Supplemental Digital Content is available in the text
Keywords: serotonin-norepinephrine reuptake inhibitors, chemotherapy, peripheral neuropathy
Abstract
Background:
To compare the efficacy of serotonin-norepinephrine reuptake inhibitors (SNRIs) treatment for chemotherapy-induced peripheral neuropathy (CIPN)
Methods:
Two authors independently searched MEDLINE, Embase, Cochran Library, and Web of Science to identify and review articles published from January 1998 until December 2018 according to selection criteria. Outcomes were expressed as mean difference, the pooled odds ratio, or relative risk in a meta-analysis model.
Results:
A total of 10 studies were included in this meta-analysis: 6 randomized-controlled studies and 4 observational studies. Meta-analysis showed that CIPN was improved after treatment with SNRI (standardized mean difference = 2.20; 95% confidence interval, 0.90–3.49; I2 = 93% in 3 randomized controlled studies). Somnolence and insomnia occurred in <15% of patients. Incidence of somnolence was lower than with pregabalin treatment, and insomnia was comparable to that in expectant management or pregabalin treatment. Incidence of nausea and vomiting was higher than in expectant management, but no significant difference was found when compared to expectant management.
Conclusion:
From the several available studies suitable for indirect comparison, SNRI shows excellent efficacy and tolerability to CIPN. SNRI could provide an important treatment option for CIPN.
1. Introduction
Peripheral neuropathy is a debilitating and painful condition that occurs with destruction and dysfunction of the motor, sensory, and autonomic peripheral nerves.[1] Certain chemotherapy classes (platinum agents, taxanes, vinca alkaloids, epothilones, immunomodulators, and proteasome inhibitors) are known neurotoxins.[2] These agents cause damage to peripheral nerves by destroying microtubules and interfering with microtubule-based axonal transport, which results in chemotherapy-induced peripheral neuropathy (CIPN).[2,3] Upward of 40% of patients receiving these chemotherapies may develop CIPN.[2] Although some patients with CIPN have complete symptom resolution over time or with discontinuation of treatment, most patients have long-term morbidity and decreased quality of life.[3]
It is known that neurotransmitters such as serotonin and norepinephrine are involved in the descending inhibitory nociceptive pathway and can amplify the effects of central sensitization.[3] Because serotonin-norepinephrine reuptake inhibitors (SNRIs) inhibit the reuptake of these neurotransmitters, synaptic concentrations increase, and prevent input to the spinal dorsal horn neurons, which results in decreased pain transmission.[3] Previous studies showed that SNRIs, specifically, venlafaxine and duloxetine, are effective treatments for painful diabetic neuropathy.[4–6] Based on these trials, some studies were conducted to show that SNRI would ameliorate CIPN pain as well.[7–9] Therefore, the objective of our study was to provide a comprehensive evaluation of the efficacy and adverse events of SNRI treatment for CIPN.
2. Materials and methods
This study is based on the Cochrane Review Methods, and reporting follows the Meta-analysis of Preferred Reporting Items for Systematic Reviews and Meta-Analyses.[10] The protocol of this study was registered on the PROSPERO website in February 2019 (registration number CRD42019119812). This was a retrospective study in which only data that were publicly available were included without any personal information of individual patients. Thus, the institutional review board concluded that the approval was not applicable for this study.
2.1. Data sources
In September of 2018, we did a comprehensive literature search. We conducted electronic searches in the MEDLINE, Embase, Cochran Library, and Web of Science. We also conducted searches in a regional electronic bibliographic database (KoreaMed). No restrictions were imposed in terms of the publication language, time, or status. The search strategy was designed for searching MEDLINE through the PubMed interface. The following keywords were used: “chemotherapy,” “peripheral neuropathy,” and “serotonin-norepinephrine reuptake inhibitors.” Electronic database searches used both free-text words and Medical Subject Headings. The search strategy was adapted as appropriate for all other databases searched, taking into account differences in indexing terms and search syntax for each database. The comprehensive search strategies are described in the supplemental file (Supplementary 1). We identified further relevant studies for possible inclusion in our review by reviewing the reference lists of the studies identified by our initial search strategies.
We used the following study-inclusion criteria: studies with patients taking SNRI for CIPN; prospective case–control studies that compared SNRI to control for CIPN; and parallel-design studies in which researchers compared outcomes of CIPN with and without SNRI treatment. The exclusion criteria were studies in which the researchers included women who were not diagnosed with CIPN, or which did not use SNRI, did not evaluate CIPN, or did not report the effect of SNRI.
2.2. Data extraction
The 2 reviewers independently did data extraction using a predefined data extraction form. Any disagreement unresolved by discussion was reviewed by a 3rd author. The following variables were extracted from the studies:
-
1.
demographic characteristics such as the number and sex of the patients,
-
2.
age at the time of treatment,
-
3.
types of cancer,
-
4.
follow-up period after treatment,
-
5.
intervention protocol,
-
6.
types of treatment drugs, and
-
7.
measurements of treatment outcomes (Table 1).
