Supplemental Digital Content is available in the text
Keywords: cell cycle arrest, IL6, p21, prostate cancer
Abstract
Background:
Polyphyllin I has been reported to possess anticancer properties in various cancer types, including prostate cancer. However, little is known about the potential of Polyphyllin I in induction of prostate cancer cell cycle arrest and its underlying mechanisms.
Methods:
The anti-proliferation activity of Polyphyllin I was tested using cell counting kit-8 assay. The cell cycle arrest effects of Polyphyllin I were confirmed by flow cytometry. Western blot was used to test the effect of Polyphyllin I on cell cycle-related protein expression. Reverse transcription-polymerase chain reaction was used to test the expression of genes regulating P21 expression.
Results:
Polyphyllin I induced prostate cancer cell cycle arrest in G0/G1 phase in concentration-dependent manner. Polyphyllin I induced cell cycle arrest by upregulating the expression of P21. Further studies showed that the upregulation of p21 expression induced by Polyphyllin I via the upregulation of IL6 expression.
Conclusion:
Polyphyllin I could induce cell cycle arrest in G0/G1 phase in prostate cancer cells by upregulating the expression of P21 and IL6.
1. Introduction
Prostate cancer is one of the most common causes of cancer-related mortality in men.[1,2] Despite advances in early diagnosis and treatment, prostate cancer morbidity remains high. The survival rates remain poor, especially among castration-resistant prostate cancer patients,[3,4] although chemotherapy has significantly improved the prognosis of prostate cancer patients. Additionally, the severe systemic toxicity of chemotherapeutic drugs has limited the application of these drugs. Moreover, patients have compromised quality of life (due to the high side effects) and pay high costs for treatment, especially in developing countries. Therefore, the development of novel therapeutic agents to improve clinical outcomes is highly desired.
Traditional Chinese medicine has a unique effect in cancer treatment, with lower side effects and costs, and may have good prospects in the treatment of prostate cancer.
Antipyretic detoxicate is one of the predominant treatments for cancer in Chinese medicine.[5–8] Paris polyphylla, a traditional antipyretic detoxicate Chinese medicinal herb, has been applied extensively for cancer treatment for nearly 2000 years.[9] Paris polyphylla has been reported to exhibit anticancer properties in various cancer types.[10–12] We previously showed that a Paris polyphylla ethanol extract exerts anticancer effects by inducing apoptosis and cell growth arrest in prostate cancer cells.[13]
Polyphyllin I (PPI), a major component isolated from Rhizoma Paridis saponins, has been reported to exhibit anticancer properties in various cancer types.[14,15] PPI significantly inhibits growth,[16] invasion and epithelial-mesenchymal transition via cellular inhibitor of PP2A/PP2A (serine/threonine protein phosphatase 2A)/extracellular regulated protein kinases signaling in prostate cancer.[17]
However, little is known about the effects of PPI on cell cycle arrest in prostate cancer. In this study, we evaluated the ability of PPI to induce prostate cancer cell cycle arrest and delineated the underlying mechanisms.
2. Materials and methods
2.1. Cell culture
PC3 cells (The Cell Bank of Chinese Academy of Sciences, Shanghai, China) were cultured in F12K (Ham‘s F 12 nutrient medium (Kaighn's)) medium HyClone (Logan, Utah, USA) supplemented with 10% fetal bovine serum (Gibco; Grand Island, NY, USA); DU145 cells (The Cell Bank of Chinese Academy of Sciences) were cultured in Roswell Park Memorial Institute 1640 medium (HyClone) supplemented with 10% fetal bovine serum (Gibco). The cells were maintained in a humidified incubator with 5% CO2 at 37°C.
2.2. Cell viability assay
Cell viability was assessed using a cell counting kit-8 (CCK-8) assay according to the manufacturer's protocol (Dojindo, Shanghai, China). Cells were seeded at 2 × 103 cells/well in 96-well plates and incubated with gradient concentrations of PPI (Chengdu Manst Biotechnology, Chengdu, China) at 37°C for 24 hours in a humidified chamber containing 5% CO2. CCK-8 solution (10 μL) was added to each well, and the plates were incubated for 1 hour at 37°C. The absorbance of the cells at 450 nm (OD450) was measured in a microplate reader (Thermo Fisher Scientific, Waltham, MA).
2.3. Colony formation assay
Cells were seeded into 6-well plates (300 cells/well) and incubated for 10 to 14 days in the presence or absence of PPI. The plates were stained with crystal violet, and the number of colonies with more than 50 cells was counted.
2.4. Cell cycle analysis
The cell cycle was assessed using a GENMED Universal periodic flow cytometry kit according to the manufacturer's protocol (GENMED SCIENTIFICS INC, Shanghai, China). Cells were seeded at 1.2 × 105 cells/well in 6-well plates and incubated with gradient concentrations of PPI at 37°C for 24 hours in a humidified chamber containing 5% CO2.
