Abstract
Background:
Erenumab is a new medicine recently approved in the United States of America for the preventive treatment of migraine among adults. We aimed to conduct a meta-analysis and evaluation of the efficacy and safety of erenumab among patients with migraine.
Methods:
The electronic databases that were searched comprised PubMed, Embase and the Cochrane library, which were independently retrieved by 2 reviewers. Only randomized controlled trials (RCTs) that compared placebo with erenumab were selected. Mean differences (MDs), pooled risk ratios (RRs), and their corresponding 95% confidence intervals (CIs) were calculated for continuous and dichotomous data, respectively.
Results:
Five RCTs representing 2928 patients were included. Pooled analysis showed significant reductions in the 50% responder rate (RR 1.55; P < .00001; I2 = 49%). In addition, the mean monthly migraine days from baseline in the erenumab group compared with placebo (MD-1.32; P < .00001; I2 = 100%) and migraine-specific medication days) from baseline (MD-1.41; P < .00001; I2 = 100%) were significantly decreased for the erenumab group as compared with placebo. Furthermore, Migraine-specific medication days from baseline in the 140 mg erenumab group were significantly reduced as compared the 70 mg group (MD = 0.55; P < .00001; I2 = 90%). Finally, there was no significant difference between the erenumab group and placebo for any adverse event and serious adverse event.
Conclusion:
Among patients with migraine, both 70 and 140 mg of erenumab were associated with reduced Migraine-specific medication days, Migraine-specific medication days from baseline, and an increased rate of a 50% reduction, in the absence of an increased risk of any serious adverse effect.
Keywords: chronic migraine, episodic migraine, erenumab, meta-analysis, RCTs
1. Introduction
Approximately 30% of adults in the age group 18 to 65 suffer from headache disorders and about 30% of these individuals have migraine.[1,2] Depending on the frequency of attacks, migraine can be broadly classified as either episodic migraine (EM), which is defined as suffering less than 15 days with headache per month during at least 3 months, or chronic migraine (CM), which is defined as suffering more than 15 days with headache per month during at least 3 months.[3]
The third edition of the International Classification of Headache Disorders volume 3 (ICHD3), indicates that CM, which is distinguished from other types of EM,[3] shares many features with, and can transform from EM.[4] Symptoms of migraine, including pain, sensitivity to light, sound, odors, and vision changes, in addition to nausea, vomiting, tingling/numbness, and language disturbances, pose significant disabling effects on the victim's physical, social, and occupational functioning.[5]
Preventive treatments that include topiramate, propranolol and valproate, β-blockers, and amitriptyline were not entirely effective and are commonly associated with side-effects that lead to negative-affect treatment outcomes and poor adherence to therapy. Thus, new and effective, and safe and tolerable preventive therapies are needed in patients that have failed existing treatment.
Calcitonin gene-related peptide (CGRP) is a 37-amino acid neuropeptide that is involved in the pathophysiology of migraine through nociceptive modulation in the trigeminal vascular system.[6,7,8] Several years ago, it was suggested that CGRP might be a valid target in the treatment of migraine.[9] There are four monoclonal antibodies developed to target CGRP as a preventive treatment of migraine, have already shown their potential in treating migraine. Erenumab, the only fully humanized monoclonal antibody targeted CGRP, the most potent vasodilator peptide known,[10] deserves special consideration.
Several randomized controlled trials (RCTs) have been conducted to investigate the efficacy of erenumab in EM and CM. Thus, we performed a meta-analysis of all RCTs and compared the dose-dependent efficacy and safety of erenumab as compared the placebo at different erenumab doses in patients with migraine. The efficacy and safety between 70 mg and 140 mg of erenumab had also be evaluated
2. Materials and methods
2.1. Ethics approval and consent to participate
All analyses were based on previously published studies, thus no ethical approval and patient consent are required.
2.2. Literature search and data source
This meta-analysis is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) Statement 2015.[11] Two investigators (CZ and JG) independently and separately performed an electronic literature search using PubMed, Embase and the Cochrane Library from inception through October 28, 2018. A third independent researcher (HX) resolved differences among assigned researchers on the inclusion of each trial. There were no language restrictions in our selected research study reviewing. “AMG 334”, “erenumab-aooe” “Aimovig” “Erenumab” are adopted as the anchoring search term for MEDLINE, EMBASE and the Cochrane Library. Studies matching all text were searched. Moreover, relevant studies and previous meta-analysis references were reviewed to determine possible qualifications. Studies were initially screened in accordance with its title and summary to determine its eligibility. The full text of the qualified study was assessed in the second step of the process before exclusion.
