Abstract
Background:
Whether the occurrence of refeeding syndrome (RFS), a metabolic condition characterized by electrolyte shifts after initiation of nutritional therapy, has a negative impact on clinical outcomes remains ill-defined. We prospectively investigated a subgroup of patients included in a multicentre, nutritional trial (EFFORT) for the occurrence of RFS.
Methods:
In this secondary analysis of a randomized-controlled trial investigating the effects of nutritional support in malnourished medical inpatients, we prospectively screened patients for RFS and classified them as “RFS confirmed” and “RFS not confirmed” based on predefined criteria (i.e. electrolyte shifts, clinical symptoms, clinical context, and patient history). We assessed associations of RFS and mortality within 180 days (primary endpoint) and other secondary endpoints using multivariable regression analysis.
Results:
Among 967 included patients, RFS was confirmed in 141 (14.6%) patients. Compared to patients with no evidence for RFS, patients with confirmed RFS had significantly increased 180-days mortality rates (42/141 (29.8%) vs 181/826 (21.9%), adjusted odds ratio (OR) 1.53 (95% CI 1.02 to 2.29), P < .05). Patients with RFS also had an increased risk for ICU admission (6/141 (4.3%) vs 13/826 (1.6%), adjusted OR 2.71 (95% CI 1.01 to 7.27), P < .05) and longer mean length of hospital stays (10.5 ± 6.9 vs 9.0 ± 6.6 days, adjusted difference 1.57 days (95% CI 0.38–2.75), P = .01).
Conclusion:
A relevant proportion of medical inpatients with malnutrition develop features of RFS upon hospital admission, which is associated with long-term mortality and other adverse clinical outcomes. Further studies are needed to develop preventive strategies for RFS in this patient population.
Keywords: effort trial, electrolyte disturbances, hypophosphatemia, malnutrition, nutritional therapy, refeeding syndrome
1. Introduction
Refeeding syndrome (RFS) is a metabolic condition characterized by severe electrolyte and fluid shifts in response to the transition from a catabolic to an anabolic state after start of nutritional therapy in malnourished patients.[1–4] The clinical presentation may vary from mild forms with few clinical signs and symptoms to severe forms with possible lethal complications.[5] The function of several organs can be affected by RFS leading to cardiac arrhythmias, heart failure, and kidney failure among other impairments.[6–10] There is currently insufficient knowledge about predisposing factors, incidence rates, and treatment options. It also remains largely unclear whether RFS is associated with worse clinical courses and has thus prognostic implications.[11] A recent systematic literature review[12] revealed a lack of large-scale, prospective studies systematically looking at the occurrence of RFS, and high heterogeneity of definition criteria used regarding electrolyte cut-offs and clinical criteria to classify patients.[12]
Importantly, recent research has questioned whether RFS has any prognostic implications regarding mortality risk. Death attributable to RFS was found in only 4 out of 260,000 hospital deaths in 1 large retrospective study from Australia looking at patients over a 20 year time period.[13] Arguably, there was a high risk for under-reporting in this investigation relying on coded data from the hospital because no accepted gold standard for RFS exists today and the diagnosis may often be missed in clinical routine.[14] Also, a prospective cohort study including 243 patients found RFS to be a relatively rare consequence of nutritional therapy and no causal relationship between RFS and adverse outcome was reported.[1] In addition, a recent systematic review found that only few studies reported data on the association between RFS and clinical outcome.[12] Due to the small number of patients and studies, the lack of statistical adjustment for confounding factors and the high heterogeneity in regard to patient population and setting, the systematic review[12] was not able to draw any firm conclusion regarding the question on whether RFS is indeed associated with adverse clinical outcome.[12] Answering this question, however, is important as it may help to justify screening and therapeutic measures in patients at risk of RFS.
Herein, our aim was to systematically study the occurrence of RFS in a large and well characterized population of malnourished patients included in a recent trial based on previously published consensus criteria[4] and to investigate whether RFS was indeed associated with adverse clinical outcomes within a follow-up of 6 months.
