Abstract
The role of atopic dermatitis (AD) in the development of colorectal cancer (CRC) has been a matter of scientific debate with mixed results. We conducted a nationwide cohort study to assess the association between AD and risk of CRC. Drawing on Taiwan's National Health Insurance Research Database, 46,703 patients with AD (the AD cohort) and 186,812 sex, age, and index year-matched patients without AD (the non-AD cohort) were identified in the period between 2000 and 2008. Follow-up time was calculated from the date of entry in the cohort until the occurrence of a first CRC diagnosis, death, or the end of the observation period (December 31, 2013), whichever occurred first. Hazards ratios (HRs) and accompanying 95% confidence intervals (CIs) derived from the Fine-Gray competing risk model were used to estimate the association between AD and CRC risk. After multivariable adjustment, AD was associated with an increased risk of CRC (adjusted HR, 1.26; 95% CI, 1.14–1.40). Of note, a significant positive association between AD and CRC risk was evident in both men and women and in all age groups. In summary, this population-based cohort study revealed that AD was associated with an increased risk of CRC in an Asian population. It will be of interest for cohort studies with prediagnostic specimens to evaluate the potential relationship between AD and CRC using biomarkers for allergy status.
Keywords: atopic dermatitis, cohort study, colorectal cancer, National Health Insurance Research Database
1. Introduction
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease with substantial effects on quality of life.[1] A recent systematic review of 69 cross-sectional and cohort studies had confirmed that AD is a worldwide phenomenon with a lifetime prevalence of >20% in many countries.[2] The hallmarks of AD are a chronic, relapsing form of skin inflammation, a disturbance of epidermal-barrier function that culminates in dry skin, and IgE-mediated sensitization to food and environmental allergens.[3] Indeed, several immunologic aberrations, both humoral and cellular, are found in patients with AD.[3] Atopic allergic conditions (AACs) such as AD, asthma, hay fever, and food allergies have been examined in connection with cancer risk. AACs may indicate a heightened immune response, which could contribute to recognize and remove malignant cells and thus reduce cancer risk.[4,5] Whereas AACs are accompanied by repeated tissue inflammation, damage, and repair, which could increase the risk of cancer.[4,5] Thus, the possibility of a promoting or protective role of AACs in carcinogenesis has been an interesting research area over the years. Previous studies have highlighted the potential inverse association between AACs and overall cancer risk with consistent findings for childhood leukemia, brain, and pancreatic cancers.[6–12] However, some other studies revealed a positive or nonsignificant association between AACs and malignant disease.[6,7,9,10,12,13] An increased risk for lung cancer, bladder cancer, lymphoma, myeloma, and prostate cancer exists among those with AACs, while studies that involve breast cancer, melanoma, and thyroid cancer have shown no association or conflicting results related to AACs.[9,10,11,13] Thus, the association between allergic conditions and malignant diseases appeared to be different according to cancer type.
To date, several epidemiologic studies have evaluated an association between AACs and colorectal cancer (CRC) risk. The exact nature of the association remains controversial. European record linkage studies comparing CRC risk in patients with asthma with that in the underlying general population reported lower risk,[14] higher risk,[15,16] and no association.[17] Of 8 other cohort studies that examined associations of CRC with AACs, 3 reported no association,[7,18,19] 4 reported an inverse association between hay fever and asthma and risk of CRC, with weaker association for those reporting only 1 of these conditions,[20–23] 1 also reported that having 2 or more atopic conditions was associated with a reduction in CRC risk in women.[24] Several case–control studies did not observe significant associations between allergy and the risk of CRC,[8,25] while other case–control studies have suggested that allergy might play a protective role in the colorectal carcinogenesis.[26,27] Recently, a meta-analysis of 9 cohort studies, including 775,178 individuals, showed no significant association between history of allergy and CRC risk.[28] Thus, the association between AACs and the risk of CRC is complex, dependent on both the study design and specific allergic condition. Taken together, results from aforementioned epidemiologic studies have provided findings of associations of allergic conditions with CRC risk in primarily white populations. A study is required to examine the association between atopic conditions and CRC risk in an Asian population, as clear racial/ethnic disparities in the risk of atopic disorders and CRC were noted.[29,30] Accordingly, we conducted a nationwide population-based cohort study to investigate the association between AD and the risk of CRC in an Asian population.
