Abstract
Objective:
To determine the association between cost-sharing and adherence to cardiac rehabilitation (CR).
Patients and Methods:
We collected detailed cost-sharing information for patients enrolled in CR at Baystate Medical Center in Springfield, MA, including the presence/absence and amounts of copays and deductibles. We evaluated the association between cost-sharing and the total number of CR sessions attended, as well as the influence of household income on CR attendance.
Results:
In 2015, 603 patients enrolled in CR had complete cost-sharing information. In total, 235 (39%) had some form of cost-sharing. Of these, 82% had copays [median copay $20 (IQR 10, 32)] and 50% had an unmet deductible [median $500 (IQR 250, 1800)]. The presence of any amount or form of cost-sharing was associated with 6 fewer sessions of CR [16 (IQR 4 to 36) vs. 10 (IQR 4 to 27), P < .001]. Patients hospitalized in November or December with deductibles that renewed in January attended 4.5 fewer sessions of CR [8.5 (3.25 to 12.5) vs. 13 (5.25 to 36) P = .049]. After adjustment for differences in baseline characteristics, every $10 increase in copay was associated with 1.5 (95% CI −2.3 to −0.7) fewer sessions of CR (P < .001). Household income did not moderate these relationships.
Conclusion:
Cost-sharing was associated with lower CR attendance and exhibited a dose-response relationship such that higher cost-sharing was associated with lower CR attendance. Given that CR is cost-effective and underutilized, insurance companies and other payers should re-evaluate their cost-sharing policies for CR.
Keywords: Cardiac Rehabilitation attendance, Cost-sharing, Copay, Deductibles
INTRODUCTION
Cardiac Rehabilitation (CR) is a multifaceted intervention that benefits patients with cardiovascular disease (CVD) and is supported by strong evidence, including meta-analyses of randomized controlled trials,1-3 such that it is given a Class I Recommendation in society guidelines. 4,5 In addition, observational studies and meta-analyses suggest that CR has a “dose-response” relationship. 6,7 Specifically, Hammill et al. found that each additional session of CR was associated with a ~1% lower risk of mortality.8,9 However, despite the strong evidence of benefits, about 75% of patients drop out before completing all 36 sessions.10,11
One important modifiable obstacle to higher levels of CR attendance may be the presence of cost-sharing, which includes co-pays, co-insurance, and/or deductibles in which the patient pays a portion of the cost of CR. While cost-sharing often minimizes inappropriate utilization of weakly-indicated or expensive clinical services, this impact may be undesirable with preventative treatments such as CR where the cost of prevention appears to be lower than that of recurrent cardiac disease. 12,13 Similarly, lack of insurance is another potential barrier to CR attendance. However, Massachusetts has nearly universal health coverage with only 2.8 % uninsured residents (vs. 9.4% in the rest of the nation in 2016) 14 and CR coverage by insurance is mandatory. 15,16 As a result, our location in Massachusetts provides an ideal environment to study the effect of cost-sharing while minimizing the potential impact of a large uninsured population.
Our study’s primary goal was to determine the association between cost-sharing and the number of CR sessions attended. We hypothesized that presence of cost-sharing would be associated with fewer CR sessions, and that the greater the cost-sharing, the larger the negative association would be.
METHODS
Study design
We performed a retrospective cohort study at Baystate Medical Center (BMC), a 684-bed academic teaching hospital that serves as the only tertiary cardiac center for a population of approximately 800,000 people living in Western Massachusetts. Data was extracted from BMC’s CR electronic database and paper records for patients who enrolled between January 1, 2015 and December 31, 2015. Follow-up continued through June 30, 2016 to allow all patients at least 6 months to attend all sessions of CR. This study was reviewed and approved by the Institutional Review Board at BMC (reference number BH-14-158).
