Supplemental Digital Content is available in the text
Keywords: diarrhea, drug-induced hypersensitivity syndrome, fecal microbiota transplantation, intestinal failure, multiple organ dysfunction syndrome
Abstract
The aim of this study was to assess effectiveness of fecal microbiota transplantation (FMT) in treating intestinal failure associated with drug-induced hypersensitivity syndrome (DIHS).
A 32-year-old Chinese woman, who developed DIHS-associated multiple organ dysfunction syndrome (MODS) manifesting as combined dysfunction of the intestine, liver, and kidney, was treated with 4 times of FMT at a frequency of once every 6 days. The structure and composition of the patient's fecal microbiota were analyzed by 16S rRNA-based molecular techniques. The clinical outcomes after FMT treatment were assessed by abdominal contrast-enhanced computed tomography (CT), characterization of fecal microbiota, measurement of serum inflammatory markers, and other clinical examinations.
After 4 rounds of FMT were administered, the patient showed dramatic improvement in MODS and severe diarrhea with these clinical conditions under control. We consistently observed significant alteration in her gut microbiota, mainly involving considerable enrichment in Firmicutes members and depletion of Proteobacteria opportunistic organisms. Moreover, this reconstituted bacterial community composition correlated with fecal output, T helper cells, and inflammatory markers. Abdominal contrast-enhanced CT scans before and after FMT indicated significant improvement in inflammation and edema within the small intestine and colon of the patient. Notably, after completion of the fourth FMT, the level of inflammation in the intestine and colon had returned to normal. Over 6 months of follow-up, the intestinal mucous remained normal.
Our results represent a breakthrough in the clinical management of MODS and suggest new therapeutic avenues to pursue for microbiota-related indications.
1. Introduction
Multiple organ dysfunction syndrome (MODS), which usually involves >2 organs with impaired function, is a common complication among patients in intensive care units (ICUs) and requires intervention for maintenance of homeostasis.[1] To date, the pathogenic mechanisms underlying MODS remain largely unclear. It has been suggested that the development MODS is closely correlated with the translocation of intestinal flora, ischemia reperfusion, dysregulated expression of immune system factors, among others.[2] Of these risk factors, the translocation of intestinal bacteria translocation was considered as possibly a causative source of systemic inflammatory that could subsequently initiate MODS.[3,4] In 1986, Carrico et al indicated, for the first time, that the dysfunction on the gut could trigger MODS, suggesting an important role for the intestinal tract in response to a variety of stressors including trauma, burn, and infection, and that the gut is regarded an initiating organ of MODS.[5] Therefore, an insightful investigation into the role of the intestinal tract may help decipher the pathogenic mechanism and develop novel approaches for the prevention, intervention, and treatment of MODS.
In fact, the gut has long been hypothesized to be “the motor” of MODS.[4] It is of note that the microbiome is markedly altered in patients admitted to ICUs.[6] A recent study of 115 stool samples from critically ill patients revealed that levels of Firmicutes and Bacteroidetes—bacteria that are prevalent in the healthy intestine—were significantly decreased in comparison with those in controls while increases were seen in opportunistic Proteobacteria.[7] Thus, maintenance of balanced intestinal microbiota, blocking of the translocation of intestinal flora, and avoidance of systematic infection, is of great value in the prevention and intervention of the development of MODS.
Our clinical research team has long-standing interest in the application of fecal microbiota transplantation (FMT) to restore the balance of intestinal microbiota, especially in patients with MODS possibly resulting from dysfunction of the gut with unbalanced intestinal microbiota. In our recent clinical practice, we successfully modified the intestinal microbiota by using FMT in patients who were diagnosed with MODS and diarrhea following severe sepsis.[6] In that study, MODS and diarrhea were greatly alleviated in the 2 patients following FMT procedures,[6] and FMT was proved to be an effective therapy for MODS with mainly intestinal failure that was induced by severe infection.
In the present study, we describe a rare case of MODS in a Chinese woman with drug-induced hypersensitivity syndrome (DIHS) who presented with intestinal failure with dysfunction on the small and the entire colon and was successfully treated with FMT. We further investigated the alterations in the intestinal microbiota before and after FMT and profiled immunological markers in the patient.
2. Patient and methods
2.1. Patient and healthy donor
A 32-year-old Chinese woman was admitted to our hospital with fever, rash (lasting >30 days), and diarrhea (lasting >20 days). Thirty-five days before admission, she developed fever (peak body temperature 39.2°C) and cough, which was treated with cephalosporins cefalexin for 5 days. Three weeks after the cefalexin was stopped, an itchy rash appeared all over the body. As the fever returned and persisted, the patient was hospitalized and diagnosed with erythema multiforme. Two years ago, she started using a weight-loss capsule, commonly called “Likeshou.” The patient's medical history showed no allergy to medications.