Table 1.
Characteristics of the included studies.
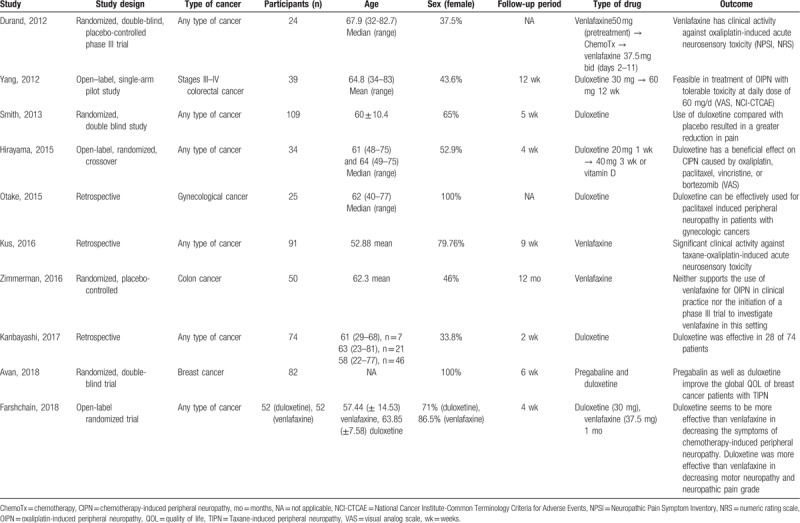
The outcomes of SNRIs treatment for CIPN that were used in the meta-analysis were as follows: percentage of patients who experienced reduction of pain after treatment; the change of neuropathic pain score by a scoring system that quantifies the degree of pain; and percentage of patients who complained of somnolence, insomnia, or nausea and vomiting after treatment.
Of the 10 studies, 6 were prospective randomized clinical trials (RCTs) and 4 were retrospective observational studies. All 6 RCTs reported the results based on the scoring system before and after treatment, whereas Durand et al and Zimmerman et al did not show standard deviations or errors, and therefore they were not included in the quantitative analysis.[7,11]
2.3. Assessment of methodologic quality
2.3.1. Assessment of risk of bias
We assessed quality with different tools appropriate for the design of each study. RCTs were assessed with the Cochrane risk of bias assessment tool, and observational studies were assessed with the Newcastle-Ottawa scale.[12] The Cochrane risk of bias assessment tool grades each type of risk as low, high, or unclear. Types of risk include random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential source of bias. We considered studies without a high risk of bias in any category to be of good quality, and considered studies with 1 high risk or 2 unclear risks to be of fair quality. The rest were considered to be of poor quality.
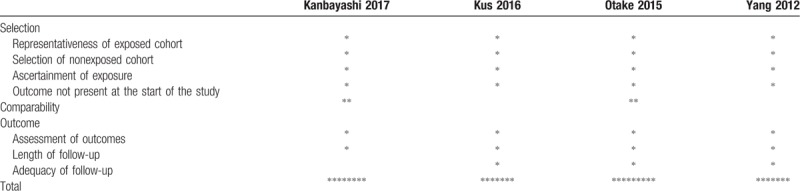
The Newcastle-Ottawa scale has three domains: selection, comparability, and outcome. In the selection domain, 1 star can be given to each category: representativeness of the exposed cohort, selection of the nonexposed cohort, ascertainment of exposure, and demonstration that the outcome of interest was not present at the start of the study. In comparability, a maximum of 2 stars can be given according to the comparability of cohorts on the basis of the analysis. In outcome, 1 star can be given to each category: outcome assessment, adequacy of the length of follow-up, and the follow-up of cohorts. Studies with more than three stars in the selection domain, 1 or 2 stars in the comparability domain, and more than 2 stars in the exposure/outcome domain were considered to be of good quality.
2.4. Statistical analysis
We carried out statistical analysis with RevMan software (version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We calculated dichotomous outcomes with risk ratios (RRs) or odds ratios (ORs) with a 95% confidence interval (CI). We calculated continuous outcomes with mean difference or standardized mean difference (SMD) with 95% CIs. We used an inverse variance weighting approach to evaluate the difference before and after SNRI treatment. When data were reported with median and range or interquartile range, we calculated the mean and standard deviation.[13] Heterogeneity was calculated with I2 statistics in which an I2 value exceeding 50% was considered to reveal substantial heterogeneity. We used a random-effect model when there was substantial heterogeneity, but otherwise used a fixed-effect model. We did subgroup analysis according to the study designs and other heterogeneity due to differences in study protocol. We did not use a Funnel plot or other tools such as Egger test for assessing publication bias, because there were few included studies: 6 RCTs[7,9,11,14–16] and 4 retrospective cohort studies.[17–20] We carefully discussed the possible effect of publication bias on the outcomes.