2. Methods
2.1. Study design and setting
This is a pre-planned secondary analysis of the prospective EFFORT trial (Effect of early nutritional support on Frailty, Functional Outcomes, and Recovery of malnourished medical inpatients Trial). EFFORT was a pragmatic, investigator-initiated, open-label, multicentre randomized controlled trial, which recruited patients from April 2014 to February 2018 in 8 Swiss hospitals. The ethical committee of the Northwestern part of Switzerland (EKNZ; 2014_001) approved the study protocol and all patients or their authorized representatives provided written informed consent. The trial was registered at ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT02517476). The main aim of EFFORT was to assess the effects of early nutritional therapy on patient outcomes in the medical inpatient setting. The rationale for the trial, design details, and eligibility features have been published previously[15] as have the main results of the trial.[16]
2.2. Patient population
EFFORT enrolled consecutive patients at nutritional risk (defined by a Nutritional Risk Screening total score (NRS 2002) ≥3 points)[17,18] with an expected length of hospital stay ≥5 days if they were willing to provide informed consent. Patients were excluded if initially admitted to intensive care units or surgical units, unable to ingest oral nutrition, already receiving nutritional support on admission, with a terminal condition (i.e., end-of-life situation), hospitalized because of anorexia nervosa, acute pancreatitis, acute liver failure, cystic fibrosis or stem cell transplantation, after gastric bypass surgery, or with contraindications for nutritional support, and patients previously included in the trial. While EFFORT included a total of 2088 patients, this secondary analysis includes 967 medical inpatients recruited in 2 of the 8 participating centers (Medical University Department, Kantonsspital Aarau and University Hospital Bern), which took part in this sub-study between February 2015 and December 2017.
2.3. Assessment of RFS and management of patients
Assessment of patients regarding RFS was done prospectively according to a pre-defined checklist based on recent consensus criteria for RFS.[4] In brief, included patients were screened daily by a study dietician for possible RFS based on new occurrence or worsening of laboratory parameters (hypophosphatemia, hypokalemia, hypomagnesemia) or clinical parameters (peripheral oedema). In case of possible RFS, a more detailed assessment of patients was done by the study dietician and the treating physician. Patients meeting either ≥2 minor criteria (phosphate <0.81 mmol/l, magnesium <0.74 mmol/l, potassium <3.6 mmol/l, peripheral oedema) or ≥1 major criteria (phosphate <0.32 mmol/l, magnesium <0.5 mmol/l, potassium <2.5 mmol/l) underwent further clinical examination focusing on clinical symptoms such as peripheral oedema, tachycardia, and tachypnea. Based on these parameters, the clinical context (i.e., other explanations for oedema or electrolyte shifts) and the overall patient history, a final classification regarding RFS was done and patients were prospectively classified as “RFS confirmed” or “RFS not confirmed”. This classification was primarily done by the study physician, and in case of doubt a senior physician was asked for a final judgment. Treatment of RFS in patients with confirmed RFS was up to the treating physician team, without interference of the study team, and included substitution of electrolytes and B-complex vitamins as well as a gradually increase of energy targets, as appropriate.
2.4. Outcome measures
The primary endpoint of the EFFORT trial was defined as all-cause long-term mortality within 180 days. To verify survival status as well as other clinical outcomes, we performed telephone interviews at 30 and 180 days in all patients. If patients or their relatives could not be reached, we verified outcome data with the patient's primary care provider. Secondary endpoints were short-term (i.e., within 30 days) mortality, admission to intensive care unit, non-elective hospital readmission after discharge, major complications (including adjudicated nosocomial infection, respiratory failure, a major cardiovascular event or pulmonary embolism, acute renal failure, gastro-intestinal events, or a decline in functional status of 10% or more from admission to day 30) and length of hospital stay of the index hospital stay. A more detailed description of endpoints has previously been published in the study protocol.[15]
2.5. Statistical analysis
Statistical analyses were performed using STATA 12.1 (STATA Corp, College Station, TX, USA). We used descriptive statistics including mean with standard deviation to describe the study population as appropriate. Categorical variables are expressed as percentages (numbers) and continuous variables as medians (interquartile ranges (IQRs)).
We did 2 main analyses, first focusing on all patients and comparing patients with confirmed RFS to patients with no RFS. Second, we focused on the subgroup of patients with possible RFS during screening and compared patients with confirmed RFS to patients with not confirmed RFS. For primary and secondary endpoints we calculated odds ratios (ORs) and 95% confidence intervals (CIs) using univariable and multivariable logistic regression analysis.[19] We adjusted the analysis for important prognostic factors such as patient age, gender, and BMI. All tests were carried out at 5% significance levels.
3. Results
3.1. Patient population
This analysis includes 967 patients that were screened for RFS during their index hospital stay in 2 study centers participating in this sub-study. A total of 353 (36.5%) of these patients had electrolyte changes or clinical signs possibly related to RFS and were reassessed in more detail for RFS. Among these, 141 patients were classified as “RFS confirmed” and 212 patients were classified as “RFS not confirmed”. Thus, as shown in Figure 1, RFS was confirmed in 141 (14.6%) patients whereas 826 (85.4%) patients had no RFS (212 with abnormalities during screening, but no RFS confirmation; and 614 with no abnormalities at screening).
Figure 1.