2. Methods
2.1. Data sources
The present study was a population-based retrospective cohort study using medical claims data from the Taiwan National Health Insurance Research Database (NHIRD). Taiwan's National Health Insurance (NHI) provides reimbursements for healthcare costs for 99% of the population in Taiwan (approximately 23 million people). The NHIRD contains comprehensive healthcare information, including demographic data of insured individuals, outpatient visits, hospital admission, disease diagnostic codes, and prescription details. The diagnostic codes used in the NHIRD follow the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). The NHIRD has previously been used for high-quality epidemiologic studies[31–33] and information on diagnoses, prescriptions, and hospitalizations have been shown to be of good validity.[34–36] Cheng et al reported that 97.85% of cases with ischemic stroke identified from the NHIRD were confirmed by radiology examination and clinical presentation.[34] In addition, Kao et al documented that the positive predictive value of cancer diagnoses in the NHIRD was 94% for all cancers and the positive predictive value of the 10 common cancers ranged from to 82% (cervical cancer) to 100% (colon cancer).[36] These data files are de-identified by scrambling the identification codes of all beneficiaries and information obtained from the databases was entirely anonymous. The data of this study were obtained from the Longitudinal Health Insurance Database (LHID 2000). LHID 2000 is a cohort data set of original medical claims data that includes 1 million beneficiaries randomly sampled from the registry of NHIRD dated between January 1, 2000 and December 31, 2013. The LHID data set contains historical ambulatory and inpatient care data for 2 million beneficiaries and allows researchers to follow the medical service utilization history of these beneficiaries. The claims data found in the LHID 2000 and NHIRD do not differ significantly in age, sex, and healthcare costs.[37] Since the data set was released for research purposes and included only scrambled information on insured individuals, the requirement for written or verbal consent from patients for study was waived, while the present study has been approved by the Institutional Review Board of Fu-Jen Catholic University (FJU-IRB NO: C104014).
2.2. Study cohorts
Patients who had a primary diagnosis of AD (ICD-9-CM codes 691 and 691.8) during the period from January 1, 2000 to December 31, 2008 were identified for the AD cohort and were compared with a comparison cohort comprised of patients who had never been diagnosed with AD (hereafter, non-AD cohort). The diagnosis had to be made by a specialist on the disease and the related specialists were dermatologists and allergists. To identify patients with AD with sufficient accuracy, all AD cases had at least 2 records of outpatient diagnosis. To enhance the statistical power, we applied frequency matching at a ratio of 1:4 for AD cohort to the matched non-AD cohort with matching for sex, age, and index year. We designated the index date as the date of primary diagnosis of AD for the subjects with AD (the AD cohort) and the matched date of physician visits for subjects without AD (the non-AD cohort). All newly diagnosed patients with AD in the LHID 2000 between January 1, 2000 and December 31, 2008 were recruited (n = 79,611). After excluding patients age <20 years (n = 29,758), sex unknown (n = 170) or with a cancer diagnosis (ICD-9-CM 140-210) before enrollment (n = 2980), 46,703 patients with AD formed the AD cohort. For each patient diagnosed with AD, we randomly selected 4 without AD as the comparison cohort from the same database. Accordingly, 186,812 patients without AD formed the non-AD cohort. Given the planned enrollment for the study of 233,515 patients: 46,703 patients with AD and 186,812 patients without AD, power analysis showed an 90% chance of detecting a strength of association between AD and CRC of 1.5 at a 2-sided significance level of P = .05. To enhance the comparability of AD and non-AD cohorts, we defined comorbidities, including allergy rhinitis (ICD-9-CM codes 477.0, 477.1, 477.2, 477.8, and 477.9), asthma (ICD-9-CM code 493.0), and hay fever (ICD-9-CM code 477.0), and included these comorbidities as covariates for adjustment. These comorbidities were determined by at least 2 outpatient visits 1 year before enrollment.
2.3. Ascertainment of CRC
The primary clinical outcome was the incidence of CRC. We determined patients with CRC as having primary diagnosis of the ICD-9-CM codes of 153, 153.0, 153.1, 153.2, 153.3, 153.4, 153.5, 153.6, 153.7, 153.8, 153.9, 154, 154.0, 154.1, 154.2, 154.3, and 154.8. To identify patients with CRC with sufficient accuracy, CRC diagnosis was defined according to the Registry for Catastrophic Illness Patient Database (RCIPD), a subpart of the NHIRD. The diagnosis of CRC needs histologic confirmation to be reported in the RCIPD. All patients were followed up from the index date until a first primary CRC diagnosis, death (as indicated by withdrawal from the NHI), or the end of the observation period (December 31, 2013), whichever occurred first (Fig. 1).