3. Results
3.1. Identification of studies
The database searches produced 935 articles (Fig. 1), and 134 duplicated articles were excluded. Of the remaining 801 articles, we excluded 760 publications because it was clear from the title and abstract that they did not meet the inclusion criteria. We obtained full manuscripts for the remaining 39 articles, and after scrutiny of these, we identified 10 potentially relevant studies: 6 RCTs[7,9,11,14–16] and 4 observational studies.[17–20]
Figure 1.
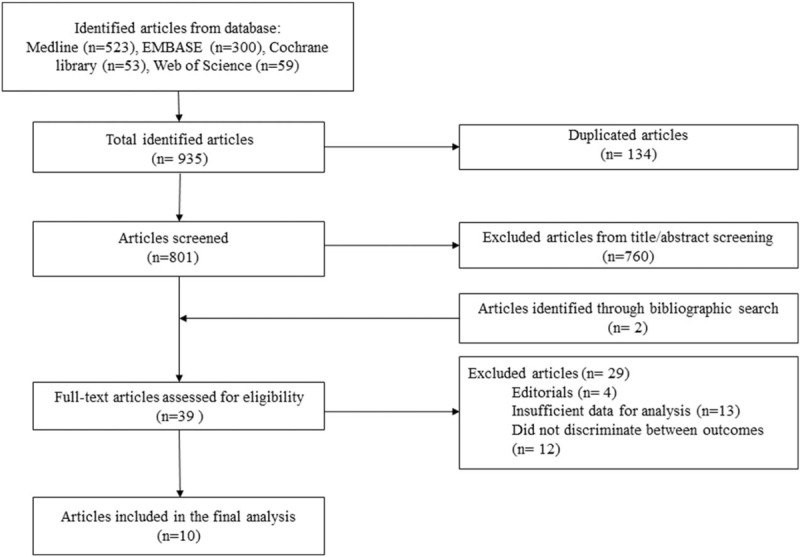
Flow chart outlining the selection of patients.
Characteristics of the included studies are listed in Table 1. A total of 632 patients who were treated with SNRI for CIPN were included, with 229 patients from the 4 observational studies. The authors of the included studies used different methods to report the women's ages (i.e., mean with SD for each group; mean with SD for all included women; median with range for each group; median with range for all included women; range for each group). In most of the studies, mean or median age of the patients was 60 to 65 years. Types of cancer were heterogenous among the studies: any type of cancers in 6 studies, colorectal cancer in 2 studies, breast cancer in 1 study, and gynecologic malignancies in 1 study. Types of chemotherapy agents were also heterogenous among the studies depending on the type of cancer. Two types of SNRI were used to treat CIPN: duloxetine in 6 studies and venlafaxine in 3 studies. One study compared the effects of venlafaxine and duloxetine on CIPN. Quality assessment of all studies is described in Tables 2 and 3. Among the 6 RCTs, 4 were of good quality and 2 were of poor quality (Table 2). All of the 4 observational studies were of fair quality (Table 3).
Table 2.
Risk of bias of included randomized controlled study using the Cochrane risk of bias assessment tool.
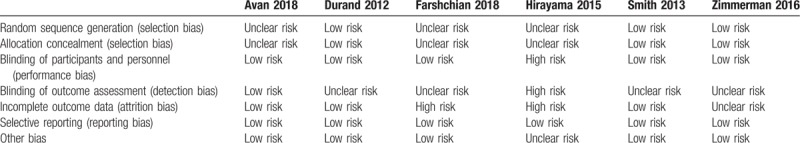
Table 3.
Quality assessment of included cohort studies using the Newcastle-Ottawa scale.
3.2. Meta-analysis of the outcomes of SNRI for CIPN
3.2.1. Primary outcome: efficacy of SNRI
Eight trials[9,14–20] involving 558 patients (329 patients in RCTs and 229 patients in observational studies) measured the efficacy of SNRI in terms of reducing neuropathic pain. Among those, 4 observational studies and 1 RCT reported the number of patients who experienced reduction of neuropathic pain after SNRI treatment. The percentage of patients with pain reduction were 63.6%,[17] 56%,[18] 51.9%,[19] 45.2%,[20] and 58.8%.[9] Three RCTs showed a difference in pain scores between before and after SNRI treatment. Avan et al used EORTC-QLQ-C30, which scores pain in a range from 0 (no pain) to 100 (worst pain).[15] Hirayama et al used visual analog scale, which scores pain from 0 (no pain) to 10 (worst pain).[16] Smith et al used the Brief Pain Inventory-Short Form, which also has similar 0 to 10 scoring.[14] As shown in Figure 2, CIPN was significantly improved after treatment with SNRI (SMD = 2.20; 95% CI, 0.90–3.49; I2 = 93%).