2.5. Western blotting
Protein sample preparation and Western blotting were performed as previously described.[18] Blots were incubated with primary antibodies against CDK2, CDK4, CDK6, CyclinD1, P21, p-RB, p-STAT3, and P27 (Cell Signaling Technology, Danvers, Massachusetts, USA) overnight at 4°C, followed by an incubation with appropriate peroxidase-conjugated secondary antibodies. β-Actin served as an internal control. The immunocomplexes were visualized by using an enhanced chemiluminescence detection system (Millipore, Billerica, Massachusetts, USA), followed by exposing the blots to X-ray film.
2.6. Quantitative polymerase chain reaction analysis
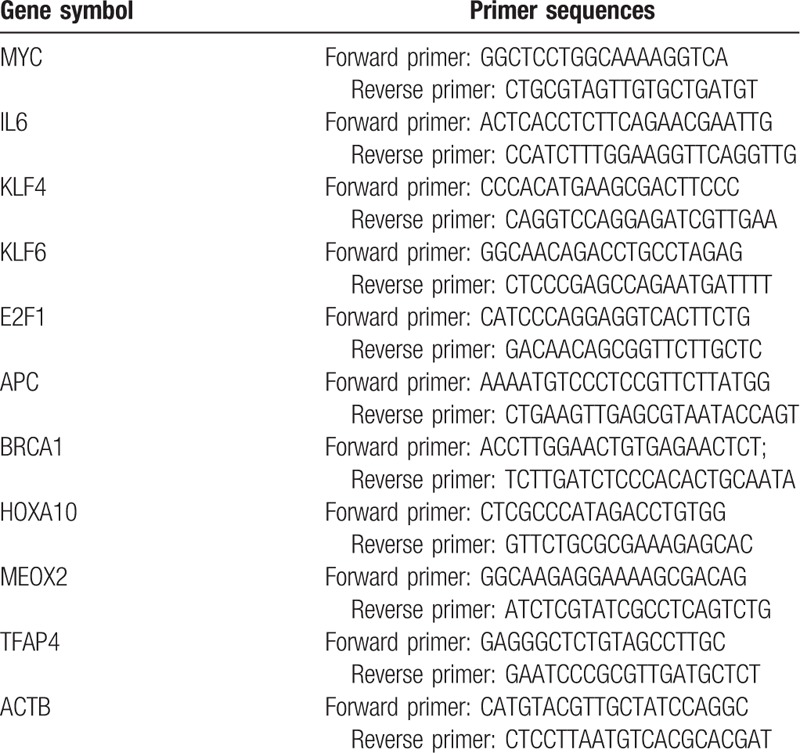
Total RNA was prepared from PC3 and DU145 cells by using an RNAiso plus kit (TaKaRa, Dalian, China). For quantitative polymerase chain reaction (qPCR) assays, complementary DNA was synthesized using a ReverTra Ace qPCR RT kit (Toyobo, Osaka, Japan). qPCR was performed using a Roche QRT-PCR System. The messenger RNA (mRNA) levels of target genes were normalized to the mRNA level of β-actin. The sequences of the primers used are shown in Table 1.
Table 1.
The sequences of the primers.
2.7. Statistical analysis
Student t test was used for the analysis of statistical significance for differences between the control and treated groups. The comparative data were expressed as the mean ± standard deviation of at least 3 independent experiments. P < .05 was considered statistically significant.
2.8. Ethical approval
Not necessary. This study does not involve animal and human ethics.
3. Results
3.1. PPI treatment inhibits the growth and clonogenic potential of prostate cancer cells in a dose-dependent manner
To study the effect of PPI on the growth of human prostate cancer cells, PC3 and DU145 cells were treated with various concentrations of PPI (0, 0.11, 0.33, 1, 3, or 9 μg/mL) for 24 hours, and cell viability was determined using a CCK-8 assay. We found that the half-maximal inhibitory concentration (IC50) of PPI for PC3 cells was 1 μg/mL and the IC50 of PPI for DU145 cells was 1.5 μg/mL (Fig. 1A). Because the clonogenic ability of tumor cells reflects their oncogenic capacity to some extent, we further determined whether PPI inhibits the colony formation ability of prostate cancer cells. PC3 and DU145 cells were incubated with PPI at 0.15 or 0.2 μg/mL, and a significant reduction in the number of colonies derived from these cells was observed with 0.15 μg/mL PPI, while their clonogenic potentials were significantly lower when treated with 0.2 μg/mL PPI (Fig. 1B and C). These results collectively suggest a strong inhibitory effect of PPI on prostate cancer cell growth and clonogenic ability.
Figure 1.