She was administered antiallergic treatment such as prednisone (40 mg q.d.) and levofloxacin plus cefuroxime. Six days later, the patient developed yellowish, watery diarrhea (700–1000 mL average volume, 3–4 bowel movements per day). A diagnosis of infectious diarrhea was considered, and the patient was treated with Bifidobacterium. However, the symptoms persisted, and the diarrhea got worse (approximately 1200 mL, with 10–20 bowel movements per day). The patient was transferred to the intensive care unit (ICU) due to signs of the development of MOF, a progressive and critical condition that is characterized by combined dysfunction of several major organs, first the intestine, then the liver and the kidney.
A 23-year-old, male graduate student who was willing to participate in this study, was chosen to donate fecal microbiota. Before the study, he tested negative for both blood-borne diseases and common stool pathogens including but not limited to HAV-IgM, HBsAg, HCV-IgG, HDV-Ab, HEVIgM, HIV, CMV, syphilis, roundworm, pinworm, hookworm, amoeba, and duovirus, among others, in his fecal samples.
The procedures performed in the patient and the healthy donor were reviewed and approved by the Administrative Panel for Medical Research on Human Subjects of Daping Hospital (the ethics committee of our hospital). The patient and healthy control individual gave us their written informed consent to undergo the procedure.
2.2. Clinical examination
When the patient came to our hospital, physical examination revealed fever (38.7°C) and percussion tenderness in the left lower quadrant, without any abdominal mass. Laboratory tests showed a total white blood cell (WBC) count of 9.94 × 109 cells/L; neutrophil count (NPC) of 8.67 × 109 cells/L; C-reactive protein (CRP) level of 47 mg/L; erythrocyte sedimentation rate (ESR) of 37 mm/h; interleukin-6 (IL-6) of 80.93 pg/mL; and procalcitonin (PCT) of 1.90 ng/mL. We tested whether the patients were possibly infected by the virus. The patient tested negatively for HHV-6, but the EBV DNA was elevated at 5.68 × 105 (the normal level is <500). The CMV DNA level was 7.19 × 104 (the normal level is <500).
Contrast-enhanced computed tomography (CT) of the abdomen demonstrated thickening of the walls of the small intestine (jejunum and ileum) and colon with adjacent fat stranding in relation to a diverticulum, hepatosplenomegaly, and normal-sized abdominal lymph nodes (Fig. 1). Findings suggested early signs of MOF.
Figure 1.
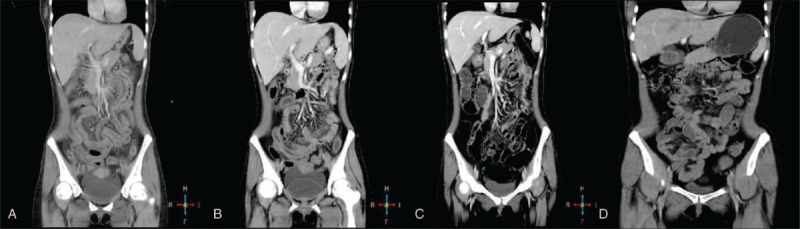
Abdominal computed tomography (CT) scan images of the patient. (A) Pre-treatment CT image showing thickening of the small intestine wall and enlarged liver and spleen; (B) CT image during treatment after initial fecal microbiota transplantation (FMT) procedure suggesting relief of inflammatory and swollen intestines; (C) CT image after the fourth FMT showing significant improvement; and (D) CT scan 6 months after recovery demonstrating normal small and large intestine with no signs of inflammation.
According to the diagnostic criteria for DIHS established by the Japanese group, based on the combination of rash, fever, impairment of multiple organs, and prolonged clinical symptoms with administration of causative drugs and presence of EBV/CMV infection, the patient was finally diagnosed with DIHS.
The examination of intestinal flora indicated significantly decreased levels of beneficial bacteria, including Bifidobacterium, Lactobacillus, and Clostridium, which suggested an imbalance in gut flora or flora disturbance III (Fig. 2). In view of these findings, antibiotic treatment was stopped and FMT was considered. A multidisciplinary team was involved in management of this patient. The treatment plan included FMT once every week, high-dose intravenous methylprednisolone pulse therapy with an initial dose of 40 mg/kg/day, intravenous immune globulin pulse therapy for 3 days, and anti-EBV and CMV treatment with ganciclovir.
Figure 2.
