Abstract
Objectives
To evaluate changes in tremor severity and motor/emotion-processing circuits in response to cognitive behavioral therapy (CBT) delivered as treatment for functional tremor (FT), the most common functional movement disorder in adults.
Methods
Fifteen patients with FT underwent fMRI with motor, basic-emotion, and intense-emotion tasks before and after 12 weeks of CBT. Baseline fMRI was compared to those of 25 healthy controls (HCs). The main clinical endpoint was the tremor score (sum of severity, duration, and incapacitation subscores) adapted from the Rating Scale for Psychogenic Movement Disorders (PMDRS) assessed by a blinded clinician. CBT responders were defined as those with PMDRS score reduction >75%. Anatomic and functional brain images were obtained with a 4T MRI system. Generalized linear model and region-of-interest analyses were used to evaluate before-versus-after treatment-related changes in brain activation.
Results
CBT markedly reduced tremor severity (p < 0.01) with remission/near remission achieved in 73.3% of the cohort. Compared to HCs, in those with FT, a functionally defined fMRI region of interest in the anterior cingulate/paracingulate cortex showed increased activation at baseline and decreased activation after CBT during basic-emotion processing (p = 0.012 for CBT responders). Among CBT responders, the change in anterior cingulate/paracingulate was more significant in those with more severe baseline depression (r = 0.75, p < 0.01).
Conclusions
Tremor severity improved significantly after CBT. The improvement was associated with changes in the anterior cingulate/paracingulate activity, which may represent a marker of emotional dysregulation in FT and a predictor of treatment response.
Classification of evidence
This study provides Class III evidence that CBT significantly improves tremor severity in patients with functional tremor.
A phenotype-specific diagnosis of functional (psychogenic) tremor (FT) is achieved with clinically definite certainty when a tremor exhibits all of the following characteristics: (1) entrainment or full suppressibility; (2) distractibility; (3) tonic coactivation at tremor onset; (4) pause of tremor during contralateral ballistic movements; and (5) variability in frequency, axis, or distribution.1 Although FT is the most common functional movement disorder in adults, its pathophysiologic underpinnings remain poorly understood. Recent therapeutic studies have focused on motor rehabilitation (specialist physiotherapy in a cohort of functional motor disorders, including 17% patients with FT2 and tremor retrainment/biofeedback in 10 patients3) but not on cognitive or behavioral interventions tailored to its neurobiology. There is no specific biomarker to help in the selection of patients for physical or psychological interventions, and the long-term prognosis of patients with FT remains poor.4
Cognitive behavioral therapy (CBT) is increasingly used to treat FT given the emergent evidence of efficacy in other functional neurologic disorders.5 CBT is a structured, time-limited therapy that helps patients identify how thinking affects emotional states or specific behaviors with an aim of inducing change in cognitions and behaviors around movements and interpersonal functioning. While typically manualized,5 this treatment can be tailored to individual patients on the basis of their experiences and cognitive frameworks.
Functional neuroimaging studies have suggested that functional neurologic disorders are network disorders and that the symptoms associated with the disruption of various parts of the networks may explain the observed variation in clinical symptoms.6 The neurobiological basis of FT has been examined only in small heterogeneous cohorts, largely in the resting state.7,8 In a recent fMRI study, we showed, using a basic-emotion task comparing FT with healthy controls (HCs), that FT is associated with alterations in several brain regions, including the anterior cingulate/paracingulate cortex.9 In that study, in response to emotional stimuli, patients with FT demonstrated alterations in functional connectivity in networks involved in emotion processing and theory of mind.9 However, it is unclear whether these alterations in fMRI signals or connectivity could constitute a biomarker of treatment response or normalize after a successful therapeutic intervention.10
In this prospective study of CBT, we sought to obtain data on the feasibility and efficacy of CBT for patients with clinically definite FT using cognitive elements from the model for intervening in psychogenic nonepileptic seizures (PNES)5 and to determine the extent to which neuronal circuits associated with emotion processing change in response to CBT in patients with FT. We hypothesized that CBT would reduce the tremor burden in at least half of the participants with FT and that activations in brain regions in response to emotional stimuli would change among those benefiting from treatment and possibly normalize or return to the level observed in HCs. Thus, the primary behavioral outcome measure was reduction in tremor, and the primary imaging outcome measure was reduction of activation in response to therapy in the a priori anterior cingulate/paracingulate region of interest (ROI). Although the network involved in the generation and maintenance of functional neurologic disorders is large,6 the anterior cingulate/paracingulate region was selected because of its known role in emotion processing, in theory of mind, and in the hyperactivity in FT vs HCs shown previously (from a study admittedly designed concurrently).9
Methods
Participants
We prospectively recruited 15 consecutively consenting patients over a 2-year period with clinically definite unilateral or asymmetric FT affecting the upper limbs according to established clinical criteria.1 We chose this number of participants on the basis of prior sample size calculations suggesting that for a liberal significance threshold of 0.05, a minimum of 12 participants is required to achieve 80% power at the single-voxel level for typical activations while accounting for intraparticipant and interparticipant variability.11 Tremor needed to be absent or minimal at rest to avoid interference with the scanning procedure. Patients were excluded if they did not understand and accept the FT diagnosis, had any comorbid functional movement or neurologic disorder, or were on benzodiazepines and were unable to discontinue them at the time of fMRI scanning. We also prospectively recruited 25 age- and sex-matched HCs with no history of neurologic or medical disorders. Patients with FT were consented for participation into a 12-week CBT program and an fMRI protocol before and after treatment. HCs underwent fMRI only once and were not subjected to the intervention. This study was approved by the local institutional review board, and all participants provided informed consent.
To reach the recruitment goal, we screened 22 patients with FT, of whom 7 were not recruited for the following reasons: 1 malingering (rather than conversion), 1 not meeting criteria for FT, 1 unwilling to provide consent, 1 unacceptance of diagnosis, 1 unable to undergo MRI due to prior neurosurgical procedure, and 2 (excluded after providing consent) with excessive tremor during scanning and inability to get comfortable in the scanner. Data excluded from final analyses due to noncompletion of the task or for data-quality issues were for basic-emotion processing task (1 HC) and intense-emotion processing task (1 FT, 1 HC).
