Abstract
The aim of this study was to assess the effect of preoperative sleep quality on acute postoperative pain in breast cancer patients.
The Pittsburgh Sleep Quality Index questionnaire (PSQI) was used to assess the overall sleep status of women scheduled for unilateral modified radical mastectomy in the past month. Based on the responses, patients were allocated to good sleep group or poor sleep group. Postoperatively, acute pain was assessed using the numerical rating score in the first 24 hours; in addition, the requirement of analgesics and the incidence of postoperative complications were recorded.
A total of 108 breast surgery patients were enrolled. Based on the PSQI results, 55 (51%) patients were allocated to poor sleep group and 53 (49%) to good sleep group. Pain scores were similar in the 2 groups at the end of surgery (P = .589); however, poor sleep group reported higher postoperative pain scores than the good sleep group at 2 (P = .002), 6 (P < .001), 12 (P < .001), and 24 (P = .002) hours after surgery. The incidence of severe pain in the poor sleep group was higher than that in the good sleep group (27% vs 8%, P = .018), and the ratio of participants who required rescued analgesics was greater in the poor sleep group (52% vs 22%, P = .002). In addition, patients with poor sleep quality had more postoperative complications and longer hospital stay.
In this study, breast cancer patients with poor preoperative sleep quality reported more severe postoperative pain, required more analgesics, experienced more complications, and had longer hospital stay.
Keywords: breast cancer surgery, numerical rating score, postoperative pain, sleep quality
1. Introduction
Acute postoperative pain after surgery is associated with increased postoperative complications, lower patient satisfaction, prolonged hospitalization, and greater medical expenditure[1,2]; in addition, it also has an effect on the incidence of chronic pain and postoperative consumption of analgesics at home, especially in patients receiving breast surgery, coronary artery bypass surgery, and thoracotomy.[3,4] Better management of postoperative pain complies with the notion of comfort medicine and enhances postoperative recovery; in addition, it also decreases the incidence of persistent postoperative pain and helps improve the long-term quality of life.[5] Currently, several modalities are available for effective management of postoperative pain[6,7]; however, identification of patients who are more sensitive to pain and those who are likely to experience severe postoperative pain is still difficult.[8] Therefore, it is important to identify the determinants of acute postoperative pain in order to improve the protocol for postoperative pain management.
An increasing body of evidence in the past 2 decades has corroborated the reciprocal relationship between pain and sleep.[9–11] Pain and analgesic use has been shown to disturb sleep[12,13]; on the other hand, sleep disturbance also induces hyperalgesia.[13,14] Impaired sleep has been shown to predict new pain incidents and aggravation of chronic pain; in addition, sleep impairment is a more consistent predictor of pain than pain is of sleep.[15–18] Experimental disturbance of sleep such as full sleep deprivation, partial sleep deprivation,[19,20] and sleep fragmentation[21] have all been shown to increase both clinical pain and responses to sensory tests; however, there is considerable interindividual variability in this respect.[22] Nonetheless, the link between sleep quality and the intensity of acute postsurgical pain is not well characterized. One study used Actiwatch for evaluation of sleep on just the night before breast-conserving surgery; they found a significant negative relationship between sleep efficiency on the night before surgery and the severity of postoperative pain.[23] However, assessment of sleep only on the night before operation is not enough to reflect the overall sleep status of patients.
As we know, perioperative sleep disturbance is a common problem that is frequently neglected by clinicians. In recent years, postoperative sleep disturbance has evoked much attention.[24–26] But it is evident that many patients have already been suffering from poor sleep quality for long before surgery; this phenomenon is especially seen among breast cancer patients because most breast cancer manifests in mid life.[27] Approximately 30% to 75% of newly diagnosed or recently treated breast cancer patients reportedly suffer from chronic poor sleep quality.[28] Thus, examining the effect of chronically preoperative sleep disturbance on acute postoperative pain in breast cancer patients receiving total mastectomy surgery is a key imperative. This may help improve the management of acute postoperative pain, which is important to decrease the incidence of persistent chronic pain after mastectomy surgery. Therefore, we designed this prospective cohort study to investigate the relationship between sleep quality of 1 month before surgery and acute postoperative pain in women scheduled for modified radical mastectomy. We assumed that poor sleep quality before surgery will increase the intensity of acute postoperative pain and postoperative analgesic consumption.
2. Methods
2.1. Study design and setting
This study was registered before patient enrollment in the Chinese Clinical Trial Registry (ID ChiCTR-RPC-17012922). The study was conducted at the Liao-cheng People's Hospital between April 2017 and June 2018. Ethics approval was provided by the ethical committee of Liao-cheng people's hospital. Written informed consent was obtained from all participants prior to their enrolment.
2.2. Subjects
Inclusion criteria: women aged 18 to 65 years scheduled to undergo unilateral modified radical mastectomy; American Society of Anesthesiologists physical status classification I or II.