Figure 1.
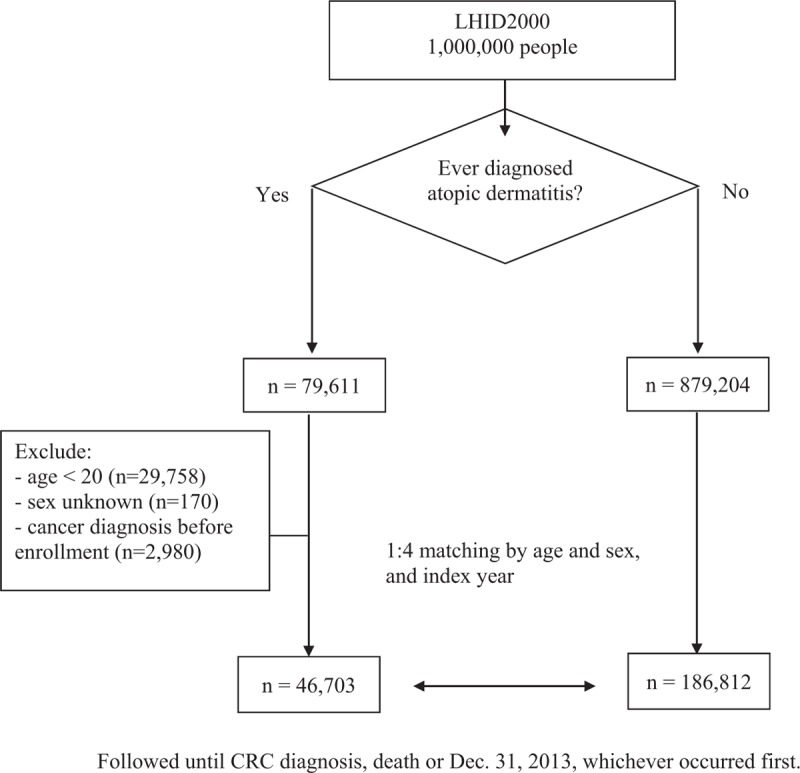
Study flowchart. CRC = colorectal cancer, LHID = longitudinal health insurance database.
2.4. Statistical analyses
Chi-squared and t tests were used to examine the differences in the distributions of the categorical and continuous variables, respectively, between cohorts. To estimate the effect of AD on the risk of CRC, the Fine-Gray competing risk model was fitted.[38] The Fine-Gray model was adjusted for potential confounders considering the risk of CRC as the event of interest, and deaths from other causes as competing risk. The potential confounding covariates included sex, age, income, and comorbidities. In this study, we used income as a surrogate measure related to CRC screening, comorbidities of chronic obstructive pulmonary disease (ICD-9-CM codes 490-496), and tobacco dependency (ICD-9-CM codes 305.1 and 649.0) as surrogate measures of smoking, as well as obesity (ICD-9-CM code 278), hypertension (ICD-9-CM codes 401–405), diabetes (ICD-9-CM code 250), and hyperlipidemia (ICD-9-CM codes 272.0–272.2 and 272.4) as proxy indicators of obesity. These surrogate covariates were included in the regression models for adjustment. In addition, it has been noted that individuals with AACs have higher endoscopy rates than those without AACs.[23] As endoscopy usage has the potential to both reduce the risk of CRC and increase the rate of CRC capture, we also included the frequency of endoscopic examinations (defined as colonoscopy [ICD-9-CM code 4523] or sigmoidoscopy [ICD-9-CM codes 4524 and 4823]) as an adjusted covariate in the regression analysis. Furthermore, data on annual clinical visits associated with AD, mainly due to either follow-up or continuous treatment, were retrieved from outpatient records and were examined its possible association with CRC risk. Difference in the cumulative risk of CRC between AD and non-AD cohorts was estimated using the Kaplan–Meier method with the log-rank test. A 2-tailed P-value <.05 was considered statistically significant. All analyses were performed using the SAS 9.4 package (SAS Institute, Cary, NC).