2.3. Study selection
The following inclusion criteria were adopted to select trials:
-
(1)
RCTs about erenumab for migraine prevention;
-
(2)
At least one of the following outcomes was reported: mean monthly migraine days from baseline and a ≥50% responder rate in migraine days per month, migraine-specific medication days from baseline, adverse event and serious adverse events; and
-
(3)
no limitations regarding the country, time, or the printed language of the publications.
Exclusion criteria were as follows:
-
(1)
duration of the follow-up was less than seven days, and the duration of the double-blind treatment phase was less than three months; and
-
(2)
Erenumab observational trials on migraine patients. The search process is shown in Figure 1.
Figure 1.
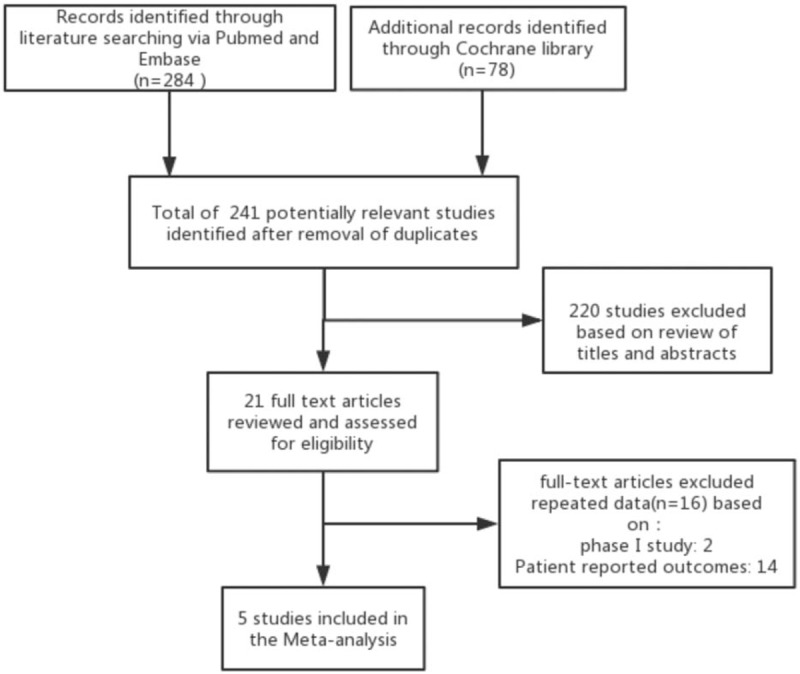
Flow diagram of literature search and study selection based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) recommendation.
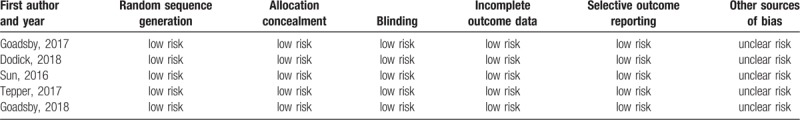
In order to assess the risk of bias in RCTs, we used the Cochrane collaboration tool to perform a quality assessment for the included RCTs. The risk of bias table including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcomes data, selective reporting, and other biases were used to assess all included RCTs (Table 1).
Table 1.
Assessment of the methodological aspects of the included studies.
2.4. Data extraction
The data were independently and separately extracted by 2 reviewers (JG and HX) into a predesigned form from the included RCTs. A third investigator (CZ) resolved any disagreements. The information extracted from each study included the following: lead author, participant characteristics, study design, sample size, outcomes, clinical follow-up, publication year and countries of origin.
2.5. Outcomes
The subjects of our study included CM and EM patients, which leads to different baselines for mean monthly migraine days and migraine-specific medication days from one study[12] to another.[13,14,15,16] As a result, a ≥50% responder rate in migraine days per month, which is a metric that was less affected by different baselines, was considered more appropriate as the primary endpoint for weeks 9 to 12.
The secondary endpoints were mean monthly migraine days from baseline and migraine-specific medication days from baseline. Safety outcomes were any adverse (major or minor) event that was defined as per every included RCT.
2.6. Statistical analysis
The weighted mean differences (MDs) and 95% confidence intervals (CIs) were reported by using the inverse variance (IV) test for continuous data. For dichotomous data, pooled risk ratios (RRs) and 95% CIs were cultivated by using the Mantel–Haenszel method. The Cochrane's Q statistics and I2 test were used to measure the heterogeneity of the study. The between-study heterogeneity was evaluated by a random-effects model. Sensitivity analysis was performed by excluding any single hazard ratio from the analysis. We spent 6 months to analyze our results. For different dosages of erenumab may have impact on the result of therapy to evaluate the efficacy and safety of different doses of erenumab against CGRP for migraine prevention, we also compared efficacy and safety between 70 and 140 mg erenumab. We also used RevMan v.5.3 for Windows to analyze data. Statistical significance was judged only for an alpha value of P < .05.