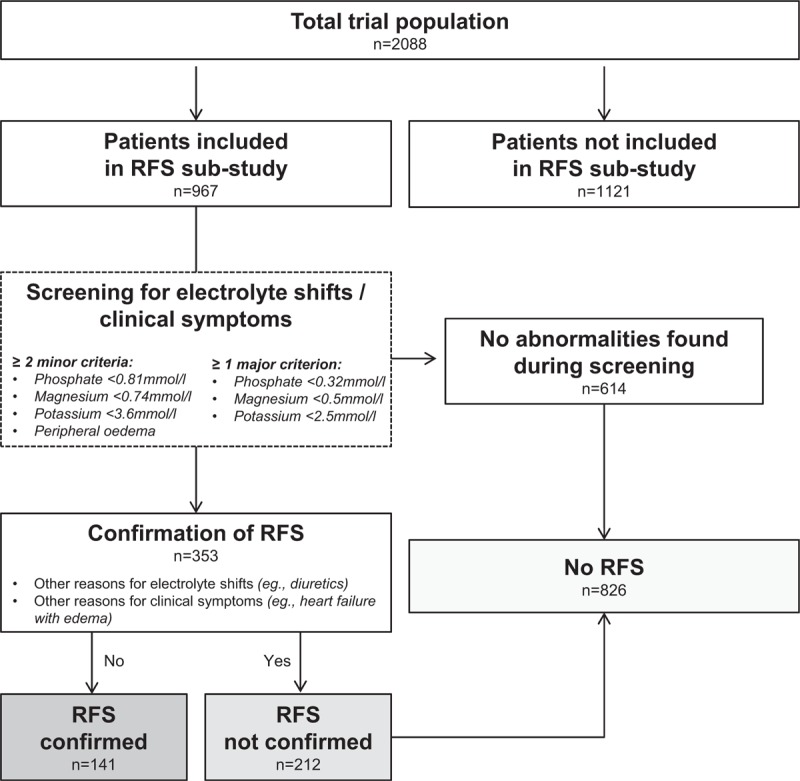
Flow chart. Patient Assessment of Refeeding Syndrome after inclusion in the EFFORT trial in 2 of the 8 participating centres (Medical University Department, Kantonsspital Aarau and University Hospital Bern, which took part in this sub-study). RFS = Refeeding Syndrome.
In a first step, we compared baseline results from patients with RFS to all patients with no RFS including patients with no abnormalities during screening (Table 1 ). Results were similar and showed differences in regards to initial randomization, some comorbidities and more frequent use of oral nutrition supplements in patients with confirmed RFS.
Table 1.
Baseline characteristics in patients with confirmed RFS and no RFS.
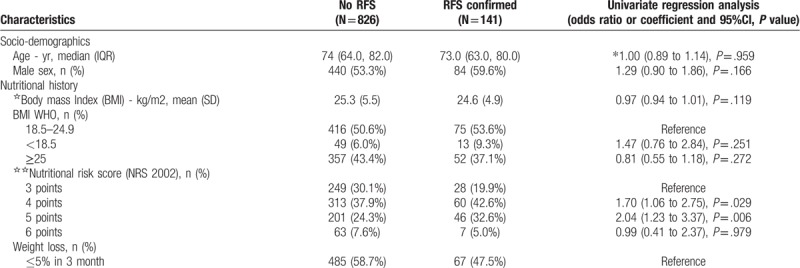
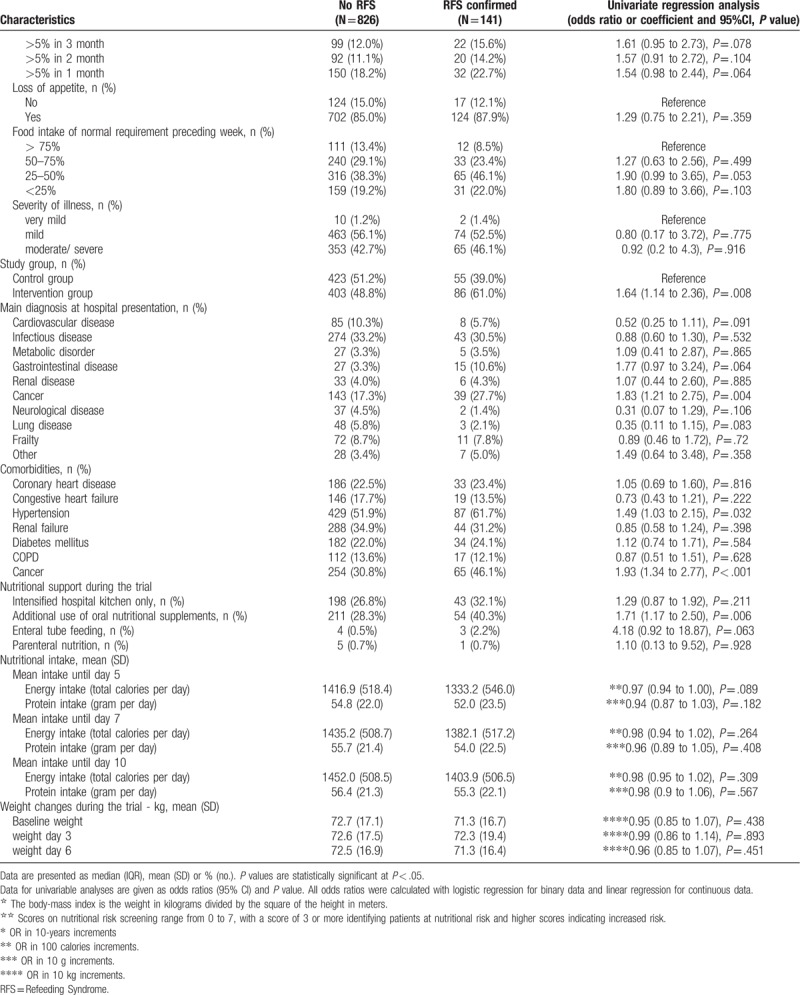
Second, we focused on patients with abnormalities during screening and compared patients with confirmed RFS (n = 141) to patients where RFS was not confirmed (n = 212). Table 2 shows baseline characteristics of these patients stratified according to RFS classification. Compared to patients without confirmed RFS, patients with confirmed RFS had a higher rate of appetite loss at baseline (87.9% vs 78.8%; OR 1.97, 95% CI 1.07–3.60, P = .028). Patients in the intervention group of the EFFORT trial also had a higher likelihood of RFS (47.2% vs 61%, adjusted OR 1.75 (95%CI 1.14–2.7), P = .011). Comorbidities such as hypertension and cancer were significantly different in both groups. Nutritional support among both groups was similar, but patients with confirmed RFS received more often oral nutrition supplements (26.4% vs 40.3%, adjusted OR 1.88 (95%CI 1.18–2.98), P = .008).
Table 1 (Continued).
Baseline characteristics in patients with confirmed RFS and no RFS.
3.2. Laboratory findings
