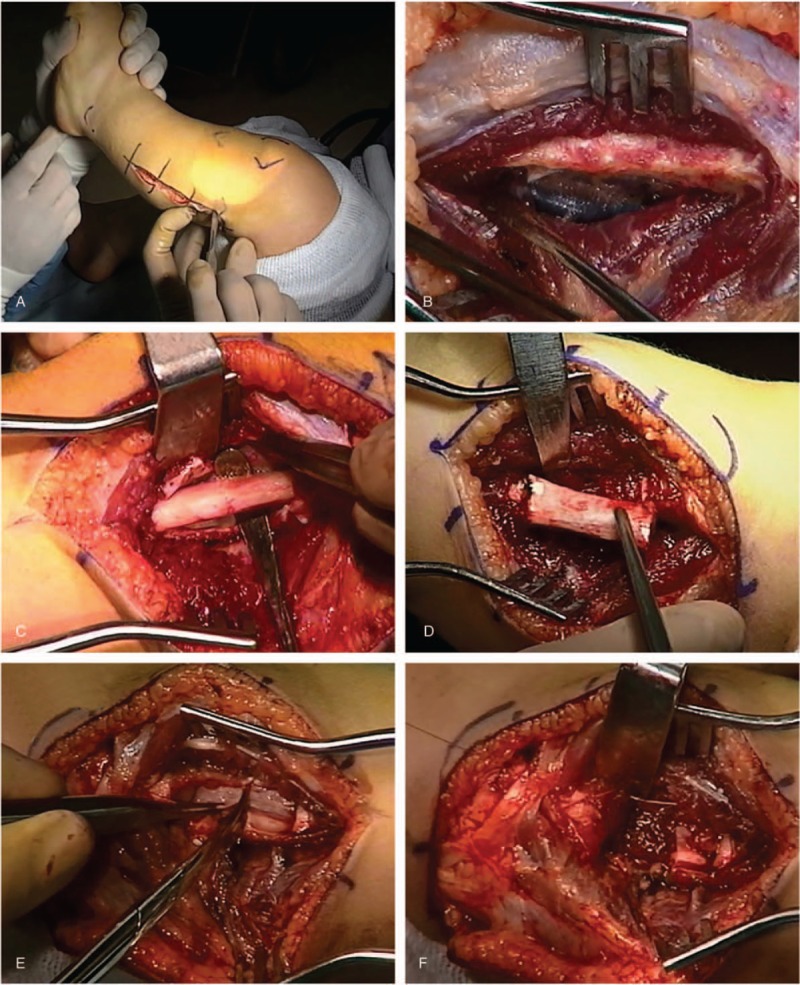
Images from surgical procedure depicting the following: (A) skin incision; (B) exposure of fibula with periosteum intact; (C) sub-periosteal exposure of the fibula; (D) excision of fibula; (E) splitting of the periosteum, and; (F) suturing of periosteum and muscle fibers over bone end.
A hockey-stick shaped incision is made transversely starting in the popliteal space and proceeding along the course of the common peroneal nerve aiming at the fibular head. The fibular portion of the incision then takes a longitudinal course on the postero-lateral aspect of the leg following the fibula to mid-shaft level. A small but constant vein is found perforating the common fascia in its course from the subcutaneous fat to the interval between soleus and peroneus longus. A fasciotomy in line with this vein leads to this interval and subsequently to the proximal fibular shaft.
The periosteum should remain intact during the next surgical step since the object at this time is an extraperiosteal dissection around the bone. Care is taken to protect the deep peroneal nerve, the belly of the extensor digitorum, and the anterior tibial artery. The origin of the peroneus longus muscle is detached from the proximal fibular metaphysis from lateral to medial, by dissecting it away from the anterior tibial branch of the peroneal nerve toward cephalad. This horizontal nerve branch is observed 2 to 3 mm distal and parallel to the detaching incision as it takes its course from the common peroneal nerve laterally to the tibialis anterior muscle medially. The common peroneal nerve is then dissected and mobilized so it may be retracted without tension from the middle of the popliteal space to the fibular neck where it enters under the origins of the peroneus longus.