To evaluate the efficacy and safety of different dose of erenumab for episodic migraine prevention, we specified subgroups based on the dosage of erenumab. Then, the efficacy and safety of 140 mg and 70 mg erenumab had also been evaluated.
3. Results
3.1. Synthesis summary
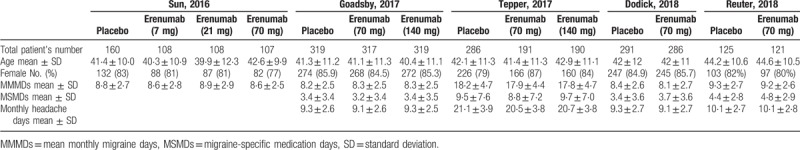
In sum, 362 studies were revealed by a thorough literature search of the selected electronic databases. After review, five RCTs that compared erenumab with placebo in patients with migraine were included.[13,14,15,12,16] Details of the selection process used for identifying the included RCTs are described in Figure 1. The summarized characteristics of the included studies are explained in Table 2. Based on the quality assessment performed by the reviewers, all included studies exhibited a low risk of bias. The average age of participants was 39.9 to 44.6 years. There were 2928 patients in total from all trials that were identified as being double-blinded in design. Four different erenumab dosages including 7 mg, 21 mg, 70 mg, and 140 mg were used in the included clinical trials which are all privately funded by the pharmaceutical industry; 70 mg and 140 mg each month were the most frequently used doses. The follow-up period was 12 weeks for all studies. The baseline characteristics of the included patients are described in Table 3.
Table 2.
Summary of included studies.
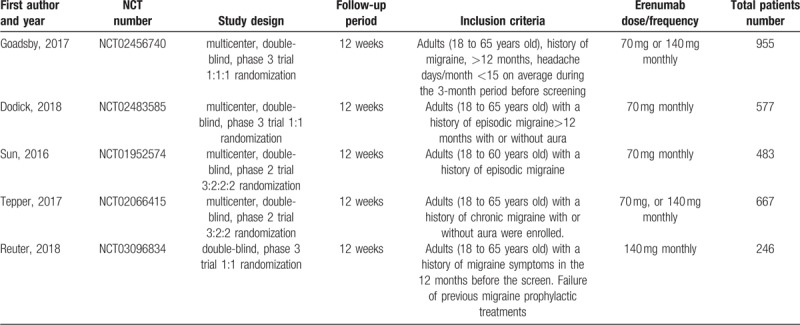
Table 3.
Baseline characteristics of the study population reported for the overall population in each study.
3.2. Primary endpoint
The outcomes of this meta-analysis indicated that erenumab significantly increased the ≥50% responder rate in migraine days per month, as compared with the placebo group (RR = 1.55; 95% CI: 1.35–1.77; P < .00001; I2 = 49%) (Fig. 2). The heterogeneity might have resulted from, those groups that were given different erenumab doses.
Figure 2.
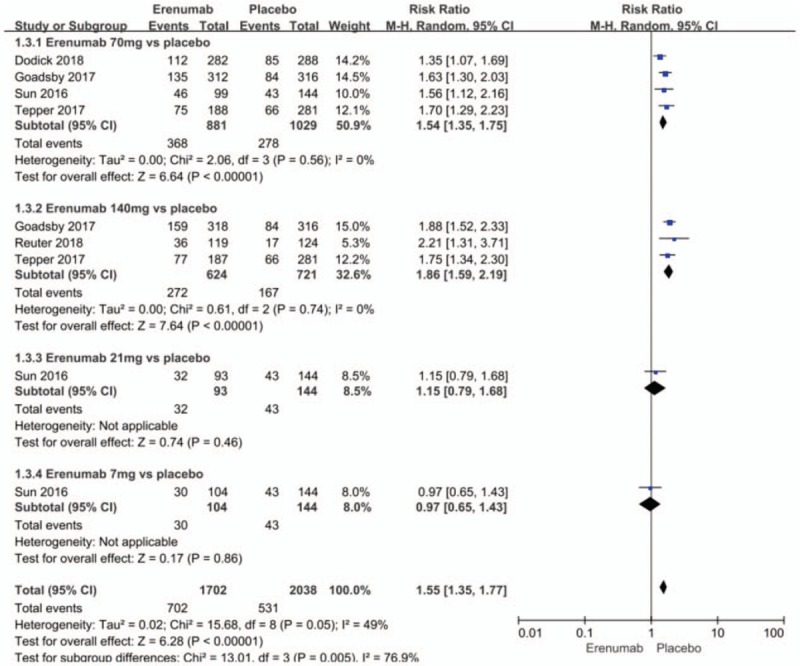
Forest plot of ≥50% responder rate (Erenumab vs placebo).
