Abstract
For early diagnosis and treatment of invasive candidiasis (IC), the well-known risk factors may not apply in the intensive care unit (ICU). This retrospective study identified the risk factors predicting IC and candidemia in cancer patients under intensive care after gastrointestinal surgery.
Enrolled were 229 cancer patients admitted to our oncology surgical ICU after gastrointestinal surgery between January 1, 2010 and October 31, 2014.
The most common types of solid gastrointestinal cancers were gastric (49.8%), colon (20.1%), and esophageal (18.3%). The percentage of patients with corrected Candida colonization index (CCI) ≥0.4 was 31.9%. IC was confirmed in 19 patients (8.3%), and the ICU mortality was 15.8%. Candida albicans accounted for 52.6% of the total number of pathogenic Candida isolates. Among patients with CCI ≥0.4, the cancers with the highest prevalence were cardiac (45%) and gastric (36%), with ICU mortalities of 20% and 4.9%, respectively. For the diagnosis of candidemia, (1-3)-β-D-glucan (BDG) ≥80 pg/mL showed a sensitivity and specificity of 25% and 82.7%, respectively, positive and negative predictive values 6.7% and 95.7%, and area under the receiver operating characteristic curve 0.512. CCI ≥0.4 was the only significant predictor of IC, and number of organ failures was the only predictor of candidemia (P = .000 and .026).
CCI ≥0.4 was the only significant risk factor predicting IC, with greater prediction of intra-abdominal candidiasis but failure to predict candidemia. Blood culture and BDG detection are recommended to supplement diagnosis. Patients may have multifocal and high-grade Candida colonization after cardiac surgery, and; therefore, are at high risk of IC, which should be taken seriously.
Keywords: 1,3-β-D-glucan; cancer; corrected Candida colonization index; gastrointestinal surgery; intensive care unit
1. Introduction
Invasive candidiasis (IC), due to infection of fungal yeast Candida spp., may involve the bloodstream (candidemia) or deep-seated tissue. Over recent decades, the prevalence of IC in nonneutropenic patients in the intensive care unit (ICU) has been stable or rising, with mortality rates reported between 29.9% and 70.3%.[1–9] While the delay or inappropriate initiation of antifungal therapy in the ICU is an independent risk factor of mortality, the lack of a prompt and accurate method of diagnosis makes such delay inevitable.[10–12] As a result, clinicians prefer to strategize therapies (pre-emptive, presumptive, or empirical) based on risk factors or nonculture tests. The pre-emptive includes the (1-3)-β-D-glucan (BDG) test for invasive fungal infections, based on the guidelines of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Infectious Diseases Society of America (IDSA). However, to our best knowledge, no study has shown any outcome benefits associated with these methods.[12–17]
Based on our experience in an oncology surgical ICU, after abdominal surgery patients are uniquely at high early risk for candidiasis, especially those with recurrent gastrointestinal perforation and anastomotic leaks. Studies are rare, and it is not known if the risk varies according to surgeries for solid tumors of the esophagus, stomach, colon, or rectum. It would be helpful to identify patients who are most likely to benefit from early antifungal treatment after different abdominal operations, rather than provide empirical therapy for all.
The present study investigated the specific risk factors of IC associated with different gastrointestinal surgery sites. In addition, the corrected Candida colonization index (CCI), BDG, and procalcitonin were compared for predicting IC.
2. Materials and methods
2.1. Study design and setting
This was a retrospective, cohort, single-center observational study conducted in the 11-bed surgical ICU of Tianjin Medical University Cancer Institute and Hospital, a 2400-bed hospital in Tianjin, China. Permission was obtained from the Ethics Commission of Tianjin Medical University Cancer Institute and Hospital to review and publish information from patients’ records. All necessary written consent from the patients involved in the study was received.
All critically ill cancer patients admitted between January 1, 2010 and October 31, 2014 to the oncology surgical ICU were evaluated. Patients who met all the following criteria were included: aged ≥18 years; acute physiology and chronic health evaluation (APACHE) II score >10[18]; undergone surgery for solid tumors of the esophagus, stomach, colon, or rectum; and without neutropenia. The epidemiological, clinical, and laboratory data collected from patients’ medical records and reports included: gender; age; height; weight; risk factors for IC; and ICU and in-hospital mortality. The patient's APACHE II score was calculated from the worst values of physiological variables in the first 24 hours upon ICU admission.
The analysis of risk factors included the underlying disease (ie, chronic heart failure, diabetes mellitus, hypertension, chronic renal failure, or chronic bronchitis), type of solid cancer, and operative modality. In addition, the analysis included the patients’ treatment histories (chemotherapy, radiotherapy, antifungal treatment, antibiotics, and steroids), and the presence of central vascular catheters, total parenteral nutrition, and mechanical ventilation and renal replacement therapy >48 hours. Also taken into account were the presence of sepsis, severe sepsis, septic shock, the number of organ failures, results of BDG (Beijing Gold Mountainriver Tech Development, Beijing, China) and procalcitonin, and length of ICU stay.
