Supplemental Digital Content is available in the text
Keywords: abiraterone (AA), adverse event (AE), enzalutamide (Enza), high-grade adverse event, overall survival (OS), progression-free survival (PFS), prostate-specific antigen (PSA) response
Abstract
Background:
Previous evidence directly evaluating the efficacy and safety of abiraterone and enzalutamide treatment for castration-resistant prostate cancer (CRPC) is limited. We aim to include more randomized controlled trials (RCTs) to comprehensively assess the efficacy and safety of abiraterone and enzalutamide treatment.
Methods:
PubMed, Embase, and ClinicalTrial.gov were systematically searched. Pooled hazard ratios (HRs) were calculated using Stata 12.0 software. The comparison of the prostate-specific antigen (PSA) response rate and adverse events (AEs) between the treatment and control groups were performed using RevMan 5.3 software.
Results:
Eight eligible RCTs with 6,490 patients were selected. Pooled HRs were 0.72 for overall survival, 0.45 for radiographic progression-free survival (rPFS), and 0.36 for PSA PFS. abiraterone and enzalutamide could significantly increase the PSA response rate OR = 8.67, 95%CI 4.42–17.04) and any AE occurrence (OR = 1.98, 95%CI 1.46–2.68). The treatment group had more occurrence of fatigue (OR = 1.34, 95%CI 1.20–1.49), back pain (OR = 1.15, 95%CI 1.01–1.15), hot flush (OR = 1.76, 95%CI 1.50–2.06), diarrhea (OR=1.22, 95%CI 1.07–2.40) and arthralgia (OR = 1.34, 95%CI 1.16–1.54). Particularly, AEs of special interest including any grade hypertension (OR = 2.06, 95%CI 1.71–2.47), hypokalemia (OR = 1.80, 95%CI 1.42–2.30) and fluid retention or edema (OR = 1.38, 95%CI 1.17–1.63) also occurred less in the control group. Moreover, a higher incidence of high-grade hypertension (OR = 2.60, 95%CI 1.79–3.79) and extremity pain (OR = 4.46, 95%CI 2.81–7.07) was observed in the treatment group.
Conclusion:
The survival benefits of abiraterone and enzalutamide for CRPC were evident and promising, while the risk of AE occurrence was also acceptably higher in the treatment group than in the placebo group.
1. Introduction
Prostate cancer is one of the most common cancers in men worldwide.[1,2] Regardless of the indolent course of many tumors and excellent prognosis of localized prostate cancer, advanced prostate cancer is fatal. Androgen deprivation therapy is the standard treatment for advanced prostate cancer.[3] Most patients initially respond to castration, but the disease will eventually progress[4]; a tumor escape mechanism is believed to be employed by castration-resistant prostate cancer (CRPC) to overcome androgen deprivation therapy and cause subsequent mortality.[5]
Abiraterone and enzalutamide are two new androgen receptor (AR) inhibitors for the treatment of CRPC. As a selective inhibitor of androgen biosynthesis, abiraterone acetate can irreversibly and potently block CYP17, a crucial enzyme in testosterone and estrogen synthesis, resulting in virtually undetectable serum and intratumoral androgens,[6,7] while enzalutamide can reduce the efficiency of its nuclear translocation and impair both DNA binding to androgen response elements and recruitment of coactivators.[7,8] With different mechanisms, both AR inhibitors are new options in CRPC treatment. The COU-AA-301,[9] COU-AA-302,[10,11] AFFIRM,[12] and PREVAIL[13] trials have promoted the use of abiraterone and enzalutamide, and several subsequent trials also evaluated their efficacy. However, few meta-analyses have included all related randomized clinical trials (RCTs) and directly pooled the hazard ratios (HRs) of overall survival (OS) and progression-free survival (PFS) of these two agents. Additionally, although some previous studies have attempted to investigate the efficacy and safety of abiraterone and enzalutamide, pooled evidence was limited, and their analyses were either indirect or subjected to bias caused by non-RCTs.[14–17] Moreover, the comparison of the occurrence of high-grade adverse events (AEs) (grade ≥ 3) between the AR inhibitor and control groups has been insufficient.
In this meta-analysis, we aim to confirm the efficacy and safety of AR axis-signaling targeting agents by directly calculating the pooled HRs of OS and PFS and pooled odds ratio (OR) of prostate-specific antigen (PSA) response rate in the abiraterone and enzalutamide treatment group compared with those in the control group. Meanwhile, the occurrence of AEs (overall and high-grade) was compared between the AR inhibitor and control groups. Furthermore, a subgroup analysis was conducted to compare abiraterone and enzalutamide treatments.