Subgroup analysis indicated that a dose of 70 mg (RR = 1.54; 95% CI: 1.35–1.75; P < .00001; I2 = 0%) and a dose of 140 mg (RR = 1.86; 95% CI, 1.59–2.19; P < .00001; I2 = 0%) for the preventive treatment of migraine significantly increased the ≥50% responder rate in migraine days per month, as compared with the placebo group. There was no significant heterogeneity for both subgroups. The dose of 21 mg (RR = 1.15; 95% CI, 0.79–1.68; P = .46) and the dose of 7 mg (RR = 0.97; 95% CI, 0.65–1.43; P = .86) has no significantly efficacy in increasing the ≥50% responder rate in migraine days per month, as compared with the placebo group. Analyses between the 70 mg and 140 mg groups did not show any differences when increasing the 50% responder rate (RR = 0.9, 0.78–1.03; P = .46; I2 = 0%) (Fig. 3).
Figure 3.
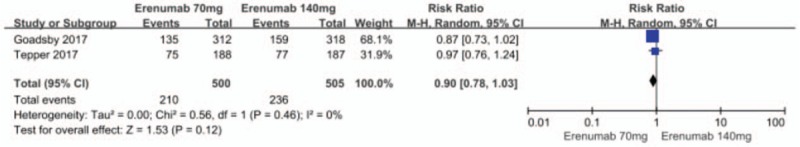
Forest plot of ≥50% responder rate (70 mg vs 140 mg Erenumab).
3.3. Secondary endpoints
3.3.1. Mean monthly migraine days from baseline
Pooled analysis showed significant reductions in mean monthly migraine days from baseline in the erenumab group as compared with the placebo group (MD −1.32; 95% CI −1.73 to −0.91; P < .00001; I2 = 100%). Subgroup analysis implemented to assess the influence of different doses showed that erenumab at a dose of 70 mg (MD −1.50; 95% CI −1.93 to −1.07; P < .00001; I2 = 100%) and 140 mg (MD −1.97; 95% CI −2.34 to −1.59; P < .00001; I2 = 99%) (Fig. 4) for preventive treatment of migraine as compared with the placebo group significantly reduced the mean monthly migraine days from baseline. Significant hetero-geneities were found in these results.
Figure 4.
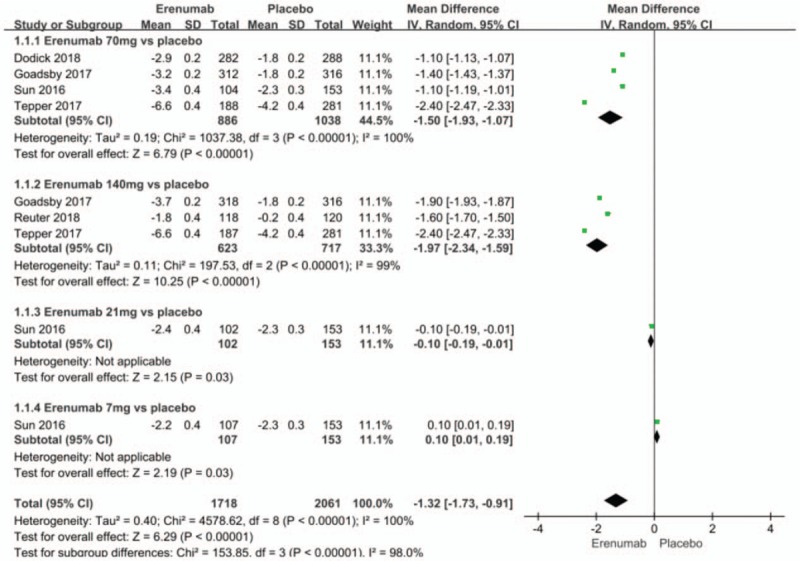
Forest plot of mean monthly migraine days from baseline (Erenumab vs placebo).
By sequentially removing trials and conducted a sensitivity analysis, it was revealed that after the Goadsby (2017) and Tepper (2017) studies were removed, the 70 mg erenumab subgroup vs placebo group heterogeneity was reduced significantly (P = 1; I2 = 0%). It suggested that:
(1) As already mentioned, the patients enrolled were different between Tepper (2017) (enrolled CM patients) and the other four articles (enrolled EM patients), and the difference in baseline and reduced range for different research studies might lead to heterogeneity; and
(2) The different approaches for categorization of groups in each study might also lead to heterogeneity.