Clinical measurements
The primary clinical endpoint for CBT was the tremor score (sum of severity, duration, and incapacitation subscores) adapted from the Rating Scale for Psychogenic Movement Disorders (PMDRS; range 0–128 [higher is worse])12 through an offline video-based rating by a clinician blinded to treatment timing. Both participants with FT and HCs underwent a 15-minute structured diagnostic interview based on the Mini International Neuropsychiatric Interview (MINI)13 to screen for axis I DSM-IV and ICD-10 psychiatric disorders.14 We also administered the 17-item Hamilton Depression Scale (HAM-D)15 and the 14-item Hamilton Anxiety Scale (HAM-A)16 to evaluate for psychic and somatic anxiety. These scales were administered as part of a structured interview.
Cognitive behavioral therapy
CBT is a structured, time-limited therapy administered to help patients identify how thinking affects emotional states that express clinically as either physical symptoms or behaviors. On the basis of preliminary experience by the authors,5 patients with FT received 12 weekly CBT outpatient sessions with the following characteristics: structured 1-hour sessions were led by a social worker with 25 years of experience with CBT delivery (S.R.) with a preset agenda at the onset of each session; the therapist undertook an active and engaged stance, applying Socratic questioning (thoughtful inquiring by the therapist to elicit engaged dialog to examine underlying thoughts); and each session was followed by a specific homework assignment, building on the gains from the session. The primary structure used was consistent with Aaron Beck's model of cognitive therapy, making extensive use of thought monitoring, identification of cognitive distortions, and thought restructuring. The CBT sessions were adapted from the protocol developed previously for the management of PNES5 and included the same elements that were tailored to individual patients on the basis of the results of clinical interview performed before initiation of the intervention. Emphasis was placed on periods of tremor exacerbation between research visits, using these as opportunities to capture related automatic thoughts (thought monitoring) and to examine potential cognitive distortions that may have preceded them. The anxiety and depression scales were separately collected by the principal investigator (A.J.E.), but all patients received an initial orientation to the treatment process and the intervention, as well as education on relapse prevention (techniques to manage reemergence of symptoms after completion of treatment) in combined sessions with the principal investigator and CBT provider. We defined responders as patients with complete remission or >75% reduction in PMDRS scores and partial responders as those with less benefit than responders.
fMRI procedure: tasks
The finger-tapping motor task
The finger-tapping motor task was made up of a 30-second block of right-only finger tapping, followed by a 30-second block of left-only tapping, followed by a 30-second block of inactivity (rest), all repeated in the same sequence 4 times. A visual prompt was presented every second, and participants were instructed to move a lever using their right or left index finger, according to whether the R or L was flashing, at the once-per-second rate. Compliance with tapping procedure was monitored visually by study personnel. The total task duration was 6 minutes; right and left tapping conditions were modeled separately with the blocks of inactivity (rest) treated as baseline in the analyses. This task was designed to assess and monitor the integrity of the motor system, hypothesized to be unaffected.
The basic-emotion face recognition task
The basic-emotion face recognition task presented participants with 120 different faces over 14 minutes, corresponding to unique (nonrepeating) facial identities depicting a particular emotion (sadness, happiness, or fear) or a neutral expression.17 This task was designed to assess response to basic-emotional stimuli. The processing of emotional expressions is thought to occur subliminally and automatically. However, it is dependent on attention to the task,18 which is engaged by asking participants to decide the sex of each face by pressing 1 of 2 buttons with the right thumb. Thirty prototypically happy, 30 sad, 30 fearful, and 30 neutral expressions were selected from the NimStim set of facial expressions19 and presented in random order. Each stimulus was presented for 2 seconds with variable interstimulus interval of 3.9 ± 2.4 seconds, during which a fixation cross appeared. The event-related design with variable interstimulus delay (jitter) was used to minimize habituation of activation, which may occur in block designs with highly repetitive and predictable stimulus presentation.20 It is well recognized that activation by faces in some brain areas is strongly affected by attentional condition, while in other brain areas, it is not (e.g., amygdala response to fearful stimuli).21
The intense-emotion task
The intense-emotion task (continuous performance task with emotional and neutral distracters) used a series of offensive or disgusting images to engage intense-emotional circuitry.22 A visual oddball paradigm was constructed for this task in which 70% of the cues were squares, 10% were circles (targets), 10% were emotionally unpleasant pictures, and 10% were emotionally neutral pictures. Pictures originated from the International Affective Picture System (University of Florida, Gainesville) and were selected according to previously used criteria.23 Participants used the same response buttons as for the basic-emotion task and were asked to press with the right thumb a 2 for circles and 1 for all other images. There were 158 total items with a constant display time of 2.75 seconds and a 0.25-second interval with fixation cross. Items were split over 2 runs of the task in the imaging session with the content of each run being the same.
The behavioral experiments were programmed in E-Prime (version 1; pstnet.com). Stimuli were presented via magnetic resonance–compatible VGA goggles and headphones (Resonance Technologies, Inc, Los Angeles, CA). In each scanning session, echo-planar imaging was acquired during behavioral paradigms with a T2*-weighted gradient-echo planar imaging pulse sequence (repetition/echo time 3000/29 milliseconds, field of view 256 × 256 mm, matrix 64 × 64, slice thickness 4 mm, flip angle 75° with the behavioral paradigms precisely timed to image acquisition). To correct for geometric distortion and Nyquist ghost artifacts, a multiecho reference scan was performed. A T1-weighted 3-D anatomic high-resolution scan using modified equilibrium Fourier transform sequence (repetition/echo time 13/6 milliseconds, T[MD] 1.1 seconds, field of view 192 × 256 × 256 mm, matrix 192 × 256 × 265, slice thickness 1 mm, flip angle 20°) was acquired.