Next, attention is directed to the posterior aspect of the fibula. The sheath of the soleus muscle is longitudinally incised and the muscle is carefully detached from the posterior periosteum of the fibula. Immediately distal to it, the origins of the flexor hallucis longus are also partially detached. Once the peroneal vessels are successfully detached from the posterior fibular periosteum, the extraperiosteal dissection has been accomplished except for the interosseous membrane still attached to the fibular periosteum.
A longitudinal midlateral incision 4 cm in length is made in the fibular periosteum within the area previously cleared by the extraperiosteal dissection. Three transverse incisions are made from medial to lateral in the anterior periosteum which ends in the middle and at both ends of the longitudinal incision. The periosteum is elevated circumferentially around the fibula while preserving its continuity lengthwise.
For the fibular ostectomy, a 2.5 to 3.0 cm long segment of fibular shaft is resected. The bone ends are smoothed and plugged with bone wax. The periosteum is then divided transversely and used to cover the remaining bone ends. The surrounding muscles are sutured in the fibular gap. Commonly the fibular bone ends approximated immediately suggesting release of the tension (“spring”) effect.
The patient was usually hospitalized for 2 days and discharged with a long leg bandage. At the 10th postoperative day, full weight-bearing without restrictions was usually allowed. Follow up examination generally took place at 3 months and then at yearly intervals until skeletal maturity was achieved.
3. Results
3.1. Demographics (Table 1)
Table 1.
Demographic information of patients included in this study.
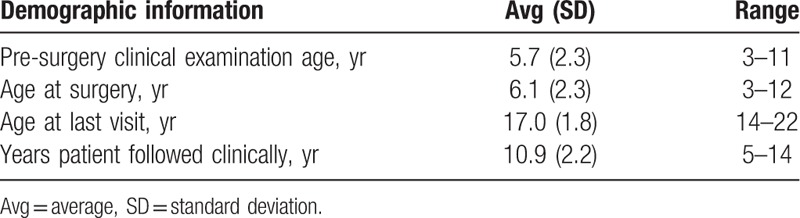
The retrospective review identified 93 limbs in 48 patients with achondroplasia that underwent PFN surgery for the treatment of genu varum before age 13 and were skeletally mature with at least 2 years follow-up at last visit. Since 26 limbs (13 patients) had prior or additional tibial-fibular procedures at time of the PFN surgery, they were excluded leaving a cohort of 67 limbs (35 patients). Pre- and post-surgical data was not available for 14 limbs (8 patients), leaving 53 limbs in 27 patients (15 female, 12 male) in the study. Of the cases included, pre- and/or postoperative radiographs were available for 37 limbs (19 patients), all of which were skeletally mature as evidenced by closed growth plates on radiographic examination. The average age at surgery and the average age at last visit were 6.1 years and 17.0 years, respectively. The number of surgeries performed by child age at the time of surgery is presented in Figure 3. In 28 available radiographs used to determine the site of fibular resection, 10 cases (36%) were performed mid-fibular. It is not known how many surgeries were performed as an index surgery in patients that did not achieve skeletal maturity.
Figure 3.