3. Results
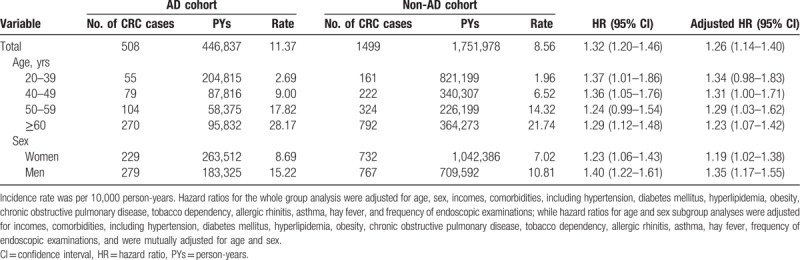
The demographics and clinical characteristics of the 2 cohorts are summarized in Table 1. The study subjects were predominantly female both in the AD (n = 27,702, 59.32%) and non-AD cohorts (n = 110,808, 59.32%), and the mean age was 44.8 and 44.6 years, respectively. There were no significant differences in the distributions of age and sex between the AD cohort and the non-AD cohort due to the matching scheme. However, the AD cohort had a significant higher proportion of low income status than that in the non-AD cohort. In addition, the AD cohort had significantly higher proportions of comorbidities, including hypertension, diabetes mellitus, hyperlipidemia, chronic obstructive pulmonary disease, allergic rhinitis, asthma, and hay fever than those in the non-AD cohort. Similarily, the AD cohort had a significant higher proportion of obesity and tobacco dependency than that in the non-AD cohort.
Table 1.
Baseline demographics and comorbidities in the atopic dermatitis (AD) cohort and the non-AD cohort.

During the follow-up of 446,837 person-years among patients with AD, there were 508 newly diagnosed patients with CRC. The corresponding incidence rate was 11.37 per 10,000 person-years. Comparatively, there were 1499 patients with CRC in the non-AD cohort during the follow-up period of 1,751,978 person-years. The corresponding incidence rate was 8.56 per 10,000 person-years. The Kaplan–Meier curves for the cumulative risk of CRC among the 2 cohorts are shown in Figure 2. The cumulative incidence of CRC was significantly higher in the AD cohort than in the non-AD cohort. The log-rank test revealed a significant difference over the entire Kaplan–Meier curve (P < .001) and the longer the follow-up, the larger the difference between the 2 cohorts. Compared with those without AD, patients with AD had a significantly elevated risk of CRC after adjustment for potential confounding covariates (adjusted hazards ratio [HR], 1.26; 95% confidence interval [CI], 1.14–1.40) (Table 2). Of note, a significant association between AD and CRC risk was evident in both men and women and in all age groups (Table 2).
Figure 2.
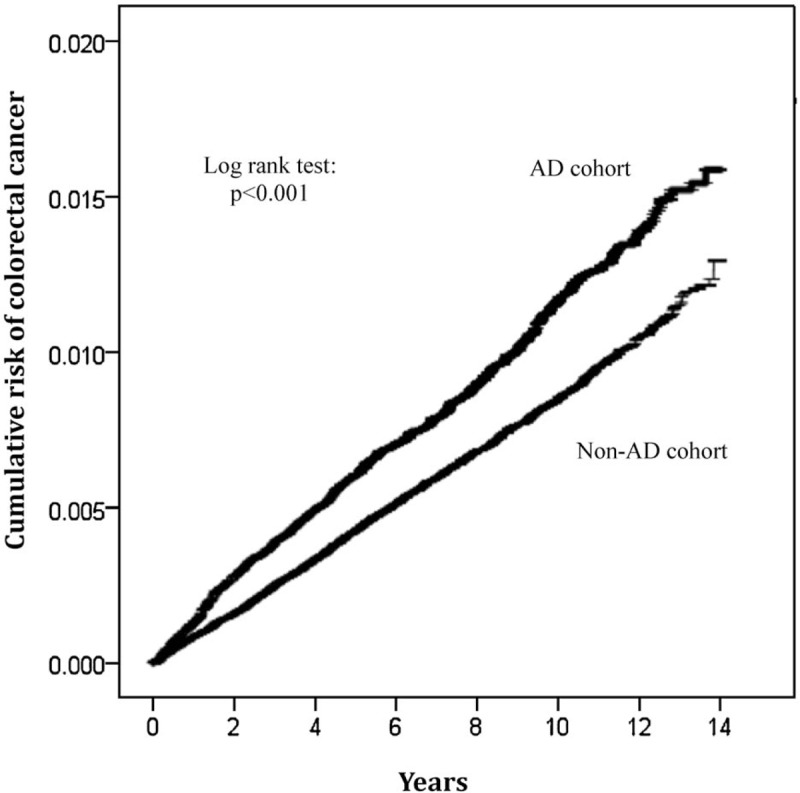
Kaplan–Meier curves of the cumulative incidence of colorectal cancer in the atopic dermatitis (AD) cohort and the non-AD cohort.
Table 2.