Anatomic data
We used fslreorient2std from the FMRIB Software Library (FSL) to rotate data to standard orientation. Next, we performed bias correction and brain extraction on T1 anatomic scans using FSL's FAST24 and BET,25 respectively. Images were spatially normalized with FSL's FLIRT26 to the 2-mm isotropic Montreal Neurological Institute ICBM 152 nonlinear sixth-generation template.27 Subcortical segmentation was performed with FSL's FIRST.28
Functional data
For each fMRI task data, typical preprocessing steps, including reorientation, slice timing correction, and brain extraction, were carried out with FSL's fslreorient2std, slicetimer, and BET,25 respectively. We detected outlying functional volumes based on motion and intensity using FSL's fsl_motion_outliers. Motion correction of the blood oxygenation level–dependent time series was carried out next with FLIRT.26 The functional file was interpolated to 2 × 2 × 2–mm voxel size and aligned to the Montreal Neurological Institute template27 by coregistering it with the participant's T1 using FSL's FLIRT.26,29 Next, the motion related artifacts were regressed from the data by setting up a general linear model design using 24 motion parameters (6 motion parameters, the 6 motion parameters squared, a first-order autoregressive model of the 6 motion parameters, and a first-order autoregressive model of the 6 motion parameters squared) plus an additional parameter for each detected outlier.30 A CompCor regression was also implemented using the eigenvectors from the first 5 of 10 principal components within a white matter and CSF mask, respectively.31
Image processing and statistical analysis
Reconstruction of the raw data was performed with 3D Hamming filter using in-house software developed in IDL (ittvis.com). First-level fMRI data processing was carried out using functions from FSL and from Analysis of Functional Neuroimages (AFNI) with the most recent updates incorporated to minimize the chance of false positives.32 For the finger-tapping task, right tapping > inactivity (rest) and left tapping > inactivity (rest) were used to model motor-related activity. In the basic-emotion task, activation related to face processing was assessed via the comparison between all faces of any emotional valence compared to the neutral expression that served as a baseline. In addition, happy, sad, and fearful faces were each contrasted with neutral expression to assess regions of activation associated with visual processing of each type of emotional expression. In the intense-emotion task (continuous performance task with emotional and neutral distracters), emotional pictures were compared to neutral pictures, as well as to the baseline of solid squares, to identify regions of activation associated with intense-emotion processing. The residuals from the general linear model were high-pass filtered in accordance with the task timing, 0.008 Hz for finger tapping and 0.04 Hz for the basic-emotion and intense-emotion tasks, and smoothed with a 6-mm full-width half-maximum filter with AFNI's 3dBandpass. For the finger-tapping task, we assessed group differences between pre-CBT fMRI in FT and the baseline HC fMRI (2-sample t test) and pre-/post-CBT fMRI (paired t test) in patients with FT. All group results were corrected for multiple comparisons at the whole-brain level with FSL's threshold-free cluster enhancement (TFCE),33 a nonparametric permutation test, with 5,000 permutations.
Small volume correction
Given the hypothesized involvement of the anterior cingulate/paracingulate, a small volume correction was implemented over these regions to correct the fMRI task–based statistical results for multiple comparisons. The small volume mask was defined anatomically with the Harvard-Oxford Cortical Atlas. FSL's randomize was used to implement small volume correction with the nonparametric TFCE method with 5,000 permutations.
ROI analysis
An ROI analysis was conducted to examine the change in activation associated with CBT in regions that were maximally different between HCs and patients with FT before CBT in our previous study.9 The larger sample size in this previous cross-sectional nonintervention study allowed us to select ROIs on the basis of results corrected for multiple comparisons. Specifically, for each task contrast, ROIs were selected from the group-level contrast using a z threshold of 2.3 and p < 0.05 corrected (using FSL's nonparametric TFCE method). These ROIs were in the right cerebellum for the motor task; in the anterior cingulate/paracingulate cortex, left Heschl gyrus, and left precentral gyrus for the basic-emotion task; and in the parahippocampal gyrus, brainstem, and anterior cingulate/paracingulate cortex for the intense-emotion task.9 Mean activation values were extracted from these functionally defined ROIs, and then t tests were performed to determine pre-to post-CBT changes in activation. The HAM-D scores were then correlated with the difference in activation (post-CBT activation minus pre-CBT activation). These results were not subject to small volume correction.
Clinical data statistical analysis
Quantitative data were expressed as mean ± SD and median and categorical data as frequency and proportion as appropriate. The sample characteristics were compared between FT and HCs using either the unpaired t test or Fisher exact test. The effect of CBT on PMDRS was evaluated with paired t test with effect size summarized using mean change along with 95% confidence interval and percent relative change with SD. The Shapiro-Wilk test was used to assess gaussian distributions. All results based on parametric analyses were validated with nonparametric analyses using Wilcoxon signed-rank tests. Spearman rank correlation analysis was used to examine correlations of change in PMDRS with baseline characteristics. The change in PMDRS (pre-post CBT) was also compared according to dichotomized age, sex, disease duration, affected body region and movement type, MINI, HAM-A, and HAM-D using a 2 sided Student t test. Values of p ≤ 5% level of significance were considered statistically significant. To visually summarize the treatment effects and the distribution of before-versus-after change in PMDRS, box-whisker plots and bar plots were constructed.
Results
Population characteristics
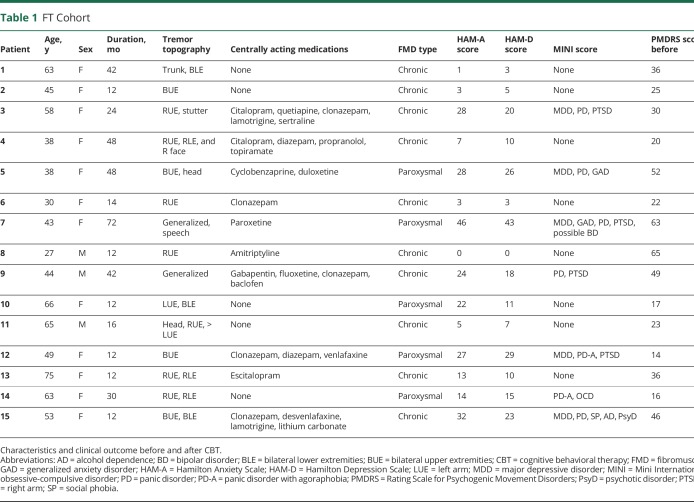
We included 15 patients with FT (age 50.5 ± 14.2 years, 12 women) and 25 HCs (43.6 ± 14.6 years, 21 women). With 1 exception (recorded as unknown), all participants were right-handed. Average disease duration in the FT cohort was 2.3 ± 1.6 (range, 1–4) years (table 1). Age and sex were not statistically different between the groups.