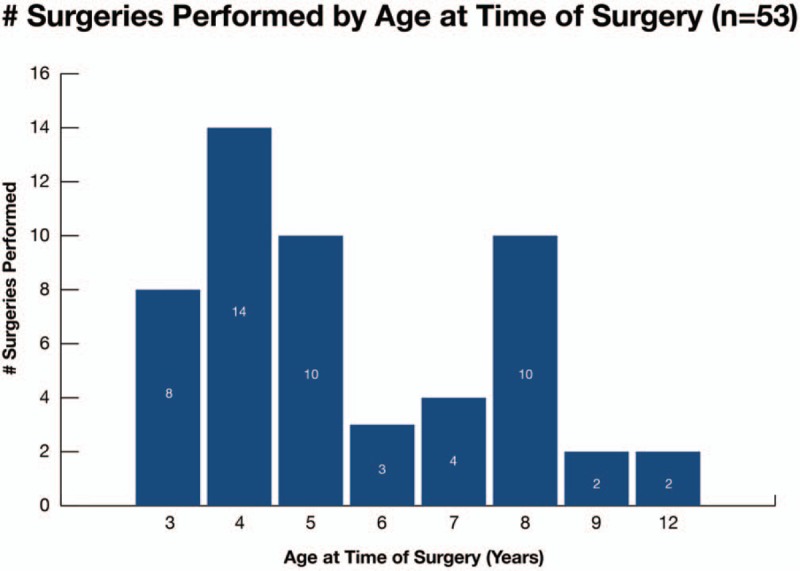
Graph depicting the number of surgeries performed by age at time of surgery.
3.2. Radiographic outcomes (Table 2)
Table 2.
Comparison of average pre- and post-surgery radiographic measures.
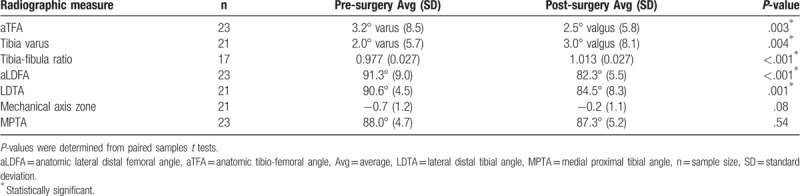
Of the 53 cases included in the study, 34 (64%) did not require subsequent tibia or fibular surgery and had acceptable or improved clinical alignment. For the cases that did not require subsequent surgery, almost all radiographic measures improved pre- to post-surgery. Compared to average pre-surgical radiographic measures, there were significant improvements in post-surgical measures of aTFA (3° varus to 2° valgus, P = .003), tibia varus (2° varus to 3° valgus, P = .004), and the tibia-fibula ratio (0.977 to 1.013, P < .001). At last radiographic examination, nonunion was maintained in 22 (88%) of the 25 successful cases. Representative pre- and post-surgical radiographic images and those from a recent surgical case are presented in Figures 4 and 5, respectively.
Figure 4.
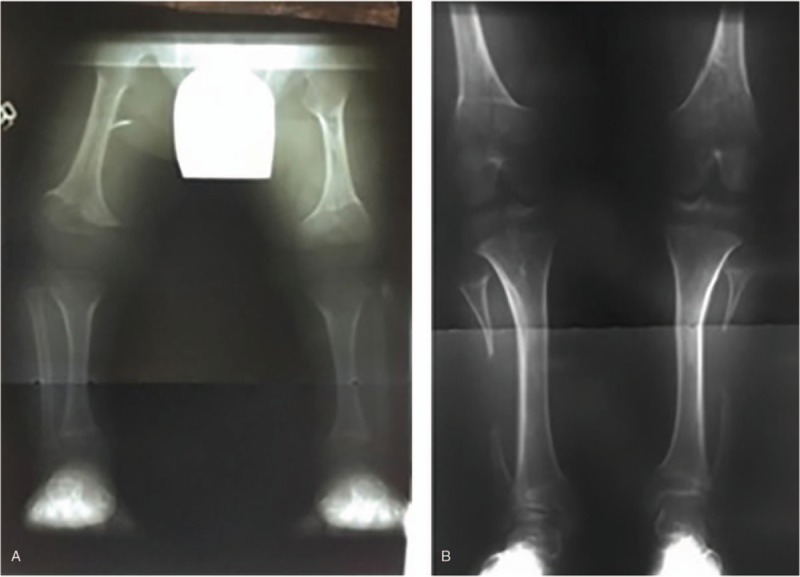
Representative radiographs (A) pre-surgery and (B) post-surgery. The pre-surgery radiographs were taken when the child was 3 years old, the surgery was performed at age 4 years, and the post-surgery radiographs at age 16 years. The pre-surgery clinical plumb-line classification was “C” for the right limb and “B” for the left and post-surgery “AK” bilaterally.