2. Patients and methods
2.1. Search strategy and inclusion criteria
This systematic review and meta-analysis was conducted according to the PRISMA guidelines.[18] Two investigators independently searched PubMed, Embase, and ClinicalTrials.gov for phase 2 and 3 RCTs published until August 1, 2018. The search strategy included the following terms: “castration-resistant prostate cancer,” “CRPC,” “androgen receptor,” “CYP17A1,” “cytochrome P450 17A1,” “abiraterone,” “enzalutamide,” “efficacy,” “survival,” “safety,” and “adverse.” References cited by the finally selected articles were also reviewed.
RCTs assessing the efficacy and safety of AR inhibitors, CYP17A1 inhibitor in patients with CRPC, and providing data on HRs with 95% confidence intervals (95% CIs) were potentially eligible. The detailed inclusion criteria were as follows:
1) RCT;
2) evaluation of abiraterone and/or enzalutamide efficacy against a control;
3) use of abiraterone or enzalutamide in the experimental group rather than in the control group;
4) report of efficacy indicators, including OS and/or PFS, or AEs;
5) available HR and 95% CI on efficacy; and
6) publication in the English language.
We excluded articles
1) that were non-RCTs and not published in the English language;
2) that did not either assess the efficacy or report the AEs of abiraterone and enzalutamide;
3) in which other agents that might deviate the conclusion were used in the experimental group;
4) in which abiraterone and enzalutamide were used together in the experimental group; and
5) in which abiraterone and enzalutamide were regarded as the control group. Duplicate articles from the same RCT were screened and excluded.
2.2. Data analysis
Two investigators independently extracted data from the included articles, and all members of our team resolved the discrepancies by consensus. Data include the clinical trial code (National Clinical Trial), study name, study phase, study size, drugs used in the experimental and control groups, median age, percentage of patients with PSA decline ≥ 50%, definition of PFS, and median OS and PFS.
The primary outcome was the efficacy of abiraterone and enzalutamide on the enrolled patients, for which pooled HRs of PFS and OS were calculated using the random-effects model. Moreover, PSA response rate (defined as at least 50% PSA decline from baseline) was compared between the treatment and control groups. A subgroup analysis of PFS according to the type of AR inhibitors was also conducted. Heterogeneity between different AR inhibitors was assessed. Pheterogeneity < .05 was regarded as statistically significant.
The second outcome was the comparison of aggregated AE statistics (any AE, high-grade AE [grade ≥ 3], AE leading to death, AE leading to discontinuation, and any severe AE) and the occurrence of any grade AE, AE of special interest, and high-grade AE (grade ≥ 3) between the treatment and control groups. To maximally eliminate the potential bias caused by various reporting standards of AE occurrence in these selected trials, we extracted data on AEs that occurred in at least 10% of either the treatment or control group. Furthermore, to make our data more statistically powerful and the conclusion more convincing, AEs reported in at least 3 trials were included for comparison. In the forest plot for comparison, when the points of the diamond did not overlap the vertical line, a significant difference between the treatment and control groups was indicated.
Q test and I2 were employed to evaluate the heterogeneity between studies. When a high heterogeneity (I2 > 50%) was noted between studies, a sensitivity analysis was conducted to determine the source of heterogeneity using the following steps: remove each trial in the analysis and recalculate the heterogeneity. When the heterogeneity remained high after trials were individually removed, we analyzed the trials themselves and determined the reasonable sources of high heterogeneity.
All mentioned analyses were conducted using Stata software (version 12.0), and images were processed with Photoshop (version CS6).
2.3. Quality assessment
The methodological quality of the included trials was assessed using the Jadad ranking system.[19] An RCT could be given a Jadad score of between 0 (poor) and 5 (optimal) according to the quality of randomization, double-blinding, and follow-up.
3. Results
3.1. Study characteristics
From 963 retrieved publications, we selected 84 potentially eligible articles for abstract and full article review. Eventually, 8 RCTs with 10 articles, which were published from 2012 to 2017, met the inclusion criteria[9–13,20–24] (Fig. 1 and Table 1). Two trials were phase 2 while 6 trials were phase 3. Five were first-line trials, and three were second-line trials (Table 1). For each trial, the number of patients with CRPC ranged from 214 to 1199, and a total of 6490 patients were included. A total of 1639 patients (25.3%) were enrolled in the abiraterone plus prednisone group and 2053 (31.6%) in the enzalutamide group, while 1165 patients (17.9%) were enrolled in the prednisone plus placebo group, 398 patients (6.1%) were enrolled in the bicalutamide group, and 1244 patients (19.1%) were administered only the placebo. The median age of the enrolled patients varied from 68 to 74 years, with two trials reporting mean ages.
Figure 1.