Table 1.
FT Cohort
Psychiatric features
Depression (HAM-D score 14.9 ± 11.8 vs 0.76 ± 1.4, p < 0.001) and anxiety (HAM-A score 16.9 ± 13.9 vs 0.76 ± 2.0, p < 0.001) scores were higher in the FT group compared to HCs. Major depression was ascertained in 5 of 15 patients with FT and posttraumatic stress disorder in 4 of 15 per MINI screen. HAM-D and HAM-A scores were highly correlated (Pearson r = 0.9498).
Clinical changes
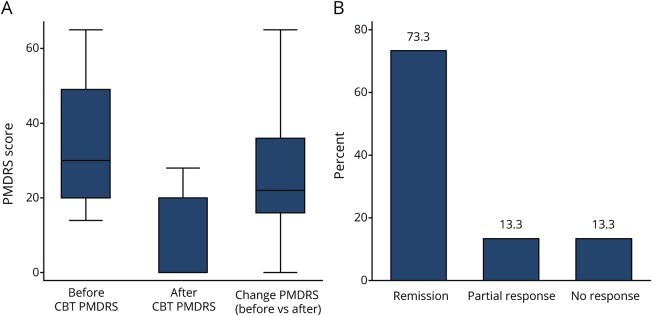
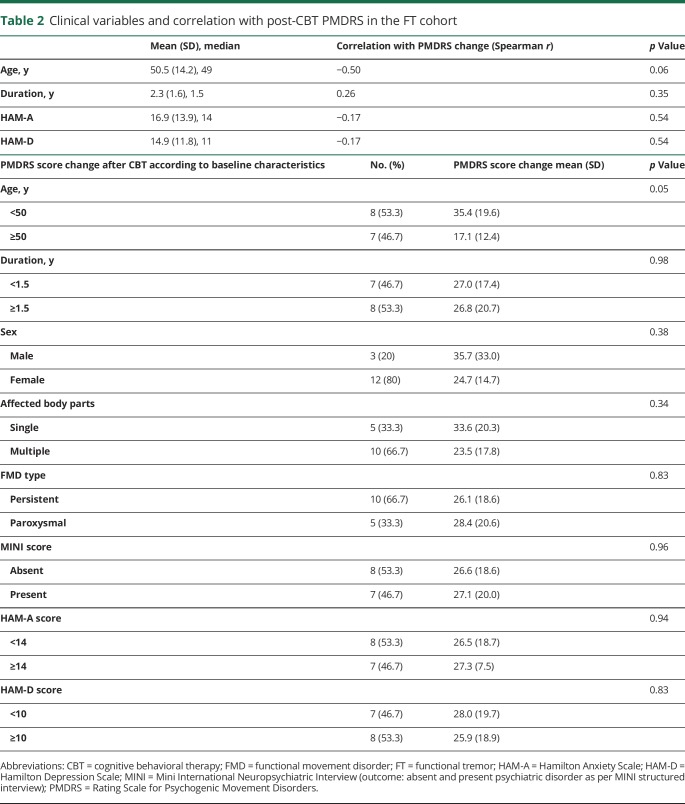
The change in PMDRS score was normally distributed (Shapiro-Wilk test, p = 0.16). CBT reduced the PMDRS score from 34.3 ± 17.1 to 7.4 ± 10.8 (78.7 ± 33.8%, mean change 26.87; 95% confidence interval 16.6–37.2, p < 0.001) with 9 of 15 patients experiencing complete resolution of their tremor and 2 of 15 experiencing near-complete (>75%) resolution at study endpoint (figure 1). The effect of CBT on PMDRS score was not influenced by any baseline variable except for age (correlation = −0.5, p = 0.06), with greater improvement in patients below the mean age (<50 years; 35.4 ± 19.6 vs 17.1 ± 12.4, p = 0.05) compared to those ≥50 years of age. Neither disease duration nor any other clinical variable, including depression or anxiety scores, predicted changes in PMDRS score (table 2). The results remained unchanged for each outcome after the data were analyzed with nonparametric tests.
Figure 1. Clinical changes after CBT.
(A) Box-whisker plots visually summarize the distribution of responses before and after cognitive behavioral therapy (CBT). (B) Distribution of response categories based on change in Rating Scale for Psychogenic Movement Disorders (PMDRS) score after CBT.
Table 2.
Clinical variables and correlation with post-CBT PMDRS in the FT cohort
Finger-tapping fMRI task
As expected, there were no significant differences in pre- vs post-CBT activation between participants with FT and HCs after adjustment for multiple comparisons at the whole-brain level.
Basic-emotion fMRI task
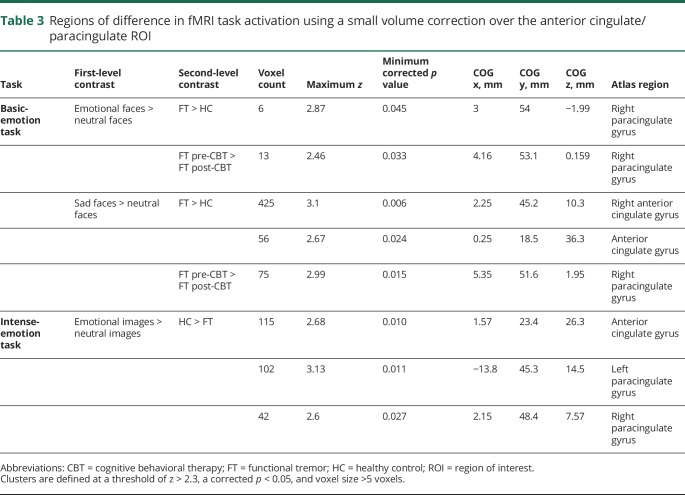
In general, compared to HCs, the anterior cingulate/paracingulate cortex showed increased activation at baseline and decreased activation after CBT during basic-emotion processing (p = 0.012 for CBT responders). At baseline, small volume correction applied to the cingulate/paracingulate region revealed greater activation in the FT group compared to HCs in the right cingulate/paracingulate gyri for the emotional faces > neutral faces contrast. For the sad faces > neutral faces contrast, small volume correction showed greater activation in the FT group in the anterior cingulate and left and right paracingulate regions (figure 2, top, and table 3). For the pre- vs post-CBT activation, small volume correction revealed a reduction in activation in the right paracingulate gyrus for both the sad faces > neutral faces contrast and the emotional faces > neutral faces contrast (figure 2, bottom, and table 3).