Figure 5.
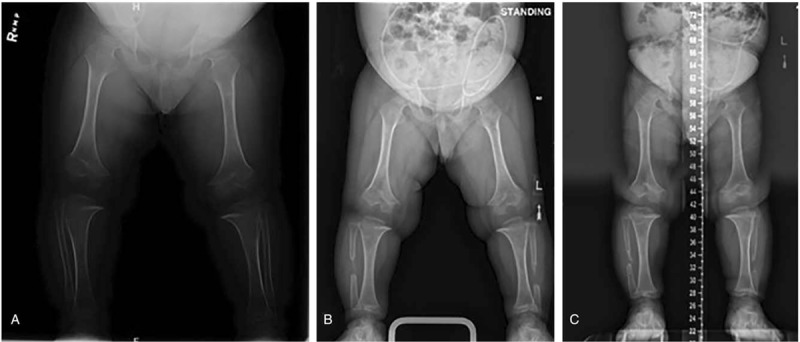
Representative radiographs from a recent surgery at the following time periods: (A) pre-surgery, (B) 7 wk post-surgery, and (C) 7 mo post-surgery. Varus was measured as 2 fingerbreadths pre-surgery, 1.5 fingerbreadths 7 wk post-surgery, and straight 7 mo after surgery.
3.3. Clinical outcomes
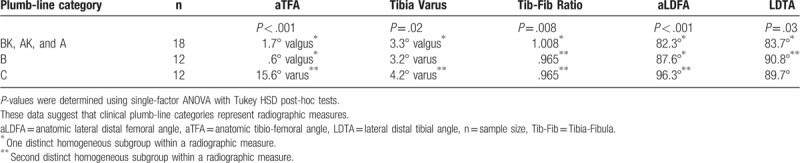
Clinically, 32 cases (60%) demonstrated significant improvement by translating into a more valgus alignment, 9 (17%) remained the same, and 12 (23%) were considered failures (Fig. 6). This represented a significant clinical improvement in post-surgical grade via McNemar test (P < .01). The clinical plumb-limb assessment compared to select radiographic measures identified statistically significant distinct homogenous subgroups giving further credibility to the clinical assessment (Table 3).
Figure 6.
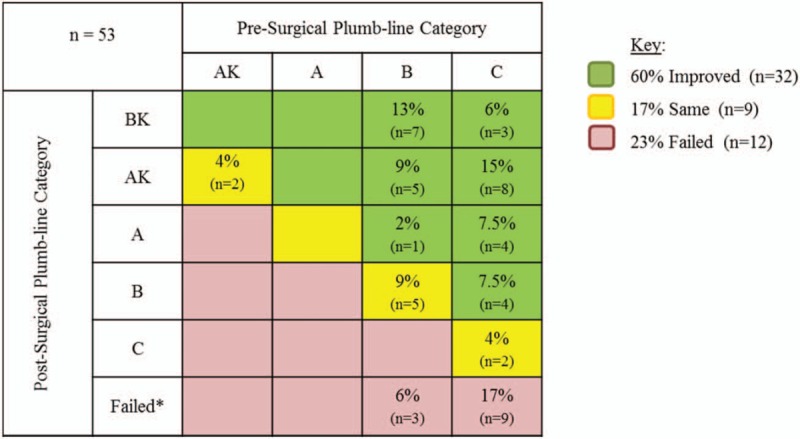
Grid depicting the % and number of cases that improved, remained the same, and failed by pre- to post-surgical clinical plumb-line category. Each box in the grid represents the change in the clinical plumb-line assessment pre- to post-surgery. For example, 13% of all cases (n = 7) went from a “B” pre-surgery plumb-line category to a “BK” post-surgery plumb-line category, which represented an improvement. The improvement in post-surgical grade via McNemar test was significant (P < .01). n = sample size, ∗ = failed PFN surgeries were defined as requiring subsequent surgery or transitioning into a more varus clinical alignment. PFN = planned fibular nonunion.