Exclusion criteria: previous breast or axillary surgery, pregnant women, women affected by anxiety or depression disorders (Self-rating Anxiety Scale score ≥50 or Self-rating Depression Scale score ≥53, respectively), history of chronic pain including different type of headache, sleep apnea syndrome, psychiatric disorder, detectable metastatic diseases, and cardiac diseases, and inability to complete Chinese test questionnaires.
2.3. Preoperative questionnaires
A researcher, who was not involved in the treatment and follow-up of patients, performed preoperative interview on the day prior to surgery to collect demographic information and submitted questionnaires of sleep quality and mental state to the enrolled patients; all participants completed the questionnaires preoperatively.
2.3.1. Sleep quality evaluation
Preoperative subjective sleep quality: The Pittsburgh Sleep Quality Index (PSQI) questionnaire was used to evaluate the participants’ subjective sleep quality of the past month. PSQI is a standard instrument with a high reliability and validity to assess the sleep quality of patients over a 1-month period,[29] which consists of 19 individual items grouped into 7 subscale scores: sleep duration; sleep latency (time needed to fall asleep); subjective sleep quality index; habitual sleep efficiency (proportion between total sleep time and time in bed); sleep disturbances (waking up during the night); use of sedatives; and daytime dysfunction. Each of these subscales is weighted equally on a 4-point scale generating a total score ranging from 0 to 21. Total score ≥5 is generally considered indicative of poor sleep quality; higher scores reflect a greater decline in sleep quality. In this study, preoperative sleep quality during the past month was assessed using the PSQI Chinese version on the day of surgery. Patients whose PSQI score were ≥5 were allocated to the poor sleep group, while those with PSQI score <5 were assigned to the good sleep group.
2.3.2. Preoperative mental state
Preoperative mental state was evaluated on the day of surgery using the Chinese-language versions of the Self-rating Anxiety Scale (SAS) and Self-rating Depression Scale (SDS). SAS and SDS are self-report questionnaires for a comprehensive assessment of anxiety and depression status of patients.[30,31] The SAS and the SDS comprised of 20 items. Each item was scored on a 4-point scale (0 – not at all; 1 – somewhat; 3 – moderately so; and 4 – very much so); the total score for each questionnaire was multiplied by 1.25 to convert it into a standardized score (range, 25–100; higher scores indicate higher levels of anxiety and depression). According to Zung severity levels classification criteria, the SDS score greater than 53 was used by Chinese psychiatric professionals as a cutoff point for depression-related symptom severity, and the cutoff values have been widely used in Chinese depression studies.[32,33] Patients with SAS score ≥50 were considered to suffer from anxiety, and patients with SDS score ≥53 were considered to suffer from depression in this study. Patients with anxiety and depression were excluded from this study.
2.4. Anesthesia and postoperative analgesia
All enrolled patients were monitored with pulse oximetry (SPO2), electrocardiography, noninvasive blood pressure, acceleromyography, and bispectral index monitor (BIS LoC2 Channel; COVIDINE IIc, Mansfiled, MA). General anesthesia was induced for all patients with propofol 2 to 3 mg/kg and fentanyl 2 μg/kg with no premedication. Endo-tracheal intubation was facilitated by 0.15 mg/kg cisatracurium IV; 7-mm tracheal tube was inserted by visual laryngoscopy. Anesthesia was maintained with an intravenous infusion of propofol (6–8 mg/kg/hour) and remifentanil (0.05–0.1 μg/kg/minute) to maintain the bispectral index within the range of 40 to 60. The presence of hypertension or tachycardia (>20% of baseline) during anesthesia was attributed to insufficient analgesia and fentanyl 1 μg/kg was administered while BIS was 40 to 60. Cisatracurium 0.03 mg/kg was administered intermittently at a train-of-four count of ≥1. Mechanical ventilation was maintained with a tidal volume of 8 mL/kg, and the ventilatory frequency was adjusted to maintain a normal end-tidal carbon dioxide concentration with an air/oxygen mixture (fraction of inspired oxygen, 0.5). Intraoperatively, Ephedrine was administered when blood pressure drops to 20% of the baseline and atropine was administered when heart rate drops to 20% of the baseline. The mid-esophageal temperature was maintained at 36 to 37 °C during surgery. Ketorolac 30 mg was injected intravenously 30 minutes before the end of the operation and neostigmine 0.05 mg/kg plus atropine 0.02 mg/kg were used to reverse possible residual neuromuscular blockade at the end of surgery. All patients were administered ondansetron 8 mg in order to prevent postoperative nausea and vomiting (PONV). The tracheal tube was removed when the patients regained their consciousness and were able to breathe spontaneously; subsequently, the patients were transferred to the postanesthesia care unit (PACU).
In the PACU, severity of pain was assessed using the numerical rating score (NRS). Fentanyl (50 μg) was administered if the NRS score was >4 points or if the patients demanded analgesics. All participants were discharged from the PACU to the ward when the Steward Recovery Score exceeded 4 points and NRS was less than 4.