Multivariable Cox proportional hazards regression analysis of the association between atopic dermatitis (AD) and risk of colorectal cancer (CRC) based on the whole group and sex and age subgroups.
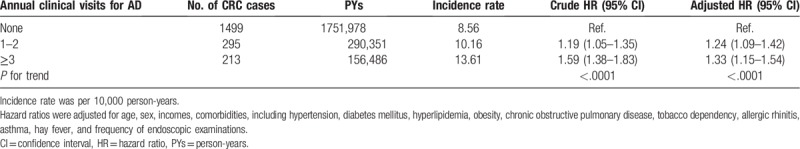
A differential pattern of association between AD and the risk of CRC was demonstrated according to the frequency of clinical visits. Relative to patients without AD, a higher HR was noted among patients having the highest frequency of clinical visits (≥3) for AD (adjusted HR, 1.33; 95% CI, 1.15–1.54). A trend with a significant increase in HRs with increasing frequency of clinical visits for AD was noted (P < .001) (Table 3).
Table 3.
The risk of colorectal cancer (CRC) in relation to annual clinical visits for atopic dermatitis (AD).
4. Discussion
In this nationwide population-based longitudinal study of an Asian population, we found an association between AD and increased risk of CRC. Our findings were robust in secondary analyses that a significant positive association between AD and CRC risk was evident in both men and women and in all age groups.
Although the association between allergic conditions and CRC risk has long been investigated, no clear conclusion has been made so far. Results from previous case–control and cohort studies have not been entirely consistent. Several case–control studies did not observe significant associations between allergy and the risk of CRC.[8,25] Whereas a number of case–control studies have suggested an inverse association between allergy and CRC risk.[26,27] Findings from cohort studies are also inconsistent. An epidemiologic study within the Cancer Prevention Study II cohort reported a nonsignificant 10% decrease in CRC incidence for individuals who have both hay fever and asthma.[21] A prospective study by Talbot-Smith et al reported a nonsignificant reduction in the risk of CRC in association with a history of asthma or hay fever.[20] Prizment et al found that having 2 or more atopic conditions was associated with a 42% reduction in CRC risk in older women in the Iowa Women's Health cohort.[24] Whereas Vesterinen et al observed an increased risk of colon cancer for patients with asthma,[16] while other cohort studies did not find any significant associations.[7,18,19] Findings in the current study indicated that AD was associated with an increased risk of CRC in both men and women. The inconsistency in study results could be explained by different study designs, various allergic conditions under study, absence of uniform definition of allergy, and failure to control for confounders. The vast majority of previous studies use self-administered questionnaire to report allergic conditions, while some used hospital discharge register. Little is known about the accuracy of reporting lifetime allergic history. Possible reporting differences may exist due to recentness or severity of allergic symptoms. Studies using hospital discharge registries or allergy medication history may have studied selected populations, as the registries covered only those whose symptoms were severe enough to require treatment.[14,16] With the help of the nationwide medical claims database used in the present study, we were able to acquire a large sample size while minimizing recall and selection biases. In addition, several studies examined individual allergic conditions while some assessed combinations of allergic conditions. Specific allergic conditions may have different effects on cancer risk. Indeed, conflicting results have provided support for 2 distinct and contradictory hypotheses. Studies reporting inverse associations between allergic conditions and CRC risk support an “enhanced immune surveillance theory,” with stimulated immune systems being better able to detect and destroy malignant cells. In contrast, studies indicating allergic conditions to be associated with an increased risk of CRC support an “antigenic stimulation theory,” with chronic immune stimulation leading to continuous tissue inflammation, damage, and repair, which increases the risk of CRC.[20,39] Indeed, several immunologic aberrations are found in AD, including impaired cellular-mediated immunity, elevated serum IgE and eosinophil levels, and IgE-bearing Langerhans cells.[3] Furthermore, colonization of the more-or-less chronically inflamed lesions by microbes may contribute to the perpetuation of the lesions.[3] Chronic inflammation and microbial colonization in combination with the immune impairments may lead to proliferative epidermal changes; hence, the enhancement to cancer development.
