Abstract
Introduction:
The efficacy of neoadjuvant buparlisib for breast cancer remains controversial. We conduct a systematic review and meta-analysis to explore the influence of neoadjuvant buparlisib versus placebo for breast cancer.
Methods:
We search PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases through May 2019 for randomized controlled trials (RCTs) assessing the efficacy and safety of neoadjuvant buparlisib versus placebo for breast cancer. This meta-analysis is performed using the random-effect model.
Results:
Four RCTs are included in the meta-analysis. Overall, compared with control group for breast cancer, neoadjuvant buparlisib can substantially reduce progressive disease (risk ratios [RR] = 0.66; 95% confidence interval [CI] = 0.52–0.82; P = .0003) and improve stable disease (RR = 1.29; 95% CI = 1.02–1.64; P = .04), but has no notable influence on overall response rate (RR = 1.32; 95% CI = 0.84–2.06; P = .22), clinical benefit rate (RR = 1.06; 95% CI = 0.79–1.43; P = .69). Neoadjuvant buparlisib results in the increase in adverse grade 3/4 adverse events including increased alanine aminotransferase (ALT) (RR = 11.87; 95% CI = 5.65–24.90; P < .00001), increased aspartate aminotransferase (AST) (RR = 6.50; 95% CI = 4.14–10.21; P < .00001) and hyperglycaemia (RR = 36.65; 95% CI = 10.44–128.68; P < .00001), as well as serious adverse events (RR = 1.47; 95% CI = 1.23–1.76; P < .0001) compared to placebo. Deaths is found to be similar between two groups (RR = 0.88; 95% CI = 0.75–1.04; P = .13).
Conclusions:
Neoadjuvant buparlisib may provide some efficacy for breast cancer, but leads to the increase in serious adverse events.
Keywords: breast cancer, buparlisib, efficacy, randomized controlled trials, safety
1. Introduction
Approximately 75% of breast cancers have the positive expression of human epidermal growth factor receptor-2 (HER2).[1–3] Previous studies reveal that HER2-targted drugs such as trastuzumab, lapatinib, and pertuzumab, are combined with chemotherapy to improve pathological complete response rates in patients with HER2+ early breast cancer.[4–7] Dual HER2-targeted strategy is found to potentially increase the efficacy than a single HER2-targeted agent.[7–9] The majority of patients with HER2+ breast cancer respond well to HER2-targeted therapy, but it is still a challenge for the resistance to HER2-targeted therapy in some breast cancer patients.[10–13]
One of the mechanisms responsible for HER2 treatment resistance is associated with the activation of phosphoinositide 3 kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) [PI3K/AKT/mTOR] pathway.[14–16] Alterations in the PI3K/AKT/mTOR pathway, such as phosphatidylinostitol 3-kinase catalytic subunit (PIK3CA) mutations, lead to the resistance to HER2-targeted agents, and PI3K inhibitors are found to hold the promise in reversing this resistance. Targeting the PI3K pathway in combination with HER2 targeting may improve the outcomes for HER2+ breast cancer.[17–19] Buparlisib, serves as an orally bioavailable pan-PI3K inhibitor targeting all the known isoform of PI3K (p110α, β, γ, and δ), and shows the synergistic growth inhibitory activity in combination with HER2-targeted agents in preclinical studies.[20] Some clinical trials have confirms the efficacy of buparlisib for breast cancer.[21–23]
Current evidence is insufficient for routine clinical use of neoadjuvant buparlisib for breast cancer. Recently, several studies have investigated the efficacy and safety of neoadjuvant buparlisib for these patients, but the results are conflicting.[24–26] This systematic review and meta-analysis of randomized controlled trials (RCTs) aim to assess the efficacy and safety of neoadjuvant buparlisib versus placebo for breast cancer.
2. Materials and methods
This systematic review and meta-analysis are performed based on the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement and Cochrane Handbook for Systematic Reviews of Interventions.[27,28] No ethical approval and patient consent are required because all analyses are based on previous published studies.
2.1. Literature search and selection criteria
We systematically search several databases including PubMed, EMbase, Web of science, EBSCO, and the Cochrane library from inception to May 2019 with the following keywords: buparlisib and breast cancer. The reference lists of retrieved studies and relevant reviews are also hand-searched and the process above is performed repeatedly in order to include additional eligible studies.
The inclusion criteria are presented as follows:
-
1.
study design is RCT,
-
2.
patients are diagnosed as breast cancer, and
-
3.
intervention treatments are neoadjuvant buparlisib versus placebo.