Analyses between the 70 mg and 140 mg groups did not show any difference in reducing mean monthly migraine days from baseline (MD = 0.25; 95% CI, -0.24 to 0.74; P = .31) (Fig. 5).[14,12] There was also a significant heterogeneity in the result (P < .00001, I2 = 99%). The presumed reasons for this heterogeneity are identical to those described above.
Figure 5.
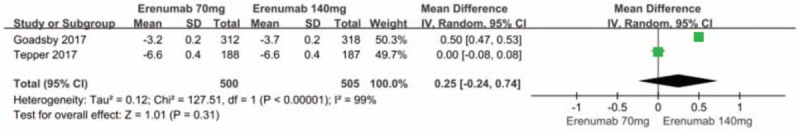
Forest plot of mean monthly migraine days from baseline (70 mg vs 140 mg Erenumab).
3.3.2. Migraine-specific medication days from baseline
The merged analysis showed significant reductions in migraine-specific medication days from baseline in the erenumab group compared with the placebo group (MD −1.41; 95%CI, −1.80 to −1.02; P < .00001; I2 = 100%). Subgroup analysis indicated that, when compared with placebo group, erenumab 70 mg (MD −1.13; 95%CI, −1.58 to −0.69; P < .00001) and 140 mg (MD -1.63; 95%CI, −2.23 to −1.02; P < .00001) (Fig. 6) for preventive treatment of migraine, significantly reduced the migraine-specific medication days from baseline. There was significant heterogeneity in the results. The sensitivity analysis did not substantively alter the overall result, we still presume that the significant heterogeneity results from the different standard of enrollment and distribution in each study are because migraine-specific medication days from baseline is as sensitive as mean monthly migraine days from baseline and affected by different baselines and other factors. When compared to the 140 mg erenumab group, a dose of 70 mg significantly reduced migraine-specific medication days from baseline (MD 0.55; 95% CI: 0.54–0.66; P < .00001) (Fig. 7). There was significant heterogeneity in the result (P = .002; I2 = 90%), a/n observation that might result from the different standards of enrollment and distributions that were adopted for both studies.[14,12]
Figure 6.
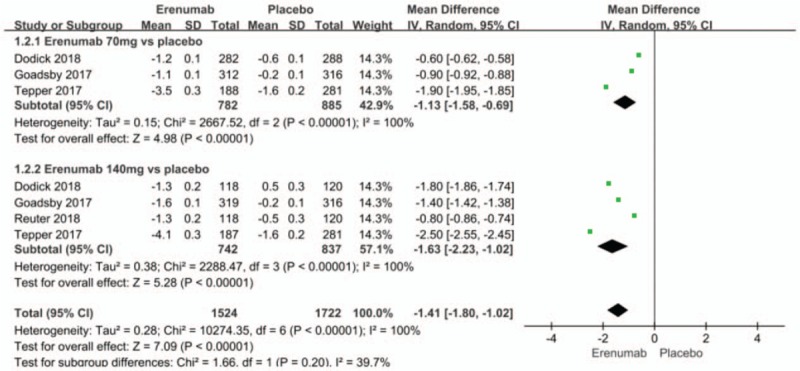
Forest plot of migraine-specific medication days from baseline (Erenumab vs placebo).
Figure 7.
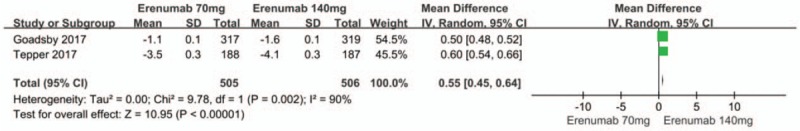
Forest plot of migraine-specific medication days from baseline (Erenumab 70 mg vs 140 mg).
3.4. Safety outcomes
The safety outcomes in weeks 9 to 12 included any adverse events, whether minor or major. There were also no significant differences between the erenumab and placebo groups with regard to the risk of any adverse event (RR 0.97; 95% CI: 0.89–1.06; P = .49; I2 = 41%) (Fig. 8). Sensitivity analysis did not substantively alter the overall result. It infers that the standard of enrollment and distribution may contribute to the observed heterogeneity. Meanwhile, serious adverse events (RR 0.95; 95% CI: 0.57–1.57; P = .84; I2 = 0%) (Fig. 9) showed no significant differences between the erenumab and placebo groups.
Figure 8.