2.2. CCI measurement
Mycological screening was performed on each patient upon admittance to the surgical ICU and then once each week afterward until discharge from the ICU. Screening included 5 biological samples: gastric and tracheal aspirates, urine collection, and oropharyngeal and rectal swabs. Specimens were cultured on Sabouraud chloramphenicol agar (JinZhangKeJi, Tianjin, China) at 35 °C for 24 to 48 hours, dependent on the results of gram staining. Species identification was performed on VITEK 2 Compact (bio-Merieux SA, Marcy l’Etoile, France). Results were rated as positive when at least 1 colony of Candida sp. was found on a culture plate. Limits of detection were 1 colony-forming unit (CFU) for swabs, 100 CFU/mL for gastric juice and urine, and 104 CFU/mL for tracheal aspirates. Results were rated as highly positive when the threshold was reached of 100 CFU for rectal and oropharyngeal swabs, and 105 CFU/mL for gastric, tracheal aspirate, and urine. The CCI (ratio of highly positive samples to the total number of samples cultured) was calculated at admittance and once per week for each patient. CCI ≥0.4 was defined as multifocal and high-grade Candida colonization.[19]
2.3. Definitions
IC was diagnosed when patients showed clinical signs of infection (in the opinion of the treating physician) and met at least 1 of the 3 diagnostic criteria, following. First, histopathological, cytopathological, or direct microscopic confirmation of yeast cells in a specimen obtained by needle aspiration or biopsy from a normally sterile site (other than mucous membranes). Second, at least 1 peripheral blood culture positive for Candida. Third, a positive Candida culture from a sample obtained by sterile technique from a normally sterile site (eg, cerebrospinal, pleural, peritoneal, or peritoneal abscess fluid).
Patients were excluded from this study for any of the following: a positive blood culture from a catheter but negative peripheral blood cultures; IC diagnosed before ICU admission; or non-IC (eg, candiduria). In addition, patients were excluded for a positive culture from a normally sterile site that could not be confirmed as uncontaminated (eg, after placement of a drainage tube, culture from a peritoneal abscess obtained by paracentesis or post-placement of a drainage tube, or ascitic fluid obtained via drainage tube after surgery for intestinal perforation or anastomosis). All eligible patients were included in the study.[9] Candidemia was considered when there was at least 1 positive blood culture in the days preceding or succeeding the diagnosis of sepsis.[12]
Only insulin-treated patients were considered to have diabetes mellitus. Chronic renal failure was recorded in patients requiring hemodialysis or peritoneal dialysis at the time of admission to the hospital. Chronic heart failure was defined as New York Heart Association grades III and IV.[20] Chronic bronchitis was the presence of a productive cough or expectoration for >90 days per year (although on separate days) and for more than 2 consecutive years, provided that a specific disorder responsible for these symptoms was not present.
A history of using steroids was considered a daily dose equivalent of 15 to 20 mg prednisone for at least 4 weeks, or a cumulative dose of 700 mg or more before isolation of Candida in cultures. Antibiotic or antifungal treatment was defined as the use of broad-spectrum antibiotics or antifungal agents within 10 days before ICU admission. Sepsis was the presence of infection together with systemic manifestations of infection. The diagnosis for severe sepsis and septic shock depended on sepsis 2.0 of the Guidelines for Management of Sepsis and Septic Shock.[21]
2.4. Statistical analysis
Statistical analyses were performed using SPSS version 19.0 software (SPS, Chicago, IL). Numerical variables are described using frequency statistics. Continuous variables are reported as median with interquartile range (IQR, 25P–75P) according to the normality of distribution verified by the Kolmogorov–Smirnov test. The between-group associations of demographic and clinical variables were examined using the Chi-squared (χ2) test for categorical variables, the independent t test or t test for randomly distributed continuous variables, and the Mann–Whitney U test for non-normally distributed continuous variables. A logistic regression model was used to analyze the independent risk factors for prognosis in the ICU, incidence of IC, and candidemia. The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using the Cox proportional hazards model, to examine the effect of multiple factors on prognosis with regard to survival in the hospital and ICU. All tests were 2-sided, and P ≤ .05 was considered statistically significant. Variables yielding P ≤ .2 by univariate analysis, and those considered clinically relevant, were entered into the multivariate analysis to estimate the independent association of each covariate with the dependent variable.