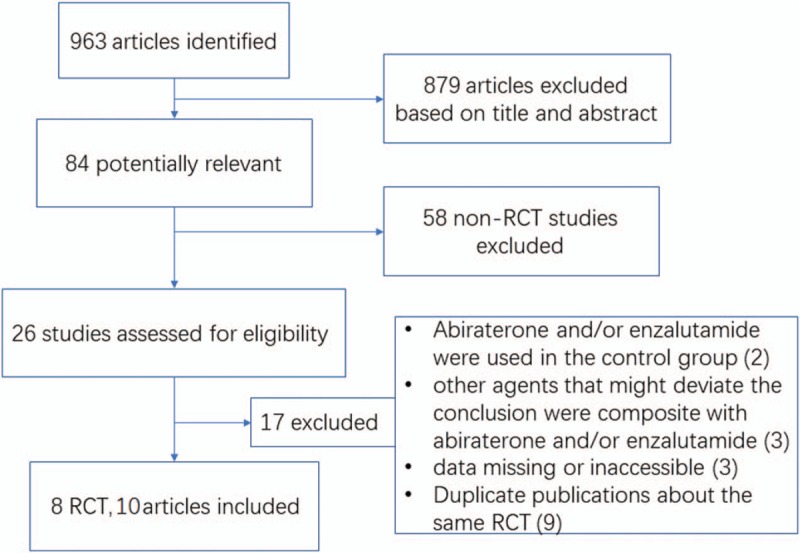
PRISMA flowchart of study selection with excluding reasons.
Table 1.
Characteristics of the included clinical trials.
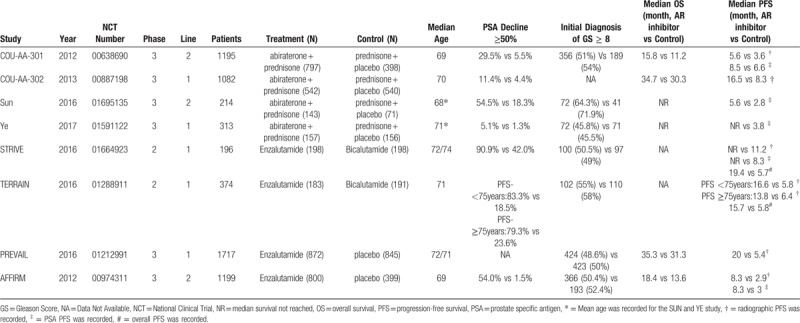
The superiority of the experimental group over the control group was shown for PSA response rate (PSA decline ≥ 50% from baseline) in seven studies. The PSA decline ≥ 50% in the experimental group ranged from 5.1% to 90.9% compared with 1.3% to 42.0% in the control group. The median OS was reported in four studies, and the prolonged duration in the experimental group varied from 4.0 to 4.6 months compared with the control group. Overall PFS, radiographic PFS (rPFS), and PSA PFS were asymmetrically reported in these trials. However, the median PFS was generally longer in the AR inhibitor group with respect to either overall PFS, rPFS, or PSA PFS.
3.2. OS, PFS, and PSA response rate
Figure 2 shows the efficacy of abiraterone and enzalutamide in all enrolled participants. HR with 95% CI for OS was assessed in 5 trials, while HR with 95% CI for rPFS and PSA PFS were both assessed in six trials. Patients treated with abiraterone and enzalutamide had greater survival benefits with pooled HR of OS (HR = 0.72; 95% CI, 0.67–0.78), rPFS (HR = 0.45; 95% CI, 0.42–0.48), and PSA PFS (HR = 0.36; 95% CI, 0.32–0.40). The PSA response rates are presented in Figure 3. Patients treated with AR inhibitors had a significantly higher PSA response rate (OR = 10.15; 95% CI, 8.07–12.28; P < .001) than the control group.
Figure 2.
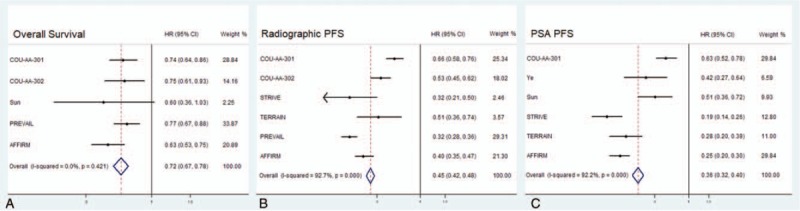
Pooled hazard ratios for overall survival, radiographic progression-free survival, prostate-specific antigen progression-free survival by androgen receptor signaling-axis Inhibitors.
Figure 3.
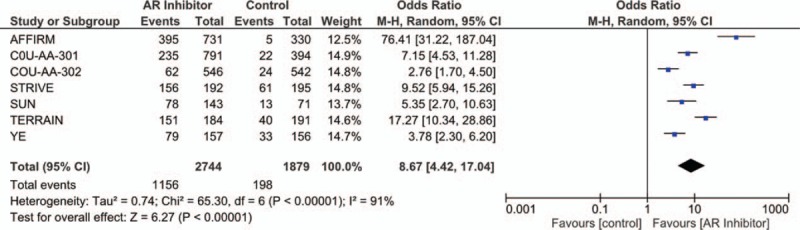
Forest plot for prostate-specific antigen response rate.