2.5. Follow-up
In the ward, all participants were followed up by the acute pain service (APS) team blinded to group allocation. NRS was used to evaluate postoperative peak pain at 0 (T0), 2 (T1), 6 (T2), 12 (T3), and 24 (T4) hours after surgery. In this study, NRS score ≥7 was defined as severe pain. NRS score ≥4 (effect on general activity, mood, and postoperative mobility)[34] was considered indicative of clinically relevant moderate pain, which is comparable with other literature.[35,36] Patients were encouraged to request analgesic medication at any time. Analgesics were administered once the NRS exceeded 4 or on the patient's request for analgesia. Ibuprofen 400 mg (oral) was the first choice. Nefopam 20 mg (intramuscular injection) was the rescue medication, if Ibuprofen did not help. If these NSAIDs were still inadequate, 2 mg morphine was injected intravenously.
2.6. Main outcome
The incidence of severe pain within the 24-hour postoperative period was the primary endpoint. Secondary endpoints included the maximum NRS score, the number of patients who used analgesic medication, and the number of patients who required rescued analgesic medication within the same time.
2.7. Postoperative complications and length of hospital stay
PONV, fever, and the length of hospital stay (from the day of surgery up to discharge) were also collected.
2.8. Sample size and statistical analysis
In our preliminary analysis of data from 60 patients (30 patients with good sleep quality and 30 patients with poor sleep quality), the incidence rate of severe postoperative pain was 7% and 30%, respectively. A sample size of 88 (44 in each group) was required to detect a between-group difference, with anticipated α error (2 tailed) of 0.05 and β error 0.20 (power: 0.8). Finally, a sample size of 106 (53 in each group) was required to account for 20% attrition.
All statistical analyses were performed using IBM SPSS for Windows version 22.0 (SPSS, Chicago, IL). Normality of data distribution was assessed using the Kolmogorov–Smirnov test. Demographic data are presented as mean (standard deviation [SD]) or median (interquartile range); categorical variables are presented as frequencies (percentages). Between-group differences with respect to normally distributed continuous variables were assessed using the Student t test, while those with respect to nonnormally distributed continuous variables were assessed using the Mann–Whitney U test. Fisher exact Chi-squared test, Pearson Chi-squared test, or Cochran–Mantel–Haenszel test were used to assess between-group differences with respect to categorical variables, as appropriate. A linear mixed-effects model was used to analyze the change of NRS score during the 24-hour postoperative period. A 2-sided P value < .05 was considered indicative of statistically significant difference.
3. Results
Between April 2017 and June 2018, 232 female patients were assessed for eligibility and 108 patients were enrolled in this study. Among the 108 women, 53 patients (49%) had good sleep quality while 55 (51%) patients had bad sleep quality. Three dropped out of the study during follow-up in each group. Finally, a total of 102 patients were included in the final analysis (Fig. 1). The average PSQI score was 3.4 ± 1.3 in the good sleep group and 11.1 ± 2.6 in the poor sleep group. There was no significant between-group difference with respect to baseline demographic characteristics including age, height, weight, BMI, marital status, and the scores of SAS and SAD (Table 1).
Figure 1.
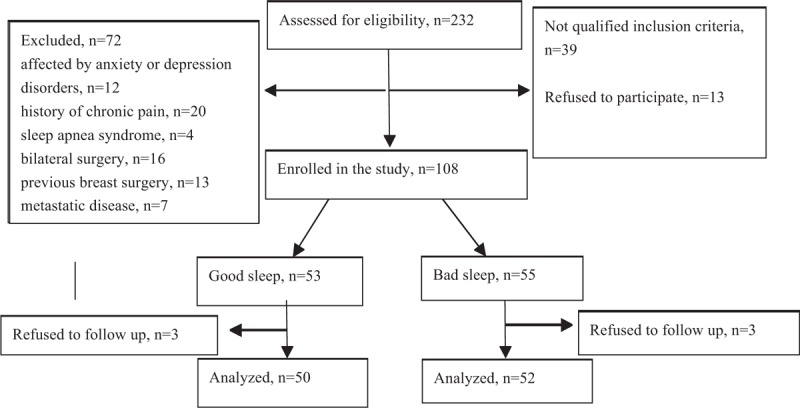
Schematic illustration of the study design and the patient-selection criteria.
Table 1.
Demographic characteristics, preoperative mental scores, and surgical and anesthetic parameters.