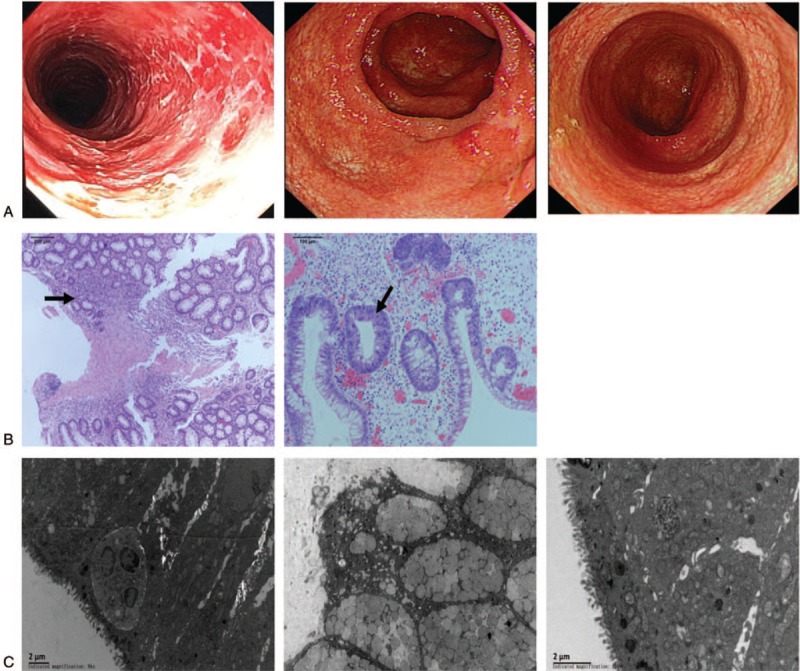
Serial clinical features of the patient at different time points. (A) Enteroscopic images at different time points: pre-fecal microbiota transplantation (FMT): mucosal exfloliation, erosions, congestion and swelling in the small intestine, colon and rectum; after the 4th FMT treatment: signs of disease resolution with nearly normal mucosal surface of the ileocecal, ascending colon, and rectum; and 6-month following discharge from the hospital: complete recovery of mucosal surface. (B) Histopathological examination of ascending colon: mucosal erosion, decreased numbers of goblet cells, increased numbers of lymphocytes, and small amounts of eosinophils in the lamina propria, as well as diffuse eosinophilic infiltration can be observed. (C) Histopathological examination of colon mucosa under a transmission electronic microscope: epithelial cell damage, enlarged gap between cells, shortened microvillus, and disordered crypt structure can be seen.
2.3. Characterization of fecal microbiota
The fecal microbiota of the patient was analyzed using 16S rRNA gene-based sequencing as described previously.[6,8] In brief, genomic DNA was isolated from the studied fecal samples. Following a standard purification procedure, the concentration and the quality of the DNA samples were examined on a NanoDrop spectrophotometer (ThermoFisher Scientific, Wilmington, DE) and by agarose gel electrophoresis, respectively. The variable V3V4 regions of the 16S rRNA genes were amplified by polymerase chain reaction (PCR) using the following primers: 5’-GTACTCCTACGGGAGGCAGCA-3’ and 5’-GTGGACTACHVGGGTWTCTAAT-3’. The purified PCR products were used to prepare the sequencing library using the TruSeq DNA Kit from Illumina following the manufacturer's manual, which were subjected to gene sequencing using Reagent Kit v3 on the MiSeq sequencer (Illumina, SanDiego, CA).[9,10] With the Quantitative Insights into Microbial Ecology (QIIME) pipeline,[11] we processed raw sequences into tags based on the overlapping relationship, and separated reads with barcodes of each studied sample with removing the reads with low quality. Subsequently, we clustered the processed tags and assigned the operational taxonomic units (OTUs) to taxa by matching to the Greengenes database.[12]
2.4. Procedures for FMT
After the severe disturbance of the fecal microbiota was revealed in the patient and with our previous experience in successful treatment of microbiota disorders using FMT, we scheduled a FMT treatment plan for the patient. The procedures used for FMT were similar to those that we described in 2 patients with diarrhea following severe sepsis.[6] Briefly, the infusion of donor feces was conducted in the patient on day 0, and she was given a total of 4 times of FMT at frequency of once every 6 days.
2.5. Analysis of serum inflammatory markers
The blood samples were collected from the patients for analysis of several serum inflammatory markers. Serum levels of IL-6 were examined using an enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems (Abingdon, UK). Analysis of inflammatory markers including PCT, CRP, and the ESR was conducted by the Department of Laboratory Testing in our hospital, as we described previously.[6]
2.6. Statistical analysis
Linear regression analysis with the Pearson test was performed to determine the relationship between 2 variables. OTU and principal component (PC) analyses were conducted using CANOCO software 4.5 from Microcomputer Power (Ithaca, NY). A P value <0.05 was considered statistically significant.