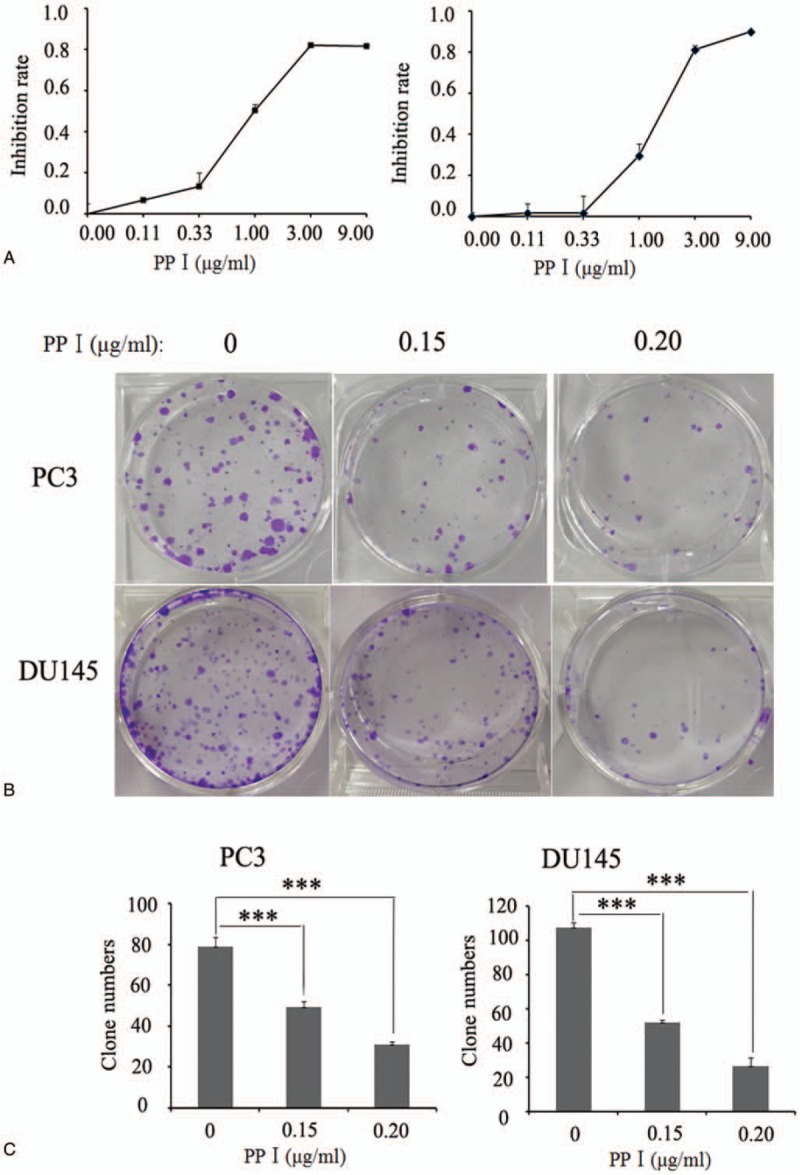
Polyphyllin I inhibit PC cell growth and clonogenic ability in a dose-dependent manner. (A) Polyphyllin I treatment significantly inhibits prostate cancer cell growth. PC3 and DU145 cells were incubated with various concentrations of Polyphyllin I for 24 h and cell viability was determined using CCK8 assay; Polyphyllin I inhibit the clonogenic potential of PC cells: cell morphology map (B); (C) clone formation statistics. CCK8 = cell counting kit-8.
3.2. PPI arrests the cell cycle in the G0/G1 phase in prostate cancer cells
To examine whether PPI inhibits cell proliferation via cell cycle arrest, we performed flow cytometry analysis to investigate the cell cycle distribution of prostate cancer cells treated with PPI. Cell cycle progression was analyzed, and the obtained results revealed that G0/G1 arrest occurred in both PC3 and DU145 cells in the presence of PPI. The proportion of cells in the G0/G1 phase reached 59.37% ± 1.56% for PC3 cells when these cells were incubated with 1 μg/mL PPI for 24 hours (Fig. 2A), whereas the proportion of cells in the G0/G1 phase reached approximately 42.29% ± 0.45% for the control untreated PC3 cells (Fig. 2A). Similar results were obtained with treated DU145 cells (Fig. 2B). After treatment with PPI, the proportion of DU145 cells in the G0/G1 phase reached 65.11% ± 1.22%, while the proportion was 42.12% ± 1.2% for the untreated DU145 cells (Fig. 2B). In addition, we also examined the cycle changes of prostate cancer cells treated with PP1 for 48 hours and 72 hours, and obtained similar results. (Supplementary Fig. 1)
Figure 2.