Next, we focused on laboratory results that were ordered in the subgroup of patients with abnormalities during the screening process (n = 353). Table 3 shows detailed results of laboratory results stratified by RFS. At baseline, levels of phosphate (mmol/l) (0.74 vs 0.93; difference 0.09, 95% CI 0.04–0.22, P < .001), magnesium (mmol/l) (0.67 vs 0.73; difference 0.10, 95% CI 0.02–0.42, P = .002), potassium (mmol/l) (3.46 vs 3.79; difference 0.25, 95% CI 0.15–0.41, P < .001) and albumin (g/L) (25.5 vs 27.2; difference 0.94, 95% CI 0.90–0.99, P = .011) were significantly lower in patients with confirmed RFS versus patients with no confirmed RFS, respectively. In addition, laboratory results of electrolytes in RFS patients were also lower when measured during follow-up. We found no significant difference in clinical symptoms between groups except for tachycardia being more frequent in patients with confirmed RFS at follow-up (12.2% vs 3.6%; OR 3.75, 95% CI 1.43–9.85, P = .007).
Table 2.
Baseline characteristics in patients with abnormalities during RFS.

3.3. Association of RFS and adverse outcome
Regarding the primary endpoint, a total of 42/141 (29.8%) patients with confirmed RFS died within the 180 days of follow up (Table 4). This was a significantly higher number compared to all patients with no RFS (181/826 (21.9%), adjusted OR 1.53 (95% CI 1.02 to 2.29), P = .038) and also when compared to patients who presented abnormalities during screening but no confirmation of RFS (37/212 (17.5%), adjusted OR 1.97, 95% CI 1.18 to 3.29, P = .01). Figure 2 shows Kaplan–Meier curves for time to death within 6 months comparing patients with confirmed RFS to patients without RFS and patients with no confirmed RFS.
Table 3.
Laboratory and clinical findings at baseline and follow-up in patients with abnormalities during RFS screening.