3. Results
3.1. Characteristics and outcomes of the study population
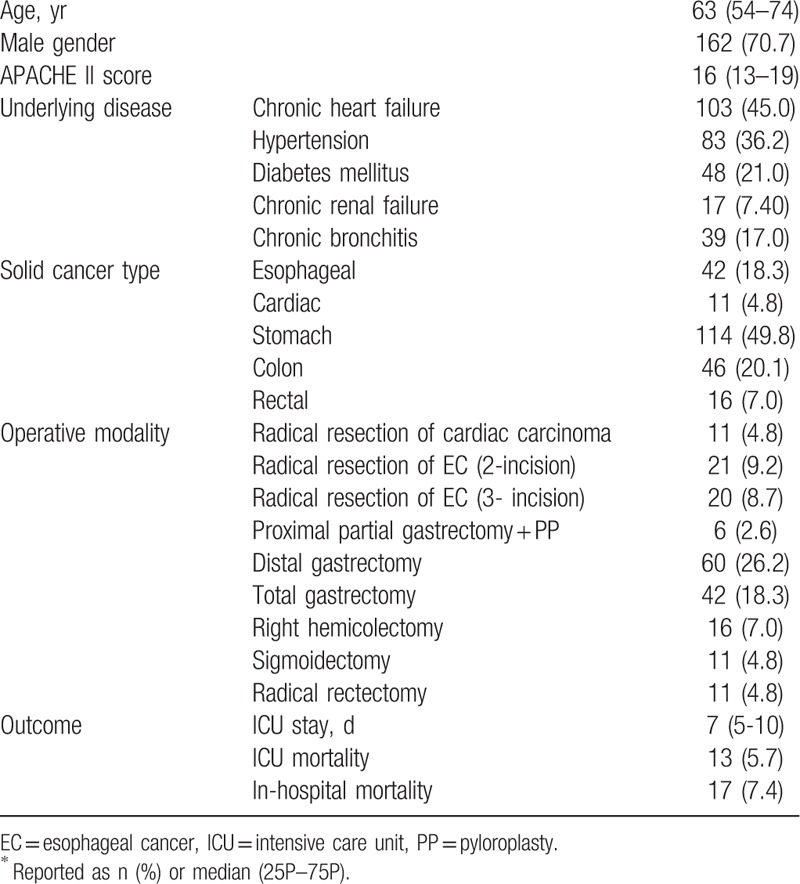
During the study period, 229 patients who met the criteria for inclusion were enrolled (Table 1). The most common types of solid gastrointestinal tumors were gastric cancer (49.8%), colon cancer (20.1%), and esophageal cancer (18.3%). The most common surgeries were distal gastrectomy plus Roux-en-Y bypass plus D2 lymph node dissection (60 patients, 26.2%), total gastrectomy (42 patients, 18.3%), and radical resection of esophageal cancer (3-incision; 20 patients, 8.7%). The median (IQR) length of ICU stay was 7 (5–10) days. ICU mortality was 5.7%, and the in-hospital mortality was 7.4% (13 and 17 of 229 patients, respectively). The causes of death in the ICU were septic shock (3.9%), abdominal hemorrhage (1.3%), and tumor progression (0.4%).
Table 1.
Characteristics and outcomes of cancer patients after gastrointestinal surgery in the surgical ICU∗.
3.2. Infective characteristics and outcomes of the study population
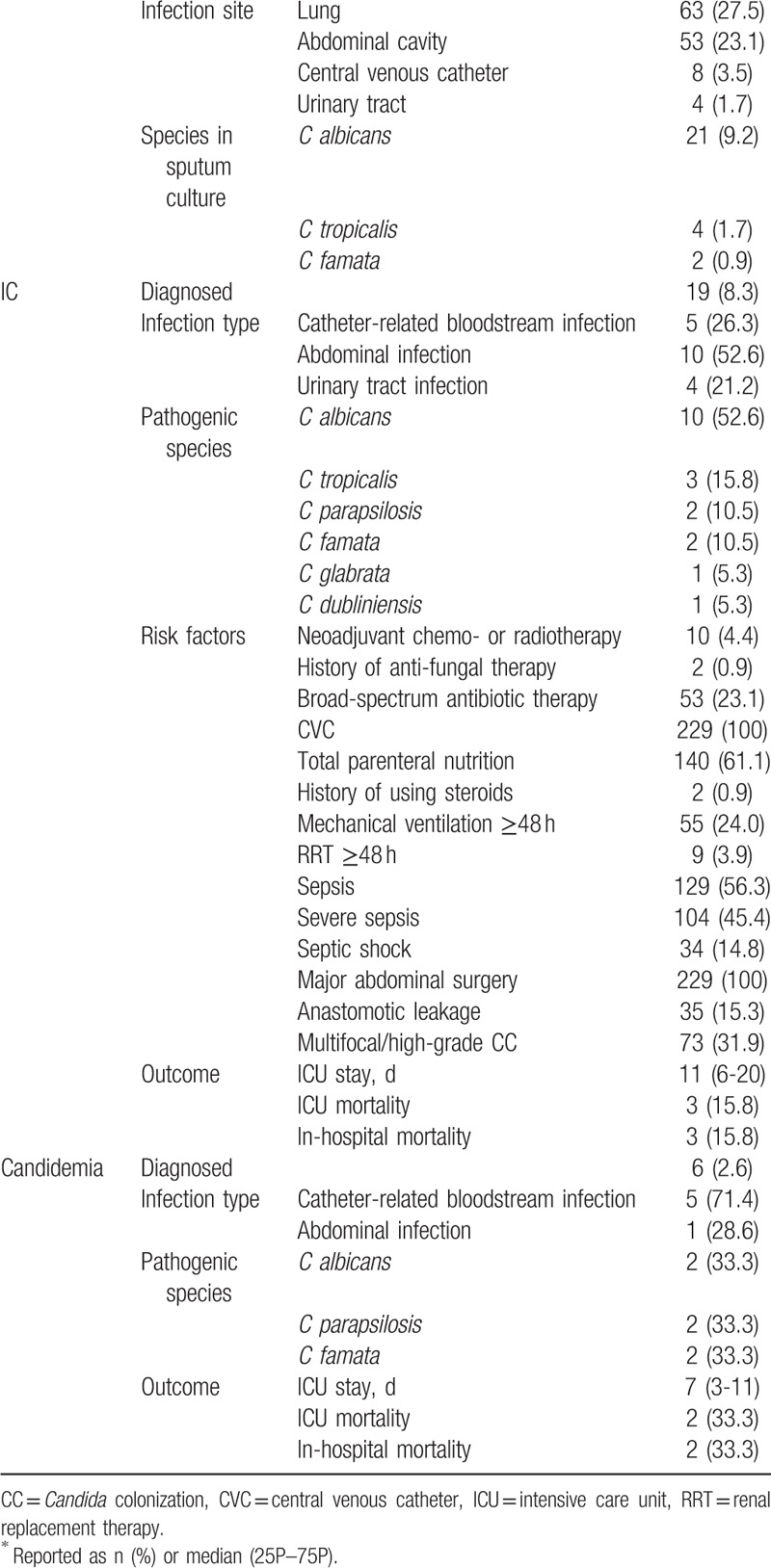
Of the total 229 patients, 129 (56.3%), 104 (45.4%), and 34 (14.8%) were diagnosed as having sepsis, severe sepsis, and sepsis shock, respectively. By definition, septic shock is a category of severe sepsis.[21] The percentage of patients with multifocal and high-grade Candida colonization (CCI ≥0.4) was 31.9%. Twenty-seven patients (11.8%) were found with Candida by sputum culture.
IC was confirmed in 19 patients (8.3%), and the ICU mortality was 15.8%. The main site of infection was the abdominal cavity (10 patients, 4.4%). Candida albicans accounted for 52.6% of the total number of pathogenic Candida isolates, while species other than C albicans were responsible for 47.3%. Specifically, the top 3 species other than C albicans were C tropicalis (3 patients, 15.8%), C parapsilosis (2 patients, 10.5%), and C famata (2 patients, 10.5%).
Six patients (2.6%) received a definite diagnosis of candidemia, and ICU mortality was 33.3%. The main sources of infection were catheter-related (5 patients, 71.4%) or abdominal (1 case, 28.6%). Infections caused by species other than C albicans were responsible for more than half of the cases (66.6%). The main species were C parapsilosis (2 patients, 33.3%) and C famata (2 patients, 33.3%).