Study patients and Covariates
We included all patients who attended ≥ 1 CR session with stable chronic heart failure, stable angina, myocardial infarction (MI), percutaneous coronary intervention (PCI), coronary artery bypass graft surgery (CABG), and heart valve surgery.17 We recorded demographics (age, gender, race, ethnicity), co-morbidities, smoking status, insurance plan, and baseline functional exercise intensity as measured by metabolic equivalents (METs) during usual exercise training 18,19 (as opposed to peak capacity on a stress test). We also noted the event date (hospitalization), date of the 1st CR session attended, and date of CR completion.
In addition, we obtained travel distance to CR and developed an estimate of household median income, both of which likely influence attendance. 20 We used the Geographic Information System (GIS) distance tool21 to calculate the distance from the patient’s home to our CR center. We estimated median home income by using the patients’ home address and then correlating this to the 2010 US census, which provided an estimated annual household median income for the census track where the patient lives.
Cost-sharing Data
Prior to enrollment, our program queried each patient’s insurance company to prepare a preliminary individualized cost-sharing report. The report contained insurance status, the presence and amount of any copays, the presence and amount of any met or unmet deductibles, and out-of-pocket fees if present (Supplement). This report was given to each patient during their first visit, informing them of anticipated cost-sharing at enrollment. Patients were asked to confirm these estimates with their insurance company. Payment was not required prior to the delivery of CR services but was rather billed at a future date, per BMC billing practice standards.
Individual cost-sharing information was complex, and most patients had plans with multiple, often overlapping tiers such as copays, deductibles and out-of-pocket maximums. Because of this, several assumptions were made to classify different components of cost-sharing into broader categories which allowed us to study relationships between those categories and attendance. All patients without copays who had met their deductibles or out-of-pocket expenses were classified as having no cost-sharing to attend CR, since they would not be incurring any out-of-pocket fee. For patient who had copays of fixed monetary value, no assumptions were made, and the copay value was recorded. For those with coinsurance, we applied their coinsurance rate percentage (or copay) to our standard session cost to obtain a numerical value. For example, a coinsurance rate of 20% applied to our standard session cost of $240 resulted in an estimate of $48 of cost-sharing per session. For patients with deductibles or out-of-pocket maximums (we treated these equivalently) we used their remaining annual deductible balance averaged over 36 sessions of CR to calculate an average session’s cost. For instance, patients who had met $1000 of their $1500 annual deductible, were given as estimated session cost of $14 ($500 divided over 36 CR sessions). Patients who had already met their annual deductibles before starting CR were categorized as not having any cost-sharing for CR. Unmet family plan deductibles were treated as individual plans: we assumed all remaining funds on the family deductible plans would be used towards the patient’s CR expenses. This conservative assumption provided the maximum out-of-pocket expense estimates possible since if other family members were to utilize services and contribute to meeting the family deductible, the patient would have no out-of-pocket expense sooner.
Study outcomes
The primary predictor was the presence (or absence) of any cost-sharing which included copays, coinsurance, unmet deductibles and out-of-pocket maximums. The primary outcome was the total number of CR sessions attended within 6 months of enrollment, with maximum session number being 36.
Secondary outcomes included enrollment into phase III (maintenance program) of CR and change in estimated metabolic equivalent of task (MET) during exercise training in CR. For patients with a high cost-sharing burden, our staff often encouraged a strategy of early transition to our low-cost phase III program after a few CR sessions (usually 6, but sometimes more or less depending on patient preference and copay cost.) Earlier transition reduces cost burden while still allowing patients to exercise in a clinical setting; however, it is not ideal since patients receive less individualized attention and monitoring. We included this outcome to assess whether patients with high cost-sharing would transition to phase III more frequently than patients without cost-sharing. Functional MET at usual exercise workloads was calculated at each session using an online calculator derived from exercise studies.18 An estimated MET change was measured as the difference between METs at the last CR session compared to METs at the 1st session. We included MET change to assess whether higher copay resulted in lower observed MET change.