Figure 2.
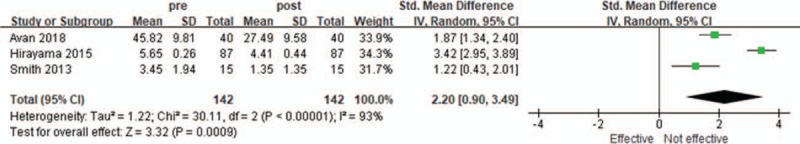
Changes in subjective assessment score of neuropathic pain after serotonin-norepinephrine reuptake inhibitors. CI = confidence interval, SD = standard deviation.
3.2.2. SNRI vs expectant management
Three RCTs compared SNRI and expectant management for CIPN in terms of pain relief.[9,14,16] Among those, Smith et al used the Brief Pain Inventory-Short Form and Hirayama et al used visual analog scale to evaluate neuropathic pain, as mentioned previously. Farshcian et al evaluated the number of patients with different grades of neuropathic pain assessed by the Radiation Therapy Oncology Group classification, which grades neuropathic pain from 0 (no pain) to 4 (most severe pain). The analysis of these studies is shown in Figure 3. CIPN was significantly decreased with SNRI more than by expectant management (SMD = −2.16; 95% CI, −3.26 to −1.06; I2 = 84%)
Figure 3.
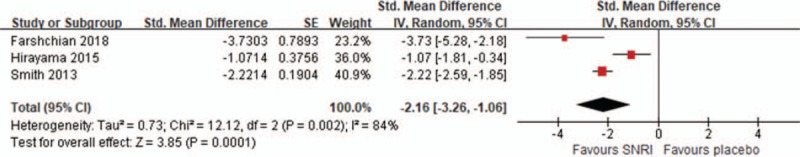
Compare serotonin-norepinephrine reuptake inhibitor (SNRI) and expectant management for chemotherapy-induced peripheral neuropathy in terms of pain relief. CI = confidence interval, SD = standard error.
3.2.3. SNRI vs pregabalin
One RCT compared SNRI and pregabalin.[15] According to Avan et al, pregabalin was significantly more effective in reducing pain assessed with EORTC-QLQ-C30 (MD = 13.78; 95% CI, 11.64–15.92, Fig. 4).
Figure 4.
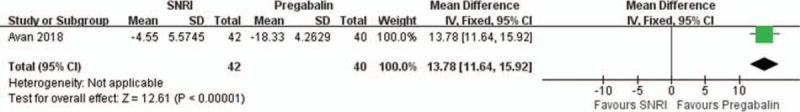
Compare serotonin-norepinephrine reuptake inhibitor (SNRI) and pregabalin treatment for chemotherapy-induced peripheral neuropathy in terms of pain relief. CI = confidence interval, SD = standard deviation.
3.2.4. Duloxetine vs venlafaxine
One RCT compared duloxetine and venlafaxine.[9] Administration of venlafaxine was more effective than duloxetine in reducing neuropathic pain (RR = 0.80; 95% CI, 0.70–0.92, Fig. 5).
Figure 5.
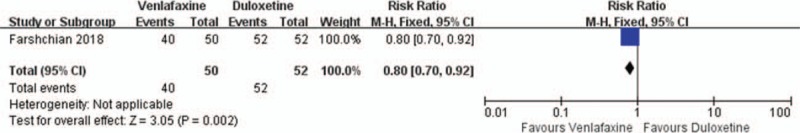
Compare duloxetine and venlafaxine treatment for chemotherapy-induced peripheral neuropathy in terms of pain relief. SNRI: serotonin-norepinephrine reuptake inhibitors. CI = confidence interval.
3.3. Secondary outcome: adverse events
3.3.1. Somnolence
The studies comparing somnolence after SNRI treatment were 2 RCTs[15,16] and 3 observational studies (Table 4).[17,18,20] Except for one study by Otake et al, in which 12% of patients complained of somnolence,[18] the percentage of patients who complained of somnolence was <10% (6.66%,[17] 5.88%,[16] 4.76%,[15] and 3.57%[20]) As shown in Figure 6, 1 RCT compared incidence of somnolence between SNRI and pregabalin treatment.[15] The rate of somnolence was significantly decreased in patients treated with SNRI compared to pregabalin (RR = 0.21; 95% CI, 0.05–0.92).
Table 4.
Percentage of patients with various types of side effects after serotonin-norepinephrine reuptake inhibitors treatment.
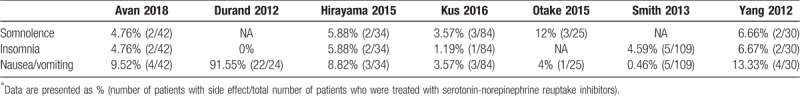
Figure 6.