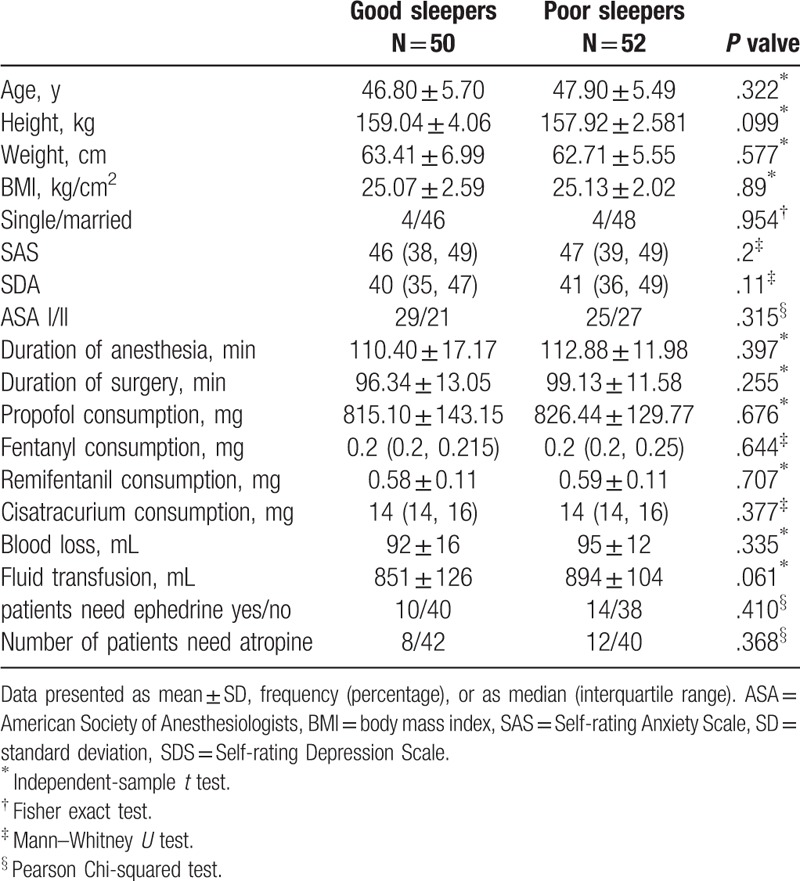
There was no significant between-group difference with respect to the American Society of Anesthesiologists grade, type of surgery, duration of surgery, duration of anesthesia, blood loss, fluid transfusion, and the consumption of propofol, fentanyl, remifentanil, cisatracurium, and vasoactive agent (Table 1).
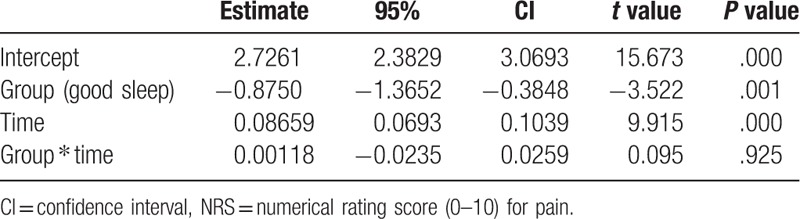
NRS score at different time-points during the first 24 hours is shown in Table 2. The pain scores were comparable in the 2 groups at T0 (P = .589); patients in the poor sleep group had significantly higher postoperative pain scores than those in the good sleep group at T1 (P = .002), T2 (P < .001), T3 (P < .001), and T4 (P = .002). A significant group effect was identified with a negative coefficient (estimate = –0.87500, P = .001), which indicated that the postoperative NRS scores in the good sleep group were significantly lower than those in the poor sleep group. Conversely, a significant effect of the postoperative time was identified with a positive coefficient (estimate = 0.08659, P < .001), which indicated that the NRS score increased within the first 24 hours postsurgery. Additionally, no significant interaction between groups and the postoperative time was found (estimate = 0.00118, P = .925), which indicated that the change in NRS scores was comparable in the 2 groups (Table 3).
Table 2.
Postoperative NRS score at different time-points during the first postoperative 24 hours.
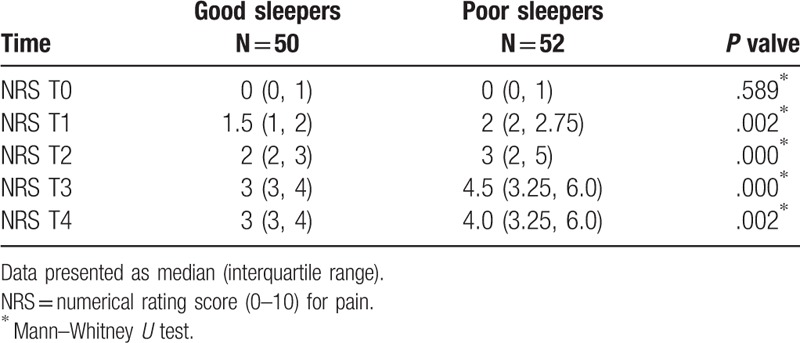
Table 3.
Effect of group and postoperative time on the change of NRS score.