Central venous catheter and abdominal surgery are considered high-risk factors of IC,[13,14] and in the present study all the patients shared these risk factors (Table 2). Other risk factors included parenteral nutritional support (220 patients, 96.1%), sepsis, and total parenteral nutrition.
Table 2.
Characteristics of infections and outcomes of cancer patients after gastrointestinal surgery in the surgical ICU∗.
3.3. Candida colonization after different gastrointestinal surgeries
The Candida colonization in 5 samples of the 229 patients was less than 50% of specimens (Table 3). The percentage of Candida colonization in oropharyngeal swabs, gastric aspirates, tracheal aspirates, urine, and rectal swabs were 33.2%, 38.9%, 41.5%, 3.9%, and 22.3%, respectively. The main strains were of C albicans, and then C tropicalis, C famata, C glabrata, and C parapsilosis. C albicans (53.8%) and C tropicalis (7.7%) were the leading colonized species.
Table 3.
Multifocal colonized Candida status and outcomes of patients after various gastrointestinal surgeries∗.
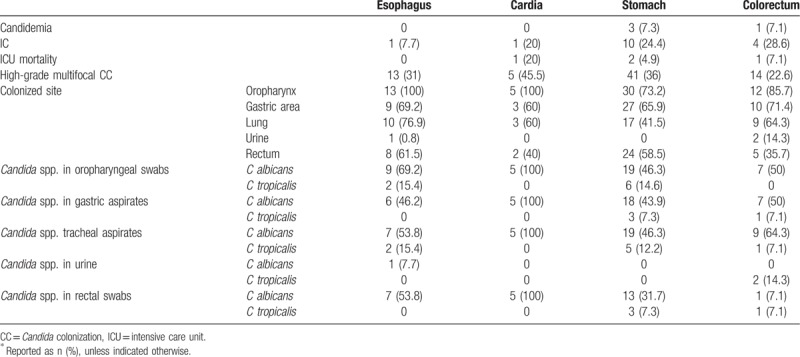
The rate of multifocal and high-grade Candida colonization in patients after cardiac surgery was 45.5%, and ICU mortality was 20%; the rate in patients after gastric surgery was 36%, with ICU mortality 4.9%.
3.4. Nonculture diagnostic tests
Since this study was a retrospective analysis, the BDG test was not provided at the early stage of medical record collection, and only 85 patients with BDG test were recorded. Overall, 19 patients were diagnosed with IC, of which 9 had an available BDG test; 2 had BDG ≥80 pg/mL. Six patients were found with candidemia, of which 4 had a BDG test available; only 1 had BDG ≥80 pg/mL. Fifteen patients had BDG ≥80 pg/mL, and 2 (13.3%) and 1 (6.7%) of these received a diagnosis of IC and candidemia, respectively. Seventy patients had BDG <80 pg/mL; 7 (10.0%) had IC, and 3 (4.3%) had candidemia.