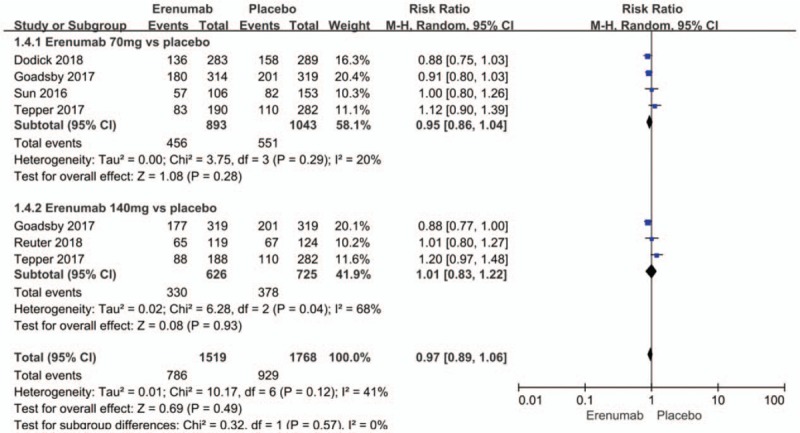
Forest plot of adverse events (Erenumab vs placebo).
Figure 9.
Forest plot of adverse events (70 mg vs 140 mg Erenumab).
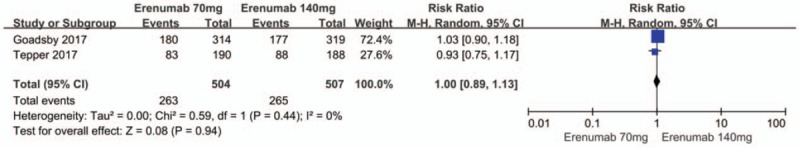
There were also no significant differences between the 70 mg and 140 mg erenumab doses with regard to the risk of any adverse event (RR 1.0; 95% CI: 0.89–1.12; P = .44; I2 = 0%) (Fig. 10) as well as any serious adverse event (RR 1.76; 95% CI: 0.74–4.17; P = .20; I2 = 0%) (Fig. 11).
Figure 10.
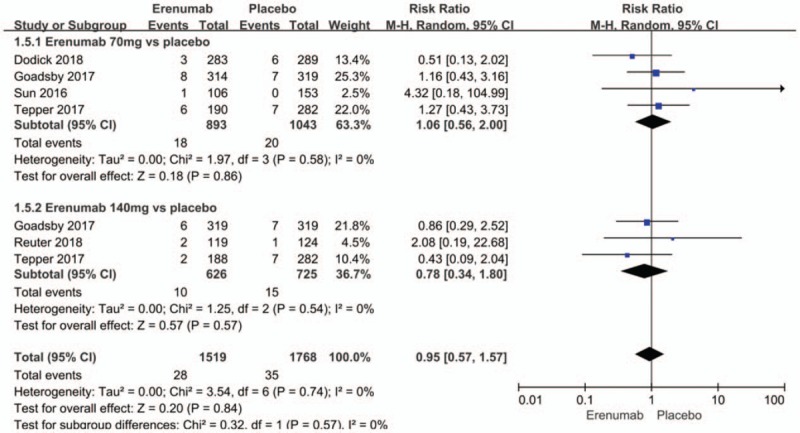
Forest plot of serious adverse events (Erenumab vs placebo).
Figure 11.
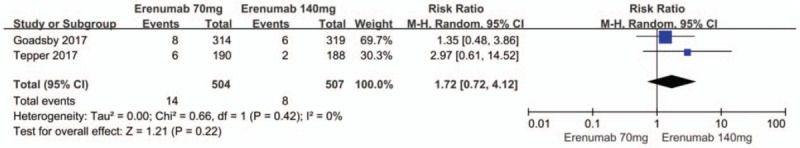
Forest plot of serious adverse events (70 mg vs 140 mg Erenumab).
4. Discussion
We compared 70 mg and 140 mg dose erenumab group to the placebo group as well as 70 mg to 140 mg group. Our meta-analysis revealed some major findings. First, erenumab significantly increased the 50% responder rate when compared to the placebo. The increased level was not significantly different when comparing the 70 mg and 140 mg dose groups of erenumab. Second, when compared with the 70 mg dose group, the effect of erenumab at 140 mg on migraine did not significantly reduce mean monthly migraine days, although it significantly reduced migraine-specific medication days from baseline. Third, at both the 70 mg and 140 mg dose groups of erenumab were associated with reduced mean monthly migraine days and migraine-specific medication days from baseline to the 12th week when compared with the placebo group. However, migraine patients gained no benefits when administered erenumab at the 7 mg and 21 mg doses as compared with placebo. Fourth, there were no significant differences between the erenumab groups and the placebo groups, or when comparing the 70 mg and 140 mg dose groups of erenumab with regard to safety outcomes.