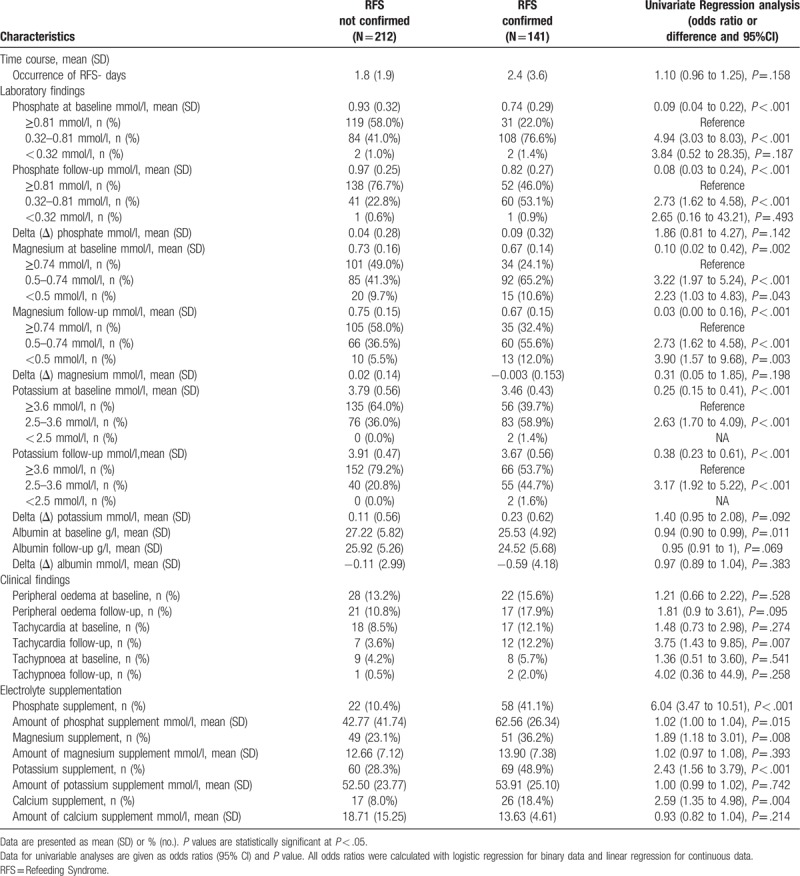
Figure 2.
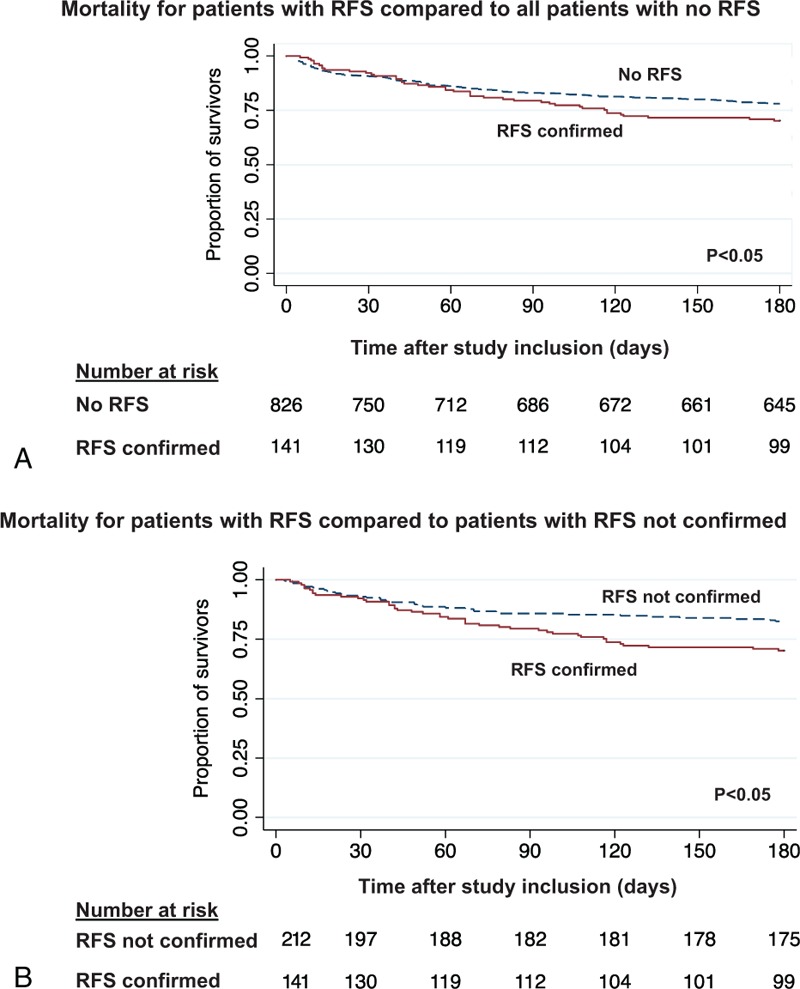
Time to mortality analysis over 180 days of follow-up. Plots showing the association between the primary endpoint all-cause long-term mortality within 180 days and patients with RFS compared to those with no RFS or not confirmed RFS. RFS = Refeeding Syndrome.
Table 4.
Primary and Secondary Endpoints according to positive RFS screening.