For the diagnosis of IC (candidemia), the sensitivity of BDG ≥80 pg/mL in 2 consecutive samples was 22.2% (25.0%), specificity was 82.9% (82.7%), the positive and negative predictive values were 13.3% (6.7%) and 90% (95.7%), respectively, and the area under the receiver-operating curve was 0.517 (0.512).
The correlation analysis showed no significant association between the detection of procalcitonin and candidemia or IC.
3.5. Multivariate analysis for the risk factors of IC
Univariate comparisons of the risk factors of IC and clinical outcomes of the diagnosed and undiagnosed IC groups in the surgical ICU are shown in Table 4. The data for age, APACHE II score, and number of organ failures were not normally distributed in the undiagnosed IC group, as verified by the Kolmogorov–Smirnov test. The Mann–Whitney U test was used for between-group comparisons. The Chi-squared test was applied for other categorical variables.
Table 4.
Risk factors and clinical outcomes of diagnosed and undiagnosed IC in cancer patients in ICU after gastrointestinal surgery.
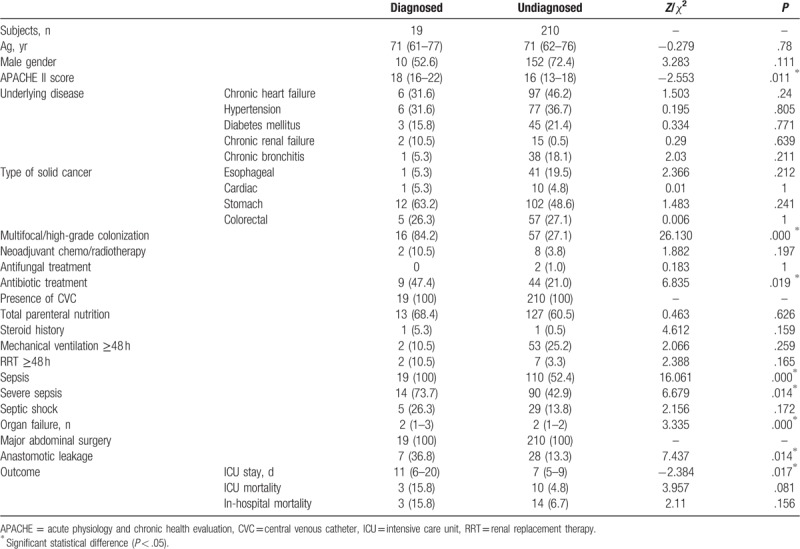
The diagnosed IC group had a significantly higher APACHE II score compared with the undiagnosed group, and significantly longer ICU stay (Table 4). In addition, the diagnosed group had significantly higher rates of multifocal and high-grade Candida colonization, applied broad-spectrum antibiotic therapy, anastomotic leakage, sepsis, severe sepsis, and number of organ failures.
Only multifocal and high-grade Candida colonization remained a significant predictor of IC (adjusted OR 66.411, 95% CI 5.971–738.652, P = .000), after adjusting for APACHE II score, multifocal and high-grade Candida colonization, broad-spectrum antibiotic therapy, sepsis, severe sepsis, number of organ failures, anastomotic leakage, and other variables yielding P ≤ .2.
3.6. Multivariate analysis for the risk factors of candidemia
The risk factors and clinical outcomes of diagnosed and undiagnosed candidemia are listed in Table 5. The data for age, APACHE II score, number of organ failures, and length of ICU stay of the undiagnosed candidemia group were not normally distributed, as verified by the Kolmogorov–Smirnov test. The Mann–Whitney U test was used for between-group comparisons.
Table 5.
Risk factors and clinical outcomes of diagnosed and undiagnosed candidemia in cancer patients in ICU after gastrointestinal surgery.
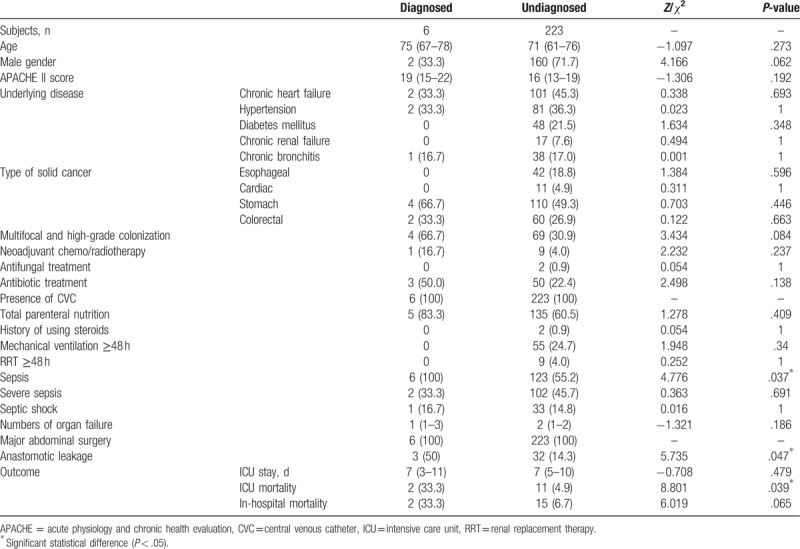
The diagnosed group had significantly higher rates of sepsis, anastomotic leakage, and ICU mortality, compared with the undiagnosed group (Table 5). After adjusting for sepsis, anastomotic leakage, and other variables yielding P ≤ .2, the number of organ failures remained a significant predictor of candidemia (adjusted OR 0.184, 95% CI 0.042–0.815, P = .026).