Because deductibles renew at the beginning of the calendar year, we also evaluated the relationship between having an insurance policy with a deductible and CR attendance, regardless of whether the deductible was met at the time of CR enrollment. We further evaluated the association between deductibles among patients with clinical events in November and December who often met their annual deductible due to costs incurred during the preceding hospitalization. However, unlike patients with hospitalizations earlier in the calendar year, this cohort would need to pay a renewed deductible in January, by which point they likely had either not yet enrolled or completed CR,
Statistical Analysis
Summary statistics (means, standard deviations and proportions) were calculated to describe patients' baseline demographics and clinical presentation. Baseline demographics were compared using Chi-squared test for categorical values and T-tests for mean group comparisons. Multi-variable linear regression analysis testing was done to determine the association between cost-sharing, copay amount, and total number of sessions, adjusting for differences in baseline patient characteristics and co-morbidities. We then tested for effect size and interactions between estimated median income, cost-sharing, and CR sessions attended. All univariable factors with a P < .20 were included in the modeling but we only retained independent predictors with a P <.05. This was done through a combination of backward selection and clinical reasoning using key predictors from prior publications about cardiac rehabilitation adherence.11,22 Statistical significance was defined as a two-sided α = 0.05. All testing was performed on JMP version 12.0.1 (SAS Cary, NC).
RESULTS
Of the 742 patients enrolled in CR during our study period, 603 (81.2%) had complete cost-sharing information and were included in data analysis. The mean age of participants was 63.7 years-old; the majority was non-Hispanic white (86%) and most were male (70%). Most common qualifying diagnoses were myocardial infarction (34.8%), percutaneous coronary intervention (20.2%) and coronary artery bypass graft surgery (17.2%). Patients with cost-sharing were generally younger and had higher estimated average functional exercise capacity at baseline compared to those without cost-sharing (Table 1).
Table 1:
Patient Baseline Characteristics
| Cost-Sharing N = 235 (%) |
No Cost-sharing N = 368 (%) |
p-value | |
|---|---|---|---|
| Age (years ± SD) | 61.6 ± 9.9 | 65.0 ± 12.6 | <.001 |
| Sex (male) | 183 (78%) | 239 (65%) | <.001 |
| Race | .37 | ||
| Caucasian | 208 (89%) | 311 (85%) | |
| Black | 9 (4%) | 21 (6%) | |
| Other | 18 (7%) | 36 (9%) | |
| Diagnosis | .03 | ||
| Valve Repair/Replacement | 29 (12%) | 45 (12%) | |
| PCI | 46 (20%) | 76 (21%) | |
| MI | 89 (38%) | 121 (32%) | |
| CHF | 10 (4%) | 45 (12%) | |
| CABG/Valve | 10 (4%) | 21 (6%) | |
| CABG | 48 (20%) | 56 (15%) | |
| Angina | 3 (1%) | 4 (1%) | |
| Risk Factors | |||
| Smoker | 31 (13 %) | 60 (16 %) | .30 |
| Hyperlipidemia | 179 (76%) | 303 (82%) | .07 |
| Hypertension | 166 (71%) | 303 (82%) | <.001 |
| Diabetes Mellitus | 62 (26%) | 132 (36%) | .02 |
| Family History of Heart Disease | 111 (47%) | 191 (52%) | .26 |
| Other Characteristics | |||
| Functional METs at Baseline | 2.9 ± 0.9 | 2.5 ± 0.8 | <.001 |
| Left Ventricular Ejection Fraction (%) | 53.4 ± 13.1 | 51.0 ± 15.1 | .06 |
| Body Mass Index (kg/m2) | 28.9 ± 5.1 | 29.6 ±6.6 | .17 |
| Distance to CR (miles, IQR) | 5.9 (4.1, 9.1) | 4.5 (2.5, 6.9) | <.001 |
| Median Income ($, IQR)) | 70,956 (51,173 to 87,727) | 52,797 (35098 to 73451) | <.001 |
| AACVPR Risk Category* (n= 597) | <.001 | ||
| Low | 57 (34%) | 46 (13%) | |
| Moderate | 77 (33%) | 110 (30%) | |
| High | 98 (42%) | 208 (57%) |
American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) risk score was determined using the AACVPR Stratification Algorithm for Risk of Event31
Of all patients, 235 (39%) had some form of cost-sharing; of these, 192(82%) had copays with a median copay amount of $20/session (IQR 10, 32); 43 (18.3%) had completely met their deductible; and 79 (33.6%) had an unmet deductible with a median amount of $500 (250, 1800). Among all patients with deductibles, the median (IQR) total annual policy amount was $1,000 (400 to 2000) and would be a new cost experienced at the beginning of the calendar year. Median (IQR) estimated annual home income was $58,967 ($42,061 to $79,338). A lower percentage of patients had cost-sharing in lower income groups compared to those in higher income groups (16%, 41%, 43% and 56% with cost-sharing for income quartiles 1, 2, 3, and 4 respectively; P < .001).