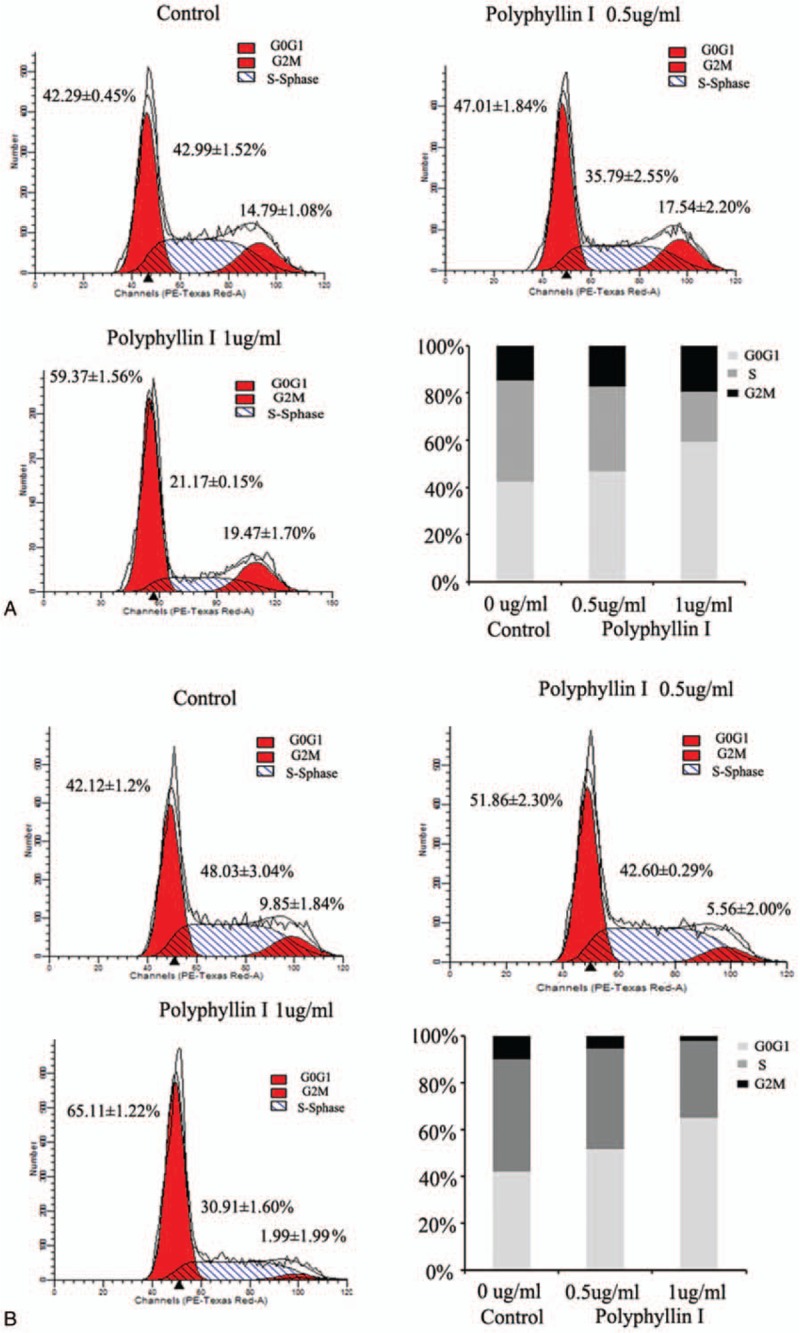
Polyphyllin I block cell cycle at G0/G1 phase in a dose-dependent manner. PC3 and DU145 cells were treated with 2 different concentrations of Polyphyllin I (0.5 and 1.0 μg/mL, respectively) and cell cycle was analyzed using flow cytometry. (A and B) Cell cycle distribution of PC3 and DU145 cells, respectively.
3.3. PPI treatment induces the upregulation of P21 expression at both the protein and mRNA levels in a dose-dependent manner
The previous experiments confirmed that PPI can induce prostate cancer cell cycle arrest in the G0/G1 phase. Next, we explored the molecular mechanism of the cell cycle arrest induced by PPI. First, we used a half lethal dose of PPI to treat PC3/DU145 cells and harvested total protein at 0, 13, 6, 12, and 24 hours. Western blot analysis was performed to determine the expression of cell cycle regulators, including P21, P27, CDK2, CDK4, CDK6, and CyclinD1. The PPI treatment significantly increased the protein expression of P21 in the PC3 and DU145 cells, while the expression of the other molecules did not change significantly (Fig. 3A and B). Further studies confirmed that PPI upregulated the expression of P21 in a concentration-dependent manner (Fig. 3C and D).
Figure 3.