4. Discussion
There is no consensus regarding the validity of each of the risk factors for IC, which makes early prediction quite difficult. Furthermore, patients with cancer after various gastrointestinal surgeries conform to many of the traditional factors that indicate high risk. Determining a predictive biomarker for these patients that might reliably lead to an early diagnosis was the problem of this study. The statistical analysis found that a CCI ≥0.4 was the only significant risk factor predicting IC. Notably, the site of abdominal surgery was not predictive. In general, not all patients with IC have candidemia, and not all patients with candidemia have IC. However, in the present study all the patients who had received a diagnosis of candidemia (ie, with at least 1 positive blood culture in the days preceding or succeeding the diagnosis of sepsis) also satisfied the criteria for IC (positive results from both blood culture from a catheter and peripheral blood). For this population with IC, the number of organ failures was the only risk factor that predicted candidemia. It is not clear whether organ failure is the cause of candidemia, or a result of the disease.
In a study of nonneutropenic patients with severe abdominal conditions, meaningful screening values for discriminating between IC, Candida colonization, or no colonization or infection could be determined by positive results to either a combination of BDG and C albicans germ tube antibody (single determination), or any of 5 biomarkers in 2 consecutive samples.[22] BDG was also considered superior to cultures, Candida score, Candida index, or CCI for anticipating diagnosis of blood culture-negative postsurgical intra-abdominal candidiasis.[19,23,24]
In the present study, only 6 (31.6%) of the 19 patients that were determined to have IC had a positive blood culture. The high sensitivity (82.7%) and negative predictive value (95.7%) of BDG ≥80 pg/mL in 2 consecutive samples for candidemia had limited value in early antifungal therapy because of the high false-positive rate. However, such a high negative predictive value means that, for patients with negative BDG, the possibility of candidemia is not high.
According to most studies, prophylactic antifungal therapy is restricted to high-risk nonneutropenic patients. Pre-emptive antifungal therapy is not considered to improve prognosis,[7,25] and controversy remains over empirical antifungal therapy. In the AmarCAND2 study of ICUs in France, delayed initiation of antifungal treatment for Candida intra-abdominal infection increased the mortality of less severely ill patients (Sequential Organ Failure Assessment <7). This result indirectly suggested that empirical antifungal therapy may improve the prognosis of patients with peritonitis.[7] Of course, there is still no direct evidence that empirical antifungal therapy can improve prognosis,[16] while starting pre-emptive or empirical early antifungal therapy based on risk factors and nonculture-based diagnostic tests according to IDSA or ESCMID guidelines may lead to overuse of antifungals.[13,14] This overuse not only contributes to a huge financial burden, but has also promoted a shift to Candida species other than the pathogenic C albicans, or Candida species with reduced susceptibility to antifungal agents.[26] In the present study, C albicans was still the main pathogen of IC (52.6%), but other species accounted for 66.6% of candidemia, which is a very dangerous signal.
In the present study, all the patients were affected by cancer, presence of central venous catheter, and major abdominal surgery. For decades other risk factors of IC have also been common, such as total parenteral nutrition and severe sepsis. In our patients, the morbidity rates of IC and candidemia were 8.3% and 2.6%, respectively, with mortalities in the ICU of 15.8% and 33.3%. Although there were no significant differences in severity, these rates were significantly lower than data provided by many large-scale studies.[1–8] We conclude that cancer and major abdominal surgery may not be inevitable risk factors of IC in oncology ICU.
The cause of the lower rates in the present study may be that these patients with cancer were otherwise relatively healthy before surgery, with mild underlying disease, and; therefore, they were able to tolerate surgery. Even if sepsis occurred postoperatively, the accepted guideline risk factors, such as abdominal surgery and anastomotic leaks, actually did not apply to our study population and these patients were not as susceptible to IC as other ICU patients. Even in cases of IC infection, if the source of infection is iatrogenic, such as a catheter, this can often be removed to facilitate treatment. In addition, C albicans was the main pathogen of IC, and reasonable susceptibility to antifungal agents might explain the lower rates.