2.2. Data extraction and outcome measures
Some baseline information is extracted from the original studies, and they include first author, number of patients, age, the number of Eastern Cooperative Oncology Group (ECOG) performance status 0, activated PI3K pathway and negative HER2, detail methods in two groups. Data are extracted independently by two investigators, and discrepancies are resolved by consensus. We have contacted the corresponding author to obtain the data when necessary.
The primary outcome is progressive disease. Secondary outcomes include stable disease, overall response rate, clinical benefit rate, the most common grade 3/4 adverse events (i.e. increased alanine aminotransferase [ALT] and aspartate aminotransferase [AST], hyperglycaemia), serious adverse events, and deaths.
2.3. Quality assessment in individual studies
The methodological quality of each RCT is assessed by the Jadad Scale which consists of three evaluation elements: randomization (0–2 points), blinding (0–2 points), dropouts and withdrawals (0–1 points).[29] One point would be allocated to each element if they have been conducted and mentioned appropriately in the original article. The score of Jadad Scale varies from 0 to 5 points. An article with Jadad score ≤ 2 is considered to be of low quality. The study is thought to be of high quality if Jadad score ≥ 3.[30]
2.4. Statistical analysis
We assess risk ratios (RR) with 95% confidence interval (CI) for dichotomous outcomes (progressive disease, stable disease, overall response rate, clinical benefit rate, the most common grade 3/4 adverse events (i.e. ALT, AST, hyperglycaemia), serious adverse events, and deaths). Heterogeneity is evaluated using the I2 statistic, and I2 > 50% indicates significant heterogeneity.[31] The random-effects model is used for all meta-analysis. We search for potential sources of heterogeneity for significant heterogeneity. Sensitivity analysis is performed to detect the influence of a single study on the overall estimate via omitting one study in turn or performing the subgroup analysis. Owing to the limited number (<10) of included studies, publication bias is not assessed. Results are considered as statistically significant for P < .05. All statistical analyses are performed using Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
3. Results
3.1. Literature search, study characteristics, and quality assessment
Figure 1 shows the detail flowchart of the search and selection results. 415 publications are searched after the initial search of databases. After the removal of duplicates, 287 publications are further evaluated. 281 papers are excluded after checking the titles/abstracts. Two studies are removed because of the study design and four RCTs are ultimately included in the meta-analysis.[24–26,32]
Figure 1.
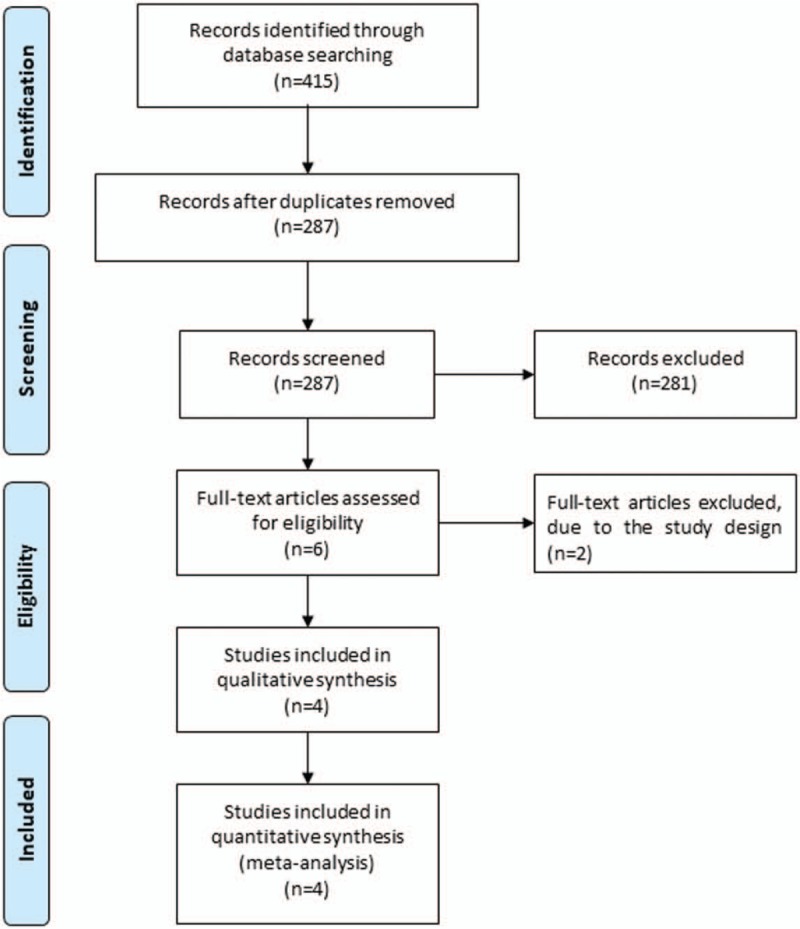
Flow diagram of study searching and selection process.