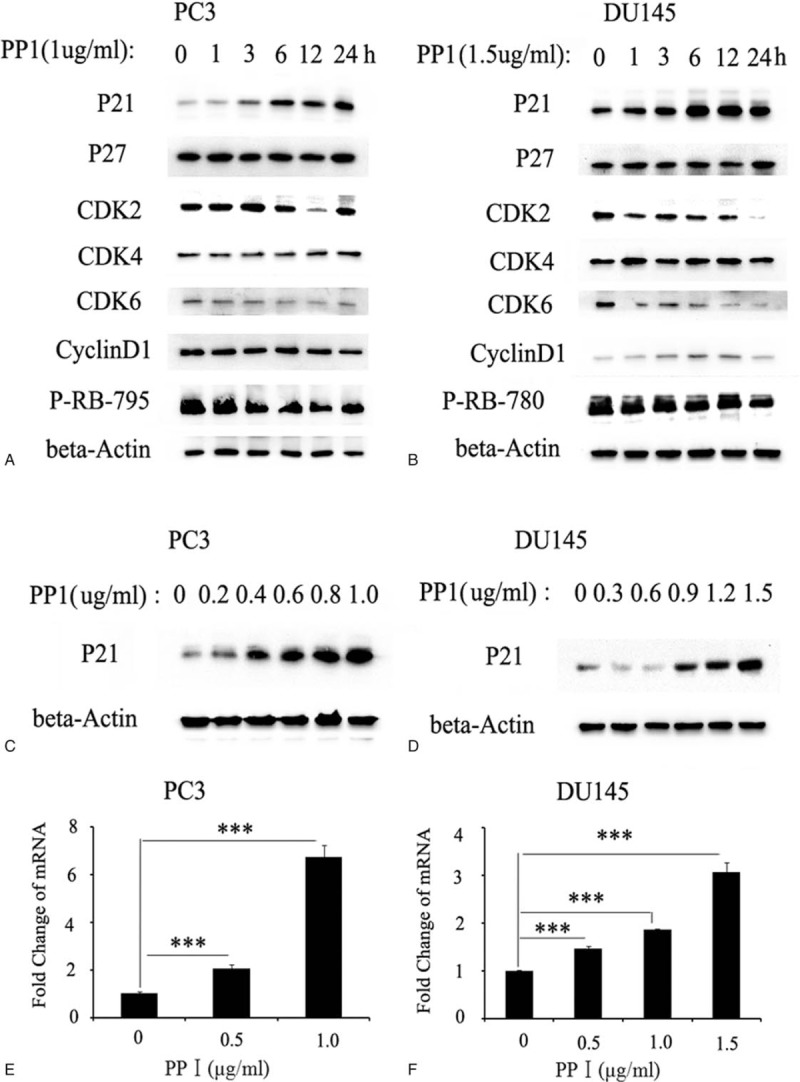
Polyphyllin I Treatment induces Up-Regulation of P21 Expression both in protein and mRNA level in a dose-dependent manner. Polyphyllin I treatment significantly increased the expression of P21 protein in PC3 cells (A) and DU145 cells (B), while other related molecules did not change significantly. Phosphorylation of RB downstream of p21 signal was inhibited by Polyphyllin I in PC3 cell (A) and DU145 cells (B). Western blot show that Polyphyllin I upregulated P21 expression in a concentration-dependent manner in PC3 cells (C) and DU145 cells (D); RT-PCR show that Polyphyllin I upregulated mRNA expression of P21 in a concentration-dependent manner in PC3 cells (E) and DU145 cells (F). mRNA = messenger RNA, RT-PCR = reverse transcription-polymerase chain reaction.
Finally, we collected RNA from prostate cancer cells treated with gradient concentrations of PPI. The qPCR results confirmed that PPI could promote the transcription of the P21 gene (Fig. 3E and F). These results suggest that PPI upregulates the mRNA and protein expression of P21, thereby inducing cell cycle arrest.
3.4. The upregulation of P21 expression may be partly due to the upregulation of IL6 expression induced by PPI
P21 is a tumor suppressor, and the regulation of its expression has been thoroughly studied. Prostate cancer cells were treated with gradient concentrations of PPI for 24 hours, and then RNA was harvested. qPCR was used to detect the expression of genes that regulate P21 expression. The results showed that the mRNA expression of interleukin 6 (IL6) in the PC3 cells was significantly increased following the PPI treatment in a concentration-dependent manner, while the expression of other genes was not significantly changed (although the expression of KLF6 was also elevated and concentration-dependent) (Fig. 4A). Similar results were obtained with the DU145 cells (Fig. 4B).
Figure 4.
