Abstract
To compare imaging indicators and clinical effects of extreme lateral interbody fusion (XLIF) using allogenic bone, autologous bone marrow + allogenic bone, and rhBMP-2 + allogenic bone as bone graft materials in the treatment of degenerative lumbar diseases.
This was a retrospective study of 93 patients with lumbar interbody fusion who underwent the extreme lateral approach from May 2016 to December 2017. According to the different bone graft materials, patients were divided into allogenic bone groups (group A, 31 cases), rhBMP-2 + allogenic bone (group B, 32 cases), and autologous bone marrow + allogenic bone (group C, 30 cases). There were no significant differences in gender, age, lesion segment, preoperative intervertebral space height, and preoperative Oswestry Dysfunction Index (ODI) and visual analogue scale (VAS) scores among the 3 groups (P > .05). Intervertebral space height, bone graft fusion rate, and ODI and VAS scores were compared immediately after surgery, and at 3, 6, and 12 months after surgery.
All groups were followed up for 12 months. The intervertebral space height was significantly higher in the 3 groups immediately after surgery and at 3, 6, and 12 months after surgery, in comparison to before surgery (P < .05). There was no significant difference in the intervertebral space height among the 3 groups immediately after surgery and at 3, 6, and 12 months after surgery (P > .05). The fusion rate of group B and C was higher than that of groups A at 3, 6, and 12 months after surgery (P < .05). In the 3 groups, the VAS and ODI scores at 3, 6, and 12 months after surgery were significantly improved compared with the preoperative scores (P < .05). The VAS and ODI scores in groups B and C were significantly higher than those in group A (P < .05), but there was no significant difference between groups B and C (P > .05).
The rhBMP-2 + allograft bone combination had good clinical effects and high fusion rate in XLIF.
Keywords: bone graft materials, extreme lumbar interbody fusion, fusion rate, rhBMP-2
1. Introduction
Lumbar fusion is one of the most common methods for the treatment of lumbar degenerative diseases, lumbar spondylolisthesis, and deformities. After a long history of development, modern lumbar fusion technology has been widely used in clinical practice with good results. Extreme lateral interbody fusion (XLIF) was first proposed by Pimenta in 2001, and quickly became popular after being promoted by Ozgur et al in 2006.[1] As an alternative to standard posterior lumbar fusion, it is a lateral retroperitoneal approach through the psoas major muscle to the intervertebral space. It allows direct visualization of the intervertebral space indirect decompression of the spinal canal and foramen by restoring intervertebral space height. Compared with traditional posterior fusion, this approach is associated with decreased trauma, shorter operation time, less bleeding, and lower risk of injury to the nerve roots and dural sac during operation. In addition, ligaments and facet joints are preserved during XLIF, inflicting little damage to the posterior stable structure of the spine, with the implanted intervertebral fusion cage spanning the whole width of the vertebral body with good intervertebral stability. In particular, the implanted intervertebral fusion has larger apertures, allowing implantation of more bone, and has a larger contact area with the vertebral body, thus promoting fusion.
Despite the advantages of XLIF, nonfusion remains a frequent complication. In order to further promote intervertebral fusion, research has focused on various bone grafting methods. Autogenous bone transplantation is generally the preferred method for intervertebral fusion. However, the use of autogenous bone as graft material in XLIF requires iliac bone extraction, which may lead to infection at the site of extraction, increased blood loss and operation time, pain at the donor site, and iliac fracture,[2] which are contrary to the original intention of the minimally invasive procedure. At present, autogenous bone transplantation has been mostly replaced by allogenic bone, which has demonstrated good bone conduction effects.[2] Bone marrow and recombinant human bone morphogenetic protein-2 (rhBMP-2) are widely used in spinal fusion surgery as auxiliary osteogenesis-inducing materials. Heymann and coworkers have reported bone marrow aspirates from the iliac crest can improve the success rate of interbody fusion.[3] Relevant studies have also shown rhBMP-2 has significant osteoinductive properties, achieving fusion rates similar to those of autologous bone.[4] XLIF is a minimally invasive technique for lumbar fusion. Whether or not vertebral fusion is an important index for evaluating the success of surgery.
In order to assess the fusion rates clinical effects of these bone grafting methods in XLIF, we studied 93 patients with lumbar degenerative diseases and spondylolisthesis who underwent this procedure with allogenic bone, autologous bone marrow + allogeneic bone, and rhBMP-2 + allogeneic bone.