3. Results
3.1. Treatment and clinical outcomes
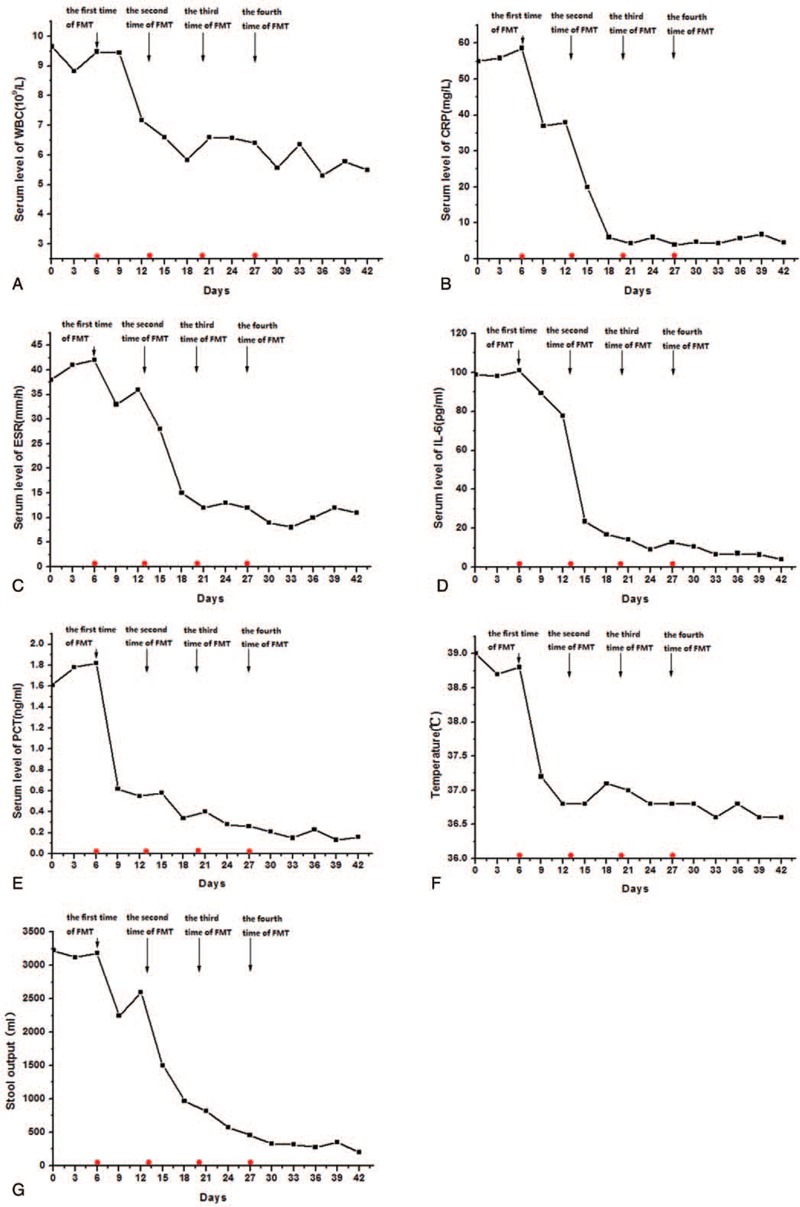
Based on the evidence of disturbed microbiota, and after administration of antibiotics and probiotics as conventional treatment strategies had proven ineffective in the patient, we used FMT to treat MODS and severe diarrhea. As expected, the response to the FMT treatment was satisfactory in the patient. On day 1 following the second round of FMT, she experienced a drop in body temperature from 38.9°C to 37.3°C (Fig. 3F). Notably, after completion of the second round of FMT, the patient had recurrent MODS symptoms, but blood cultures remained sterile. The frequency of stool output in the patient declined following the third FMT (Fig. 3 G), and her stools became well-formed. After the fourth round of FMT was complete, the frequency and volume of stools returned to normal levels. Additionally, the serum levels of inflammatory markers such as CRP, PCT, ESR, and IL-6 were significantly reduced after the second round of FMT in contrast to those at day 0 (Fig. 3A–E). Furthermore, we observed that the activities of serum aspartate aminotransferase and alanine aminotransferase gradually decreased and eventually returned to normal values in the patient (Supplemental Digital Content (Fig. 1)). Notably, the patient's level of consciousness improved as well. The EBV and CMV DNA levels were back to within normal after anti-viral treatment for 21 days. In addition, normal stool consistency and body temperature were restored, and the patient was discharged after 2 months of admission in the ICU.
Figure 3.
Serial laboratory test results for the patient at different time points. Serial lab tests at different time points: (A) WBC counts; (B) CRP; (C) ESR; (D) IL-6; (E) PCT; (F) body temperature; g) daily output of stools; and (H) stool club ratio. CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, IL-6 = interleukin-6, PCT = procalcitonin, WBC = white blood cell.