Figure 2. Differences in fMRI activation for the basic-emotion task after small volume correction.
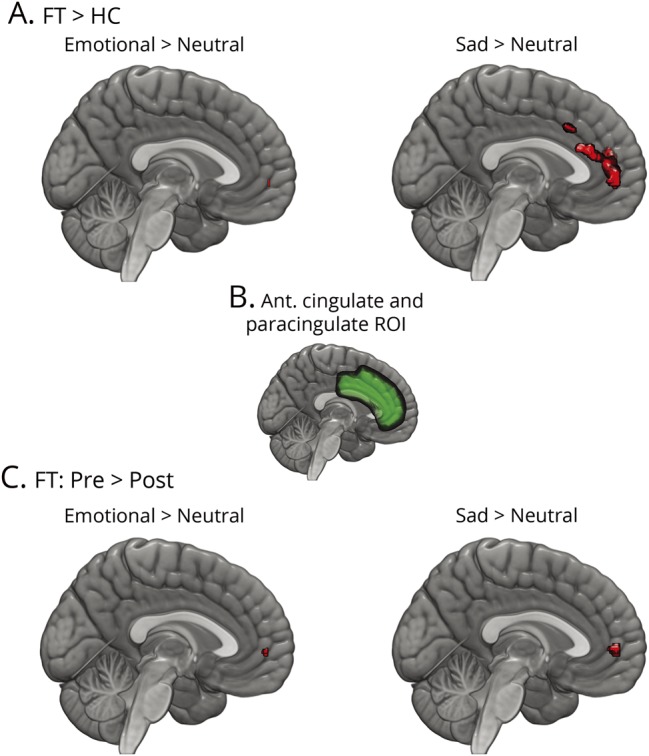
(A) Differences between participants with functional tremor (FT) and healthy controls (HCs). Regions in red showed greater activation in FT. (B) Region over which small volume correction was implemented. (C) Changes from before cognitive behavioral therapy (CBT) to after CBT in patients with FT. Regions in red showed greater activation before CBT that decreased after CBT. ROI = region of interest.
Table 3.
Regions of difference in fMRI task activation using a small volume correction over the anterior cingulate/paracingulate ROI
Intense-emotion fMRI task
The planned analysis in the cingulate/paracingulate ROI with small volume correction applied revealed greater activation for the emotional images > neutral images contrast in HCs compared to those with FT (figure 3 and table 3).
Figure 3. Differences in fMRI activation for the intense-emotion task after small volume correction.
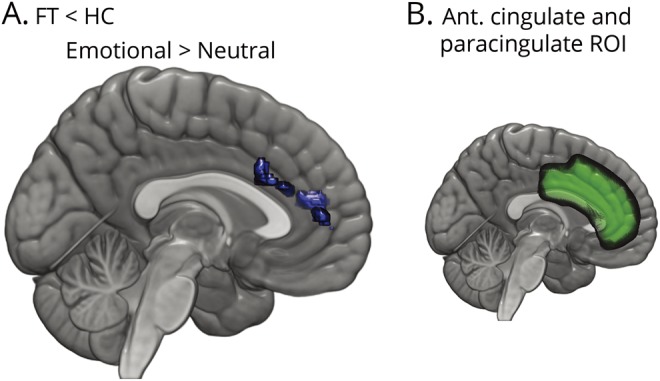
(A) Differences between participants with functional tremor (FT) and healthy controls (HCs). Regions in blue showed greater activation in HCs. (B) Region over which small volume correction was implemented.
ROI results (mean activation, without small volume correction)
The ROI in the anterior cingulate/paracingulate cortex showed a significant decrease in activation from before to after CBT during the basic-emotion task (p = 0.05 corrected). Among CBT responders, a greater change in the anterior cingulate/paracingulate activation pattern from before to after CBT was associated with higher pre-CBT depression scores (greater HAM-D score) (r2 = 0.7556, p = 0.008) (figure 4).
Figure 4. Changes in activation and depression scores.
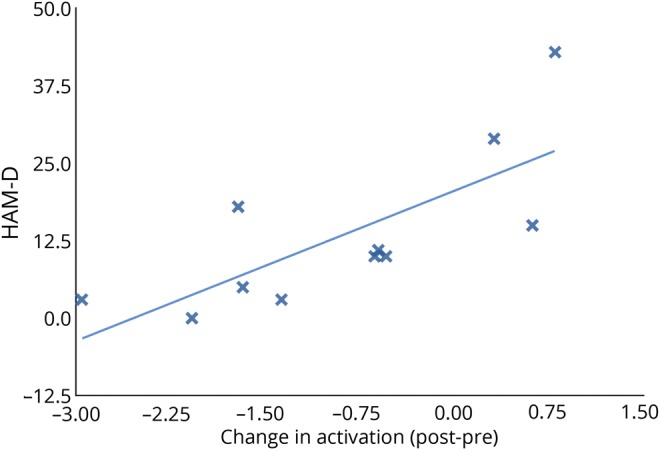
A strong correlation was found between Hamilton Depression Scale (HAM-D) scores and changes in activation, before cognitive behavioral therapy to after CBT, in the anterior cingulate/paracingulate region (r2 = 0.56, p = 0.008) among those patients with FT who responded to CBT therapy.