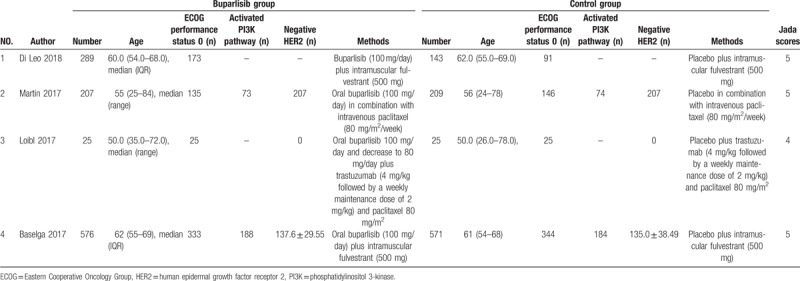
The baseline characteristics of four included RCTs are shown in Table 1. These studies are published between 2017 and 2018, and the total sample size is 2045. Among the included RCTs, buparlisib is regarded as the adjunctive therapy to fulvestrant,[24,32] paclitaxel,[25] trastuzumab, and paclitaxel.[26] Three studies report progressive disease and stable disease,[24–26] four studies report overall response rate,[24–26,32] three studies report clinical benefit rate,[24,25,32] four studies report ALT,[24–26,32] three studies report AST,[24,26,32] three studies report hyperglycaemia,[24,25,32] four studies report serious adverse events and deaths.[24–26,32] Jadad scores of the four included studies vary from 3 to 5, and all four studies have high-quality based on the quality assessment.
Table 1.
Characteristics of included studies.
3.2. Primary outcome: progressive disease
The random-effect model is used for the analysis of primary outcome. The results find that compared to control group for breast cancer, neoadjuvant buparlisib results in the significant decrease in progressive disease (RR = 0.66; 95% CI = 0.52–0.82; P = .0003), with no heterogeneity among the studies (I2 = 0%, heterogeneity P = .80, Fig. 2).
Figure 2.
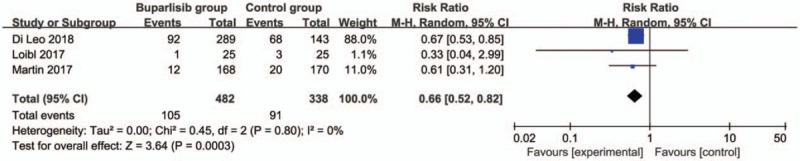
Forest plot for the meta-analysis of progressive disease.
3.3. Sensitivity analysis
There is no heterogeneity for the primary outcome, and thus we do not perform sensitivity analysis by omitting one study in each turn to detect the source of heterogeneity.
3.4. Secondary outcomes
In comparison with control intervention for breast cancer, neoadjuvant buparlisib is associated with the increase in stable disease (RR = 1.29; 95% CI = 1.02–1.64; P = .04; Fig. 3), but shows no impact on overall response rate (RR = 1.32; 95% CI = 0.84–2.06; P = 0.22; Fig. 4), clinical benefit rate (RR = 1.06; 95% CI = 0.79–1.43; P = .69; Fig. 5). The most common adverse grade 3/4 adverse events including increased ALT (RR = 11.87; 95% CI = 5.65–24.90; P < .00001; Fig. 6), increased AST (RR = 6.50; 95% CI = 4.14–10.21; P < .00001; Fig. 7) and hyperglycaemia (RR = 36.65; 95% CI = 10.44–128.68; P < .00001; Fig. 8), as well as serious adverse events (RR = 1.47; 95% CI = 1.23–1.76; P < .0001; Fig. 9) are found to be higher in neoadjuvant buparlisib group than those in control group. There is no statistical difference of deaths (RR = 0.88; 95% CI = 0.75–1.04; P = .13; Fig. 10) between two groups.
Figure 3.
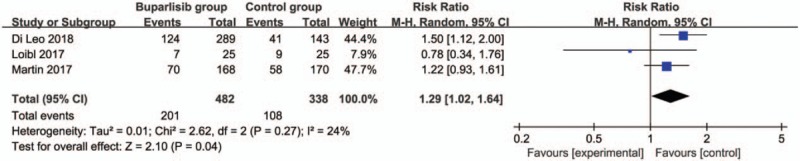
Forest plot for the meta-analysis of stable disease.