In unadjusted analyses, the presence of any cost-sharing was associated with a lower median (IQR) number of CR sessions completed [16 sessions (4 to 36) vs. 10 sessions (4 to 27), P < .001, Table 2.) Upon adjustment, we found that the number of CR sessions attended was affected by multiple independent predictors including age, surgical diagnosis, and the presence of cost-sharing (Table 3). After adjustment for age and presence of surgical procedure, copay amount was associated with attendance in a dose-dependent manner: for every $10 increase in copay, patients attended 1.5 fewer sessions of CR (95% CI, −2.3 to −0.7). Patients with any cost-sharing attended 1.9 fewer sessions compared to those without (95% CI, −3.0 to −0.80).
Table 2:
Unadjusted Outcomes
| Cost-Sharing N = 235 |
No Cost-sharing N = 368 |
p-value | |
|---|---|---|---|
| Unadjusted Outcomes | |||
| Sessions attended (mean ± SD) | 14.3 ± 13.0 | 19.1 ± 14.4 | < .001 |
| Sessions attended (median, IQR) | 10 (4, 27) | 16 (4, 36) | < .001 |
| MET change | 1.4 ± 1.4 | 1.2 ± 1.3 | .07 |
| Phase III enrollment | 16 (7%) | 31 (8%) | .47 |
MET = Metabolic Equivalent of Task
Table 3:
Adjusted Outcomes: Independent Predictors of CR Sessions Attended
| B-Coefficient (95% confidence interval) |
p- value |
|
|---|---|---|
| Cost-sharing | ||
| Intercept | 3.3 (−2.9 to 9.5) | .29 |
| Age (per 10 years) | 2.2 (1.2 to 3.1) | <.001 |
| Surgical Diagnosis | 2.0 (0.80 to 3.1) | <.001 |
| Cost-sharing | −1.9 (−3.0 to −0.80) | <.001 |
| Copay Amount | ||
| Intercept | 5.3 (1.2 to 11.8) | .11 |
| Age (per 10 years) | 2.1 (1.2 to 3.1) | <.001 |
| Surgical Diagnosis | 1.9 (0.7 to 3.1) | <.001 |
| Copay Amount (per $10 increments) | −1.5 (−2.3 to −0.7) | <.001 |
When comparing patients with an unmet deductible to those who either had no deductible or had already met it, we found no difference in the median number (IQR) of CR sessions attended [11 (4 to 33) vs. 13 (4 to 36), P = 0.41.] However, patients with a deductible in their insurance policy (regardless of it is was met) attended 3.5 fewer sessions of CR [10.5 (3.75 to 30.25) vs. 14 (4 to 36), P = .047]. This effect was stronger among patients hospitalized in November or December (n = 82), where patients with a deductible (n = 14) attended 4.5 fewer sessions of CR compared to patients with hospitalizations at different times of year [8.5 (3.25 to 12.5) vs. 13 (5.25 to 36), P = .049]. However, this association was no longer seen after adjustment for age and surgical procedure [B-coefficient, −0.8 sessions (95% CI, −2.2 to 0.6), P = .25].