2. Materials and methods
2.1. Inclusion and exclusion criteria
The inclusion criteria were the following: The main clinical manifestations were low back pain or low back and leg pain with or without intermittent claudication; Conservative treatment for more than 3 to 6 months had no obvious effect, with indications for operation; Imaging findings suggest mild lumbar instability, scoliosis, intervertebral space stenosis, or spinal canal stenosis; and In patients with multiple lumbar degenerative changes, less than 3 responsible segments were confirmed by discography or selective nerve root occlusion.
The exclusion criteria were the following: L5-SI intervertebral disc degeneration; Obvious articular process lesions, calcification of intervertebral disc, ossification of posterior longitudinal ligament, and other osteophytes; Lumbar spondylolisthesis of 2nd degree or more; Severe lumbar spinal stenosis or dissociation of the nucleus pulposus; Severe osteoporosis; Poor general health with intolerability for surgery; and Incomplete follow-up data.
According to inclusion and exclusion criteria, 93 patients with lumbar degenerative diseases who underwent XLIF surgery in our department from May 2016 to December 2017 were included in this study. Subjects were divided into 3 groups according to their bone grafting material. Group A received allogenic bone, group B received rhBMP-2 + allogenic bone, and group C received autologous bone marrow + allogenic bone as bone graft fusion material. All patients signed informed consent before inclusion in the research database, and the study was approved by the ethics committee of our hospital.
2.2. Operative method
After general anesthesia, patients were placed in the right decubitus position, and the lumbar bridge was adjusted to open the rib margin space caused by the iliac spine. The position of the incision was confirmed and marked according to preoperative fluoroscopy. The relative position and distance to the nerves were evaluated with the neuroelectrophysiological monitor to improve safety. After routine disinfection, the skin was cut, and the fingers were bluntly separated into the retroperitoneal space through the muscular layer, touching the lateral side of the vertebral body. The guide needle and primary dilator were inserted through the incision, and the position of the guide needle and primary dilator was confirmed by fluoroscopy. These were gradually inserted in the expanding canal, separating the psoas major muscle bundle to reach the side of the intervertebral disc. Then, the passage was inserted, the free arm was connected, and the working passage was fixed and opened, exposing the lateral side of the vertebral body and intervertebral disc. Next, an incision was made in the lateral fibrous ring of the diseased intervertebral disc, curetting the intervertebral disc and the upper and lower endplates step by step to the subchondral bone, penetrating the intervertebral disc to the opposite side. The model was then tested and the cage confirmed. In group A, allogenic bone was used as bone graft material; while in group B, rhBMP-2 mixed allogenic bone granules were used, and group C used autologous bone marrow mixed with allogeneic bone granules. In group C, 2 mL of bone marrow were extracted with a long suction needle and mixed with allogenic bone. The cage filled with bone graft material was implanted into the intervertebral space. Fluoroscopy confirmed the appropriate position of the fusion cage, which was then rinsed and sutured, closing the incision.
2.3. Observation items and follow-up
Preoperative general information was recorded, including age, gender, BMI, and others. The Oswestry Dysfunction Index (ODI) was used to evaluate lumbar spine function before and after operation and during follow-up. The visual analogue scale (VAS) was used to assess the severity of pain function before and after operation and during follow-up.
Postoperative X-rays and CT scans of the lumbar spine were performed at 3, 6, and 12 months after the procedure. The Dabbs and Dabbs[5] method was used to measure intervertebral space height before and after operation; the average value of the height of the anterior and posterior edges of the intervertebral space. The anterior and posterior diameters of the upper vertebral body were recorded each time during the measurement, and the magnification of the measurement was calculated to adjust for the different magnifications of each photograph. Thin-slice plain CT scans were performed to evaluate the rate of intervertebral fusion according to Williams et al.[6] The fusion was graded according to CT findings: Grade I was complete fusion, with complete ossification of implants accompanied by involvement of the upper and lower endplates; Grade II was incomplete or progressive fusion, with ossification of implants in the cage and connection of 1 or 2 endplates without definite endplates. Grade III was an ambiguous fusion, with the cage having no definite ossifying connection or connective endplate.[2] Vertebral fusion rate was the main objective of this study, with plain CT plain scans being the main evaluation method. Grade I was judged as successful integration. Vertebral fusion was assessed by the same radiologist in all patients.
2.4. Statistical methods
SPSS v22.0 was used for data analysis. Categorical data were compared with the χ2 test. Continuous data with normal distribution were expressed as means ± standard deviations (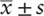 ). One-way ANOVA was used for multigroup comparisons, and LSD was used for posterior multiple comparisons. Rank sum test and median and quartile spacing were used to evaluate continuous data with nonnormal distribution. Results with P < .05 were considered statistically significant, while P < .01 was considered a very significant difference.