3.2. Comparison of abdominal CT images, colonoscopy findings, and pathological features of intestinal mucous member before and after FMT
Abdominal contrast-enhanced CT scans and colonoscopies undertaken before and after FMT at different time points indicated significant improvement in inflammation and edema of the small intestine and colon in the patient. Before FMT, abdominal CT images demonstrated signs of inflammation and edema in the small intestine and the colon. After the first FMT, intestinal mucosal inflammation and edema were markedly reduced. Notably, after completion of the fourth FMT, the intestine and colon returned to normal with regard to inflammation. During a 6-month follow-up, the intestinal mucous remained normal (Fig. 1). On colonoscopic examination before FMT, stripping of the entire colonic mucous membrane, and severe congestion, swelling, and erosions of the intestinal wall were apparent (Fig. 2A). We also performed pathological analysis of intestinal mucous membrane and found mucosal erosion, decreased numbers of goblet cells, increased numbers of lymphocytes, and small amounts of eosinophilic in the lamina propria as well as diffuse eosinophilic infiltration under a microscope (Fig. 3B). Histopathological examination of the colon mucosa under a transmission electronic microscope before FMT displayed: epithelial cell damage, enlarged gaps between cells, shortened microvillus, and a disordered crypt structure (Fig. 3C). After completion of FMT, the intestinal mucosa recovered completely.
3.3. Correction of disturbed intestinal microflora following FMT
The abundances of nonpathogenic microbiota (ie, Streptococcus, Streptococcus thermophiles, Bacillus acidi lactici, Bacillus coli, Lactobacillus plantarum) in fecal samples of the healthy donor were higher than those of pathogenic microbiota (ie, Enterobacter cloacae, Klebsiella pneumoniae, Yersinia enterocolitica,). In agreement with the results obtained from the molecular phylogenetic tree, the OUT and PC analyses (Fig. 4) revealed that the microbiome structure of the patient was largely distinct from that of the healthy donor. Notably, the gut microbiota composition in the patient exhibited a trend toward the microbiota composition of the feces of the healthy donor, especially after completion of the fourth round of FMT.
Figure 4.
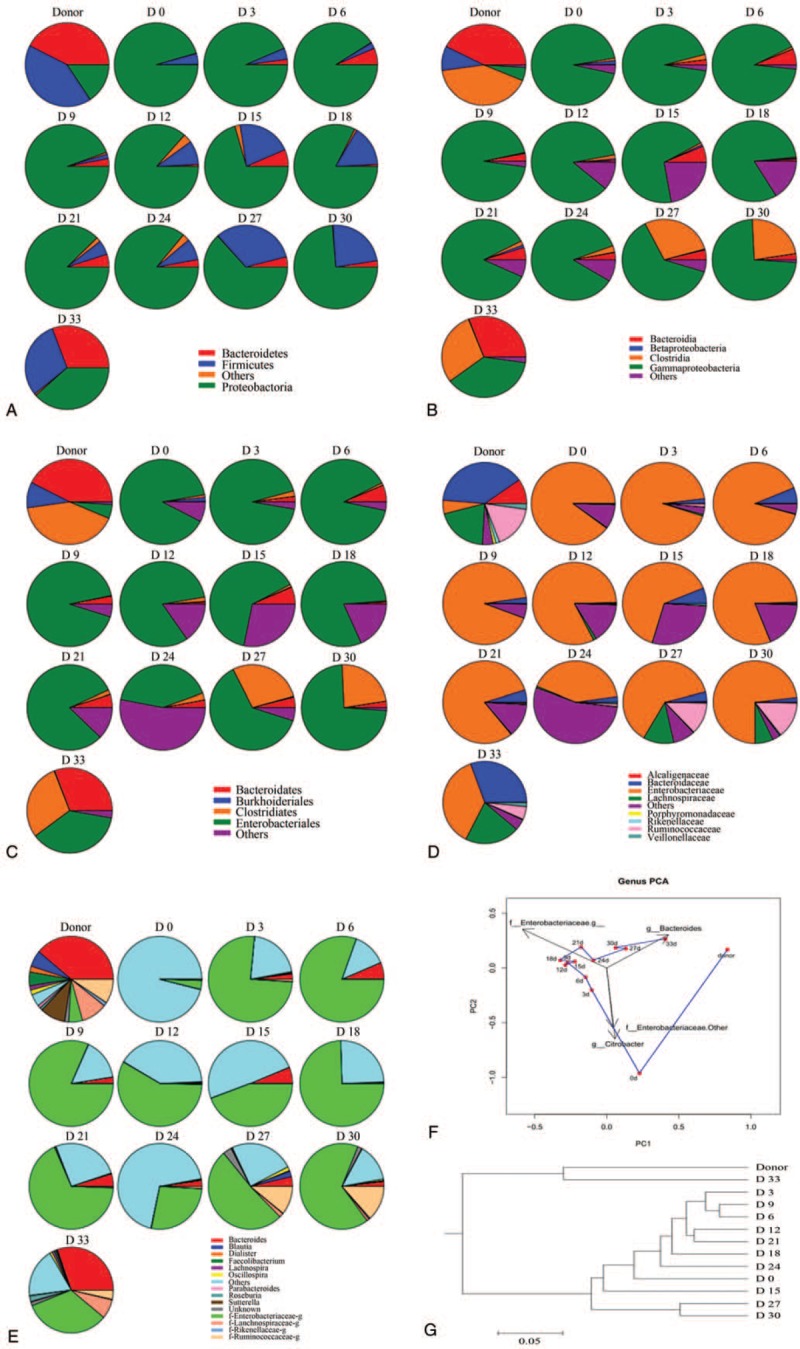
Analysis of intestinal flora of the patient. (A-E) Differences in bacterial composition between fecal samples. Colors represent bacterial composition in fecal samples. (A) phyla; (B) classes; (C) orders; (D) families, or (E) genera in the patient's fecal samples. (F) Principal component analysis of bacteria based on the relative abundance of bacterial genera. (G) Clustering tree of the patient. Levels of beneficial bacteria including, Bifidobacterium, Lactobacillus, and Clostridium were found to be significantly decreased. After the patient underwent fecal microbiota transplantation, levels of these bacteria increased gradually, returning to levels similar to those of the donor.