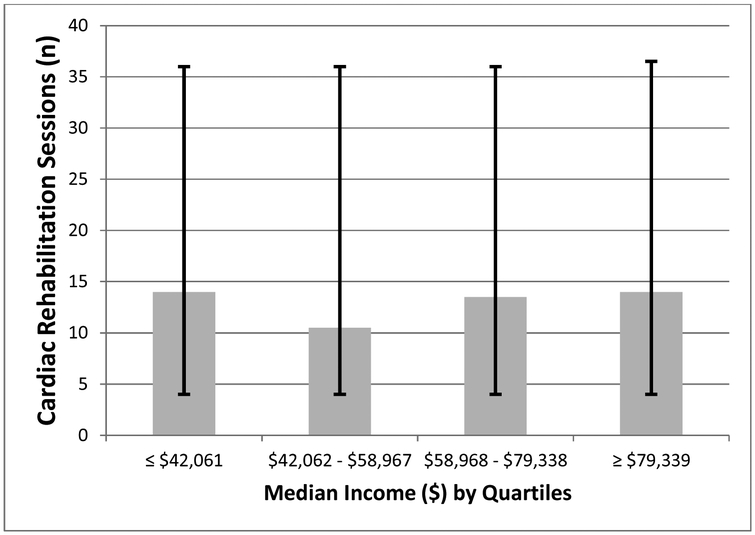
Secondary outcomes are shown in table 2. Estimated functional MET level change did not differ between patients with cost-sharing compared to those without (1.4 ± 1.4 vs. 1.2 ± 1.3, P =.07). No significant difference was found in phase III enrollment between patients with cost-sharing and those without (7% vs. 8%, P = .47, Table 2). We found no significant difference in the number of CR sessions attended when stratified by quartiles of estimated home median income (P = .78, Figure 1).
Figure 1:
Relationship between Estimated Median Income and CR Attendance
Figure 1 shows the median number of sessions with the Interquartile range within each median income strata. It shows that there was no significant difference in the number of CR sessions attended (P = .78).
DISCUSSION
In a large, single-center, retrospective cohort, we found that about 40% of our patients who qualified for CR were required to pay at least some out-of-pocket expenses to attend. The presence of any cost sharing was associated with fewer CR sessions attended. Higher cost-sharing was associated with lower CR attendance in a dose-dependent manner: each $10 increase in copay was associated with approximately 1.5 fewer sessions of CR. Furthermore, our analyses suggest that deductibles might influence CR attendance, particularly among patients with hospitalizations late in the calendar year who experience a January deductible reset, although these findings were not statistically significant after adjustment. Moreover, patients with cost-sharing did not transition to our lower cost phase III CR at any different rates than those without cost-sharing, which suggests that rather than using lower-cost exercise options, these patients may be choosing to not exercise at all. In total, our results indicate that the reduction, or even elimination, of cost-sharing could potentially increase CR adherence and outcomes.
These findings are consistent with one prior study of cost-sharing in CR. Zhang et al. found that patients with copays were 40% less likely to attend 18 sessions of CR, compared to patients without copay. However, this study was primarily evaluating predictors of CR attendance, and did not analyze the dose-response relationship with copays, the impact of deductibles, or the transition to phase III CR.22 Thus, to our knowledge, we are the first to show a “dose-response” that higher copays are associated with lower CR attendance. We suspect cost-sharing impacts CR because, in addition to being a preventive service, CR is repetitive, making it particularly vulnerable to the negative impact of cost-sharing. Similarly, cost-sharing has been previously shown to reduce use of other recommended preventive medical services, and lack of medical insurance has been associated with decreased use of preventive services.22-24 Among cardiac patients, the MI-FREE trial showed that eliminating medication copayments following myocardial infarction decreased incidence of first major cardiovascular events and decreased patient spending25.