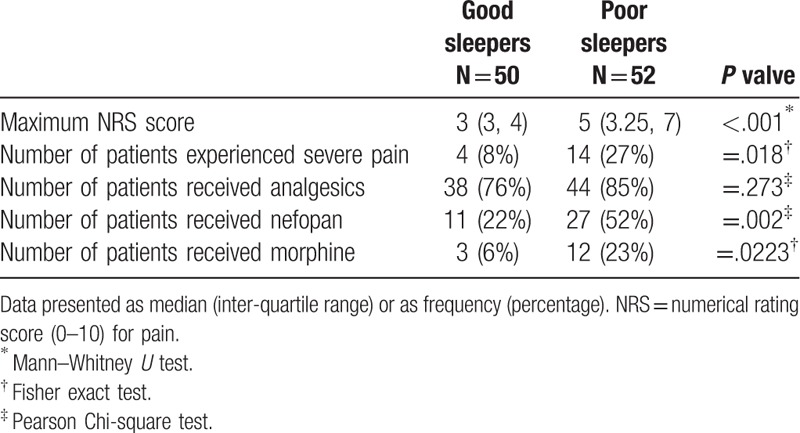
The incidence of severe pain in the poor sleep group was higher than that in the good sleep group (8% vs 27%, P = .018) (Table 4). In addition, maximum NRS pain score during the first postoperative 24 hours in the poor sleep group was higher than that in the good sleep group (5 [3.25, 7] vs 3 [3,4], P < .001). The percentage of patients who used analgesic medicine within the 24-hour postsurgical period in the good sleep group and the poor sleep group was 76% and 84.62%, respectively; there was no significant between-group difference in this respect (P = .273). However, the ratio of participants who required rescued analgesics within the same time was significantly different, 52% of participants used rescued analgesics-nefopan in the poor sleep group, compared with 22% in the good sleep group (P = .002); in addition, 23% of participants with poor sleep quality used rescued analgesics-morphine, compared with 6% in the good sleep group (P = .0223).
Table 4.
Maximum pain score, incidence of severe pain, and characterization of analgesic use during the first 24 h.
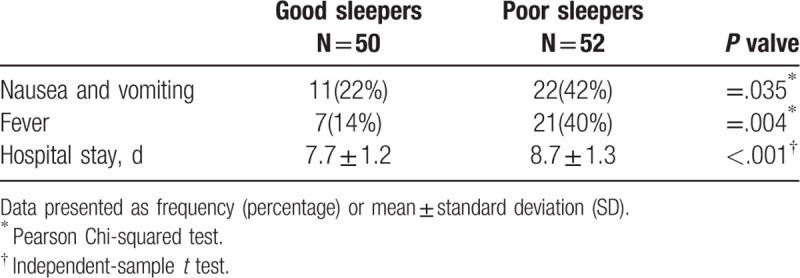
Data pertaining to postoperative complications and hospital stay are presented in Table 5. The incidence of PONV (P = .035) and postoperative fever (P = .004) in the poor sleep group during the first 24 hours was significantly higher than that in the good sleep group. In addition, patients with poor sleep quality had significantly longer hospital stay (P < .001).
Table 5.
Postoperative complications and length of hospital stay in the 2 groups.
4. Discussion
As anticipated, we found that breast cancer patients with poor sleep quality before surgery had higher incidence of severe postoperative pain, reported higher NRS scores, and demanded more rescued analgesics in the first postoperative 24 hours. What is unexpected was that the incidence of PONV and postoperative fever is higher in poor sleep group; in addition, patients with poor preoperative sleep had longer hospital stay.
In this study population, 51% of breast cancer patients scheduled for breast surgery were affected by poor sleep before surgery, which is consistent with the results of previous studies.[37,38] Adult women of all ages with breast cancer are affected by sleep disturbances. Poor sleep quality, frequent nocturnal awakenings, and insomnia are the main characteristics of sleep disorders experienced by breast cancer patients aged ≥50 years.[37] In a previous study, 53.5% of women with no preoperative breast pain reported sleep problems before breast cancer surgery as against 66.7% of patients who had preoperative breast pain.[38]
Of note, we found that patients with poor sleep quality before surgery were more likely to report higher postoperative pain scores (NRS); in addition, the incidence rate of severe pain was also higher in the poor sleep quality group. Although there was no significant between-group difference with respect to the ratio of participants received analgesics during the first postoperative 24 hours, the number of patients who required rescued analgesics was far greater in the poor sleep group. Our findings add to the growing body of evidence that impaired preoperative sleep is a consistent predictor of postoperative pain. In 2017, one feasibility study found that a prophylactic extended time in bed before joint replacement surgery in patients with moderately short sleep resulted in increased sleep time and reduced postoperative pain and analgesic use[39]; this provided an indirect evidence of the relationship of preoperative sleep with postoperative pain. There were also some direct evidences. In a cross-sectional study of adults undergoing scheduled abdominal or orthopedic surgery, self-reported chronic sleep disturbance was the only predictor of severe postoperative pain at rest; it was associated with a 2- to 3-fold higher risk of severe postoperative pain.[40] However, the authors did not use standard sleep assessment tools for a comprehensive assessment of preoperative sleep quality. Caroline et al used Actiwatch to evaluate patients’ sleep on just the night before breast-conserving surgery; they found actigraphy-determined total time in bed, sleep onset time, sleep end time, and total sleep time (sleep duration) the night before surgery were not associated with postoperative pain, but there existed a significant negative relationship between sleep efficiency on the night before surgery and the severity of postoperative pain.[23] However, assessment of sleep only on the night before operation is not enough to reflect the overall sleep status of patients; in addition, the use of Actiwatch is liable to disrupt the patient's normal sleep and is unpractical to assess all patients’ sleep condition. In clinical settings, sleep disturbance lasting for a duration of 2 to 3 weeks is typically used to differentiate between transient and persistent sleep disorders. Therefore, the use of PSQI in our study allowed us to distinguish between most transient and persistent sleep disturbances. Beyond that, PSQI is a convenient, practical, and economic tool to assess patients’ sleep condition. In addition, the brightest spot of our study is that we excluded all patients with anxiety and depression, which is an important risk factor of postoperative pain. Indeed, the anxiety and depression scores of participants in the poor sleep group were slightly higher than that in the good sleep group in our study, but there was no difference between groups. Studies suggest that sleep disturbances may impair important physiological processes such as dopaminergic signaling, opioidergic signaling, and emotional regulation, which contribute to the development of hyperalgesia and maintenance of chronic pain.[15] Since the mechanism of acute postoperative pain is different from that of chronic pain, future studies investigating the association of sleep and acute postoperative pain should also focus on the underlying mechanisms.