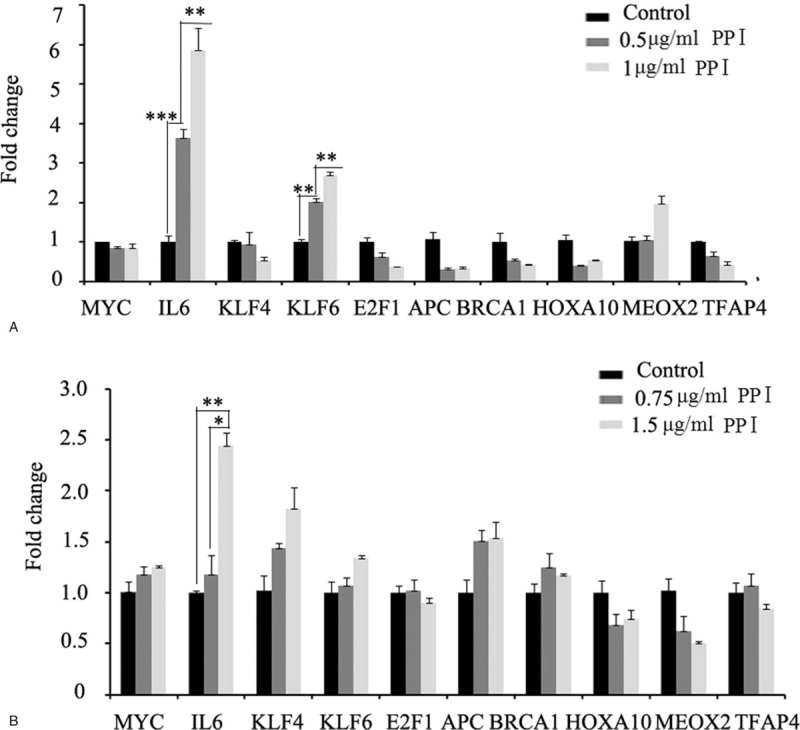
Quantitative PCR was used to detect the expression of genes regulating P21 expression. Polyphyllin I treatment significantly increased the mRNA expression of IL6 in PC3 cells (A) and DU145 cells (B), while the expression of other genes was not significantly changed. mRNA = messenger RNA, PCR = polymerase chain reaction.
We speculate that the high expression of P21 induced by PPI may be regulated by IL6. To this end, we used anti-IL6 antibodies to neutralize the IL6 protein secreted by the prostate cancer cells treated with PPI. First, cell morphology analysis showed that the PC3 cells treated with PPI alone for 24 hours became significantly narrower and sparser, while the cells treated with the anti-IL6 antibody, especially those treated with a high concentration of the anti-IL6 antibody, appeared very similar to the cells in the control group (Supplementary Fig. 2). Thus, blocking IL6 could partially reverse the effect of PPI. Western blotting (Fig. 5A and B) and qPCR (Fig. 5C and D) further confirmed that blocking IL6 could reduce the upregulation of P21 expression induced by PPI.
Figure 5.
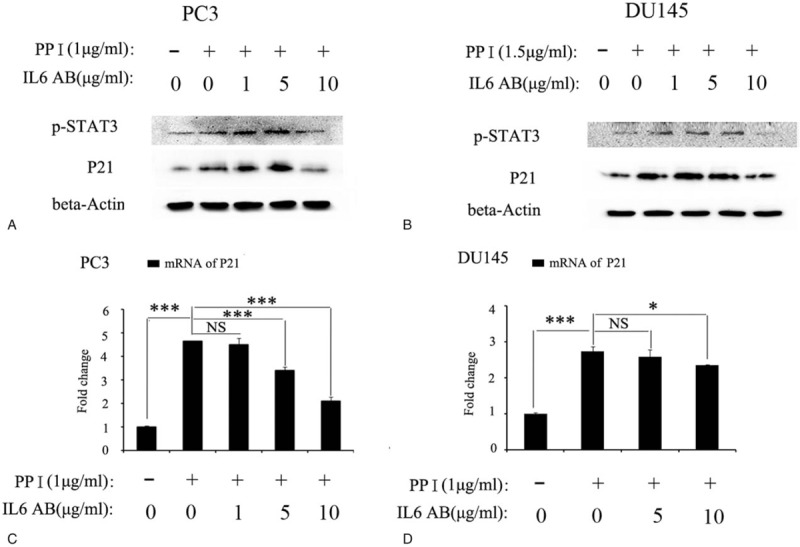
Blocking IL6 can reverse partial of the role Polyphyllin I in PC cells in the expression of P21. Western blot results confirmed that blocking IL6 could reduce the upregulation of P21 in protein level both in PC3 (A) and DU145 cells (B). RT-PCR assay confirmed that blocking IL6 could reduce the upregulation of P21 in mRNA level both in PC3 (C) and DU145 cells (D). RT-PCR = reverse transcription-polymerase chain reaction.
The above experiments suggest that the increased expression of P21 in the PPI-treated cells is at least partially regulated by IL6.