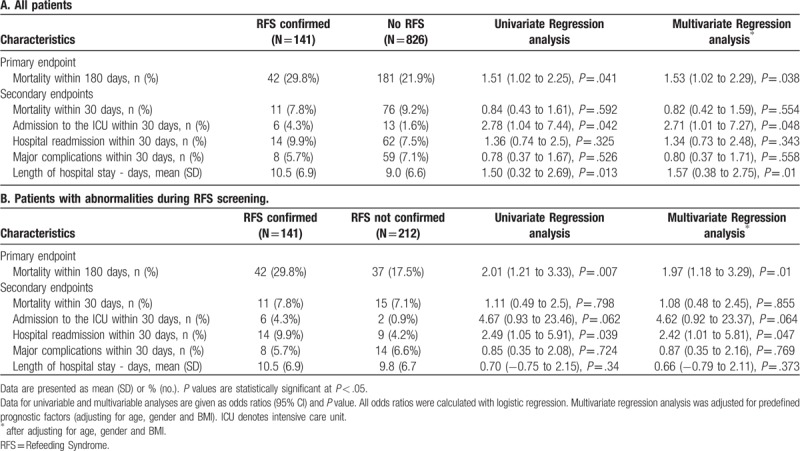
Regarding secondary endpoints, compared to patients with no RFS, patients with confirmed RFS showed an increased risk for ICU admission (4.3% vs 1.6%, adjusted OR 2.71 (95% CI 1.01–7.27), P.048) and an increase in length of hospital stay (10.5 vs 9.0 days, adjusted difference 1.57 days (95%CI 0.38–2.75), P = .01). Comparing patients with confirmed to patients with not confirmed RFS there was a doubling in 30-day readmission rate (9.9% vs 4.2%, adjusted OR 2.42 (95% CI 1.01–5.81), P = .047). There were no significant differences in other secondary endpoints (Table 4).
4. Discussion
Key results of this secondary analysis of a previous interventional trial are two-fold. First, we found that a relevant proportion of medical inpatients at risk of malnutrition develop RFS, based on the evaluation of laboratory and clinical parameters, affecting about 1 of 7 patients. Second, within this large and well-characterized population of medical inpatients at risk for malnutrition with systematic short- and long-term assessment, we found an association between the occurrence of RFS and long-term mortality within a follow-up period of 180 days. There was also an increase in unplanned hospital readmissions, as well as ICU admissions and days in the hospital when compared to the overall population of patients with no RFS. Thus these data provide empiric evidence that RFS is a potentially harmful condition that needs to be adequately identified and managed.
Early start of nutritional support has become part of standard of care treatments in many hospitals to lower the burden of morbidity associated with disease-related malnutrition.[11,18,20] NICE guidelines[21] have proposed several risk factors for RFS including low BMI, unwanted weight loss, starvation, alcohol abuse, and low levels of some electrolytes on admission. Our data largely confirms this and other previous reports about risk factors for RFS showing that loss of appetite, reduced dietary intake, use of nutritional supplements, and a history of cancer are strongly associated with RFS occurrence.[22,23] Yet, NRS[17] total score and low BMI was not associated with RFS in our analysis which may be explained by the fact that the original trial only included patients at risk of malnutrition, i.e. with a NRS of at least 3 points. In addition, we found low levels of phosphate, potassium, magnesium, and albumin at admission to be associated with an increased risk of RFS after start of nutritional support. These risk factors may help to early identify patients that will later develop RFS during hospital stay. Still, whether or not preventive measures are effective in reducing the associated risks of RFS remain unclear and cannot be answered with our study.
Importantly, our findings also confirm previous findings regarding timing of the occurrence of RFS. Similar to the results of a previous meta-analysis, we also found that in most patients RFS occurs within the first 72 hours after the start of nutritional therapy.[12] Thus, close monitoring of risk factors and clinical features of RFS during this initial vulnerable time period seems appropriate to detect patients with RFS.
The pathophysiology of RFS is still incompletely understood, but there seem to be several metabolic factors causing this condition.[24–26] During starvation and other catabolic conditions, secretion of glucagon increases while insulin secretion decreases leading to an activated gluconeogenesis and proteolysis. Additionally, electrolyte supplies and intracellular vitamin, such as thiamine, are depleted.[6,27] After the start of nutritional intake, glucose concentration rises followed by an increase in insulin and decrease of glucagon secretion. Insulin stimulates glycogen, fat, and protein synthesis, which requires minerals and cofactors, such as phosphate, magnesium, and thiamine. Insulin also leads to an uptake of glucose and potassium into the cell through the sodium-potassium ATPase symporter. Phosphate and magnesium are absorbed and water follows by osmosis.[27] The result of this transcellular shift is a redistribution of electrolytes with extracellular volume expansion resulting from increased sodium and water retention.[12] The volume overload can lead to peripheral oedema and heart failure, while the transcellular shift of electrolytes is a risk for arrhythmias or spasms.[6,10] Changes in electrolytes thus may precede clinical symptoms and screening of electrolytes derangements in at-risk patients is therefore reasonable. A recent expert consensus on the topic proposed to classify patients as imminent RFS if severe laboratory changes occur, and manifest RFS if clinical symptoms are also present.[4] Our data support these considerations showing a strong association between baseline laboratory parameters and later occurrence of RFS. Still, whether systematic treatment of patients with electrolytes and vitamins reduces RFS and its associated risks remains uncertain.