In the present study, we found that proportion of low-income insured individuals was higher in the AD cohort than that in the non-AD cohort. Several studies have reported that higher socioeconomic status is associated with increased AD prevalence, whereas lower socioeconomic status is associated with increased AD severity.[40,41] In Taiwan, Since the NHI's inception in March 1995, 99% of its 23 million populace enjoys an almost free access to healthcare services with only a small copayment required by clinics and hospitals, of which over 60% are privately operated.[42] An analysis of the NHI's immediate impact on the use of health services revealed that <1 year after its introduction, the hitherto uninsured used about twice the number of outpatient visits, hospital admissions, and emergency services as they had before the NHI began, bringing them up to par with those who had insurance before. By contrast, use rates for the previously insured group rose only slightly. If the NHI's aim was to remove financial barriers to healthcare, the program appears to have reached that goal almost immediately.[43] Accordingly, low-income AD patients could have severe disease but would have easy accessed healthcare system in Taiwan. Therefore, income was included as a covariate in regression models for adjustment in the current study.
The study had a number of strengths. The participants were drawn from a population-based and highly representative computerized database of medical records. NHIRD contains data from Taiwan's compulsory and universal healthcare system which has high coverage rate in Taiwan. This allowed us to perform our analysis in a real-life setting in an unselected patient population. In addition, patient dropout was avoided and selection bias minimized because of the use of routine database records.
Despite the strengths of our medical claims data, the results of the present study need to be interpreted wthin the context of some limitations. A major concern of our cohort study is the comparability between the AD and non-AD cohorts. It is possible for inclusion of individuals with undiagnosed AD among the non-AD cohort. Consequently, the association between AD and CRC risk could be underestimated. On the contrary, we had adjusted for baseline comorbidities of other allergic conditions, including allergic rhinitis, asthma, and hay fever, as well as the frequency of endoscopic examinations for the comparison between the AD cohort and non-AD cohort to improve the comparability between the cohorts. In addition, it has been noted that studies that are based on insurance claims or other 3rd-party data are often flawed because the information on confounding factors contained in insurance data is often limited.[44] Thus, several potential confounding covariates that are associated with CRC, such as family history of CRC, personal history of CRC screening, smoking habits, and obesity were not available in the NHIRD. Of note, the potential confounding by CRC screening could arise if patients with physician-diagnosed allergies are those with better access to healthcare and more likely to visit physicians. In the present study, we used income as a surrogate measure related to CRC screening, comorbidities of chronic obstructive pulmonary disease and tobacco dependency as surrogate measures of smoking, as well as hypertension, diabetes, and hyperlipidemia as proxy variables of obesity. These surrogate covariates were included in the regression models for adjustment. Although analyses were adjusted for potential confounding factors, we cannot rule out the possibility that the association between AD and CRC risk might have been due to residual confounding by unmeasured confounders.
In conclusion, despite its limitations, this population-based cohort study revealed that AD was associated with an increased risk of CRC in an Asian population. Findings from the present study highlight the importance of the immune system in the pathogenesis of CRC. Further studies are needed to confirm our findings. It will be of interest for cohort studies with prediagnostic specimens to evaluate the potential relationship between AD and CRC using biomarkers for allergy status.
Author contributions
Conceptualization: Wan-Yun Chou, Yu-Ching Chou, Chien-An Sun.
Data curation: Yong-Chen Chen.
Formal analysis: Wan-Yun Chou, Pin-Yu Lai, Yu-Ching Chou.
Funding acquisition: Chien-An Sun.
Investigation: Wan-Yun Chou, Je-Ming Hu, Chih-Hsiung Hsu, Yu-Feng Tian, San-Lin You, Cheng-Wen Hsiao, Yu-Ching Chou, Chien-An Sun.
Methodology: Wan-Yun Chou, Yu-Ching Chou, Chien-An Sun.
Project administration: Yu-Ching Chou, Chien-An Sun.
Supervision: Yu-Ching Chou, Chien-An Sun.
Validation: Je-Ming Hu, Chih-Hsiung Hsu, Yu-Ching Chou, Chien-An Sun.
Writing – original draft: Wan-Yun Chou.
Writing – review & editing: Wan-Yun Chou, Je-Ming Hu, Chih-Hsiung Hsu, Yong-Chen Chen, Yu-Feng Tian, San-Lin You, Cheng-Wen Hsiao, Yu-Ching Chou, Chien-An Sun.
Footnotes
Abbreviations: AACs = atopic allergic conditions, AD = atopic dermatitis, CAD = coronary artery disease, CI = confidence interval, COPD = chronic obstructive pulmonary disease, CRC = colorectal cancer, DM = diabetes mellitus, HR = hazard ratio, LHID = longitudinal health insurance database, LHID = Longitudinal Health Insurance Database, NHI = National Health Insurance, NHRI = National Health Research Institutes, PYs = person-years, RCIPD = Registry for Catastrophic Illness Patient Database.