Our data supports efforts of advocacy groups urging payers to re-evaluate their cost-sharing policies for CR coverage. 26 Specifically, given that prior studies have found a 1% lower mortality rate per session attended,8 our study suggests that the removal of copays and cost-sharing may translate into a 2-6% reduction in mortality for patients who qualify for CR. It would also likely decrease recurrence of cardiovascular events, procedures, and rehospitalizations27 at a comparable proportion to many other cardiovascular interventions, while being less expensive26. As such, we believe CR should be considered for value-based insurance designs, where insurance policies are designed to encourage high-value care, with the goal of maximizing cardiac rehabilitation utilization. To this end, we urge policymakers to either eliminate cost-sharing in CR or at least reduce cost-sharing to a single co-pay for the duration of the CR program. However, until this happens, we hope that clinicians will carefully discuss the benefits of CR with their patients, as some patients will choose to attend CR despite cost-sharing. Indeed, we noted 5 patients who attended our full CR program, despite an expected cost-sharing burden of > $1000.
We hypothesized that median household income would affect cost-sharing’s relationship with CR attendance, but our data did not support this. This may be because cost-sharing was unusual in the first quartile (16%), because these low-income patients were frequently insured by Medicaid and Mass Health which rarely impose cost-sharing. We also expected that the presence of cost-sharing would be associated with higher enrollment into phase III, but this was not seen. We suspect this is because transition to Phase III may be influenced more strongly by non-financial factors28 and these patients never develop the relationships with staff members such that they remain in the program after CR completion. Finally, we did not see any difference in changes in exercise capacity throughout CR between patients, but this is likely due to the younger age and greater baseline exercise capacity seen among patients with cost-sharing. Furthermore, the difference in number of CR sessions attended may have been too few to cause a detectable difference in average METs. Additional studies are needed to evaluate the relationship between cost-sharing and changes in both functional and peak exercise capacity as well as long-term outcomes.
Our study has a few limitations worth mentioning. First, our data can only be generalized to patients who attended at least 1 session of CR, because we did not obtain cost-sharing information on patients who were eligible for CR but never attended. Additional studies are needed among this group, particularly because they have been shown to be a high-cost, high-mortality risk group.6 Second, while we evaluated participation in phase III CR, we did not assess enrollment into other local gyms or exercise programs. Thus, it is possible some of these patients exercised in other settings. However, prior publications suggest that most of these patients don’t exercise and instead experience functional declines.29,30 Third, we did not have access to total costs or long-term outcomes such as rehospitalization or mortality. As a result, while we found lower attendance with cost-sharing, we cannot be sure if these differences in CR attendance are associated with differences in cost or patient outcomes. However, the dose-response relationship of CR attendance to long-term outcomes and cost are generally favorable.6-8 Fourth, we used cost-sharing estimates based on queries to insurance companies, rather than the actual bills sent to patients at future dates. This meant we effectively studied the psychological effect of anticipated cost-sharing, but this may not reflect actual final out-of-pocket expenses patients incurred, particularly if our insurance information was incorrect or some cost-sharing was written off by our health system.
CONCLUSION
Cost-sharing is a modifiable factor associated with CR attendance and we found an inverse dose-response relationship between the amount of cost-sharing and CR adherence. Appropriate reclassification of CR as a preventative intervention exempt from cost-sharing would likely benefit patients and policymakers alike. Further studies, including prospective cohorts and randomized clinical trials are needed to better determine how changing in cost-sharing policies might affect CR adherence, total costs, and long-term patient outcomes.
Supplementary Material
Acknowledgments
Funding: Dr. Pack, Dr. Lagu, and Dr. Lindenauer were each supported by grants from the National Heart, Lung and Blood Institute of the National Institutes of Health of Bethesda, MD under award numbers 1K23HL135440, 1K01HL114745, and 1K24HL132008 respectively.