). One-way ANOVA was used for multigroup comparisons, and LSD was used for posterior multiple comparisons. Rank sum test and median and quartile spacing were used to evaluate continuous data with nonnormal distribution. Results with P < .05 were considered statistically significant, while P < .01 was considered a very significant difference.
3. Results
3.1. General observation
Ninety-four patients in 3 groups were followed up for 12 months. There were 31 patients in group A, including 21 males and 10 females, who completed 34 operative segments; 32 patients in group B, including 20 males and 12 females, who completed 34 operative segments; and 30 patients in group C, including 18 males and 12 females, who completed 32 operative segments. There were no significant differences in age, sex, body mass index, and operative segments among the 3 groups (Table 1). One patient who used allogeneic bone as bone graft material was excluded from group A because cage displacement was found after 3 months of follow-up. (Fig. 1).
Table 1.
Basic information of group A, group B, and group C.
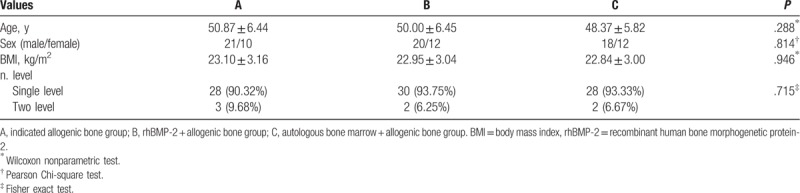
Figure 1.
A 34-year-old male patient underwent XLIF 3 months ago, and allogeneic bone was used as bone filling material during the operation. At 3 months after operation X-ray (A1) and coronal position of CT plain scan (A2) showed that cage displacement was obvious, sagittal position of CT plain scan (B1, B2) indicated that there was no osseous connection between the implant and the upper and lower endplates. The patients underwent routine posterior surgery again (C1, C2). CT = computed tomography, XLIF = extreme lateral interbody fusion.
3.2. Intervertebral space height
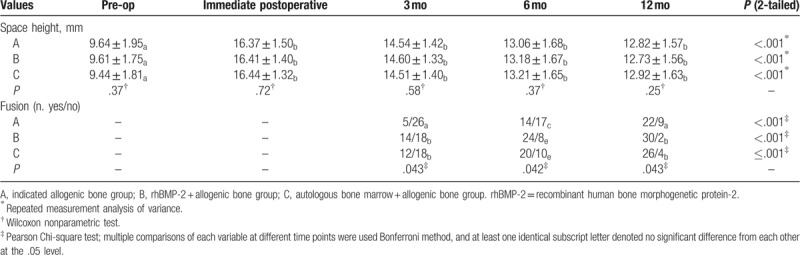
There were no significant differences in intervertebral space height among the groups immediately and at 3, 6, and 12 months after operation (P > .05). There was a significant difference in intervertebral space height among the groups immediately and at 3, 6, and 12 months after operation (P > .05) (Table 2).
Table 2.
Intervertebral space height and fusion rate among 3groups.
3.3. Fusion rate
The Williams et al[6] method was used to evaluate the rate of intervertebral fusion. The fusion rate in group A was 16.1% (5/31) at 3 months, 45.2% (14/31) at 6 months, and 71.0% (22/31) at 12 months. In group B, the fusion rate was 43.8% (14/32) at 3 months, 75.0% (24/32) at 6 months, and 93.8% (30/32) at 12 months. In group C, the fusion rate was 40.0% (12/30) at 3 months, 66.7% (20/30) at 6 months, and 86.7% (26/30) at 12 months. Thus, rhBMP-2 + bone allograft fusion rate was the highest, while the fusion rate in the autologous bone marrow + bone allograft group was higher than that of pure bone allograft (Table 2, Fig. 2).
Figure 2.
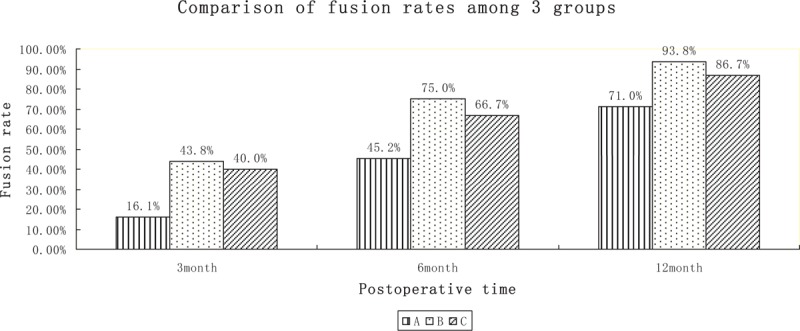
The fusion rate of the 3 groups increased with time, and the fusion rate of group B was higher than that of the other 2 groups.