How to cite this article: Chou WY, Lai PY, Hu JM, Hsu CH, Chen YC, Tian YF, You SL, Hsiao CW, Chou YC, Sun CA. Association between atopic dermatitis and colorectal cancer risk: a nationwide cohort study. Medicine. 2020;99:1(e18530).
Y-CC and C-AS contributed equally to this work.
This study was supported by a grant from the Chi-Mei Hospital (105-CM-FJU-09).
The authors have no conflicts of interest to disclose.
References
- [1].Abuabara K, Yu AM, Okhovat JP, et al. The prevalence of atopic dermatitis beyond childhood: a systematic review and meta-analysis of longitudinal studies. Allergy 2017;73:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Deckers IAG, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One 2012;7:e39803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bieber T. Atopic dermatitis. N Engl J Med 2008;358:1483–94. [DOI] [PubMed] [Google Scholar]
- [4].Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298–306. [DOI] [PubMed] [Google Scholar]
- [5].Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘immunoscore’ in the classification of malignant tumours. J Pathol 2014;232:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Eriksson NE, Holmen A, Hogstedt B, et al. A prospective study of cancer incidence in a cohort examined for allergy. Allergy 1995;50:718–22. [DOI] [PubMed] [Google Scholar]
- [7].Eriksson NE, Mikoczy Z, Hagmar L. Cancer incidence in 13811 patients skin tested for allergy. J Investig Allergol Clin Immuno 2005;15:161–6. [PubMed] [Google Scholar]
- [8].Wang H, Diepgen TL. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies. Allergy 2005;60:1098–111. [DOI] [PubMed] [Google Scholar]
- [9].Merrill RM, Isakson RT, Beck RE. The association between allergies and cancer: what is currently known? Ann Allergy Asthma Immunol 2007;99:102–17. [DOI] [PubMed] [Google Scholar]
- [10].Vojtechova P, Martin RM. The association of atopic diseases with breast, prostate, and colorectal cancers: a meta-analysis. Cancer Causes Control 2009;20:1091–105. [DOI] [PubMed] [Google Scholar]
- [11].Rittmeyer D, Lorentz A. Relationship between allergy and cancer: an overview. Int Arch Allergy Immunol 2012;159:216–25. [DOI] [PubMed] [Google Scholar]
- [12].Helby J, Bojesen SE, Nielsen SF, et al. IgE and risk of cancer in 37747 individuals from the general population. Ann Oncol 2015;26:1784–90. [DOI] [PubMed] [Google Scholar]
- [13].Karim AF, Westenberg LEH, Eurelings LEM, et al. The association between allergic diseases and cancer: a systematic review of the literature. Neth J Med 2019;77:42–66. [PubMed] [Google Scholar]
- [14].Kallen B, Gunnarskog J, Conradson TB. Cancer risk in asthmatic subjects selected from hospital discharge registry. Eur Respir J 1993;6:694–7. [PubMed] [Google Scholar]
- [15].Ji J, Shu X, Li X, et al. Cancer risk in hospitalized asthma patients. Br J Cancer 2009;100:829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vesterinen E, Pukkala E, Timonen T, et al. Cancer incidence among 78,000 asthmatic patients. Int J Epidemiol 1993;22:976–82. [DOI] [PubMed] [Google Scholar]
- [17].Gonzalez-Perez A, Fernandez-Vidaurre C, Rueda A, et al. Cancer incidence in a general population of asthma patients. Pharmacoepidemiol Drug Saf 2006;15:131–8. [DOI] [PubMed] [Google Scholar]
- [18].McWhorter WP. Allergy and risk of cancer. A prospective study using NHANES I followup data. Cancer 1988;62:451–5. [DOI] [PubMed] [Google Scholar]
- [19].Mills PK, Beeson WL, Fraser GE, et al. Allergy and cancer: organ site-specific results from the Adventist Health Study. Am J Epidemiol 1992;136:287–95. [DOI] [PubMed] [Google Scholar]
- [20].Talbot-Smith A, Fritschi L, Divitini ML, et al. Allergy, atopy, and cancer: a prospective study of the 1981 Busselton cohort. Am J Epidemiol 2003;157:606–12. [DOI] [PubMed] [Google Scholar]
- [21].Turner MC, Chen Y, Krewski D, et al. Cancer mortality among US men and women with asthma and hay fever. Am J Epidemiol 2005;162:212–21. [DOI] [PubMed] [Google Scholar]
- [22].Jacobs EJ, Gapstur SM, Newton CC, et al. Hay fever and asthma as markers of atopic immune response and risk of colorectal cancer in three large cohort studies. Cancer Epidemiol Biomarkers Prev 2013;22:661–9. [DOI] [PubMed] [Google Scholar]