Table 3.
Comparison of clinical plumb-line categories to radiographic measures.
3.4. Complications and management of PFN failures
Complications were minimal and included minor skin complications and 1 instance of peroneal nerve palsy which was successfully managed. Of the 12 failed PFN cases, 8 were salvaged by tibio-fibular osteotomies with similar surgery recommended but not performed in 4 cases.
4. Discussion
To date, the etiology of genu varum in achondroplasia patients remains unclear with no specific surgical intervention established as the best means for correcting the deformity. While there have been several previous studies proposing an etiology of bowleg development with subsequent proposed surgical interventions,[8–11] this is the first study of its kind to demonstrate that PFN significantly improved the degree of genu varum through skeletal maturity in achondroplasia patients, both radiographically and clinically.
Despite statistical significance, the radiographic (Table 2) correction of the deformity and 60% clinical improvement (Fig. 6) can be considered small for an invasive bilateral surgery with significant risk to the peroneal nerve. The literature also suggests that children with achondroplasia do not seem to be at higher risk for degenerative knee arthritis despite a substantial varus deformity.[7,17] However, the incidence of knee osteoarthritis in adults with achondroplasia and the correlation with the severity of the deformity remains unknown.[7,10] It is important to remember that the surgeon's indication for treatment of the genu varum cannot be deduced from the research and; therefore, the manuscript concerns itself only with the results of the planned fibular osteotomy. By performing the surgery at the mid-fibular level and only in children with severe varus and clinical symptoms, risks can be minimized. The authors; therefore, feel that presenting this as an alternative surgical option is reasonable.
Ponseti and others suggested a causal relationship between overgrowth of the fibula and the development of genu varum.[8–10] The results of this study support this theory. When pre- and post-surgery radiographic measurements were compared, there was an overall increase in the tibia-fibula ratio indicating correction of fibular overgrowth corresponding with correction of the genu varum. Ain et al. suggested that fibular overgrowth was not associated with the occurrence of genu varum and measured the absolute value at which the fibula grew past the tibial knee joint line proximally and ankle joint line distally.[11] It is possible the difference in these measurement techniques contributes to the conclusions drawn as to whether fibular overgrowth plays a role in genu varum. The senior author (DSW) has observed at the time of surgery that the fibular bone ends approximated during surgery suggesting release of the tension (“spring”) effect.
Krackow describes acceptable knee alignment as slightly valgus (6° valgus). (K.A. Krackow, MD, unpublished data, 2008, https://www.medschool.lsuhsc.edu/orthopaedics/docs/How%20to%20Measure%20Knee%20Alignment.pdf) This is consistent with plumb-line categories A and AK in the present study. However, there were 10 post-surgical cases of plumb-line category BK (Fig. 6). An argument could be made that this constitutes over-correction.
The presence of later fibular union or nonunion did not influence the success or failure of the correction. Of the 32 cases with available postoperative radiographs, fibular nonunion was maintained in all but 5 cases (16%). Since radiographs for each individual patient were not routinely taken at each office visit, it was impossible to determine the exact time when fibular union occurred. Despite this fibular union, the surgical correction of genu varum was maintained in 3 of the 5 cases. It is possible that the lower limbs in these patients had grown into proper alignment following surgery due to the release of tension imparted by partial fibular resection. After the limb had migrated into more appropriate alignment, the fibular segments could have re-united.
It is important to note that in the 12 failed PFN cases, 8 had salvage correction by tibio-fibular osteotomy. It appears from our data the preferred age range for PFN is between 3 and 10 years (average age 6.1 years). The site of fibular resection is likely less important in as much as 10 of the 28 cases with available radiographs were performed at mid-fibular rather than so proximal that peroneal nerve and branches have to be isolated. The current authors propose a mid-fibular location for resection, eliminating the risk associated with the peroneal nerve involvement in more proximal resections.