We found that bacteria in the Firmicutes phylum were significantly increased at 24 days after the first FMT in contrast to day 0 in this patient (Fig. 4A). Notably, bacteria in the Bacteroidetes and Firmicutes phyla also were increased to levels approximately equal to those in the donor after 33 days from the first FMT. In addition, there was a growing trend in the expression of Proteobacteria phylum genes by 9 days following FMT treatment, and then it slowly decreased. Finally, stabilization at a level approximately equivalent to that in the healthy donor occurred at 33 days after the first FMT (Fig. 4A).
The patient showed alterations in the abundances of fecal microbiota at the class and order level, and these changes were likely to follow those of the health donor (Fig. 4B and C). Additionally, we found that the bacteria in the Lachnospiraceae, Ruminococcaceae, and Veillonellaceae families were significantly enriched and contributed largely to the boom in Firmicutes after FMT treatment was implemented (Fig. 4D), whereas bacteria in the Proteobacteria, Enterobacteriaceae, and Alcaligenaceae families were significantly declined in this patient. Furthermore, analysis at the genus level showed that Roseburia, Dialister, Prevotella, Bacteroides, Oscillospira, and Faecalibacterium were increased after the patient was treated with FMT (Fig. 4E).
4. Discussion
MODS is a life-threatening and complex condition with multiple etiological factors. Of these, has been found to eventually progress to MODS and is considered the most common cause of this acute serious syndrome.[13] Until now, most previous studies and clinical case reports have focused on the control of inflammatory responses for managing patients with MODS.[14] Recently, increasing evidence has demonstrated a tight link between intestinal microflora dysregulation and the development of MODS.[6] Also recently, we have successfully corrected the unbalanced intestinal microbiota in 2 patients with MODS and diarrhea resulting from sepsis.[6] Building upon our extensive experience with application of FMT for modification of altered gut microbiota in MODS, in this study, we performed FMT in an unusual case of suspected DIHS in attempt to treat intestinal failure in MODS. To the best of our knowledge, this is the first study that has shown the effectiveness of FMT for intestinal failure in MODS following DIHS.
DIHS is a rare disorder with an immune-mediated systemic reaction to an offending drug and is mainly characterized by rash, fever, and progressive deterioration of multiple organs; the liver, kidneys, and lungs are most commonly involved.[15] To our knowledge, intestinal failure has not been reported in DIHS. We present an unusual case of suspected DIHS caused by inappropriate use of the antibiotic cefalexin. Cefalexin is a broad-spectrum, beta-lactam antibiotic in the class of cephalosporins with main adverse drug reactions similar to those of other antibiotics within the class, including anaphylactic reaction and drug eruption. Notably, severe anaphylactic reaction is most likely to cause DIHS, especially MODS, and several beta-lactam antibiotics induce DIHS, including carbamazepine, phenobarbitonen, allopurinol, salazosulfadimidine, cefalosporins, among others. Intestinal failure was successfully treated with FMT, which appeared to be highly effective in this case. Perhaps, intestinal microbiota dysregulation might play an important role in the development of DIHS. Considering this possibility, the restoration of intestinal function in our patient could be largely attributed to correction of the gut microbiota through FMT.
The diagnosis of DIHS is not straightforward due to its highly variable clinical presentation and the lack of a standard diagnostic method. Among the 3 diagnostic criteria for DIHS, including the Japanese criteria, the international Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) criteria, and Bocquet criteria, the Japanese criteria were established in 2006 by a Japanese consensus group and include the following 7 criteria[16]: maculopapular rash developing 3 weeks after starting with a limited number of drugs; clinical symptoms prolonged after discontinuation of the causative drug; fever (>38°C); liver or other organ abnormalities; at least one of leukocyte abnormality present; lymphadenopathy; and HHV-6 reactivation.