Additionally, we also conducted a subgroup analysis according to the different types of AR inhibitors (Supplementary Figures S1, S2, and S3). It seemed that enzalutamide had greater efficacy than abiraterone in either rPFS (HR, 0.36 vs 0.60; Pheterogeneity < .05), PSA PFS (HR, 0.24 vs 0.57; Pheterogeneity < .05), or PSA response rate (OR, 21.88 vs 4.69; Pheterogeneity < .05), but not in OS (HR, 0.71 vs 0.78; Pheterogeneity = .319).
3.3. Aggregated AE statistics
As shown Figure 4, the AR inhibitor group had a higher risk of occurrence of overall AE (OR = 1.98; 95% CI, 1.46–2.68; P < .001). However, no significant difference was found between the treatment and control groups regarding the occurrence of high-grade AE, AE leading to death, AE leading to discontinuation, and any severe AE, even though the AR inhibitor group had a virtually significantly lower risk of occurrence of high-grade AE (OR = 0.91; 95% CI, 0.82–1.01; P = .08).
Figure 4.
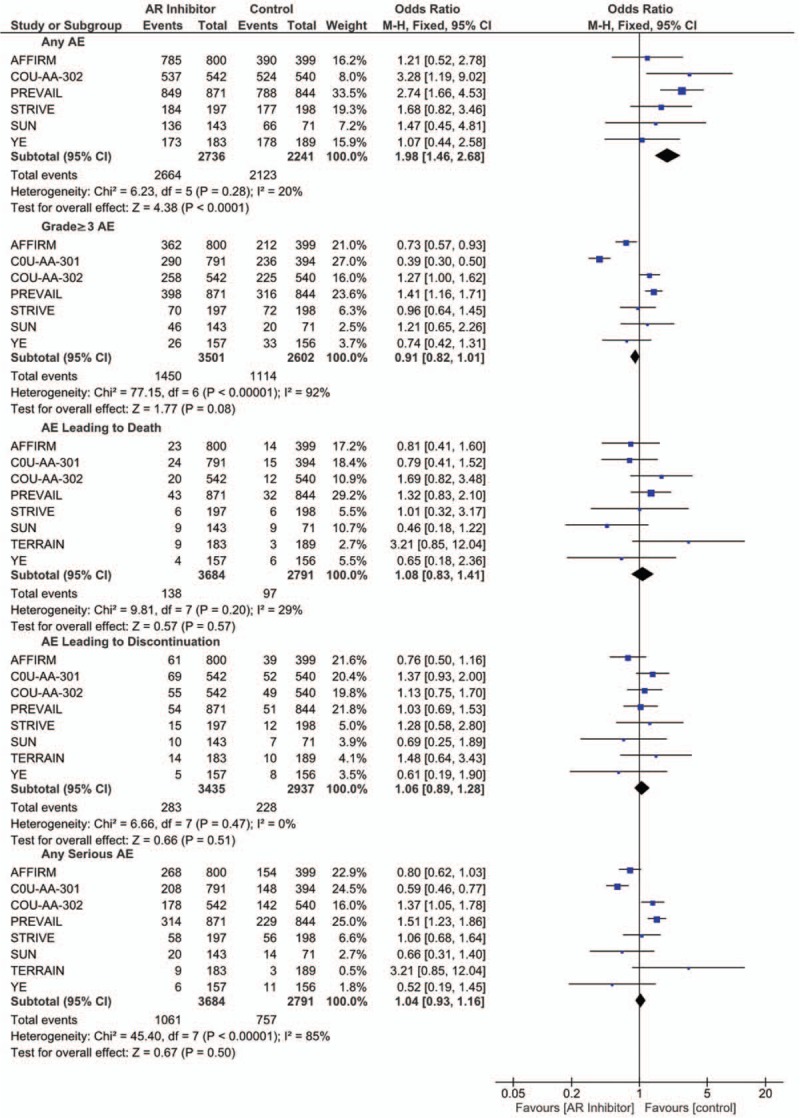
Forest plot for the occurrence of overall AE, high-grade AE, severe AE, and AE leading to death or discontinuation.
3.4. AEs
Figure 5A presents the overall AEs with significant difference between the AR inhibitor and control groups. As shown in the forest plots, patients in the treatment group had higher rate of fatigue (OR = 1.34; 95% CI, 1.20–1.49; P < .001), back pain (OR = 1.15; 95% CI, 1.01–1.15; P = .03), hot flush (OR = 1.76; 95% CI, 1.50–2.06; P < .001), diarrhea (OR = 1.22; 95% CI, 1.07–2.40; P = .003), and arthralgia (OR = 1.34; 95% CI, 1.16–1.54; P < .001). No significant difference was observed in the frequency of constipation (OR = 1.13; 95% CI, 0.98–1.29; P = .09) bone pain, extremity pain, anemia, tract infection, and nausea (Supplementary Fig. S4).