Long term studies on the clinical effect of residual genu varum in older adults with achondroplasia are currently unavailable.[7] The simplicity of the PFN procedure at mid-fibular level and its efficacy suggest it could be used for treatment of significant genu varum in achondroplasia patients particularly when young (3–10 years). It is important to remember that the surgeon's indication for treatment of the genu varum cannot be deduced from the research and therefore the manuscript concerns itself only with the results of the planned fibular osteotomy.
4.1. Limitations
Because the natural history of achondroplastic genu varus is unknown, we could not draft a similar cohort of nonsurgical patients for comparison. As in any observational retrospective review, missing data poses a concern. In this series, 53 out of 67 (79%) of known skeletally mature cases were presented. However, it is not known how many index surgeries were performed in patients that did not achieve skeletal maturity. The use of a plumb-line for clinical assessment of lower limb alignment can be problematic as it can be influenced by the amount of ligamentous laxity and abduction of the hip during stance. However, there was a significant correlation between radiographic and clinical measures of genu varum (Table 3) and the use of “plumb” for lower limb alignment is reported in the literature.[7,12] Despite a relatively small sample size, it is important to note that statistical significance was obtained both radiographically and clinically.
5. Conclusions
This is the first study to demonstrate that PFN significantly improved genu varum radiographically and clinically in achondroplasia patients at skeletal maturity with relatively few complications.
Acknowledgments
The authors would like to thank William Schrader MD and Meadow Newton BS (Akron Children's Hospital) for their help with a recent surgical case and data collection, respectively.
Author contributions
Conceptualization: Dennis S. Weiner, Melanie A. Morscher.
Data curation: Dennis S. Weiner, Gabriel J.M. Mirhaidari, Melanie A. Morscher, Mark J. Adamczyk.
Formal analysis: Dennis S. Weiner, Gabriel J.M. Mirhaidari, Melanie A. Morscher, M. David Gothard, Mark J. Adamczyk.
Funding acquisition: Dennis S. Weiner.
Investigation: Dennis S. Weiner, Gabriel J.M. Mirhaidari, Melanie A. Morscher, Mark J. Adamczyk.
Methodology: Dennis S. Weiner, Gabriel J.M. Mirhaidari, Melanie A. Morscher, M. David Gothard, Mark J. Adamczyk.
Project administration: Dennis S. Weiner, Melanie A. Morscher.
Resources: Dennis S. Weiner, Melanie A. Morscher, Mark J. Adamczyk.
Software: M. David Gothard.
Supervision: Dennis S. Weiner, Melanie A. Morscher, Mark J. Adamczyk.
Validation: Dennis S. Weiner, Gabriel J.M. Mirhaidari, Melanie A. Morscher, M. David Gothard, Mark J. Adamczyk.
Visualization: Dennis S. Weiner, Gabriel J.M. Mirhaidari, Melanie A. Morscher, M. David Gothard, Mark J. Adamczyk.
Writing – original draft: Dennis S. Weiner, Gabriel J.M. Mirhaidari, Melanie A. Morscher, M. David Gothard, Mark J. Adamczyk.
Writing – review and editing: Dennis S. Weiner, Gabriel J.M. Mirhaidari, Melanie A. Morscher, M. David Gothard, Mark J. Adamczyk.
Footnotes
Abbreviations: aLDFA = anatomic lateral distal femoral angle, aTFA = anatomic tibio-femoral angle, DSW = Dennis S. Weiner, MD, LDTA = lateral distal tibial angle, MPTA = medial proximal tibial angle, PFN = planned fibular non-union (osteotomy), SEK = Steven E. Kopits, MD.