ABBREVIATIONS:
- CR
Cardiac Rehabilitation
- CVD
Cardiovascular Disease
- MET
Metabolic equivalent
Footnotes
Disclosures: No authors have any conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Clark AM, Hartling L, Vandermeer B, McAlister FA. Meta-analysis: secondary prevention programs for patients with coronary artery disease. Ann Intern Med. 2005;143(9):659–672. [DOI] [PubMed] [Google Scholar]
- 2.Piepoli MF, Davos C, Francis DP, Coats AJS, ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ. 2004;328(7433):189. doi: 10.1136/bmj.37938.645220.EE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682–692. doi: 10.1016/j.amjmed.2004.01.009 [DOI] [PubMed] [Google Scholar]
- 4.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Members WC, Thomas RJ, Balady G, et al. 2018 ACC/AHA Clinical Performance and Quality Measures for Cardiac Rehabilitation: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Journal of the American College of Cardiology. March 2018:24587. doi: 10.1016/j.jacc.2018.01.004 [DOI] [Google Scholar]
- 6.Alter DA, Yu B, Bajaj RR, Oh PI. Relationship Between Cardiac Rehabilitation Participation and Health Service Expenditures Within a Universal Health Care System. Mayo Clin Proc. March 2017. doi: 10.1016/j.mayocp.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 7.Santiago de Araújo Pio C, Marzolini S, Pakosh M, Grace SL. Effect of Cardiac Rehabilitation Dose on Mortality and Morbidity: A Systematic Review and Meta-regression Analysis. Mayo Clin Proc. 2017;92(11):1644–1659. doi: 10.1016/j.mayocp.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 8.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121(1):63–70. doi: 10.1161/CIRCULATIONAHA.109.876383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soga Y, Yokoi H, Ando K, et al. Safety of early exercise training after elective coronary stenting in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2010;17(2):230–234. doi: 10.1097/HJR.0b013e3283359c4e [DOI] [PubMed] [Google Scholar]
- 10.Doll JA, Hellkamp A, Ho PM, et al. Participation in Cardiac Rehabilitation Programs Among Older Patients After Acute Myocardial Infarction. JAMA Intern Med. 2015;175(10):1700–1702. doi: 10.1001/jamainternmed.2015.3819 [DOI] [PubMed] [Google Scholar]
- 11.Gaalema DE, Savage PD, Rengo JL, et al. Patient Characteristics Predictive of Cardiac Rehabilitation Adherence. J Cardiopulm Rehabil Prev. 2017;37(2):103–110. doi: 10.1097/HCR.0000000000000225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fendrick AM, Chernew ME, Levi GW. Value-based insurance design: embracing value over cost alone. Am J Manag Care. 2009;15(10 Suppl):S277–283. [PubMed] [Google Scholar]
- 13.Increases in cost-sharing payments continue to outpace wage growth. Peterson-Kaiser Health System Tracker. https://www.healthsystemtracker.org/brief/increases-in-cost-sharing-payments-have-far-outpaced-wage-growth/. Accessed November 14, 2018.
- 14.Bureau UC. Health Insurance in the United States: 2016 - Tables. https://www.census.gov/data/tables/2017/demo/health-insurance/p60-260.html. Accessed October 19, 2018.
- 15.42 CFR 410.49 - Cardiac rehabilitation program and intensive cardiac rehabilitation program: Conditions of coverage. LII / Legal Information Institute; https://www.law.cornell.edu/cfr/text/42/410.49. Accessed November 14, 2018. [Google Scholar]
- 16.Cardiac rehabilitation programs ∣ Medicare. https://www.medicare.gov/coverage/cardiac-rehabilitation-programs. Accessed October 19, 2018.
- 17.Thomas RJ, King M, Lui K, et al. AACVPR/ACC/AHA 2007 performance measures on cardiac rehabilitation for referral to and delivery of cardiac rehabilitation/secondary prevention services endorsed by the American College of Chest Physicians, American College of Sports Medicine, American Physical Therapy Association, Canadian Association of Cardiac Rehabilitation, European Association for Cardiovascular Prevention and Rehabilitation, Inter-American Heart Foundation, National Association of Clinical Nurse Specialists, Preventive Cardiovascular Nurses Association, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2007;50(14):1400–1433. doi: 10.1016/j.jacc.2007.04.033 [DOI] [PubMed] [Google Scholar]
- 18.Sydó N, Abdelmoneim SS, Mulvagh SL, Merkely B, Gulati M, Allison TG. Relationship Between Exercise Heart Rate and Age in Men vs Women. Mayo Clinic Proceedings. 2014;89(12):1664–1672. doi: 10.1016/j.mayocp.2014.08.018 [DOI] [PubMed] [Google Scholar]
- 19.Fedel.Com Home Page. http://fedel.com/. Accessed January 8, 2019.