To the best of our knowledge, this is the first study that reveals that women with poor preoperative sleep quality are more likely to experience PONV. The incidence of PONV in the poor sleep group was almost twice as high as that in the good sleep group (42% vs 22%, P < .05). Indeed, women undergoing breast surgery under general anesthesia are at a particularly high risk of PONV; among patients who received no antiemetic medication, the reported incidence is 60% to 80%.[41–43] In this study, all participants received antiemetic medication at the completion of surgery, which explains the low incidence rate of PONV in our study population. In a previous study of breast cancer patients, poor sleep quality was associated with an increased risk of chemotherapy-induced nausea and vomiting[44] due to autonomic nervous system dysfunction induced by poor sleep. Although the side effect of nefopam is another factor to explain the higher incidence of PONV in the poor sleep group.[45] In the past decades, numerous studies have identified the risk factors of PONA, such as female gender, history of vestibular diseases, use of opioids, and absence of a smoking history.[46] It seems likely that preoperative sleep disturbance is another important correlate of PONV that should not be ignored. Future studies should investigate the relationship between preoperative sleep quality and PONV.
The incidence of postoperative fever was higher in the poor sleep group, which can be explained by the relationship between sleep and inflammatory system. The current viewpoint is that sleep disorder has an effect on the inflammatory system activation, which is different from direct stimulation of the immune system by infection or injury.[47,48] Levels of interleukin (IL)-1 beta, IL-6, and tumor necrosis factor (TNF)-alpha were found to be elevated after acute sleep deprivation or chronic partial sleep deprivation in healthy participants.[49–54] In addition, sleep disturbance (as assessed by validated questionnaires) was shown to be associated with increased levels of C-reactive protein and IL-6.[55] However, the mechanism by which sleep disorder activates inflammatory pathways is still unclear. Further studies are required to confirm the association between preoperative sleep quality and the change in inflammatory system in patients undergoing surgery.
Some limitations of our study need to be acknowledged. Firstly, the sleep quality was only assessed using the PSQI; we did not evaluate the sleep patterns using more objective methods such as polysomnography or actigraphy. Secondly, it is not a randomised, controlled, blinded trail. It is a single-center, relatively small population trial, a multicenter trial is necessary to verify this result. Finally, acute postoperative pain was just reported within the postoperative 24 hours, and we did not examine the duration of postoperative pain and persistent postoperative pain after breast cancer surgery.
5. Conclusions
Breast cancer patients with poor sleep quality prior to surgery reported higher postoperative pain scores in the first postoperative 24 h, required more analgesics, experienced more complications (such as PONV), and had longer hospital stay. Further studies using more objective measures of sleep are required to validate our findings. We suggest that Chinese clinicians should pay more attention to preoperative sleep quality of breast cancer patients.
Author contributions
Conceptualization: Jin-ping Wang, Su-fen Lu, Li-na Guo, Chun-guang Ren, Zong-wang Zhang.
Data curation: Jin-ping Wang, Su-fen Lu, Li-na Guo, Chun-guang Ren, Zong-wang Zhang.
Formal analysis: Jin-ping Wang.
Funding acquisition: Jin-ping Wang.
Investigation: Jin-ping Wang, Li-na Guo.
Methodology: Zong-wang Zhang.
Project administration: Zong-wang Zhang.
Resources: Chun-guang Ren, Zong-wang Zhang.
Software: Jin-ping Wang, Su-fen Lu.
Supervision: Jin-ping Wang, Su-fen Lu.
Validation: Jin-ping Wang, Su-fen Lu, Zong-wang Zhang.