The efficacy of some monoclonal antibodies in migraine patients has been demonstrated in many RCTs.[17] Two systematic reviews reported that CGRP monoclonal antibody therapy reduced mean monthly migraine days from baseline, but did not consider multiple dosage groups of erenumab for each study.[18,19] An antibody must have a prolonged long half-life and must be highly selective for the CGRP receptor, because prolonged long half-life allows for less frequent dosing, for example, once or twice monthly. And if antibodies are highly selective, allowing for highly specific targeting of either CGRP or its receptor. Indeed, erenumab is such an antibody with favorable pharmacokinetic and pharmacodynamic profiles.[20] In addition, one study had already described the development and pharmacological characteristics of erenumab.[21] A subcutaneous injection (SC) formulation of erenumab was recently approved in the United States of America (US), for the prevention of adult migraines.[22] A Phase I study indicated that single doses of erenumab displayed no clear dose-dependency when erenumab was administered at doses ≥21 mg.[23]
However, no research has seriously discussed the dosing of erenumab treatment to migraine patients. As a cost-effectiveness evaluation of erenumab for EM and CM from both the US societal and indicated payer perspectives, the use of 140 mg erenumab might represent a cost-effective approach towards preventing mean monthly migraine days among patients with CM versus botulinum toxin A. In addition, no preventive treatment is available according to societal and payer perspectives, but prescribing erenumab is considered less likely to offer good value for money for those with EM.[24] Our meta-analysis indicates that the 140 mg erenumab groups might offer greater benefit than the 70 mg groups in terms of reducing the migraine-specific medication days from baseline, which could weakly demonstrate that a dose of 140 mg erenumab could result in greater benefit. As a new RCT had already shown, in patients whose previous treatments were unsuccessful, erenumab at 70 mg and 140 mg was consistently more efficacious in terms of reducing the mean number of migraine days than the placebo, which indicates a greater clinical benefit for Erenumab at the 140 mg dose than at 70 mg.[16] However, when limited by the sample size, heterogeneity results from different baselines and different grouping strategies in these five studies. Thus, more RCTs need to be used to provide more effective evidence in determining whether a dose of 140 mg erenumab displays greater benefit than a dose of 70 mg.
5. Limitations
There are several limitations in the present meta-analysis. First, the inclusion criteria differed among the included studies. One study included CM patients, while other studies included EM patients. The difference in the standard of enrollment led to different baselines across different research studies. Second, three of the included studies represented phase 3 trials, and two were phase 2 trials. Third, erenumab was given in different doses across all included trials. Fourth, most of the patients enrolled were female, which might result from the fact that the epidemiology of migraine is 2 to 3 times more prevalent in women than it is in men.[25]
Our meta-analysis indicated that erenumab could have preventive efficacy in patients with migraine without any difference in safety outcomes. Primary discussions with regard how the dosage of erenumab might have affected the outcome were performed in our study, and the result provides an important clue to researchers that are considering the dose of erenumab in their studies. In order to be more suggestive of the use of erenumab; further, adequately powered RCTs are needed to establish an optimal regimen, especially for EM and CM.
6. Conclusions
In patients with EM and CM, doses of 70 and 140 mg erenumab were associated with reduced mean monthly migraine days, migraine-specific medication days, and a greater than 50% responder rate as compared placebo. Reduced mean monthly migraine days and a greater than or equal to 50% responder rate were seen for patients taking 70 and 140 mg erenumab; however, this was not statistically significant in a subset of patients, despite a significantly reduced frequency of migraine-specific medication days from baseline in the 140 mg erenumab groups as compared to 70 mg. Both the 70 and 140 mg erenumab groups did not show any statistically significant differences from placebo with regard to adverse and more serious events. Furthermore, adequately powered RCTs will be needed to establish an optimal erenumab dose to treat migraine. In addition, more studies should seek to evaluate the efficacy, safety, and cost of erenumab in comparison with other available monoclonal antibodies.
Acknowledgments
We thank all the participants for their support for this research. All their help has been declared in Author contributions.
Author contributions
Conceptualization: Changyu Zhu.
Data curation: Changyu Zhu, Jianmei Guan, Hua Xiao, Weinan Luo.
Formal analysis: Changyu Zhu.
Funding acquisition: Rongsheng Tong.
Investigation: Changyu Zhu.
Methodology: Changyu Zhu.
Resources: Jianmei Guan.
Supervision: Rongsheng Tong.
Writing – original draft: Changyu Zhu.
Writing – review & editing: Changyu Zhu.