Discussion
This pilot clinical and fMRI study revealed 2 important findings: (1) CBT led to remission or near remission in almost 75% of this cohort of patients with FT, an impressive magnitude of benefit for a chronic condition with progressive disability and no proven available treatment; and (2) the CBT-mediated improvement was associated with a large difference in the fMRI activity of the anterior cingulate/paracingulate region, with the magnitude of change being higher among those with more severe baseline depression. The absence of functional changes in motor regions despite the marked reduction of tremor severity after CBT indicates that the motor system itself is unaffected in FT. These findings suggest that CBT may be a promising therapeutic intervention for FT (and plausibly a range of other functional movement disorders) and that the anterior cingulate/paracingulate region is an important node in the emotion-processing network associated with FT. Furthermore, normalization of medial frontal overactivity may represent a mechanism of action for CBT, perhaps playing a greater role among those with depression.
The anterior cingulate and paracingulate regions may be important for the generation of FT and the prediction of CBT response. We recently showed that this region had increased activity in patients with FT compared to HCs during emotional faces processing after controlling for depression scores and correcting for multiple comparisons,9 which is consistent with our current results. Both small volume correction analysis and ROI analysis show that CBT elicits a normalizing effect by reducing this anterior cingulate/paracingulate activity during emotion processing. Significant activation in the cingulate/paracingulate region after emotional stimuli has been correlated with alexithymia (the inability to identify and describe emotions)34 and may represent a common psychopathologic mechanism in functional motor disorders.35 In addition, the anterior cingulate region plays a role in cognitive processing related to attention (because abnormal attention to symptoms seems key in functional motor symptoms), as explained under bayesian models of brain function.36 It is possible that changes in attention may provide a mechanism for an intervention such as CBT to exert its benefit. Furthermore, the anterior paracingulate cortex is considered a key prefrontal region subserving the theory of mind, the ability to represent mental states, which is important for the understanding of the intentions of people involved in social interactions and in predicting future intentional social interactions.37 The impaired reasoning about our own and other people's mental states has already been identified in another functional neurologic disorder, PNES.38 Theory of mind can unify functional neurologic disorders by reconciling the perception of the internal states of the body, or interoception, across a variety of emotional states.39 In the setting of altered emotion processing, the ability to predict future events is eroded.40 This impaired bayesian prediction may explain the ostensible contradictions between voluntary and unconscious motor control in functional motor disorders, resulting in abnormal predictive beliefs about movements, which are therefore experienced as involuntary, that is, without a sense of agency.41
This is the first study that measured longitudinally the neural correlates of a response to an intervention in a functional movement disorder. Prior studies on the neurobiology of FT have focused largely on cross-sectional imaging of patients in the resting state. These fMRI studies showed decreased functional connectivity between the right temporoparietal junction and bilateral sensorimotor regions, which is considered to underlie the impairment of sensory motor integration and patients' impaired self-agency, the sense of control of one's own actions.7,8 The demonstration of hypermetabolism in the medial posterior parietal lobes bilaterally by PET with 18F-deoxyglucose in the resting state of a single participant also supports an impairment of sensorimotor integration.42 In contrast to a previous resting-state study (which demonstrated temporoparietal junction hypofunction8), our fMRI protocol focused on motor and emotion-processing tasks, with particular attention to pre- versus post-CBT changes.
It has previously been postulated that patients with FT exhibit abnormalities in subcortical processing similar to those found in other functional neurologic disorders.43 One study in patients with unilateral paralysis and hemianesthesia documented a decrease in regional cerebral blood flow to passive vibratory stimulation in the thalamus and basal ganglia using single photon emission CT with (99m)Tc–ethyl cysteinate dimer after an intervention.43 Together, compared to HCs, the data suggest that abnormal processing of emotional information is associated with limbic activation via changes in connectivity between basal ganglia and thalamocortical circuits and results in a deficit in sensory or motor processing.44 Alternatively, simple emotional stimuli may instead lead to functional deafferentation due to active inhibition of somatosensory processing by limbic areas concerned with emotion and attention,45 thus resulting in the overall decreased fMRI responses to the emotion-processing paradigm. The extent to which these changes in patients with FT apply to other functional movement disorders remains unclear.
Finally, the issue of asymmetry is worth briefly highlighting because, in the basic-emotion task, the activation was greater in the right cingulate/paracingulate cortex in the FT group. Asymmetry is often observed in studies of patients with functional neurologic disorders and is not necessarily always present in the same hemisphere.6 One study argued that emotional disturbances related to right hemispheric abnormalities may be the etiology of emotional disturbances that result, as an end effect, in PNES (a functional neurologic disorder), and another study documented aberrant right uncinate fasciculus (connection between amygdala and medial frontal regions, including cingulate); this was not confirmed by other studies.
Limitations
Our study has limitations. As an open-label study, comparisons were performed before and after CBT rather than in an ideal parallel design with patients having received a placebo intervention. Because of the limited sample size, we were unable to determine the effects, if any, of symptom duration >4 years and other clinical variables, including severe and continuous resting tremor (these patients had to be excluded in order to be scanned). In addition, we used a nonmanualized form of CBT, inducing a source of variability; however, it was important to tailor the therapy to individual patients. As a result of this adjustment, it may be difficult to reproduce our findings in other settings, including clinical trials. The fMRI paradigms used in the current study have not been studied for intraindividual consistency over time, and changes in cortical activation may be altered without implying true biological changes. Furthermore, we did not measure anxiety and depression at follow up, preventing us from measuring the extent to which some of the fMRI changes may have been explained by changes in mood states.46 This concern has become especially warranted with the increasing recognition that fMRI processing may result in false positives or confounded data,47 and the technical updates we incorporated32 may not have entirely eliminated this potential shortcoming. Finally, it is also possible that at least some of the differences in activation in participants with FT may relate to their psychiatric comorbid condition and be a sign of maladaptive cognitive interpretations of emotions.48 Attenuating the potential for a direct effect of psychiatric comorbid conditions in our ROI, fluorodeoxyglucose-PET studies in patients with major depression have shown that interpersonal psychotherapy reduces glucose metabolism of the left middle anterior cingulate cortex,49 while CBT increases glucose metabolism in the dorsal anterior cingulate cortex.50
CBT significantly improved FT in this open-label study, with nearly 75% remission or near remission. Improvements were associated with decreased overactivity in the anterior cingulate/paracingulate region, especially prominent in those with greater depression at baseline. Because it is possible that changes in emotion-processing networks/anterior cingulate region may be related to changes in associated psychiatric comorbid conditions rather than changes in the severity of the movement disorder, a randomized controlled trial will need to include a control arm (such as psychoeducation) ideally matched for associated psychiatric comorbidities. Future studies should be aimed at ascertaining the long-term duration of benefits and the extent to which adjuvant interventions such as physical therapy may yield synergistic effects. If confirmed, changes in the anterior cingulate/paracingulate region may represent a marker of FT and predict CBT response.