Visualization: Jin-ping Wang.
Footnotes
Abbreviations: NRS = numerical rating score, PACU = postanesthesia care unit, PONV = postoperative nausea and vomiting, PSQI = Pittsburgh Sleep Quality Index, SAS = Self-rating Anxiety Scale, SDS = Self-rating Depression Scale.
How to cite this article: Wang Jp, Lu Sf, Guo Ln, Ren Cg, Zhang Zw. Poor preoperative sleep quality is a risk factor for severe postoperative pain after breast cancer surgery. Medicine. 2019;98:44(e17708).
Data availability: The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet 2011;377:2215–25. [DOI] [PubMed] [Google Scholar]
- [2].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- [3].Pozek JP, Beausang D, Baratta JL, et al. The acute to chronic pain transition: can chronic pain be prevented? Med Clin North Am 2016;100:17–30. [DOI] [PubMed] [Google Scholar]
- [4].Butrick CW. Persistent postoperative pain: pathophysiology, risk factors, and prevention. Female Pelvic Med Reconstr Surg 2016;22:390–6. [DOI] [PubMed] [Google Scholar]
- [5].Kraychete DC, Sakata RK, Lannes Lde O, et al. Postoperative persistent chronic pain: what do we know about prevention, risk factors, and treatment. Braz J Anesthesiol 2016;66:505–12. [DOI] [PubMed] [Google Scholar]
- [6].Argoff CE. Recent management advances in acute postoperative pain. Pain Pract 2014;14:477–87. [DOI] [PubMed] [Google Scholar]
- [7].Mitra S, Carlyle D, Kodumudi G, et al. New Advances in Acute Postoperative Pain Management. Current pain and headache reports 2018;22:35. [DOI] [PubMed] [Google Scholar]
- [8].Rawal N. Current issues in postoperative pain management. Eur J Anaesthesiol 2016;33:160–71. [DOI] [PubMed] [Google Scholar]
- [9].Doufas AG, Panagiotou OA, Ioannidis JP. Concordance of sleep and pain outcomes of diverse interventions: an umbrella review. PloS One 2012;7:e40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Koffel E, Kroenke K, Bair MJ, et al. The bidirectional relationship between sleep complaints and pain: analysis of data from a randomized trial. Health Psychol 2016;35:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Affleck G, Urrows S, Tennen H, et al. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain 1996;68:363–8. [DOI] [PubMed] [Google Scholar]
- [12].Onen SH, Onen F, Courpron P, et al. How pain and analgesics disturb sleep. Clin J Pain 2005;21:422–31. [DOI] [PubMed] [Google Scholar]
- [13].Bigatti SM, Hernandez AM, Cronan TA, et al. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum 2008;59:961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Park SJ, Yoon DM, Yoon KB, et al. Factors associated with higher reported pain levels in patients with chronic musculoskeletal pain: a cross-sectional, correlational analysis. PloS One 2016;11:e0163132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain 2013;14:1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Andreucci MA, Campbell P, Dunn KM. Are sleep problems a risk factor for the onset of musculoskeletal pain in children and adolescents? A systematic review. Sleep 2017;40: doi: 10.1093/sleep/zsx093. [DOI] [PubMed] [Google Scholar]
- [17].Bromberg MH, Gil KM, Schanberg LE. Daily sleep quality and mood as predictors of pain in children with juvenile polyarticular arthritis. Health Psychol 2012;31:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain 2008;137:202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Smith MT, Edwards RR, McCann UD, et al. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep 2007;30:494–505. [DOI] [PubMed] [Google Scholar]
- [20].Kundermann B, Spernal J, Huber MT, et al. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med 2004;66:932–7. [DOI] [PubMed] [Google Scholar]
- [21].Rosseland R, Pallesen S, Nordhus IH, et al. Effects of sleep fragmentation and induced mood on pain tolerance and pain sensitivity in young healthy adults. Front Psychol 2018;9:2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lewandowski AS, Palermo TM, De la Motte S, et al. Temporal daily associations between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain 2010;151:220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wright CE, Bovbjerg DH, Montgomery GH, et al. Disrupted sleep the night before breast surgery is associated with increased postoperative pain. J Pain Symptom Manage 2009;37:352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rosenberg J. Sleep disturbances after non-cardiac surgery. Sleep Med Rev 2001;5:129–37. [DOI] [PubMed] [Google Scholar]
- [25].Gogenur I, Wildschiotz G, Rosenberg J. Circadian distribution of sleep phases after major abdominal surgery. Br J Anaesth 2008;100:45–9. [DOI] [PubMed] [Google Scholar]
- [26].Krenk L, Jennum P, Kehlet H. Sleep disturbances after fast-track hip and knee arthroplasty. Br J Anaesth 2012;109:769–75. [DOI] [PubMed] [Google Scholar]