3.4. Clinical efficacy
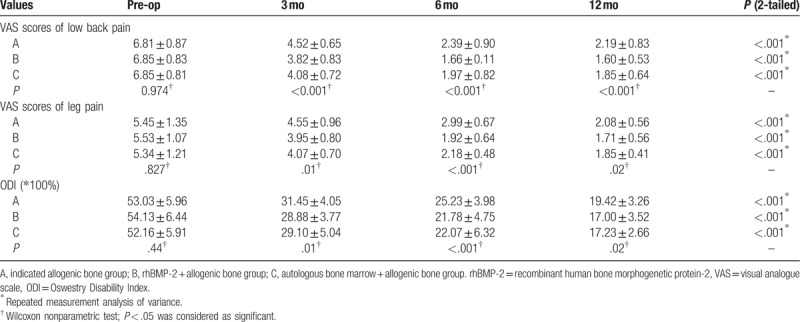
There were 15 cases of thigh numbness in segments L4–L5 in the 3 groups immediately after operation, which did not require special treatment and disappeared 2 weeks after operation. In the 3 groups, the VAS scores of low back pain and leg pain and the ODI scores at 3, 6, and 12 months after surgery were significantly improved compared with the preoperative scores (P < .05). The VAS and ODI scores in groups B and C were significantly higher than those in group A (P < .05), but there was no significant difference between groups B and C (P > .05) (Table 3).
Table 3.
Clinical efficacy among 3 groups.
4. Discussion
As a procedure with increasing popularity, XLIF differs from the traditional posterior or anterior/anterior approaches, involving a lateral approach. Aiming to protect the nerve, it allows full resection of the intervertebral disc and provides an excellent environment for fusion; also having a good indirect decompression effect. However, whether cage stand-alone can provide reliable and lasting stability to maintain intervertebral space height without affecting the fusion between vertebral bodies is also a problem in XLIF. Patients included in this study were all treated with cage stand-alone without assistant internal fixation system. Because the anterior longitudinal ligament, posterior longitudinal ligament, and facet joints are retained, multidirectional motion can be stabilized by the tension of the residual ring and ligament.[7] In addition, biomechanical analysis suggests larger fusion cages can provide higher segmental stability.[8] Some studies also suggest stand-alone may be a better choice in the absence of osteoporosis, severe segmental instability, or partial bone defects.[9] Marchi and coworkers have argued some patients with facet joint lesions need assisted posterior screw-rod fixation because moderate to severe facet joint degeneration indicates these joints are under heavy load or exercise.[10] Therefore, patients included in this study had no obvious degeneration of segmental instability, no osteoporosis or bone destruction, no obvious facet joint lesions, and no endplate injury. Assisted posterior nail-rod fixation increases the risk of anesthesia, the need to change body position during operation, and the economic burden of patients.
New materials for intervertebral bone grafting are in constant development. Although many kinds of bone graft materials are used in clinical, a common important purpose is to achieve early, long, and firm fusion of these materials. The osteoinductive ability of bone substitutes is closely related to bone fusion. rhBMP-2 was first discovered by Uris et al in 1965[11,12]; as a member of the transforming growth factor-beta superfamily, it can induce undifferentiated mesenchymal cells to differentiate into osteoblasts and chondrocytes, and promote their proliferation. It can also promote the differentiation and maturation of osteoblasts, and has strong osteogenic induction effects. Studies have shown rhBMP and bone graft has better fusion effects.[13] A multicenter study showed that rhBMP-2 was used in 143 patients with lumbar disc herniation during lumbar fusion.[14] After 2 years of follow-up, the final fusion rate was 94.5%, higher than 88.7% in the control group. Boden et al also showed that rhBMP-2 was more effective than iliac bone graft.[15] Nevertheless, some studies have reported complications such as osteolysis, heterotopic bone formation, and cage settlement or displacement.[16,17] Some studies have also shown these complications may be dose-dependent.[18] No serious complications were found in this study. Therefore, low-dose rhBMP-2 appears to be relatively safe. Some studies have also shown the addition of bone marrow to bone graft materials can promote fusion, as osteogenic progenitor cells have a strong ability to induce bone, and the bone marrow is one of the most abundant and reliable sources of osteoblasts.[3]
According to the CT evaluation at 3, 6, and 12 months after operation, the fusion rate of the bone marrow + allograft group was higher than that of bone allograft alone, while rhBMP-2 + allograft as bone graft material had the highest fusion rate among the 3 groups. Long-term follow-up studies have found failure to achieve osseous fusion between vertebral bodies can lead to instability of the spine, degeneration of the vertebral body, and increased risk of reoperation.[19] Relevant studies have also indicated rhBMP-2 can significantly reduce surgical revision rate.[20] In this study, cage displacement only occurred in 1 patient who used allogenic bone at 3 months after operation, and cage removal and fusion and internal fixation were performed in the later stage (Fig. 1). The patients recovered well after operation. We believe this case of cage displacement was related to slow fusion and inappropriate postoperative activity.