Compare adverse reaction after serotonin-norepinephrine reuptake inhibitor (SNRI) treatment in terms of somnolence. CI = confidence interval.
3.3.2. Insomnia
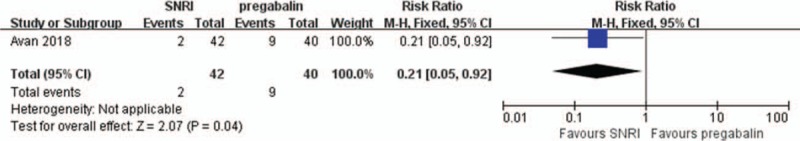
Insomnia was reported in 3 RCTs[14–16] and 3 observational studies.[7,17,20] One RCT[16] and 1 prospective observational study[17] reported that 5.88% and 6.67% of patients complained of insomnia, respectively. Other than these 2 studies, insomnia occurred in <5% of patients.[14,15,20] In 1 RCT, none of the patients complained of insomnia after SNRI treatment.[7] There was no significant difference of the rate of insomnia between SNRI and expectant treatment in 2 RCTs (RR = 0.59; 95% CI, 0.21–1.63, Fig. 7A).[7,14] There was also no significant difference of the rate of insomnia between SNRI and pregabalin treatment in 1 RCT (RR = 4.77; 95% CI, 0.24–96.34, Fig. 7B).[15]
Figure 7.
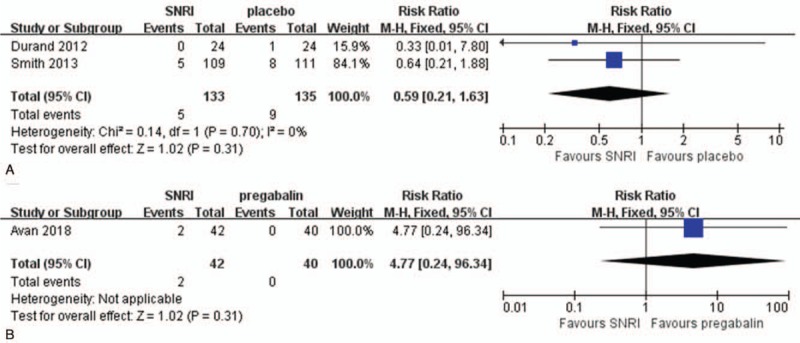
Adverse reaction in terms of insomnia (A) compare serotonin-norepinephrine reuptake inhibitor (SNRI) treatment and expectant managment (B) compare SNRI and pregabalin.
3.3.3. Nausea and vomiting
Nausea and vomiting were reported in four RCTs[7,14–16] and 3 observational studies.[17,18,20] The incidence of nausea and vomiting differed between studies. In 1 RCT, only 0.46% of patients reported nausea and vomiting,[14] but Durand et al reported that 91.66% of patients had nausea and vomiting.[7] Also, Durand et al reported nausea and vomiting were significantly increased after SNRI treatment more than by expectant management (RR = 1.57; 95% CI, 1.10–2.25, Fig. 8A).[7] There was also no significant difference of the rate of nausea and vomiting between SNRI and pregabalin treatment in 1 prospective study (RR = 8.58; 95% CI, 0.48–154.45, Fig. 8B).[15]
Figure 8.
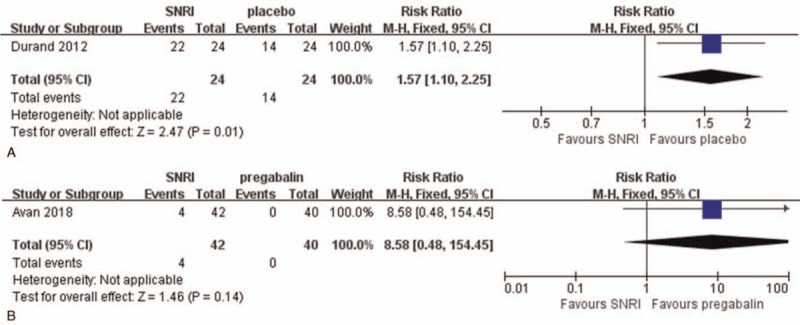
Adverse reaction in terms of nausea and vomitting (A) compare serotonin-norepinephrine reuptake inhibitor (SNRI) treatment and expectant managment (B) compare SNRI and pregabalin.