How to cite this article: Weiner DS, Mirhaidari GJ, Morscher MA, Gothard MD, Adamczyk MJ. Results through skeletal maturity of planned fibular nonunion for the treatment of genu varum in achondroplasia. Medicine. 2019;98:44(e17723).
This study was supported in part by a grant from the Women's Board of Akron Children's Hospital (to DSW).
No direct or indirect commercial financial incentive was associated with this publication.
The authors declare no conflicts of interest.
References
- [1].Daugherty A. Achondroplasia: etiology, clinical presentation, and management. Neonatal Netw 2017;36:337–42. [DOI] [PubMed] [Google Scholar]
- [2].Beery TA, Workman ML. Genetics and Genomics in Nursing and Health Care. Philadelphia, PA: E.A. Davis; 2012. [Google Scholar]
- [3].Moore KL, Persaud TVN, Torchia MG. The Developing Human: Clinically Oriented Embryology. 10th edPhiladelphia, PA: Elsevier; 2016. [Google Scholar]
- [4].Kemp WL, Burns DK, Brown TG. Kemp WL, Burns DK, Brown TG. Chapter 19. Pathology of the Bones and Joints. McGraw-Hill, Pathology: The Big Picture. New York, NY: 2008. [Google Scholar]
- [5].Saenz M, Tsai A, Manchester DK. Hay WW, Jr, Levin MJ, Deterding RR, Abzug MJ, et al. Genetics & Dysmorphology. McGraw-Hill, CURRENT Diagnosis & Treatment: Pediatrics, 22e. New York, NY: 2013. [Google Scholar]
- [6].Speer M, Mahlmann M, Caero J. Elsayes KM, Oldham SA, et al. Pediatric Radiology. McGraw-Hill, Introduction to Diagnostic Radiology. New York, NY: 2015. [Google Scholar]
- [7].Pauli RM. Achondroplasia: a comprehensive clinical review. Orphanet J Rare Dis 2019;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ponseti IV. Skeletal growth in achondroplasia. J Bone Joint Surg Am 1970;52:701–16. [PubMed] [Google Scholar]
- [9].Stanley G, McLoughlin S, Beals RK. Observations on the cause of bowlegs in achondroplasia. J Pediatr Orthop 2002;22:112–6. [PubMed] [Google Scholar]
- [10].Lee ST, Song HR, Mahajan R, et al. Development of genu varum in achondroplasia: relation to fibular overgrowth. J Bone Joint Surg Br 2007;89:57–61. [DOI] [PubMed] [Google Scholar]
- [11].Ain MC, Shirley ED, Pirouzmanesh A, et al. Genu varum in achondroplasia. J Pediatr Orthop 2006;26:375–9. [DOI] [PubMed] [Google Scholar]
- [12].Kopits SE. Genetics clinics of the Johns Hopkins Hospital. Surgical intervention in achondroplasia. Correction of bowleg deformity in achondroplasia. Johns Hopkins Med J 1980;146:206–9. [PubMed] [Google Scholar]
- [13].Beals RK, Stanley G. Surgical correction of bowlegs in achondroplasia. J Pediatr Orthop B 2005;14:245–9. [DOI] [PubMed] [Google Scholar]
- [14].Paley D. Principles of Deformity Correction. Berlin, Germany: Springer-Verlag; 2002. [Google Scholar]
- [15].Matsui Y, Yasui N, Kimura T, et al. Genotype phenotype correlation in achondroplasia and hypochondroplasia. J Bone Joint Surg Br 1998;80:1052–6. [DOI] [PubMed] [Google Scholar]
- [16].Stevens PM, Maguire M, Dales MD, et al. Physeal stapling for idiopathic genu valgum. J Pediatr Orthop 1999;19:645–9. [PubMed] [Google Scholar]
- [17].Chapple CM, Nicholson H, Baxter GD, et al. Patient characteristics that predict progression of knee osteoarthritis: a systematic review of prognostic studies. Arthritis Care Res (Hoboken) 2011;63:1115–25. [DOI] [PubMed] [Google Scholar]