Typical DIHS is indicated by the presence of the 7 criteria, whereas atypical DIHS is diagnosed if any 5 of these criteria are satisfied. In the case followed in this study, reactivation of HHV-6, which is most commonly associated with DIHS, was not detected, whereas reactivation of other types of herpes viruses, such as CMV and EBV, was detected.[17] Moreover, we also ruled out other diseases with similar presentation.
The negative result for the Clostridium difficile toxin A and B negativity made the diagnosis of antibiotic-associated diarrhea unlikely. The abdominal CT scan images ruled out lymphoma, and the diagnosis of intestinal allergic purpura was ruled out due to the absence of a prolonged course and the absence of supporting clinical findings. Therefore, the patient was eventually diagnosed with DIHS. It is noteworthy that intestinal failure occurred during the course of DIHS, which has not been reported as a complication of DIHS previously. Indeed, the patient presented characteristics of DIHS, whereas she was previously misdiagnosed mainly due to difficulties in diagnosis of DIHS, and the disease was left uncontrolled.
Although the pathogenesis of intestinal failure is not fully understood, a number of factors may have been involved such as long-term oral administration of l-carnitine (4 years), attach on the intestinal mucosa by T cells due to antibiotic- and hormone-mediated disruption, EBV and CMV infection, and secondary infections due to persistence of viral infection and disturbance of intestinal flora. In the case we present in this study, the severe attack on the patient's immune system was likely to result in disruption of the intestinal mucosal barrier of the entire intestine ranging from the small intestine to the colon, which explains why MODS with a main feature of intestinal failure subsequently occurred. A few of questions in relation to the intestinal failure in the patient still remain: what is the root cause? Is it a consequence of a supersensitive response or the use of antibiotics? Based on alterations in the intestinal mucosa (eg, edema), we postulated that a supersensitive response might largely contribute to the development of intestinal failure, whereas FMT may play a key role in modulation of intestinal immune activation, and in turn, has greatly improved the function of the intestine in the patient. Our findings suggested that integration of FMT into the treatment for DIHS would be beneficial to intervene and block damage to the intestinal mucosal barriers, repair the microbial and flora barrier, and in turn relieve the symptoms of MODS.
In this study, we attempted to elucidate a possible mechanism whereby the patient benefited from FMT. Compared to the intestinal microbiota before FMT, we identified the following significant alterations in the intestinal microbiota post FMT via 16S rRNA analysis of the gut microbiota. First, the quantities of certain types of the gut bacteria were significantly altered with increases in the aerobic Gram-positive enterococcus and Gram-negative enterobacteria, but decreases in the anaerobic Bifidobacteria, Lactobacillus, and Bacteroides. Notably, the anaerobic Bifidobacteria declined most in response to FMT. Given the fact that Bifidobacteria as a physiological bacteria plays a pivotal role in combating pathogenic bacteria, infection, and cancer, and boosting immunity, it is likely that the marked decrease in the population of Bifidobacteria impaired or disrupted the intestinal barrier function. Second, the proportions of the gut microbiota significantly changed. As an important indicator of the ecologically intestinal balance and biologically intestinal barrier, the ratios of Bifidobacteria to Escherichia coli (ie, B/E ratio) and anaerobic to aerobic bacteria were significantly altered and even reversed. These observations provide direct evidence of impairment of the intestinal barrier function in MODS, and this mechanical barrier and mucosal barrier function in our patient was apparently disrupted. After FMT, these alterations in both quantities and proportions tended to approach those of the healthy donor. Thus, we postulated that FMT is able to restore the balance of the interrupted gut microbiota, and this is probably an important mechanism, at least in part, through which FMT therapy has shown effectiveness in MODS-associated intestinal failure in DIHS. Furthermore, our findings suggested that the microecological balance of the intestinal microflora should not be neglected, and if necessary, the intestinal barrier should be protected in our care for the patient with MODS.
The consensus treatment of choice for DIHS is withdrawal of the causative drug and supportive care. However, the management of MOF in patients with DIHS is challenging. Successful treatment with FMT in this case merits attention. The role of FMT in restoring the balance of intestinal microbiota is well acknowledged.[18,19,20] We believe that the clinical outcomes in this patient were improved by the use of FMT. As demonstrated by comparison between post-FMT and pre-FMT stool samples from the recipient, the numbers of favorable bacteria were increased with numbers of unfavorable bacteria largely declining. We understand that most patients with DIHS recover completely; however, some can develop long-term sequelae. Thus, follow-ups are scheduled for the patient to continue therapy, if necessary.
5. Conclusions
In summary, intestinal failure with other organ system involvement may occur in patients with DIHS. The intestinal impairment can be severe, and FMT is strongly advocated for the treatment of such cases. FMT is capable of effectively maintaining microecological homeostasis and antagonizing pathogenic microorganisms, and therefore, shows promise as an effective and novel therapy for intestinal failure regardless of causative factors especially in the era of the abuse of antibiotics and drug resistance with the underlying mechanism to be elucidated in the future.
Author contributions
Conceptualization: Dongfeng Chen.