Footnotes
Abbreviations: CGRP = calcitonin gene-related peptide, CIs = confidence intervals, CM = chronic migraine, EM = episodic migraine, ICHD3 = International Classification of Headache Disorders 3, IV = inverse variance, MDs = Mean differences, PRISMA-P = Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols, RCTs = randomized controlled trials, RRs = risk ratios, SC = Subcutaneous injection.
How to cite this article: Zhu C, Guan J, Xiao H, Luo W, Tong R. Erenumab safety and efficacy in migraine: A systematic review and meta-analysis of randomized clinical trials. Medicine. 2019;98:52(e18483).
This study and the analyses contained herein were supported by the National Key Specialty Construction Project of Clinical Pharmacy (Gran No. 30305030698). The funding was aligned to staff compensation accounting, budgeting the design of the study, and the collection, and interpretation of data and in drafting the manuscript. Authors declare that they have no real or perceived financial or academic conflicts of interest in regard the conduct of this work, or its synthesis and publication.
References
- [1]. Headache disorders. https://www.who.int/en/news-room/fact-sheets/detail/headache-disorders [accessed date October 14, 2019]. [Google Scholar]
- [2]. Jain S, Yuan H, Spare N, et al. Erenumab in the treatment of migraine. Pain Manag 2018;8:415–26. [DOI] [PubMed] [Google Scholar]
- [3]. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- [4]. Katsarava Z, Buse DC, Manack AN, et al. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep 2012;16:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache 2015;55: Suppl 2: 103–22. quiz 123-6. [DOI] [PubMed] [Google Scholar]
- [6]. Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990;28:183–7. [DOI] [PubMed] [Google Scholar]
- [7]. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 1993;33:48–56. [DOI] [PubMed] [Google Scholar]
- [8]. Hansen JM, Hauge AW, Olesen J, et al. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 2010;30:1179–86. [DOI] [PubMed] [Google Scholar]
- [9]. Edvinsson L. Functional role of perivascular peptides in the control of cerebral circulation. Trends Neurosci 1985;8:126–31. [Google Scholar]
- [10]. Russell FA, King R, Smillie S-J, et al. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 2014;94:1099–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2017;16:425–34. [DOI] [PubMed] [Google Scholar]
- [13]. Dodick DW, Ashina M, Brandes JL, et al. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 2018;38:1026–37. [DOI] [PubMed] [Google Scholar]
- [14]. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med 2017;377:2123–32. [DOI] [PubMed] [Google Scholar]
- [15]. Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016;15:382–90. [DOI] [PubMed] [Google Scholar]
- [16]. Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. The Lancet 2018;392:2280–7. [DOI] [PubMed] [Google Scholar]
- [17]. Mitsikostas DD, Reuter U. Calcitonin gene-related peptide monoclonal antibodies for migraine prevention: comparisons across randomized controlled studies. Curr Opin Neurol 2017;30:272–80. [DOI] [PubMed] [Google Scholar]
- [18]. Hong P, Wu X, Liu Y. Calcitonin gene-related peptide monoclonal antibody for preventive treatment of episodic migraine: A meta-analysis. Clin Neurol Neurosurg 2017;154:74–8. [DOI] [PubMed] [Google Scholar]
- [19]. Zhu Y, Liu Y, Zhao J, et al. The efficacy and safety of calcitonin gene-related peptide monoclonal antibody for episodic migraine: a meta-analysis. Neurol Sci 2018;39:2097–106. [DOI] [PubMed] [Google Scholar]
- [20]. Vu T, Ma P, Chen JS, et al. Pharmacokinetic-pharmacodynamic relationship of erenumab (AMG 334) and capsaicin-induced dermal blood flow in healthy and migraine subjects. Pharm Res 2017;34:1784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Shi L, Lehto SG, Zhu DXD, et al. Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene-related peptide receptor. J Pharmacol Exp Ther 2016;356:223–31. [DOI] [PubMed] [Google Scholar]
- [22]. Markham A. Erenumab: first global approval. Drugs 2018;78:1157–61. [DOI] [PubMed] [Google Scholar]
- [23]. de Hoon J, van Hecken A S Vandermeulen##C, et al. Phase I, randomized, double-blind, placebo-controlled, single-dose, and multiple-dose studies of erenumab in healthy subjects and patients with migraine. Clin Pharmacol Ther 2018;103:815–25. [DOI] [PubMed] [Google Scholar]
- [24]. Sussman M, Benner J, Neumann P, et al. Cost-effectiveness analysis of erenumab for the preventive treatment of episodic and chronic migraine: results from the US societal and payer perspectives. Cephalalgia 2018;38:1644–57. [DOI] [PubMed] [Google Scholar]
- [25]. Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 2017;16:76–87. [DOI] [PubMed] [Google Scholar]