In the present analysis, well-known risk factors that justify empirical therapy, such as anastomotic leakage,[13,14,27] were not determined to be independent predictors. Only multifocal and high-grade Candida colonization (CCI ≥0.4) was an independent risk factor predicting IC, but this failed to predict candidemia. In the patients with confirmed IC, candidemia accounted for 31.6% of cases. The precondition of IC is multifocal and high-grade Candida colonization, caused by engraftment of local colonized Candida after the loss of mucosal barrier integrity during gastrointestinal surgery. However, candidemia may not be transmitted by this route, but enter the blood directly through the skin sinus duct of the catheter. Overall, this suggests that when critically ill patients with cancer after gastrointestinal surgery become infected, the CCI has greater predictive ability for the diagnosis of intra-abdominal candidiasis, and the good negative predictive value associated with BDG can be used to exclude candidemia. This is the most notable conclusion of the present study. Of course, blood culture and abdominal fluid should be routinely conducted at the same time.
In this study it was found that sputum, gastric fluid, and the pharynx were the most likely sites of C albicans colonization. Among the 4 types of surgery, rates of CCI ≥0.4 and mortality were the highest in patients after surgery for cardiac cancer, and then gastric surgery. This indirectly indicates that patients may have multifocal and high-grade Candida colonization after cardiac surgery, and; therefore, be at higher risk of IC. The cardiac is an anatomical junction between the esophagus and the stomach. The main controversy is over which department should perform the surgery, the esophageal or the stomach surgery department. The esophageal oncology department sometimes uses transthoracic and abdominal surgery, and surgical trauma is relatively large. It is difficult to perform surgery through thoracic surgery alone. On the other hand, the gastric oncology department only operates in the abdominal cavity, which is more difficult than the simple distal gastrectomy. Sometimes the diaphragm is damaged during the operation. In general, cardiac surgery is too traumatic, prone to complications such as chest and abdominal infections, and there is a higher probability of infection. There may be other reasons, such as the disappearance of the third physiological stenosis after the operation of the cardia, the greater damage to the mucosal physiological barrier caused by the reflux of the digestive juice, resulting in a higher rate of multifocal and high-grade Candida colonization in these patients. Yet, the multivariate statistical analysis showed that no operative modality was associated with IC or candidemia. This result may be of limited value, because there were only 11 patients who had undergone cardiac surgery altogether.
Our study had several limitations. First, this was a retrospective conducted at a single cancer center. However, to the best knowledge, it is the first report about risk factors of IC in critically ill cancer patients after gastrointestinal surgery in China. Second, the small number of patients distributed among the different gastrointestinal surgeries prevented us from investigating a link between CCI ≥0.4 and surgery type. Third, BDG monitoring was not available at the early stage of the study, which may affect the statistical results because of insufficient data. Fourth, BDG and CCI were monitored only for research purposes, but not for early diagnosis. Clinicians did not adjust the antifungal treatment based on these indicators. The significance of the prognostic data is thus limited.
5. Conclusion
In summary, in the early diagnosis and treatment of IC in the ICU, cancer and major abdominal surgery may not necessarily be the most telling risk factors. In this study, CCI ≥0.4 was the only significant risk factor in predicting IC, and not other well-accepted risk factors such as anastomotic leakage. CCI had good predictive ability for the diagnosis of intra-abdominal candidiasis, but failed to predict candidemia. Blood culture and BDG detection are recommended as auxiliary backup, because BDG ≥80 pg/mL had good negative predictive value for the diagnosis of candidemia. This can be useful for making negative clinical decisions regarding early antifungal treatment. Multifocal and high-grade Candida colonization is a risk factor after cardiac surgery, making IC more likely, and; therefore, should be taken seriously.
Acknowledgment
The authors thank Dr Ding Li for the microbiological experimental procedures.
Author contributions
Conceptualization: Rui Xia, Donghao Wang.
Data curation: Rui Xia, Donghao Wang.
Formal analysis: Rui Xia, Donghao Wang.
Funding acquisition: Rui Xia, Donghao Wang.
Investigation: Rui Xia, Donghao Wang.
Methodology: Rui Xia, Donghao Wang.
Project administration: Rui Xia.
Resources: Rui Xia, Donghao Wang.
Software: Rui Xia, Donghao Wang.
Supervision: Rui Xia, Donghao Wang.
Validation: Rui Xia, Donghao Wang.
Visualization: Rui Xia, Donghao Wang.
Writing – original draft: Rui Xia, Donghao Wang.
Writing – review & editing: Rui Xia, Donghao Wang.
Footnotes
Abbreviations: APACHE = acute physiology and chronic health evaluation, BDG = (1-3)-β-D-glucan, CCI = corrected Candida colonization index, CFU = colony-forming unit, CI = confidence interval, IC = invasive candidiasis, ICU = intensive care unit, IQR = interquartile range, OR = odds ratio.
How to cite this article: Xia R, Wang D. Risk factors of invasive candidiasis in critical cancer patients after various gastrointestinal surgeries. Medicine. 2019;98:44(e17704).
This work was supported by research funds granted by the Tianjin Medical University (2010KY38), and the Science and Technology Funds from the Tianjin Health Bureau (2013KZ094).
The authors have no conflicts of interest to disclose.