Figure 5.
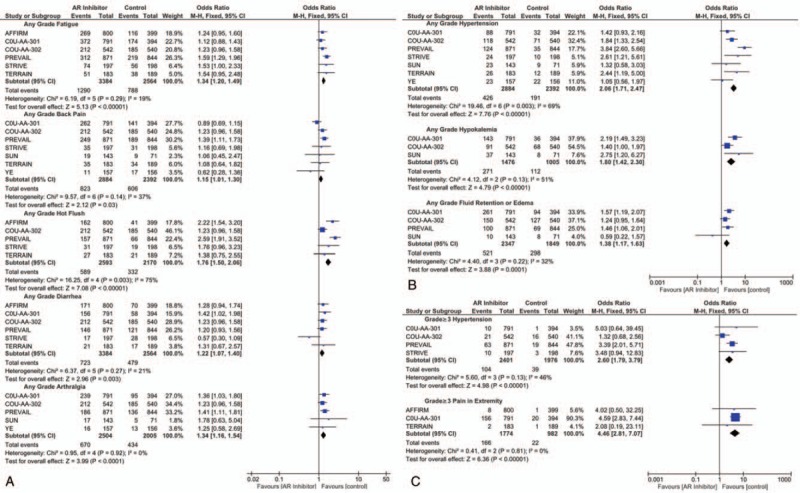
Forest plot for overall AE, high-grade AE, and AE of special interest with significant difference between the treatment and control groups.
Outcomes for AEs of special interest (Fig. 5B), including hypertension (OR = 2.06; 95% CI, 1.71–2.47; P < .001), hypokalemia (OR = 1.80; 95% CI, 1.42–2.30; P < .001), and fluid retention or edema (OR = 1.38; 95% CI, 1.17–1.63; P < .001) all significantly favored the control group.
3.5. High-grade AEs
Regarding high-grade AEs, a higher risk of hypertension (OR = 2.60; 95% CI 1.79–3.79; P < .001) and extremity pain (OR = 4.46; 95% CI 2.81–7.07; P < .001) was observed in the AR inhibitor group (Fig. 5C). There were no significant differences in high-grade fatigue, back pain, constipation, diarrhea, hot flush, fluid retention or edema, urinary tract infection, falls, or decreased appetite between the treatment and control groups (Supplementary Fig. S5).
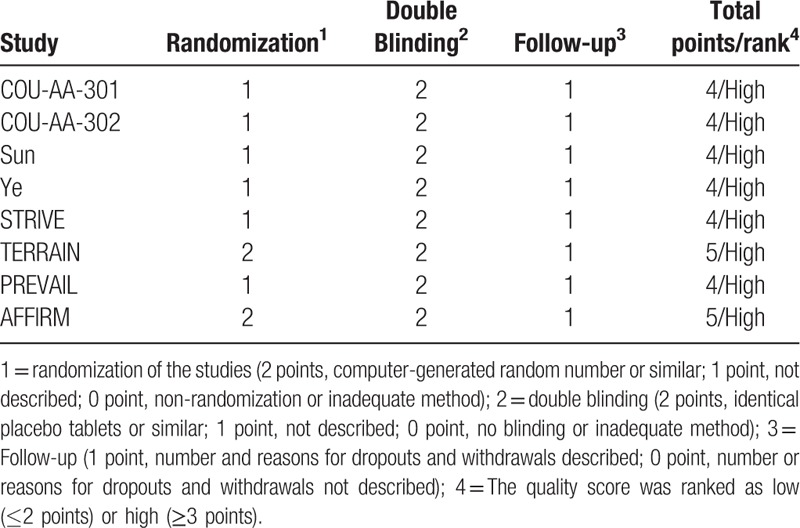
3.6. Quality assessment
All trials employed randomized treatment allocation sequences, and three trials used computer-generated random numbers or a similar fashion. All trials were double blinded. Jadad scores were provided to each trial and listed in Table 2. The mean score was 4.25, and all trials were ranked as high quality.
Table 2.
Jadad quality assessment for randomized clinical trials.
4. Discussion
In this study, the collected data showed that abiraterone and enzalutamide could provide a maximal median OS of 35.3 months, median PFS of 20 months, and PSA response rate of 90.9%. By calculating the pooled HR, we confirmed that patients diagnosed with CRPC had evidently greater survival benefits after AR inhibitor treatment compared with controls (OS, HR = 0.72; rPFS, HR = 0.45; PSA PFS, HR = 0.36). To our knowledge, this is the first meta-analysis that directly calculated the pooled HRs of OS and PFS of AR inhibitors with the original HR data provided in the trials. A previous study by Fang et al[16] also attempted to pool the HR based on median OS and PFS and a method by Cortes.[25] However, as the authors explained, the estimated HR could bring considerable uncertainty and was weakly convincing.