Glossary
- AFNI
Analysis of Functional Neuroimages
- CBT
cognitive behavioral therapy
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- FSL
FMRIB Software Library
- FT
functional tremor
- HAM-A
Hamilton Anxiety Scale
- HAM-D
Hamilton Depression Scale
- HC
healthy control
- ICD-10
International Classification of Diseases, 10th revision
- MINI
Mini International Neuropsychiatric Interview
- PMDRS
Rating Scale for Psychogenic Movement Disorders
- PNES
psychogenic nonepileptic seizures
- ROI
region of interest
- TFCE
threshold-free cluster enhancement
Appendix. Authors
Footnotes
Study funding
This study was funded by the NIH (K23 MH092735).
Disclosure
A. Espay has received grant support from the NIH (National Institute of Mental Health; K23MH092735), Great Lakes Neurotechnologies, and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, Adamas, Acadia, Acorda, Neuroderm, Impax, Sunovion, Lundbeck, Osmotica Pharmaceutical, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from USWorldMeds, Lundbeck, Acadia, Sunovion, the American Academy of Neurology, and the Movement Disorders Society. S. Ries and T. Maloney report no disclosures relevant to the manuscript. J. Vannest receives funding from the NIH. E. Neefus reports no disclosures relevant to the manuscript. A. Dwivedi is supported as a coinvestigator by the NIH (1R01HL125016-01, 1R21 HL143030-01, and 1R21 AI133207) and as a collaborator in NIH R21 AI118228 grant. He has been also serving as a statistician in Cancer Prevention and Research Institute of Texas grants (PP180003, PP170068, PP170004, PP140164, 140211, PP110156, PP150031, and PP130083), CCTST K12 (consultant) award, Coldwell (coinvestigator), and TMF (coinvestigator). J. Allendorfer has received funding from the Shor Foundation for Epilepsy Research. She serves as an associate editor of Restorative Neurology and Neuroscience. L. Wulsin reports no disclosures relevant to the manuscript. W. LaFrance has served on the editorial boards of Epilepsia, Epilepsy & Behavior, Journal of Neuropsychiatry and Clinical Neurosciences, and Journal of Neurology, Neurosurgery and Psychiatry; receives editor's royalties from the publication of Gates and Rowan's Nonepileptic Seizures, 3rd edition (Cambridge University Press, 2010) and 4th edition (2018); author's royalties for Taking Control of Your Seizures: Workbook and Therapist Guide (Oxford University Press, 2015); has received research support from the NIH (National Institute of Neurological Disorders and Stroke; 5K23NS45902 [principal investigator]), Rhode Island Hospital, the American Epilepsy Society, the Epilepsy Foundation, Brown University, the Siravo Foundation, and the Department of Defense (W81XWH-17- 1-0619 [principal investigator]); serves on the Epilepsy Foundation Professional Advisory Board; has received honoraria for the American Academy of Neurology Annual Course; has served as a clinic development consultant at University of Colorado Denver, Cleveland Clinic, Spectrum Health, and Emory University; and has provided medico-legal expert testimony. A. Lang has served as an advisor for AbbVie, Allon Therapeutics, Avanir Pharmaceuticals, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Ceregene, Cipla, InteKrin, Lilly, Medtronic, Merck, Novartis, NeuroPhage Pharmaceuticals, Teva, and UCB; received honoraria from Medtronic, Teva, UCB, and AbbVie; received grants from Brain Canada, the Canadian Institutes of Health Research, the Edmond J. Safra Philanthropic Foundation, the Michael J. Fox Foundation, the Ontario Brain Institute, National Parkinson Foundation, Parkinson Society Canada, Physicians Services Inc, Tourette Syndrome Association, and W. Garfield Weston Foundation; received publishing royalties from Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press; and has served as an expert witness in cases related to the welding industry. J. Szaflarski has received research funding from NIH, Shor Foundation for Epilepsy Research, Eisai, Epilepsy Foundation of America, Food and Drug Administration, Compumedics Neuroscan, Inc, Department of Defense, state of Alabama, and University of Alabama at Birmingham. While this research was conducted, he was supported by NIH K23 NS052468. He serves as an associate editor of Restorative Neurology and Neuroscience and Journal of Epileptology and on editorial boards of the journals Epilepsy & Behavior, Folia Medica Copernicana, and Journal of Medical Science. Go to Neurology.org/N for full disclosures.