- [23].Tambe NA, Wilkens LR, Wan P, et al. Atopic allergic conditions and colorectal cancer risk in the multiethnic cohort study. Am J Epidemiol 2015;181:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Prizment AE, Folsom AR, Cerhan JR, et al. History of allergy and reduced incidence of colorectal cancer, Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev 2007;16:2357–62. [DOI] [PubMed] [Google Scholar]
- [25].Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illness, operations, and medications: case-control results from the Melbourne Colorectal Cancer Study. Cancer Res 1988;48:4399–404. [PubMed] [Google Scholar]
- [26].La Vecchia C, D’Avanzo B, Negri E, et al. History of selected diseases and the risk of colorectal cancer. Eur J Cancer 1991;27:582–6. [DOI] [PubMed] [Google Scholar]
- [27].Negri E, Bosetti C, La Vecchia C, et al. Allergy and other selected diseases and risk of colorectal cancer. Eur J Cancer 1999;35:1838–41. [DOI] [PubMed] [Google Scholar]
- [28].Ye J, Talaiti A, Ma Y, et al. Allergies and risk of colorectal cancer: a systematic review and meta-analysis of observational studies. Oncotarget 2017;8:14646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kaufman B, Guttman-Yassky E, Alexis A. Atopic dermatitis in diverse racial and ethnic groups: variations in epidemiology, genetics, clinical presentation, and treatment. Exp Dermatol 2018;27:340–57. [DOI] [PubMed] [Google Scholar]
- [30].Ollberding NJ, Nomura AMY, Wilkens LR, et al. Racial/ethnic differences in colorectal cancer risk: The Multiethnic Cohort Study. Int J Cancer 2011;129:1899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B Virus–related hepatocellular carcinoma recurrence following liver resection. JAMA 2012;308:1906–13. [DOI] [PubMed] [Google Scholar]
- [32].Chien HC, Kao Yang YH, Bai JPF. Trastuzumab-related cardiotoxic effects in Taiwanese women: a nationwide cohort study. JAMA Oncol 2016;2:1317–25. [DOI] [PubMed] [Google Scholar]
- [33].Chang SH, Chou IJ, Yeh YH, et al. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA 2017;318:1250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011;20:236–42. [DOI] [PubMed] [Google Scholar]
- [35].Chen CC, Chen LS, Yen MF, et al. Geographic variation in the age-and gender-specific prevalence and incidence of epilepsy: analysis of Taiwanese National Health Insurance-based data. Epilepsia 2012;53:283–90. [DOI] [PubMed] [Google Scholar]
- [36].Kao WH, Hong JH, See LC, et al. Validity of cancer diagnosis in the National Health Insurance database compared with the linked National Cancer Registry in Taiwan. Pharmacoepidemiol Drug Saf 2018;27:1060–6. [DOI] [PubMed] [Google Scholar]
- [37].Tsan YT, Lee CH, Wang JD, et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol 2012;30:623–30. [DOI] [PubMed] [Google Scholar]
- [38].Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. JASA 1999;94:496–509. [Google Scholar]
- [39].Wang H, Rothenbacher D, Low M, et al. Atopic diseases, immunoglobulin E and risk of cancer of the prostate, breast, lung and colorectum. Int J Cancer 2006;119:695–701. [DOI] [PubMed] [Google Scholar]
- [40].Lee KS, Rha YH, Oh IH, et al. Socioeconomic and sociodemographic factors related to allergic diseases in Korean adolescents based on the Seventh Korea Youth Risk Behavior Web-based Survey: a cross-sectional study. BMC Pediatr 2016;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chung J, Simpson EL. The socioeconomics of atopic dermatitis. Ann Allergy Asthma Immunol 2019;122:360–6. [DOI] [PubMed] [Google Scholar]
- [42].Kreng VB, Yang CT. The equality of resource allocation in health care under the National Health Insurance System in Taiwan. Health Policy 2010;100:203–10. [DOI] [PubMed] [Google Scholar]
- [43].Cheng SH, Chiang TL. The effect of universal health insurance on health care utilization in Taiwan. JAMA 1997;278:89–93. [DOI] [PubMed] [Google Scholar]
- [44].Hyman J. The limitations of using insurance data for research. JADA 2015;146:283–5. [DOI] [PubMed] [Google Scholar]