3.3.4. Other adverse events
Other adverse events such as dizziness, headache, fatigue, dysgeusia, and constipation after SNRI treatment have been reported. Avan et al reported higher incidence of dizziness after duloxetine treatment compared to pregabalin treatment (17.5% vs 0%, respectively).[15] However, Kus et al reported only 2.4% of patients experienced dizziness after duloxetine treatment.[20] Similarly, Durand et al reported 2% of patients complained dizziness after venlafaxine treatment, which was even lower than placebo group (4.9%).[7] Headache after venlafaxine treatment was only reported by Durand et al, and the incidence was comparable to the placebo group (2% in venlafaxine group vs 1.6% in placebo group).[7] Incidence of dysgeusia and constipation was reported to be 4% after duloxetine treatment in the study by Otake et al.[18] However, Durand et al reported no incidence of dysgeusia or constipation after venlafaxine treatment.[7]
4. Discussion
The development of chemotherapeutic agents has increased the survival period of patients with malignancies. However, a significant increase of CIPN due to toxic chemotherapeutic agents has become a major factor that lowers the quality of life for cancer patients. Treatment of painful CIPN continues to be a challenge, because most drugs tested to date have fallen short of providing adequate pain relief.[21–24] To treat CIPN, SNRIs that decrease pain transmission via inhibition of serotonin and norepinephrine have been suggested by many studies. Durand et al showed that venlafaxine was effective in reducing CIPN.[25,26] Matsuoka et al showed duloxetine was effective for cancer patients with CIPN who are not responsive to pregabalin,[27] and several randomized trials are ongoing to prove the effect of duloxetine for patients with CIPN.[28,29] Based on these findings, we quantitatively analyzed 6 prospective and four retrospective studies to elucidate the efficacy of SNRI for painful CIPN. In this study, SNRI was significantly effective for the relief of CIPN. The incidence of adverse effects such as somnolence and insomnia after SNRI treatment was <15% and was comparable to that from expectant management or pregabalin treatment. The incidence of nausea and vomiting was diverse in this meta-analysis; 1 RCT reported a high incidence of nausea and vomiting that was significantly increased compared to expectant management.[15,25] However, the percentages of patients reported by other studies were <15%, and 1 study reported a decreased incidence of nausea and vomiting compared to pregabalin treatment.[15] Therefore, we recommend SNRI as a rescue therapy for neuropathic pain after neurotoxic chemotherapy.
Only 1 randomized controlled trial compared SNRI to pregabalin.[15] That study reported that pregabalin was significantly more effective in reducing neuropathic pain.[15] However, both pregabalin and duloxetine equally improved the global QOL of patients over a 6-week trial in that study.[15] Although improvement of pain and insomnia domain of QOL was better with pregabalin, duloxetine was superior in improvement on the emotional functioning subscale of QOL. Moreover, other studies reported that the efficacy of duloxetine was 1.27 times that of pregabalin in the improvement of diabetic neuropathic pain.[30,31] Therefore, further studies are needed to confirm the efficacy of SNRI and pregabalin for CIPN.
Previous studies reported that several adverse events occurred in at least 5% of duloxetine-treated participants; these common adverse events included somnolence, nausea, dizziness, malaise, and vomiting.[32,33] In this study, adverse effects such as somnolence, and insomnia were not common after SNRI treatment. Except for the study by Otake et al,[18] the incidence of somnolence was reported to be about 5%.[15–17,20] Likewise, the incidence of insomnia was reported to be about 5%,[14–17,20] and none of the patients complained about insomnia after treatment in Durand et al (0%).[7] The incidence of nausea and vomiting differed greatly between the included studies. Durand et al reported a high incidence of nausea and vomiting (91.66%), which was significantly more than for expectant management.[7] However, the percentages of patients reported by other studies were <15%,[7,14–18,20] with the lowest incidence of 0.46%.[14] In Avan et al, the incidence of nausea and vomiting was less than from pregabalin treatment.[15] Although SNRI appears to be feasible compared to expectant management or pregabalin treatment in terms of somnolence and insomnia, more research is needed to see the effect of SNRI on nausea and vomiting.
In this study, only one randomized controlled trial compared duloxetine and venlafaxine and reported that duloxetine was more effective than venlafaxine in decreasing motor neuropathy and neuropathic pain grade, since duloxetine produced better reduction of cranial, motor, sensory neuropathy, and neuropathic pain.[9] However, 1 limitation of this study was the small sample. This limitation can justify some of the insignificant comparisons. Therefore, we recommend that further studies with larger samples be conducted to confirm these results.
This study has several limitations. First, we included only a few studies, and second, the studies included were heterogeneous not only in study designs, but also in chemotherapy agents, cancer type, and type of SNRIs. Third, there may be some exaggerated effect of SNRI due to publication bias. We acknowledge that some exaggeration of the effect of SNRI on pain reduction might be present. We decided not to use a Funnel plot or other tools such as Egger test for assessing publication bias, because there were few included studies, according to the opinion of statisticians about the statistical analysis. However, this is the 1st meta-analysis to evaluate the efficacy of SNRI in patients with CIPN and did not apply any restrictions according to study types, language, or time, to draw conclusions not skewed to one side.