The PSA response rate was another commonly used marker as an efficacy measure for CRPC response, although the clinical significance of the PSA response rate was not completely clear.[26] Smith reported that a higher PSA response rate was associated with longer survival,[27] even though the meta-analysis by Zheng[14] found inconsistent results. Due to insufficient data in our study, we could not evaluate the correlation between PSA response rate and survival. However, given that our study found that the AR inhibitor group had a significantly higher PSA response rate, we concluded that the efficacy of AR inhibitors for CRPC is quite promising.
Factors associated with response therapy were previously investigated.[28] One of the reported predictive factors of AR inhibitor therapy for CRPC was HSD3B1 gene, the variant type of which was claimed by Shiota[29] to be correlated with lower progression risk and lower all-cause mortality in patients with CRPC receiving abiraterone treatment. Ryan[10] and Penson[22] reported that a higher Gleason score (GS) at initial diagnosis and baseline serum PSA level could also indicate higher risk of disease progression after therapy. Other serum parameters, including lactate dehydrogenase and alkaline phosphatase, were also associated with therapy response, but the outcomes were inconsistent.[9,12,22] Higher scores in scale systems, including Eastern Cooperative Oncology Group (ECOG) performance status and Brief Pain Inventory Short-Form, could also predict higher risk of all-cause mortality. However, it is noted that only the HR for each subgroup with the abovementioned parameters was presented, but the P value was not provided.[9,10,12,22]
The reliability of the efficacy of abiraterone was confirmed by Zhou,[15] and our subgroup outcomes suggested similar conclusions. Moreover, several previous studies had insights into the comparison between abiraterone and enzalutamide. To confirm their conclusions, we also performed a subgroup analysis and evaluated the heterogeneity between abiraterone and enzalutamide. With a limited number of included RCTs, Zhang[17] indirectly compared the OS, PSA PFS, rPFS, and PSA response rate of abiraterone with those of enzalutamide. Consistent to our findings, the study showed that enzalutamide outperformed abiraterone with respect to PSA PFS, rPFS, and PSA response rate. However, there was no significant difference with regard to OS. Similarly, Zheng[14] also found that enzalutamide had greater benefits in PFS but not in OS, although it is an indirect comparison and only two trials were included.
Moreover, we comprehensively explored the safety of abiraterone and enzalutamide by showing that AR inhibitors could lead to higher rates of overall AE occurrence, virtually significantly lower rates of high-grade AE, and similar rates of AE leading to death or discontinuation. Zheng's study[14] also evaluated the safety of abiraterone and enzalutamide, although less AEs occurred. Furthermore, given that only the COU-AA-302 and PREVAIL trials were included in the analysis, the statistical power was relatively low. Our meta-analysis suggested that patients treated with AR inhibitors had a more frequent occurrence of fatigue, back pain, hot flush, diarrhea, arthralgia, hypertension, hypokalemia, fluid retention, or edema. Regarding high-grade AE, hypertension and extremity pain were associated with AR inhibitors. However, the safety of abiraterone and enzalutamide seemed acceptable and controlled, since those AEs could be generally managed by appropriate medical monitoring[15] and our meta-analysis also suggested that they would not lead to more frequent death. Still, those AEs were less fatal compared with AEs caused by cytotoxic therapy.[30] Measures, including a higher dosage of antihypertensive drugs, oral potassium supplementation, and analgesic use, are required to manage these AEs while on AR inhibitor treatment.
Notably, inter-study heterogeneity was generally low, except only in the analyses of PFS, hot flush, hypertension, and hypokalemia, which perhaps could be explained by the different lines of treatment and heterogeneity between abiraterone and enzalutamide.[31,32] Considering that the limitation of our study is relying on published results rather than on the original individual patients’ data, some important baseline characteristics of the patients, that is, age, bone lesion, visceral disease, and ECOG performance status score, along with GS, might also play a crucial role in this substantial heterogeneity. It is likely that other unknown patient characteristics would also cause substantial heterogeneity.
One advantage of this study is the employment of pooled HR to assess the efficacy of AR inhibitors. Compared with the median values of OS and PFS, HR considers both time and cohort size. Furthermore, all data in the analysis were collected from a large population in high quality RCTs. Therefore, our outcomes are more statistically powerful and convincing. Concerning the multiple definitions of PFS, to eliminate the potential bias and make the outcomes more accurate, we categorized PFS into radiographic and PSA-related, which actually had clinical significance. Moreover, as a host of meta-analyses have been conducted to compare abiraterone and enzalutamide, we did not only assess AR inhibitors as a whole but also performed a subgroup analysis to analyze them separately. Moreover, unlike previous meta-analyses, we did not only evaluate the safety of abiraterone and enzalutamide by overall AEs but also focused on high-grade AEs, which is in fact critical and practical since high-grade AEs are more likely to cause mortality, even if we found AR inhibitors to be only associated with more frequent occurrence of high-grade hypertension and extremity pain.