Data curation: Hao Gong.
Formal analysis: Yanling Wei, Hanyang Xing, Hao Gong.
Funding acquisition: Ning Li, Hao Gong.
Investigation: Ning Li, Hanyang Xing, Tianjiao Guo.
Methodology: Ning Li, Hanyang Xing, Tianjiao Guo, Hao Gong.
Project administration: Yanling Wei, Ning Li, Hanyang Xing.
Resources: Yanling Wei.
Supervision: Dongfeng Chen.
Validation: Dongfeng Chen.
Visualization: Dongfeng Chen.
Writing – original draft: Yanling Wei, Dongfeng Chen.
Writing – review & editing: Yanling Wei, Dongfeng Chen.
Supplementary Material
Footnotes
Abbreviations: BT = bacteria translocation, CRP = C-reactive protein, CT = computed tomography, DIHS = drug-induced hypersensitivity syndrome, ESR = erythrocyte sedimentation rate, FMT = fecal microbiota transplantation, ICUs = intensive care units, IL-6 = interleukin-6, MODS = multiple organ dysfunction syndrome, NPC = neutrophil count, PCT = procalcitonin, WBC = white blood cell.
How to cite this article: Wei Y, Li N, Xing H, Guo T, Gong H, Chen D. Effectiveness of fecal microbiota transplantation for severe diarrhea after drug-induced hypersensitivity syndrome. Medicine. 2019;98:52(e18476).
This study was supported by the following grants: Science and Technology Innovation Project, the Military Scientific Committee of the People's Liberation Army of China (17-163-12-ZT-002-060-01); Medical Science and Technology Pilot Project for Youth Investigators, the Military Scientific Committee of the People's Liberation Army of China (16QNP098); Funds for clinical medical research, the Daping Hospital & Research Institute of Surgery Clinical Research affiliated to the Third Military Medical University (2014YLC05); Chongqing Social Livelihood Project (cstc2015shmszx120047).
All authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1]. Kirchhoff C, Biberthaler P, Mutschler WE, et al. Early down-regulation of the pro-inflammatory potential of monocytes is correlated to organ dysfunction in patients after severe multiple injury: a cohort study. Crit Care 2009;13:R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Rossaint J, Zarbock A. Pathogenesis of multiple organ failure in sepsis. Crit Rev Immunol 2015;35:277–91. [DOI] [PubMed] [Google Scholar]
- [3]. Nieuwenhuijzen GA, Deitch EA, Goris RJ. The relationship between gut-derived bacteria and the development of the multiple organ dysfunction syndrome. J Anat 1996;189 (pt 3):537–48. [PMC free article] [PubMed] [Google Scholar]
- [4]. Klingensmith NJ, Coopersmith CM. The gut as the motor of multiple organ dysfunction in critical illness. Crit Care Clin 2016;32:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Carrico CJ, Meakins JL, Marshall JC, et al. Multiple-organ-failure syndrome. Arch Surg 1986;121:196–208. [DOI] [PubMed] [Google Scholar]
- [6]. Wei Y, Yang J, Wang J, et al. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit Care 2016;20:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Meng M, Klingensmith NJ, Coopersmith CM. New insights into the gut as the driver of critical illness and organ failure. Curr Opin Crit Care 2017;23:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 2006;55:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Caporaso JG, Lauber CL, Costello EK, et al. Moving pictures of the human microbiome. Genome Biol 2011;12:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America 2011;108 suppl 1:4516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 2006;72:5069–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Lin SM, Chung FT, Kuo CH, et al. Circulating angiopopietin-1 correlates with the clinical course of multiple organ dysfunction syndrome and mortality in patients with severe sepsis. Medicine 2015;94:e878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Gao YL, Lu B, Zhai JH, et al. The parenteral vitamin c improves sepsis and sepsis-induced multiple organ dysfunction syndrome via preventing cellular immunosuppression. Mediators Inflamm 2017;2017:4024672. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [15]. Cheng CY, Su SC, Chen CH, et al. HLA associations and clinical implications in T-cell mediated drug hypersensitivity reactions: an updated review. J Immunol Res 2014;2014:565320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Shiohara T, Iijima M, Ikezawa Z, et al. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol 2007;156:1083–4. [DOI] [PubMed] [Google Scholar]
- [17]. Hamm RL. Drug-hypersensitivity syndrome: diagnosis and treatment. J Am Coll Clin Wound Spec 2011;3:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Aroniadis OC, Brandt LJ. Intestinal microbiota and the efficacy of fecal microbiota transplantation in gastrointestinal disease. Gastroenterol Hepatol 2014;10:230–7. [PMC free article] [PubMed] [Google Scholar]
- [19]. Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011;9:1044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Borody TJ, Campbell J. Fecal microbiota transplantation: current status and future directions. Expert Rev Gastroenterol Hepatol 2011;5:653–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