In our initial EFFORT paper, we reported low rates of RFS and did not find a higher risk for RFS according to randomization, while in the current analysis RFS was diagnosed more frequently and nutritional intervention was associated with the occurrence of RFS. Importantly, we used a different definition for RFS in the initial trial and we did not perform routine RFS screening in all patients, but only patients with clinically apparent RFS reached the safety endpoint. In this sub-study, we systematically screened and assessed all patients for possible RFS based on a consensus definition. Thus, underreporting is likely in our EFFORT trial and also in other previous studies relying on routine data and reporting low number of RFS and lack of association with outcomes.[1,12–14] Interestingly, mortality was significantly lower in intervention group patient compared to controls in EFFORT although the intervention seems to be associated with risk for RFS. Possibly, the use of micronutrients as part of the intervention had a preventive effect in the trial. This hypothesis, however, needs further confirmation in future research.
The strength of our research is the prospective screening and assessment of a large population of patients, with predefined diagnostic criteria and long-term follow-up. However, we are aware of some limitations. We relied our RFS definition on a previous consensus statement because no gold standard currently exists. Laboratory parameters were not routinely available on a daily basis in all patients but were ordered systematically on admission and on day 2 of hospital stay, and were based on the clinical situation. We only screened patients in 2 out of 8 participating hospitals due to limited resources. Nevertheless, the cohort with a total of 967 screened patients is among the largest studies investigating RFS. Further, based on our trial, we only focused on medical inpatient at nutritional risk and thus generalizability to other populations and settings is uncertain.
5. Conclusion
The prognostic importance of RFS has been questioned mainly because clinical data showing strong associations of RFS with worse clinical outcomes have been largely lacking. Herein, our data provide strong evidence that patients classified as RFS based on a recent consensus definition have a doubling in mortality risk, and an increase also in other adverse clinical outcomes. Our data illustrate that a relevant proportion of medical patients with malnutrition develop RFS upon hospital admission, which puts them at increased risk for adverse clinical outcomes. Future studies should investigate preventive measures to improve outcomes in this patient population.
Acknowledgments
We are grateful to the staff and patients who participated in the EFFORT trial and the follow-up data collection.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, EFFORT = effect of early nutritional support on frailty, functional outcomes and recovery of malnourished medical inpatients trial, EKNZ = The ethical committee of the Northwestern part of Switzerland, HR = hazard ratio, ICU = intensive care unit, IQR = interquartile range (25th-75th percentiles), NRS = nutritional risk screening, OR = odds ratio, RFS = refeeding syndrome, SD = standard deviation.
How to cite this article: Friedli N, Baumann J, Hummel R, Kloter M, Odermatt J, Fehr R, Felder S, Baechli V, Geiser M, Deiss M, Tribolet P, Gomes F, Mueller B, Stanga Z, Schuetz P. Refeeding syndrome is associated with increased mortality in malnourished medical inpatients: Secondary Analysis of a Randomized Trial. Medicine. 2020;99:1(e18506).
NF, JB, RH, and MK contributed equally to the manuscript.
The EFFORT Trial was investigator-initiated and supported by a grant from the Swiss National Science Foundation to P.Schuetz (SNSF Professorship, PP00P3_150531) and the Forschungsrat of the Kantonsspital Aarau (1410.000.058 and 1410.000.044). The Institution of P. Schuetz has previously received unrestricted grant money unrelated to this project from Neste Health Science and Abbott Nutrition. The institution of Z. Stanga received speaking honoraria and research support from Neste Health Science, Abbott Nutrition and Fresenius Kabi.
All other authors report no conflicts of interest.