In this study, the VAS and ODI scores at 3, 6, and 12 months after operation were significantly improved in each group compared with those before operation (P < .05). The excellent and good rates of VAS and ODI scores in groups B and C were significantly higher than in group A (P < .05), but there was no significant difference between groups B and C (P > .05). In summary, the application of allogenic bone in combination with bone marrow or rhBMP was associated with enhanced early postoperative improvement after XLIF in comparison with allogenic bone alone, with no significant difference between the 2 alternatives. However, this study did not compare the clinical effects of the 3 groups at long term. Assessment of the final clinical effects of the 3 groups requires a longer follow-up study.
We found intervertebral space height improved significantly in all groups; decreasing slightly at 3, 6, and 12 months after operation in comparison to immediately after operation, but without symptoms and no significant difference among the 3 groups (P > .05). Research has shown rhBMP has a significant osteolytic effect, leading to dissolution of the endplate, thereby increasing cage sedimentation rate and reducing the intervertebral space height.[21] However, no significant differences were found regarding intervertebral space height among the 3 groups. This may be one of the advantages of XLIF compared with other spinal fusion operations. A larger cage can be implanted into the opening system of the approach, spanning the entire vertebral body width, so that the cage can ride across the strongest epiphyseal ring on both sides of the vertebral body to restore intervertebral space height. The load stress of the endplate is dispersed, thus the settlement of the cage is reduced. Marchi and coworkers consider the small settlement of cage does not affect the fusion rate and clinical effects.[10] However, we believe the assisting internal fixation system remains an effective way to stabilize the spinal segment and avoid cage collapse and displacement.
In this study, we assessed the BMI of the included cases, finding significant differences in the surgical effects and fusion rates between obese patients and nonobese patients. Nevertheless, during the operation, obesity may pose a difficulty, due to the depth of operation site, poor illumination, low clarity of the operation field, and complicated operation of the endplate.
This study shows the use of rhBMP-2 + allogenic bone as fusion material in XLIF can achieve higher fusion rates and better clinical effects than that of bone allograft alone and bone marrow + allogenic bone in early stages. Although the clinical effect of bone marrow + allogeneic bone is comparable to that of rhBMP-2 + allogeneic bone, bone marrow extraction during operation requires puncture from iliac bone, which increases the risk of infection and pain, and has inferior fusion effects in comparison to rhBMP-2 + allogeneic bone.
In this study, although the combination of allogeneic bone and rhBMP-2 can achieve higher fusion rate and better clinical efficacy in XLIF, there is no detailed large sample study on the possible complications of allogeneic bone + rhBMP-2 in XLIF. Secondly, rhBMP-2 causes the settlement of fusion cage in other fusion operations. Whether it will affect the clinical effect after XLIF remains to be further studied. In addition, the sample size of this study is small, and long-term large sample size research and observation follow-up are needed.
5. Conclusions
In conclusion, the application of rhBMP-2 + allogeneic bone as bone graft material in extreme lateral interbody fusion (XLIF) can achieve higher fusion rate and better clinical results.
Author contributions
Conceptualization: Wei Zhang.
Data curation: Yuan Gao, Jiaqi Li, Hao Cui.
Formal analysis: Yuan Gao, Yapeng Sun, Zeyang Li, Wenyuan Ding, Yong Shen.
Methodology: Yuan Gao, Fei Zhang.
Writing – original draft: Yuan Gao.
Writing – review & editing: Wei Zhang.