4. Discussion
Our previous work demonstrated that a Paris polyphylla ethanol extract showed a profound inhibitory effect on human prostate cancer cells in vitro and in vivo by inducing apoptosis and cell cycle arrest with few toxic side effects in the host.[13] The main components of the Paris polyphylla ethanol extract are PPI I, II, VI, and VII. A CCK-8 assay was used to detect the effects of PPI I, II, VI, and VII on the viability of prostate cancer cells, and the IC50 of PPI was lower than the IC50s of the other components. The results showed that PPI inhibited cell survival in a concentration-dependent manner in PC3 and DU145 cells, with IC50 values of 1.0 to 1.5 μg/mL. Similar results have been reported in lung cancer,[19] osteosarcoma,[20] and breast cancer.[15]
Cell cycle deregulation is a hallmark of cancer cells.[21] Targeting the aberrant expression of critical proteins involved in cell cycle progression has become a novel strategy for cancer intervention. Fluorescence activated cell sorting analyses showed that PPI dose-dependently induced G0/G1 arrest in both PC3 and DU145 cells. PPI can induce apoptosis in prostate cancer cells, as mentioned in other studies.[15] However, PPI induces apoptosis in prostate cancer cells without good selectivity. Therefore, no studies on apoptosis have been carried out in this paper.
To elucidate the mechanism of PPI-induced prostate cell cycle arrest, we detected key molecules involved in G0/G1 phase regulation, including p21, P27, CDK2, 4, and 6, using Western blotting. The results showed that the P21 levels gradually increased with increasing concentrations and treatment times of PPI. Other studies have revealed that the expression of p-RB, a molecule downstream of p21,[22] decreases gradually in response to increasing P21 expression, suggesting that the PPI-mediated p21 induction is functional. P21 is an important tumor suppressor in the prostate. Promoting the expression of P21 may inhibit tumorigenesis, which is likely an important molecular mechanism of PPI.
Another important finding of this study is that for the first time, PPI was shown to upregulate the expression of IL-6 coupled with the P21 expression increase. Using the literature,[23] 10 factors were identified that might be involved in the regulation of P21 expression by PPI, and these factors were screened. PPI significantly promotes the expression of IL6, thereby positively regulating the expression of P21. Our study demonstrated that an anti-IL-6 blocking antibody could partially reverse the upregulation of P21 expression induced by PPI and even reverse the alteration in cell morphology. The antitumor activity of PPI, a natural product, has been confirmed, but further studies are required to understand the underlying mechanism, poor absorption in vivo and other clinically related issues. Therefore, clarifying the targets of PPI and reformulating PPI to reduce toxicity and increase efficiency will be important in the future.
In conclusion, this study demonstrated that PPI induces cell cycle arrest in the G0/G1 phase in prostate cancer cells in a dose-dependent manner, and this effect was achieved by upregulating the expression of P21 and IL6.
Acknowledgments
We wish to acknowledge the Shandong Innovation Center of National Human Genetic Resources sharing service platform [YCZYPT(2018)01-006].
In addition, we also wish to acknowledge the Engineering Laboratory of Shandong Province for Structure and Functional Reconstruction of Urinary Organs sharing service platform.
Author contributions
Data curation: Denglu Zhang, Shuai Liu.
Formal analysis: Zhiyong Liu, Chenchen Ma.
Investigation: Zhaomin Lin, Peng Wang, Jianye Zhang.
Methodology: Chao Sun, Kailin Li, Guangshang Cao.
Software: Yuehua Jiang.
Supervision: Dawei Xu, Feng Kong, Shengtian Zhao.
Writing – review and editing: Dawei Xu, Shengtian Zhao.
Supplementary Material
Footnotes
Abbreviations: CCK-8 = cell counting kit-8, PPI = Polyphyllin I, qPCR = quantitative polymerase chain reaction.
How to cite this article: Zhang D, Liu S, Liu Z, Ma C, Jiang Y, Sun C, Li K, Cao G, Lin Z, Wang P, Zhang J, Xu D, Kong F, Zhao S. Polyphyllin I induces cell cycle arrest in prostate cancer cells via the upregulation of IL6 and P21 expression. Medicine. 2019;98:44(e17743).