References
- [1].Rio A, Whelan K, Goff L, et al. Occurrence of refeeding syndrome in adults started on artificial nutrition support: prospective cohort study. BMJ Open 2013;3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aubry E, Aeberhard C, Leuenberger MS, et al. Refeeding syndrome: a consensus-based algorithm for inpatients. Aktuell Ernährungsmed 2018;43:1–0. [Google Scholar]
- [3].Aubry E, Friedli N, Schuetz P, et al. Refeeding syndrome in the frail elderly population: prevention, diagnosis and management. Clin Exp Gastroenterol 2018;11:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Friedli N, Stanga Z, Culkin A, et al. Management and prevention of refeeding syndrome in medical inpatients: an evidence-based and consensus-supported algorithm. Nutrition 2018;47:13–20. [DOI] [PubMed] [Google Scholar]
- [5].Preiser JC, van Zanten AR, Berger MM, et al. Metabolic and nutritional support of critically ill patients: consensus and controversies. Crit Care (London, England) 2015;19:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boateng AA, Sriram K, Meguid MM, et al. Refeeding syndrome: treatment considerations based on collective analysis of literature case reports. Nutrition 2010;26:156–67. [DOI] [PubMed] [Google Scholar]
- [7].Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition 2001;17:632–7. [DOI] [PubMed] [Google Scholar]
- [8].Solomon SM, Kirby DF. The refeeding syndrome: a review. JPEN J Parenter Enteral Nutr 1990;14:90–7. [DOI] [PubMed] [Google Scholar]
- [9].Brooks MJ, Melnik G. The refeeding syndrome: an approach to understanding its complications and preventing its occurrence. Pharmacotherapy 1995;15:713–26. [PubMed] [Google Scholar]
- [10].Mehler PS, Winkelman AB, Andersen DM, et al. Nutritional rehabilitation: practical guidelines for refeeding the anorectic patient. J Nutr Metab 20102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Merker M, Gomes F, Stanga Z, et al. Evidence-based nutrition for the malnourished, hospitalised patient: one bite at a time. Swiss Med Wkly 2019;149:w20112. [DOI] [PubMed] [Google Scholar]
- [12].Friedli N, Stanga Z, Sobotka L, et al. Revisiting the refeeding syndrome: results of a systematic review. Nutrition 2017;35:151–60. [DOI] [PubMed] [Google Scholar]
- [13].Matthews KL, Capra SM, Palmer MA. Throw caution to the wind: is refeeding syndrome really a cause of death in acute care? Eur J Clin Nutr 2017. [DOI] [PubMed] [Google Scholar]
- [14].Schuetz P, Zurfluh S, Stanga Z. Mortality due to refeeding syndrome? You only find what you look for, and you only look for what you know. Eur J Clin Nutr 2018;72:307–8. [DOI] [PubMed] [Google Scholar]
- [15].Schuetz P, Fehr R, Baechli V, et al. Design and rationale of the effect of early nutritional therapy on frailty, functional outcomes and recovery of malnourished medical inpatients trial (EFFORT): a pragmatic, multicenter, randomized-controlled trial. Int J Clin Tri 2018;5:142–50. [Google Scholar]
- [16].Schuetz P, Fehr R, Baechli V, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet (London, England) 2019;393:2312–21. [DOI] [PubMed] [Google Scholar]
- [17].Kondrup J, Rasmussen HH, Hamberg O, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003;22:321–36. [DOI] [PubMed] [Google Scholar]
- [18].Bounoure L, Gomes F, Stanga Z, et al. Detection and treatment of medical inpatients with or at-risk of malnutrition: suggested procedures based on validated guidelines. Nutrition 2016;32:790–8. [DOI] [PubMed] [Google Scholar]
- [19].Thompson SG, Turner RM, Warn DE. Multilevel models for meta-analysis, and their application to absolute risk differences. Stat Methods Med Res 2001;10:375–92. [DOI] [PubMed] [Google Scholar]
- [20].Gomes F, Schuetz P, Bounoure L, et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr 2018;37:336–53. [DOI] [PubMed] [Google Scholar]
- [21].Nutrition support for adults: oral nutrition support, enteral tube feeding and parenteral nutrition [Internet]. Nat Collab Centre Acute Care 2006;[cited November 3, 2019]. Available from: nice.org.uk/guidance/cg32. [PubMed] [Google Scholar]
- [22].Gonzalez Avila G, Fajardo Rodriguez A, Gonzalez Figueroa E. The incidence of the refeeding syndrome in cancer patients who receive artificial nutritional treatment. Nutr Hosp 1996;11:98–101. [PubMed] [Google Scholar]
- [23].Hernandez-Aranda JC, Gallo-Chico B, Luna-Cruz ML, et al. [Malnutrition and total parenteral nutrition: a cohort study to determine the incidence of refeeding syndrome]. Rev Gastroenterol Mex 1997;62:260–5. [PubMed] [Google Scholar]
- [24].McCray S, Walker S, Parrish C. Much ado about refeeding. Pract Gastroenterol 2005;26:31–3. 37-40, 43-44. [Google Scholar]
- [25].Reber E, Gomes F, Vasiloglou MF, et al. Nutritional risk screening and assessment. J Clin Med 2019;8:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Reber E, Gomes F, Bally L, et al. Nutritional management of medical inpatients. J Clin Med 2019;8:1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. Bmj 2008;336:1495–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