In conclusion, from the several available studies suitable for indirect comparison, SNRI shows excellent efficacy and tolerability for CIPN. Therefore, SNRI could provide an important treatment option for CIPN.
Acknowledgment
The authors thank professor In-Seon Kwon for statistical consults.
Author contributions
Conceptualization: Soo Youn Song, Young Bok Ko, Geon Woo Lee, Jung Bo Yang, Sun Yeul Lee, Heon Jong Yoo.
Data curation: Soo Youn Song, Young Bok Ko, Geon Woo Lee, Jung Bo Yang, Ha Kyun Chang, Sang Mi Kwak, Sun Yeul Lee, Heon Jong Yoo.
Formal analysis: Soo Youn Song, Young Bok Ko, Hyeun Kim, Ha Kyun Chang, Jaeyun Jung, Sun Yeul Lee, Heon Jong Yoo.
Investigation: Soo Youn Song, Hyeun Kim, Geon Woo Lee, Jung Bo Yang, Sang Mi Kwak, Sun Yeul Lee, Heon Jong Yoo.
Methodology: Soo Youn Song, Young Bok Ko, Hyeun Kim, Geon Woo Lee, Jung Bo Yang, Ha Kyun Chang, Sang Mi Kwak, Sun Yeul Lee, Heon Jong Yoo.
Project administration: Soo Youn Song.
Resources: Soo Youn Song, Jaeyun Jung, Heon Jong Yoo.
Software: Soo Youn Song, Sang Mi Kwak.
Supervision: Soo Youn Song, Young Bok Ko, Jung Bo Yang, Ha Kyun Chang, Jaeyun Jung, Sun Yeul Lee, Heon Jong Yoo.
Validation: Soo Youn Song, Heon Jong Yoo.
Writing – original draft: Soo Youn Song, Young Bok Ko, Siyeo Lee, Sun Yeul Lee, Heon Jong Yoo.
Writing – review & editing: Young Bok Ko, Siyeo Lee, Sun Yeul Lee, Heon Jong Yoo.
Heon Jong Yoo orcid: 0000-0003-4808-3450.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CIPN = chemotherapy-induced peripheral neuropathy, ORs = odds ratios, RCTs = randomized clinical trials, RRs = risk ratios, SMD = standardized mean difference, SNRIs = serotonin-norepinephrine reuptake inhibitors.
How to cite this article: Song SY, Ko YB, Kim H, Lee GW, Yang JB, Chang HK, Kwak SM, Jung J, Lee S, Lee SY, Yoo HJ. Effect of serotonin-norepinephrine reuptake inhibitors for patients with chemotherapy-induced painful peripheral neuropathy: a meta-analysis. Medicine. 2020;99:1(e18653).
SYS and YBK contributed equally to the work and considered as co-first authors.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03032588).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 2010;85:S3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst 2008;13:27–46. [DOI] [PubMed] [Google Scholar]
- [3].Pachman DR, Barton DL, Watson JC, et al. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther 2011;90:377–87. [DOI] [PubMed] [Google Scholar]
- [4].Sindrup SH, Gram LF, Brosen K, et al. The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain 1990;42:135–44. [DOI] [PubMed] [Google Scholar]
- [5].Sindrup SH, Bjerre U, Dejgaard A, et al. The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy. Clin Pharmacol Ther 1992;52:547–52. [DOI] [PubMed] [Google Scholar]
- [6].Chambliss ML. Are serotonin uptake inhibitors useful in chronic pain syndromes such as fibromyalgia or diabetic neuropathy? Arch Fam Med 1998;7:470–1. [DOI] [PubMed] [Google Scholar]
- [7].Durand JP, Deplanque G, Montheil V, et al. Efficacy of venlafaxine for the prevention and relief of oxaliplatin-induced acute neurotoxicity: results of EFFOX, a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol 2012;23:200–5. [DOI] [PubMed] [Google Scholar]
- [8].Takenaka M, Iida H, Matsumoto S, et al. Successful treatment by adding duloxetine to pregabalin for peripheral neuropathy induced by paclitaxel. Am J Hosp Palliat Care 2013;30:734–6. [DOI] [PubMed] [Google Scholar]
- [9].Farshchian N, Alavi A, Heydarheydari S, et al. Comparative study of the effects of venlafaxine and duloxetine on chemotherapy-induced peripheral neuropathy. Cancer Chemother Pharmacol 2018;82:787–93. [DOI] [PubMed] [Google Scholar]
- [10].Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- [11].Zimmerman C, Atherton PJ, Pachman D, et al. MC11C4: a pilot randomized, placebo-controlled, double-blind study of venlafaxine to prevent oxaliplatin-induced neuropathy. Support Care Cancer 2016;24:1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015;8:2–10. [DOI] [PubMed] [Google Scholar]