References
- 1.Espay AJ, Lang AE. Phenotype-specific diagnosis of functional (psychogenic) movement disorders. Curr Neurol Neurosci Rep 2015;15:556. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen G, Buszewicz M, Stevenson F, et al. Randomised feasibility study of physiotherapy for patients with functional motor symptoms. J Neurol Neurosurg Psychiatry 2017;88:484–490. [DOI] [PubMed] [Google Scholar]
- 3.Espay AJ, Edwards MJ, Oggioni GD, et al. Tremor retrainment as therapeutic strategy in psychogenic (functional) tremor. Parkinsonism Relat Disord 2014;20:647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKeon A, Ahlskog JE, Bower JH, Josephs KA, Matsumoto JY. Psychogenic tremor: long-term prognosis in patients with electrophysiologically confirmed disease. Mov Disord 2009;24:72–76. [DOI] [PubMed] [Google Scholar]
- 5.LaFrance WC Jr, Baird GL, Barry JJ, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry 2014;71:997–1005. [DOI] [PubMed] [Google Scholar]
- 6.Szaflarski JP, LaFrance WC Jr. Psychogenic nonepileptic seizures (PNES) as a network disorder: evidence from neuroimaging of functional (psychogenic) neurological disorders. Epilepsy Curr 2018;18:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurer CW, LaFaver K, Ameli R, Epstein SA, Hallett M, Horovitz SG. Impaired self-agency in functional movement disorders: a resting-state fMRI study. Neurology 2016;87:564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology 2010;74:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espay AJ, Maloney T, Vannest J, et al. Impaired emotion processing in functional (psychogenic) tremor: a functional magnetic resonance imaging study. Neuroimage Clin 2018;17:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voon V, Cavanna AE, Coburn K, Sampson S, Reeve A, LaFrance WC Jr. Functional neuroanatomy and neurophysiology of functional neurological disorders (conversion disorder). J Neuropsychiatry Clin Neurosci 2016;28:168–190. [DOI] [PubMed] [Google Scholar]
- 11.Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods 2002;118:115–128. [DOI] [PubMed] [Google Scholar]
- 12.Hinson VK, Cubo E, Comella CL, Goetz CG, Leurgans S. Rating Scale for Psychogenic Movement Disorders: scale development and clinimetric testing. Mov Disord 2005;20:1592–1597. [DOI] [PubMed] [Google Scholar]
- 13.Pinninti NR, Madison H, Musser E, Rissmiller D. MINI International Neuropsychiatric Schedule: clinical utility and patient acceptance. Eur Psychiatry 2003;18:361–364. [DOI] [PubMed] [Google Scholar]
- 14.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(suppl 20):22–33; quiz 34–57. [PubMed] [Google Scholar]
- 15.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry 1988;45:742–747. [DOI] [PubMed] [Google Scholar]
- 16.Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord 1988;14:61–68. [DOI] [PubMed] [Google Scholar]
- 17.Szaflarski JP, Allendorfer JB, Heyse H, Mendoza L, Szaflarski BA, Cohen N. Functional MRI of facial emotion processing in left temporal lobe epilepsy. Epilepsy Behav 2014;32:92–99. [DOI] [PubMed] [Google Scholar]
- 18.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA 2002;99:11458–11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 2009;168:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breiter HC, Etcoff NL, Whalen PJ, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron 1996;17:875–887. [DOI] [PubMed] [Google Scholar]
- 21.Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res 2002;15:31–45. [DOI] [PubMed] [Google Scholar]
- 22.Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci USA 2002;99:11447–11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strakowski SM, Eliassen JC, Lamy M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry 2011;69:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 2001;20:45–57. [DOI] [PubMed] [Google Scholar]
- 25.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 27.Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv 2006;9(pt 2):58–66. [DOI] [PubMed] [Google Scholar]
- 28.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011;56:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001;5:143–156. [DOI] [PubMed] [Google Scholar]
- 30.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med 1996;35:346–355. [DOI] [PubMed] [Google Scholar]
- 31.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007;37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI clustering in AFNI: false-positive rates redux. Brain Connect 2017;7:152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 34.Deng Y, Ma X, Tang Q. Brain response during visual emotional processing: an fMRI study of alexithymia. Psychiatry Res 2013;213:225–229. [DOI] [PubMed] [Google Scholar]
- 35.Demartini B, Petrochilos P, Ricciardi L, Price G, Edwards MJ, Joyce E. The role of alexithymia in the development of functional motor symptoms (conversion disorder). J Neurol Neurosurg Psychiatry 2014;85:1132–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhns AB, Dombert PL, Mengotti P, Fink GR, Vossel S. Spatial attention, motor intention, and bayesian cue predictability in the human brain. J Neurosci 2017;37:5334–5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walter H, Adenzato M, Ciaramidaro A, Enrici I, Pia L, Bara BG. Understanding intentions in social interaction: the role of the anterior paracingulate cortex. J Cogn Neurosci 2004;16:1854–1863. [DOI] [PubMed] [Google Scholar]
- 38.Schonenberg M, Jusyte A, Hohnle N, et al. Theory of mind abilities in patients with psychogenic nonepileptic seizures. Epilepsy Behav 2015;53:20–24. [DOI] [PubMed] [Google Scholar]
- 39.Shah P, Catmur C, Bird G. From heart to mind: linking interoception, emotion, and theory of mind. Cortex 2017;93:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartsch K, Estes D. Why we assume it's all good: the role of theory of mind in early inherent feature inferences. Behav Brain Sci 2014;37:482. [DOI] [PubMed] [Google Scholar]
- 41.Newby R, Alty J, Kempster P. Functional dystonia and the borderland between neurology and psychiatry: new concepts. Mov Disord 2016;31:1777–1784. [DOI] [PubMed] [Google Scholar]
- 42.Hedera P. Metabolic hyperactivity of the medial posterior parietal lobes in psychogenic tremor. Tremor Other Hyperkinet Mov 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vuilleumier P, Chicherio C, Assal F, Schwartz S, Slosman D, Landis T. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain 2001;124(pt 6):1077–1090. [DOI] [PubMed] [Google Scholar]
- 44.Harvey SB, Stanton BR, David AS. Conversion disorder: towards a neurobiological understanding. Neuropsychiatr Dis Treat 2006;2:13–20. [PMC free article] [PubMed] [Google Scholar]
- 45.Black DN, Seritan AL, Taber KH, Hurley RA. Conversion hysteria: lessons from functional imaging. J Neuropsychiatry Clin Neurosci 2004;16:245–251. [DOI] [PubMed] [Google Scholar]
- 46.Beauregard M. Functional neuroimaging studies of the effects of psychotherapy. Dialogues Clin Neurosci 2014;16:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 2016;113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrison NA, Critchley HD. Neuroimaging and Emotion, 2nd ed. Oxford: Elsevier; 2007. [Google Scholar]
- 49.Brody AL, Saxena S, Stoessel P, et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry 2001;58:631–640. [DOI] [PubMed] [Google Scholar]
- 50.Goldapple K, Segal Z, Garson C, et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 2004;61:34–41. [DOI] [PubMed] [Google Scholar]