However, limitations of our study also exist. First, the number of included RCTs still seems insufficient, especially in the subgroup analysis comparing abiraterone and enzalutamide, although we have systematically searched available databases and cross-referenced the identified articles. Second, the variations in the included studies, for instance, the baseline PSA level and GS, different inclusion and exclusion criteria, and follow-up therapy, could affect the individual survival outcomes and subsequent pooled outcomes. Third, a substantial heterogeneity was found in some analyses. In our meta-analysis, six trials had first-line setting, and only two had second-line setting. However, Zhang[17] investigated the optimal treatment sequencing using the same second-line setting trials included in our studies, so we did not perform the same analysis. Last, data loss was common when we performed analysis for AE occurrence due to different reporting standards in trials. To minimize the potential bias, we only extracted data strictly meeting our inclusion criteria, causing many AEs to be excluded from the analysis.
Accordingly, in future clinical practice, AR inhibitors should be considered as an efficacious and safe treatment option for patients with CRPC, even though practitioners should pay special attention to the AEs mentioned in our study, particularly high-grade AEs. Moreover, it would be meaningful for investigators conducting the original studies to use a uniformed AE reporting standard for further and deeper data analysis.
5. Conclusion
The survival benefits of abiraterone and enzalutamide for CRPC were evident and promising, while the risk of AE occurrence was also acceptably higher in the treatment group than in the placebo group.
Author contributions
Conceptualization: Lu Yang, Qiang Wei, Jianzhong Ai.
Data curation: Xiaonan Zheng, Xiaohui Zhao, Hang Xu, Ruilin Peng.
Formal analysis: Xiaohui Zhao, Hang Xu.
Funding acquisition: Lu Yang, Qiang Wei, Jianzhong Ai.
Investigation: Xin Han, He Xu, Xin Dong.
Methodology: Xiaonan Zheng.
Project administration: Lu Yang, Qiang Wei, Jianzhong Ai.
Resources: Xin Han, He Xu, Xin Dong.
Software: Xiaonan Zheng, Xin Han, He Xu, Xin Dong.
Supervision: Xiaonan Zheng, Xiaohui Zhao, Hang Xu.
Visualization: Xiaonan Zheng.
Writing – original draft: Xiaohui Zhao, Hang Xu.
Writing – review & editing: Xiaohui Zhao, Hang Xu.
Supplementary Material
Footnotes
Abbreviations: AA = abiraterone, AE = adverse event, AR = androgen receptor, CRPC = castration-resistant prostate cancer, ECOG = Eastern Cooperative Oncology Group, Enza = enzalutamide, GS = Gleason score, HR = hazard ratios, OS = overall survival, PFS = progression-free survival, PSA = prostate-specific antigen, RCT = randomized controlled trials, rPFS = radiographic progression-free survival.
How to cite this article: Zheng X, Zhao X, Xu H, Han X, Xu H, Dong X, Peng R, Yang L, Wei Q, Ai J. Efficacy and Safety of Abiraterone and Enzalutamide for castration-resistant prostate cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine. 2019;98:44(e17748).
XZ, XZ, and HX contributed equally to this work.
This research was supported by the National key research and development program of China (Grant No. SQ2017YFSF090096), the National Natural Science Foundation of China (Grant No. 81370855, 81702536, 81770756), Programs from Science and Technology Department of Sichuan Province (Grant No. 2017HH0063, 2018HH0153).
The authors have no conflicts of interest to disclose.
Take home message :Our study suggested the survival benefits by abiraterone and enzalutamide for CRPC were evident and promising at the cost of acceptably higher risk of AEs occurrence.