- [27].Van Onselen C, Paul SM, Lee K, et al. Trajectories of sleep disturbance and daytime sleepiness in women before and after surgery for breast cancer. J Pain Symptom Manage 2013;45:244–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Otte JL, Carpenter JS, Manchanda S, et al. Systematic review of sleep disorders in cancer patients: can the prevalence of sleep disorders be ascertained? Cancer Med 2015;4:183–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- [30].Zung WW. A Self-Rating Depression Scale. Arch Gen Psychiatry 1965;12:63–70. [DOI] [PubMed] [Google Scholar]
- [31].Zung WW. A rating instrument for anxiety disorders. Psychosomatics 1971;12:371–9. [DOI] [PubMed] [Google Scholar]
- [32].McDowell I. Measuring Health: A Guide to Rating Scales and Questionnaires. USA: Oxford University Press; 2006. [Google Scholar]
- [33].Yin W, Pang L, Cao X, et al. Factors associated with depression and anxiety among patients attending community-based methadone maintenance treatment in China. Addiction (Abingdon, Engl) 2015;110Suppl 1:51–60. [DOI] [PubMed] [Google Scholar]
- [34].Peters ML, Sommer M, de Rijke JM, et al. Somatic and psychologic predictors of long-term unfavorable outcome after surgical intervention. Ann Surg 2007;245:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Onishi E, Murakami M, Nishino R, et al. Analgesic effect of double-level retrolaminar paravertebral block for breast cancer surgery in the early postoperative period: a placebo-controlled, randomized clinical trial. Tohoku J Exp Med 2018;245:179–85. [DOI] [PubMed] [Google Scholar]
- [36].Bruce J, Thornton AJ, Scott NW, et al. Chronic preoperative pain and psychological robustness predict acute postoperative pain outcomes after surgery for breast cancer. Br J Cancer 2012;107:937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Enderlin CA, Coleman EA, Cole C, et al. Subjective sleep quality, objective sleep characteristics, insomnia symptom severity, and daytime sleepiness in women aged 50 and older with nonmetastatic breast cancer. Oncol Nurs Forum 2011;38:E314–25. [DOI] [PubMed] [Google Scholar]
- [38].Van Onselen C, Aouizerat BE, Dunn LB, et al. Differences in sleep disturbance, fatigue and energy levels between women with and without breast pain prior to breast cancer surgery. Breast 2013;22:273–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Roehrs TA, Roth T. Increasing presurgery sleep reduces postsurgery pain and analgesic use following joint replacement: a feasibility study. Sleep Med 2017;33:109–13. [DOI] [PubMed] [Google Scholar]
- [40].Mamie C, Bernstein M, Morabia A, et al. Are there reliable predictors of postoperative pain? Acta Anaesthesiol Scand 2004;48:234–42. [DOI] [PubMed] [Google Scholar]
- [41].Oddby-Muhrbeck E, Jakobsson J, Andersson L, et al. Postoperative nausea and vomiting. A comparison between intravenous and inhalation anaesthesia in breast surgery. Acta Anaesthesiol Scand 1994;38:52–6. [DOI] [PubMed] [Google Scholar]
- [42].Sadhasivam S, Saxena A, Kathirvel S, et al. The safety and efficacy of prophylactic ondansetron in patients undergoing modified radical mastectomy. Anesth Analg 1999;89:1340–5. [DOI] [PubMed] [Google Scholar]
- [43].Hammas B, Thorn SE, Wattwil M. Superior prolonged antiemetic prophylaxis with a four-drug multimodal regimen - comparison with propofol or placebo. Acta Anaesthesiol Scand 2002;46:232–7. [DOI] [PubMed] [Google Scholar]
- [44].Jung D, Lee KM, Kim WH, et al. Longitudinal association of poor sleep quality with chemotherapy-induced nausea and vomiting in patients with breast cancer. Psychosom Med 2016;78:959–65. [DOI] [PubMed] [Google Scholar]
- [45].Koh HJ, Joo J, Kim YS, et al. Analgesic effect of low dose nefopam hydrochloride after arthroscopic rotator cuff repair: a randomized controlled trial. J Clin Med 2019;8: pii: E553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Apfel CC, Laara E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999;91:693–700. [DOI] [PubMed] [Google Scholar]
- [47].Mullington JM, Simpson NS, Meier-Ewert HK, et al. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab 2010;24:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry 2016;80:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dinges DF, Douglas SD, Hamarman S, et al. Sleep deprivation and human immune function. Adv Neuroimmunol 1995;5:97–110. [DOI] [PubMed] [Google Scholar]
- [50].Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun 2007;21:1050–7. [DOI] [PubMed] [Google Scholar]
- [51].Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep 2007;30:1145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 2004;43:678–83. [DOI] [PubMed] [Google Scholar]
- [53].Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol 2001;107:165–70. [DOI] [PubMed] [Google Scholar]
- [54].van Leeuwen WM, Lehto M, Karisola P, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PloS One 2009;4:e4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Burgos I, Richter L, Klein T, et al. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun 2006;20:246–53. [DOI] [PubMed] [Google Scholar]