This work was supported by the key clinical projects of the Ministry of Health (Study on the risk factors for prostate cancer and establishment of screening model for high-risk population, Project number: 76) and the Funds for Discipline Construction of High-Level talents in the Affiliated Hospital of Shandong University of Traditional Chinese Medicine.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Gomella LG. Prostate cancer statistics: anything you want them to be. Can J Urol 2017;24:8603–4. [PubMed] [Google Scholar]
- [3].Nakano K, Komatsu K, Kubo T, et al. External validation of risk classification in patients with docetaxel-treated castration-resistant prostate cancer. BMC Urol 2014;18:14–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].O’Callaghan ME, Raymond E, Campbell J, et al. Tools for predicting patient-reported outcomes in prostate cancer patients undergoing radical prostatectomy: a systematic review of prognostic accuracy and validity. Prostate Cancer Prostatic Dis 2017;20:378–88. [DOI] [PubMed] [Google Scholar]
- [5].Kuo YT, Liao HH, Chiang JH, et al. Complementary Chinese herbal medicine therapy improves survival of patients with pancreatic cancer in Taiwan: a nationwide population-based cohort study. Integr Cancer Ther 2018;17:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang L, Ren B, Zhang J, et al. Anti-tumor effect of Scutellaria barbata D. Don extracts on ovarian cancer and its phytochemicals characterisation. J Ethnopharmacol 2017;206:184–92. [DOI] [PubMed] [Google Scholar]
- [7].Huang M, Xin W. Matrine inhibiting pancreatic cells epithelial-mesenchymal transition and invasion through ROS/NF-(B/MMPs pathway. Life Sci 2018;192:55–61. [DOI] [PubMed] [Google Scholar]
- [8].Zhu H, Zhao H, Zhang L, et al. Dandelion root extract suppressed gastric cancer cells proliferation and migration through targeting lncRNA-CCAT1. Biomed Pharmacother 2017;93:1010–7. [DOI] [PubMed] [Google Scholar]
- [9].Qin XJ, Ni W, Chen CX, et al. Seeing the light: shifting from wild rhizomes to extraction of active ingredients from above-ground parts of Paris polyphylla var. yunnanensis. J Ethnopharmacol 2018;224:134–9. [DOI] [PubMed] [Google Scholar]
- [10].Yao N, Ren K, Wang Y, et al. Paris polyphylla suppresses proliferation and vasculogenic mimicry of human osteosarcoma cells and inhibits tumor growth in vivo. Am J Chin Med 2017;45:575–98. [DOI] [PubMed] [Google Scholar]
- [11].Wang CW, Tai CJ, Choong CY, et al. Aqueous extract of Paris polyphylla (AEPP) inhibits ovarian cancer via suppression of peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1alpha. Molecules 2016;21:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li FR, Jiao P, Yao ST, et al. Paris polyphylla Smith extract induces apoptosis and activates cancer suppressor gene connexin26 expression. Asian Pac J Cancer Prev 2012;13:205–9. [DOI] [PubMed] [Google Scholar]
- [13].Zhang D, Li K, Sun C, et al. Anti-cancer effects of Paris polyphylla ethanol extract by inducing cancer cell apoptosis and cycle arrest in prostate cancer cells. Curr Urol 2018;11:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yu S, Wang L, Cao Z, et al. Anticancer effect of Polyphyllin Ι in colorectal cancer cells through ROS-dependent autophagy and G2/M arrest mechanisms. Nat Prod Res 2018;32:1489–92. [DOI] [PubMed] [Google Scholar]
- [15].Li GB, Fu RQ, Shen HM, et al. Polyphyllin I induces mitophagic and apoptotic cell death in human breast cancer cells by increasing mitochondrial PINK1 levels. Oncotarget 2017;8:10359–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xiang S, Zou P, Tang Q, et al. HOTAIR-mediated reciprocal regulation of EZH2 and DNMT1 contribute to polyphyllin I-inhibited growth of castration-resistant prostate cancer cells in vitro and in vivo. Biochim Biophys Acta Gen Subj 2017;1862:589–99. [DOI] [PubMed] [Google Scholar]
- [17].Liu X, Sun Z, Deng J, et al. Polyphyllin I inhibits invasion and epithelial-mesenchymal transition via CIP2A/PP2A/ERK signaling in prostate cancer. Int J Oncol 2018;53:1279–88. [DOI] [PubMed] [Google Scholar]
- [18].Zhang D, Cui Y, Niu L, et al. Regulation of SOD2 and (-arrestin1 by interleukin-6 contributes to the increase of IGF-1R expression in docetaxel resistant prostate cancer cells. Eur J Cell Biol 2014;93:289–98. [DOI] [PubMed] [Google Scholar]
- [19].Li L, Wu J, Zheng F, et al. Inhibition of EZH2 via activation of SAPK/JNK and reduction of p65 and DNMT1 as a novel mechanism in inhibition of human lung cancer cells by polyphyllin I. J Exp Clin Cancer Res 2016;35:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chang J, Li Y, Wang X, et al. Polyphyllin I suppresses human osteosarcoma growth by inactivation of Wnt/β-catenin pathway in vitro and in vivo. Sci Rep 2017;7:7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Malumbres M, Carnero A. Cell cycle deregulation: a common motif in cancer. Prog Cell Cycle Res 2003;5:5–18. [PubMed] [Google Scholar]
- [22].Moser J, Miller, Carter D, et al. Control of the restriction point by Rb and p21. Proc Natl Acad Sci U S A 2018;115:E8219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 2009;9:400–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