References
- [1].Calandra T, Roberts JA, Antonelli M, et al. Diagnosis and management of invasive candidiasis in the ICU: an updated approach to an old enemy. Crit Care 2016;20:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bassetti M, Peghin M, Timsit JF. The current treatment landscape: candidiasis. J Antimicrob Chemother 2016;71:ii13–22. [DOI] [PubMed] [Google Scholar]
- [3].Colombo AL, Guimaraes T, Sukienik T, et al. Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: an analysis of five multicenter studies sequentially conducted over a 9-year period. Intensive Care Med 2014;40:1489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kett DH, Azoulay E, Echeverria PM, et al. Extended prevalence of infection in ICUSGoI. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med 2011;39:665–70. [DOI] [PubMed] [Google Scholar]
- [5].Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39:309–17. [DOI] [PubMed] [Google Scholar]
- [6].Lortholary O, Renaudat C, Sitbon K, et al. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002-2010). Intensive Care Med 2014;40:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leroy O, Bailly S, Gangneux JP, et al. Systemic antifungal therapy for proven or suspected invasive candidiasis: the AmarCAND 2 study. Ann Intensive Care 2016;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shahin J, Allen EJ, Patel K, et al. Predicting invasive fungal disease due to Candida species in non-neutropenic, critically ill, adult patients in United Kingdom critical care units. BMC Infect Dis 2016;16:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gong X, Luan T, Wu X, et al. Invasive candidiasis in intensive care units in China: risk factors and prognoses of Candida albicans and non-albicans Candida infections. Am J Infect Control 2016;44:e59–63. [DOI] [PubMed] [Google Scholar]
- [10].Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 2006;43:25–31. [DOI] [PubMed] [Google Scholar]
- [11].De Rosa FG, Trecarichi EM, Montrucchio C, et al. Mortality in patients with early- or late-onset candidaemia. J Antimicrob Chemother 2013;68:927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Montravers P, Perrigault PF, Timsit JF, et al. Antifungal therapy for patients with proven or suspected Candida peritonitis: Amarcand2, a prospective cohort study in French intensive care units. Clin Microbiol Infect 2017;23:117e111–8. [DOI] [PubMed] [Google Scholar]
- [13].Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of Candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis 2016;62:e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cornely OA, Bassetti M, Calandra T, et al. ESCMID∗ guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 2012;18Suppl 7:19–37. [DOI] [PubMed] [Google Scholar]
- [15].Knitsch W, Vincent JL, Utzolino S, et al. A randomized, placebo-controlled trial of preemptive antifungal therapy for the prevention of invasive candidiasis following gastrointestinal surgery for intra-abdominal infections. Clin Infect Dis 2015;61:1671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Timsit JF, Azoulay E, Schwebel C, et al. Empirical micafungin treatment and survival without invasive fungal infection in adults with ICU-acquired sepsis. Candida colonization, and multiple organ failure: the EMPIRICUS randomized clinical trial. JAMA 2016;316:1555–64. [DOI] [PubMed] [Google Scholar]
- [17].Piarroux R, Grenouillet F, Balvay P, et al. Assessment of preemptive treatment to prevent severe candidiasis in critically ill surgical patients. Crit Care Med 2004;32:2443–9. [DOI] [PubMed] [Google Scholar]
- [18].Lee H, Lim CW, Hong HP, et al. Efficacy of the APACHE II score at ICU discharge in predicting post-ICU mortality and ICU readmission in critically ill surgical patients. Anaesth Intensive Care 2015;43:175–86. [DOI] [PubMed] [Google Scholar]
- [19].Pittet D, Monod M, Suter PM, et al. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg 1994;220:751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Goldman L, Hashimoto B, Cook EF, et al. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation 1981;64:1227–34. [DOI] [PubMed] [Google Scholar]
- [21].Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;43:304–77. [DOI] [PubMed] [Google Scholar]
- [22].Leon C, Ruiz-Santana S, Saavedra P, et al. Contribution of Candida biomarkers and DNA detection for the diagnosis of invasive candidiasis in ICU patients with severe abdominal conditions. Crit Care 2016;20:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tissot F, Lamoth F, Hauser PM, et al. Beta-glucan antigenemia anticipates diagnosis of blood culture-negative intraabdominal candidiasis. Am J Respir Crit Care Med 2013;188:1100–9. [DOI] [PubMed] [Google Scholar]
- [24].Leon C, Ruiz-Santana S, Saavedra P, et al. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med 2006;34:730–7. [DOI] [PubMed] [Google Scholar]
- [25].Ostrosky-Zeichner L, Shoham S, Vazquez J, et al. MSG-01: a randomized, double-blind, placebo-controlled trial of caspofungin prophylaxis followed by preemptive therapy for invasive candidiasis in high-risk adults in the critical care setting. Clin Infect Dis 2014;58:1219–26. [DOI] [PubMed] [Google Scholar]
- [26].Eggimann P, Pittet D. Candida colonization index and subsequent infection in critically ill surgical patients: 20 years later. Intensive Care Med 2014;40:1429–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maseda E, Rodriguez AH, Aguilar G, et al. EPICO 3.0: recommendations on invasive candidiasis in patients with complicated intra-abdominal infection and surgical patients with ICU extended stay. Rev Iberoam Micol 2016;33:196–205. [DOI] [PubMed] [Google Scholar]