- 20.De Vos C, Li X, Vlaenderen I, et al. Participating or not in a cardiac rehabilitation programme: factors influencing a patient’s decision. European journal of preventive cardiology. 2012;20. doi: 10.1177/2047487312437057 [DOI] [PubMed] [Google Scholar]
- 21.Angier H, Likumahuwa S, Finnegan S, et al. Using geographic information systems (GIS) to identify communities in need of health insurance outreach: An OCHIN practice-based research network (PBRN) report. J Am Board Fam Med. 2014;27(6):804–810. doi: 10.3122/jabfm.2014.06.140029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Sobolev M, Piña IL, Prince DZ, Taub CC. Predictors of Cardiac Rehabilitation Initiation and Adherence in a Multiracial Urban Population. J Cardiopulm Rehabil Prev. 2017;37(1):30–38. doi: 10.1097/HCR.0000000000000226 [DOI] [PubMed] [Google Scholar]
- 23.Peters Anthony E, Keeley Ellen C Trends and Predictors of Participation in Cardiac Rehabilitation Following Acute Myocardial Infarction: Data From the Behavioral Risk Factor Surveillance System. Journal of the American Heart Association. 7(1):e007664. doi: 10.1161/JAHA.117.007664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trivedi AN, Moloo H, Mor V. Increased ambulatory care copayments and hospitalizations among the elderly. N Engl J Med. 2010;362(4):320–328. doi: 10.1056/NEJMsa0904533 [DOI] [PubMed] [Google Scholar]
- 25.Choudhry NK, Avorn J, Glynn RJ, et al. Full Coverage for Preventive Medications after Myocardial Infarction. New England Journal of Medicine. 2011;365(22):2088–2097. doi: 10.1056/NEJMsa1107913 [DOI] [PubMed] [Google Scholar]
- 26.Babu AS, Lopez-Jimenez F, Thomas RJ, et al. Advocacy for outpatient cardiac rehabilitation globally. BMC Health Services Research. 2016;16(1):471. doi: 10.1186/s12913-016-1658-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunlay SM, Pack QR, Thomas RJ, Killian JM, Roger VL. Participation in Cardiac Rehabilitation, Readmissions, and Death After Acute Myocardial Infarction. The American Journal of Medicine. 2014;127(6):538–546. doi: 10.1016/j.amjmed.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganga Harsha V, Jantz Jennifer, Choudhary Gaurav, Wu Wen-Chih. Abstract P310: Predictors of Phase III Enrollment in Participants Completing Phase II Cardiac Rehabilitation in a Large Urban Cardiac Rehabilitation Center. Circulation. 2016;133(suppl_1):AP310–AP310. doi: 10.1161/circ.133.suppl_1.p310 [DOI] [Google Scholar]
- 29.Reid RD, Morrin LI, Pipe AL, et al. Determinants of physical activity after hospitalization for coronary artery disease: the Tracking Exercise After Cardiac Hospitalization (TEACH) Study. Eur J Cardiovasc Prev Rehabil. 2006;13(4):529–537. doi: 10.1097/01.hjr.0000201513.13343.97 [DOI] [PubMed] [Google Scholar]
- 30.Leung YW, Ceccato N, Stewart DE, Grace SL. A prospective examination of patterns and correlates of exercise maintenance in coronary artery disease patients. J Behav Med. 2007;30(5):411–421. doi: 10.1007/s10865-007-9117-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.AACVPR Stratification Algorithm for Risk of Event. https://www.aacvpr.org/Portals/0/Registry/Cardiac%20Registry/Cardiac%20Registry%20User%20Resources/AACVPR%20Risk%20Stratification%20Algorithm.pdf. Accessed February 28, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.