- [13].Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 2013;309:1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Avan R, Janbabaei G, Hendouei N, et al. The effect of pregabalin and duloxetine treatment on quality of life of breast cancer patients with taxane-induced sensory neuropathy: a randomized clinical trial. J Res Med Sci 2018;23:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hirayama Y, Ishitani K, Sato Y, et al. Effect of duloxetine in Japanese patients with chemotherapy-induced peripheral neuropathy: a pilot randomized trial. Int J Clin Oncol 2015;20:866–71. [DOI] [PubMed] [Google Scholar]
- [17].Yang YH, Lin JK, Chen WS, et al. Duloxetine improves oxaliplatin-induced neuropathy in patients with colorectal cancer: an open-label pilot study. Support Care Cancer 2012;20:1491–7. [DOI] [PubMed] [Google Scholar]
- [18].Otake A, Yoshino K, Ueda Y, et al. Usefulness of duloxetine for paclitaxel-induced peripheral neuropathy treatment in gynecological cancer patients. Anticancer Res 2015;35:359–63. [PubMed] [Google Scholar]
- [19].Kanbayashi Y, Inagaki M, Ueno H, et al. Predictors of the usefulness of duloxetine for chemotherapy-induced peripheral neuropathy. Med Oncol 2017;34:137. [DOI] [PubMed] [Google Scholar]
- [20].Kus T, Aktas G, Alpak G, et al. Efficacy of venlafaxine for the relief of taxane and oxaliplatin-induced acute neurotoxicity: a single-center retrospective case-control study. Support Care Cancer 2016;24:2085–91. [DOI] [PubMed] [Google Scholar]
- [21].Hammack JE, Michalak JC, Loprinzi CL, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain 2002;98:195–203. [DOI] [PubMed] [Google Scholar]
- [22].Kautio AL, Haanpaa M, Leminen A, et al. Amitriptyline in the prevention of chemotherapy-induced neuropathic symptoms. Anticancer Res 2009;29:2601–6. [PubMed] [Google Scholar]
- [23].Rao RD, Flynn PJ, Sloan JA, et al. Efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled trial, N01C3. Cancer 2008;112:2802–8. [DOI] [PubMed] [Google Scholar]
- [24].Aziz MT, Good BL, Lowe DK. Serotonin-norepinephrine reuptake inhibitors for the management of chemotherapy-induced peripheral neuropathy. Ann Pharmacother 2014;48:626–32. [DOI] [PubMed] [Google Scholar]
- [25].Durand JP, Brezault C, Goldwasser F. Protection against oxaliplatin acute neurosensory toxicity by venlafaxine. Anticancer Drugs 2003;14:423–5. [DOI] [PubMed] [Google Scholar]
- [26].Durand JP, Alexandre J, Guillevin L, et al. Clinical activity of venlafaxine and topiramate against oxaliplatin-induced disabling permanent neuropathy. Anticancer Drugs 2005;16:587–91. [DOI] [PubMed] [Google Scholar]
- [27].Matsuoka H, Makimura C, Koyama A, et al. Pilot study of duloxetine for cancer patients with neuropathic pain non-responsive to pregabalin. Anticancer Res 2012;32:1805–9. [PubMed] [Google Scholar]
- [28].Matsuoka H, Ishiki H, Iwase S, et al. Study protocol for a multi-institutional, randomised, double-blinded, placebo-controlled phase III trial investigating additive efficacy of duloxetine for neuropathic cancer pain refractory to opioids and gabapentinoids: the DIRECT study. BMJ Open 2017;7:e017280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Battaglini E, Park SB, Barnes EH, et al. A double blind, placebo controlled, phase II randomised cross-over trial investigating the use of duloxetine for the treatment of chemotherapy-induced peripheral neuropathy. Contemp Clin Trials 2018;70:135–8. [DOI] [PubMed] [Google Scholar]
- [30].Roy MK, Kuriakose AS, Varma SK, et al. A study on comparative efficacy and cost effectiveness of pregabalin and duloxetine used in diabetic neuropathic pain. Diabetes Metab Syndr 2017;11:31–5. [DOI] [PubMed] [Google Scholar]
- [31].Tanenberg RJ, Irving GA, Risser RC, et al. Duloxetine, pregabalin, and duloxetine plus gabapentin for diabetic peripheral neuropathic pain management in patients with inadequate pain response to gabapentin: an open-label, randomized, noninferiority comparison. Mayo Clin Proc 2011;86:615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yasuda H, Hotta N, Kasuga M, et al. Efficacy and safety of 40 mg or 60 mg duloxetine in Japanese adults with diabetic neuropathic pain: results from a randomized, 52-week, open-label study. J Diabetes Investig 2016;7:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yasuda H, Hotta N, Nakao K, et al. Superiority of duloxetine to placebo in improving diabetic neuropathic pain: Results of a randomized controlled trial in Japan. J Diabetes Investig 2011;2:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