References
- [1].Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- [3].Lam JS, Leppert JT, Vemulapalli SN, et al. Secondary hormonal therapy for advanced prostate cancer. J Urol 2006;175:27–34. doi: 10.1016/s0022-5347(05)00034-0. [DOI] [PubMed] [Google Scholar]
- [4].Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 1998;339:1036–42. doi: 10.1056/nejm199810083391504. [DOI] [PubMed] [Google Scholar]
- [5].Higano CS, Crawford ED. New and emerging agents for the treatment of castration-resistant prostate cancer. Urol Oncol 2011;296 Suppl:S1–8. doi: 10.1016/j.urolonc.2011.08.013. [DOI] [PubMed] [Google Scholar]
- [6].O’Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer 2004;90:2317–25. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science (New York, NY) 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Guerrero J, Alfaro IE, Gomez F, et al. Enzalutamide, an androgen receptor signaling inhibitor, induces tumor regression in a mouse model of castration-resistant prostate cancer. Prostate 2013;73:1291–305. doi: 10.1002/pros.22674. [DOI] [PubMed] [Google Scholar]
- [9].Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012;13:983–92. doi: 10.1016/s1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- [10].Ryan CJ, Smith MR S de Bono##JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16:152–60. doi: 10.1016/s1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- [12].Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- [13].Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer: extended analysis of the Phase 3 PREVAIL Study. European urology 2017;71:151–4. doi: 10.1016/j.eururo.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zheng H, Chen J, Qiu W, et al. Safety and efficacy of first-line treatments for chemotherapy-naive metastatic castration-resistant prostate cancer: a systematic review and indirect comparison 2017. Biomed Res Int 2017;3941217.doi: 10.1155/2017/3941217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhou ZR, Liu SX, Zhang TS, et al. Abiraterone for treatment of metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. APJCP 2014;15:1313–20. [DOI] [PubMed] [Google Scholar]
- [16].Fang M, Nakazawa M, Antonarakis ES. Efficacy of abiraterone and enzalutamide in pre- and postdocetaxel castration-resistant prostate cancer: a trial-level meta-analysis 2017. Prostate Cancer 2017;8560827.doi: 10.1155/2017/8560827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang W, Wu TY, Chen Q, et al. Indirect comparison between abiraterone acetate and enzalutamide for the treatment of metastatic castration-resistant prostate cancer: a systematic review. Asian J Androl 2017;19:196–202. doi: 10.4103/1008-682x.178483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA Statement. JAMA 2018;319:388–96. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- [19].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Contrd Clin Trial 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [20].Sun Y, Zou Q, Sun Z, et al. Abiraterone acetate for metastatic castration-resistant prostate cancer after docetaxel failure: A randomized, double-blind, placebo-controlled phase 3 bridging study. Int J Urol 2016;23:404–11. doi: 10.1111/iju.13051. [DOI] [PubMed] [Google Scholar]
- [21].Ye D, Huang Y, Zhou F, et al. A phase 3, double-blind, randomized placebo-controlled efficacy and safety study of abiraterone acetate in chemotherapy-naive patients with mCRPC in China, Malaysia, Thailand and Russia. Asian J Urol 2017;4:75–85. doi: 10.1016/j.ajur.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the strive trial. J Clin Oncol 2016;34:2098–106. doi: 10.1200/jco.2015.64.9285. [DOI] [PubMed] [Google Scholar]
- [23].Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol 2016;17:153–63. doi: 10.1016/s1470-2045(15)00518-5. [DOI] [PubMed] [Google Scholar]
- [24].Siemens DR, Klotz L, Heidenreich A, et al. Efficacy and safety of enzalutamide vs bicalutamide in younger and older patients with metastatic castration resistant prostate cancer in the TERRAIN trial. J Urol 2018;199:147–54. [DOI] [PubMed] [Google Scholar]
- [25].Cortes J, Gonzalez JA, Campbell MJ, et al. A hazard ratio was estimated by a ratio of median survival times, but with considerable uncertainty. J Clin Epidemiol 2014;67:1172–7. doi: 10.1016/j.jclinepi.2014.05.017. [DOI] [PubMed] [Google Scholar]
- [26].Song G, Lee C, You D, et al. Prostate-specific antigen response rate of sequential chemotherapy in castration-resistant prostate cancer: the results of real life practice. Prostate Int 2013;1:125–32. doi: 10.12954/pi.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Smith DC, Dunn RL, Strawderman MS, et al. Change in serum prostate-specific antigen as a marker of response to cytotoxic therapy for hormone-refractory prostate cancer. J Clin Oncol 1998;16:1835–43. doi: 10.1200/jco.1998.16.5.1835. [DOI] [PubMed] [Google Scholar]
- [28].Cormio L, Lucarelli G, Selvaggio O, et al. Absence of bladder outlet obstruction is an independent risk factor for prostate cancer in men undergoing prostate biopsy. Medicine 2016;95:e2551.doi: 10.1097/md.0000000000002551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shiota M, Narita S, Akamatsu S, et al. Association of missense polymorphism in HSD3B1 with outcomes among men with prostate cancer treated with androgen-deprivation therapy or abiraterone. JAMA Network Open 2019;2:e190115.doi: 10.1001/jamanetworkopen.2019.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Loriot Y, Bianchini D, Ileana E, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann Oncol 2013;24:1807–12. doi: 10.1093/annonc/mdt136. [DOI] [PubMed] [Google Scholar]
- [32].Azad AA, Eigl BJ, Murray RN, et al. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur Urol 2015;67:23–9. doi: 10.1016/j.eururo.2014.06.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