Footnotes
Abbreviations: ODI = Oswestry Dysfunction Index, rhBMP-2 = recombinant human bone morphogenetic protein-2, VAS = visual analog scale, XLIF = extreme lateral interbody fusion.
How to cite this article: Gao Y, Li J, Cui H, Zhang F, Sun Y, Li Z, Ding W, Shen Y, Zhang W. Comparison of intervertebral fusion rates of different bone graft materials in extreme lateral interbody fusion. Medicine. 2019;98:44(e17685).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435–43. [DOI] [PubMed] [Google Scholar]
- [2].Tohmeh AG, Blake W, Mirna T, et al. Allograft cellular bone matrix in extreme lateral interbody fusion: preliminary radiographic and clinical outcomes. Sci World J 2012;2012:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Romih M, Delécrin J, Heymann D, et al. The vertebral interbody grafting site's low concentration in osteogenic progenitors can greatly benefit from addition of iliac crest bone marrow. Eur Spine J 2005;14:645–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Siddiqui MMA, Ana ARPS, Yeo W, et al. Bone morphogenic protein is a viable adjunct for fusion in minimally invasive transforaminal lumbar interbody fusion. Asian Spine J 2016;10:1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dabbs VM, Dabbs LG. Correlation between disc height narrowing and low-back pain. Spine (Phila Pa 1976) 1990;15:1366–8. [DOI] [PubMed] [Google Scholar]
- [6].Williams AL, Gornet MF, Burkus JK. CT evaluation of lumbar interbody fusion: current concepts. Am J Neuroradiol 2005;26:2057–66. [PMC free article] [PubMed] [Google Scholar]
- [7].Oliveira L, Marchi L, Coutinho E, et al. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35Supplement:S331–7. [DOI] [PubMed] [Google Scholar]
- [8].Goh JC, Wong HK, Thambyah A, et al. Influence of PLIF cage size on lumbar spine stability. Spine (Phila Pa 1976) 2000;25:35–40. [DOI] [PubMed] [Google Scholar]
- [9].Ahmadian A, Bach K, Bolinger B, et al. Stand-alone minimally invasive lateral lumbar interbody fusion: Multicenter clinical outcomes. J Clin Neurosci 2015;22:740–6. [DOI] [PubMed] [Google Scholar]
- [10].Abdala, Nitamar, Marchi, et al. Radiographic and clinical evaluation of cage subsidence after; stand-alone lateral interbody fusion clinical article. J Neurosurg Spine 2013;19:110–8. [DOI] [PubMed] [Google Scholar]
- [11].Urist MR, Iwata H, Ceccotti PL, et al. Bone morphogenesis in implants of insoluble bone gelatin. Proc Natl Acad Sci 1973;70:3511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Urist MR, Mikulski AJ, Lietze A. Solubilized and insolubilized bone morphogenetic protein. Proc Natl Acad Sci U S A 1979;76:1828–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J 2009;9:623–9. [DOI] [PubMed] [Google Scholar]
- [14].Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech 2002;15:337–49. [DOI] [PubMed] [Google Scholar]
- [15].Boden SD, Zdeblick TA, Sandhu HS, et al. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine (Phila Pa 1976) 2000;25:376–81. [DOI] [PubMed] [Google Scholar]
- [16].Mroz TE, Wang JC, Hashimoto R, et al. Complications related to osteobiologics use in spine surgery. Spine (Phila Pa 1976) 2010;359 Suppl:S86–104. [DOI] [PubMed] [Google Scholar]
- [17].Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 2011;11:0–491. [DOI] [PubMed] [Google Scholar]
- [18].Boden SD, Martin GJ, Horton WC, et al. Laparoscopic anterior spinal arthrodesis with rhBMP-2 in a titanium interbody threaded cage. J Spinal Disord 1998;11:95–101. [PubMed] [Google Scholar]
- [19].Videbaek TS, Christensen FB, Soegaard R, et al. Circum-ferential fusion improves outcome incomparison with in-strumented posterolateral fusion: long-term results of arandomized clinical trial. Spine (Phila Pa 1976) 2006;31:2875–80. [DOI] [PubMed] [Google Scholar]
- [20].Yao Q, Cohen J, Buser Z, et al. Analysis of recombinant human bone morphogenetic protein-2 use in the treatment of lumbar degenerative spondylolisthesis. Global Spine J 2016;6:749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vaidya R. Transforaminal interbody fusion and the “off-label” use of recombinant human bone morphogenetic protein-2. Spine J 2009;9:667–9. [DOI] [PubMed] [Google Scholar]