Figure 4.
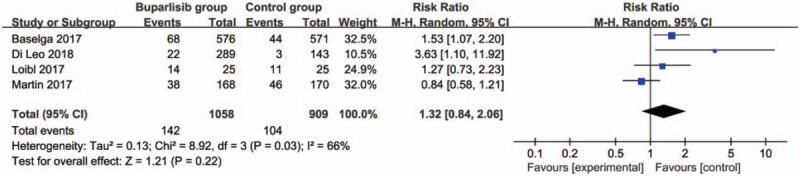
Forest plot for the meta-analysis of overall response rate.
Figure 5.
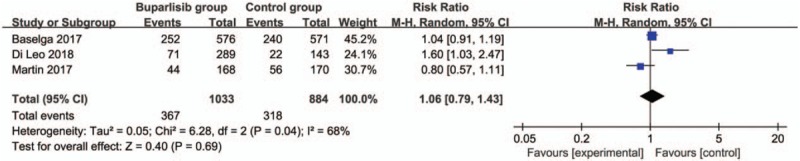
Forest plot for the meta-analysis of clinical benefit rate.
Figure 6.
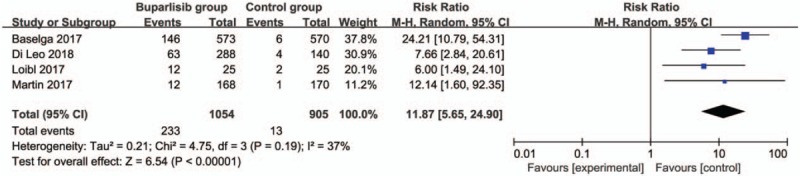
Forest plot for the meta-analysis of increased ALT.
Figure 7.
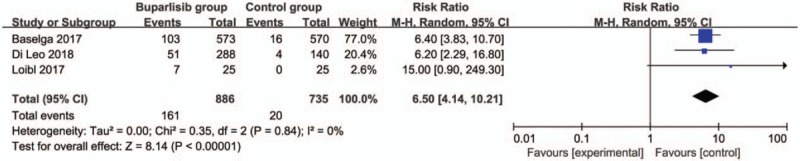
Forest plot for the meta-analysis of increased AST.
Figure 8.
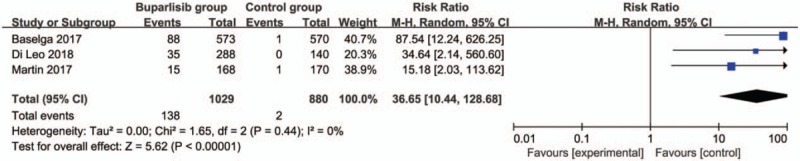
Forest plot for the meta-analysis of hyperglycaemia.
Figure 9.
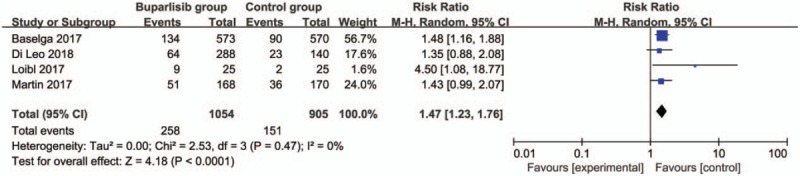
Forest plot for the meta-analysis of serious adverse events.
Figure 10.
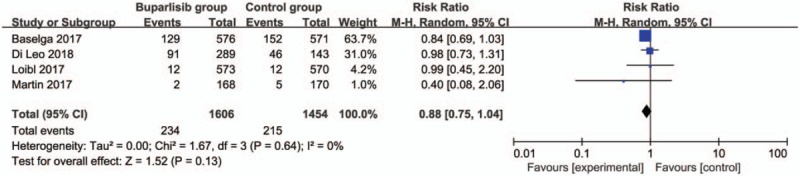
Forest plot for the meta-analysis of deaths.
4. Discussion
In one RCT study for hormone-receptor-positive, HER2-negative, advanced breast cancer, combining buparlisib with fulvestrant can significantly improve progression-free survival and overall response compared with that for placebo plus fulvestrant.[24] While in postmenopausal women with aromatase inhibitor-resistant, hormone-receptor positive, HER2-negative, advanced breast cancer, combination of buparlisib plus fulvestrant can significantly improve progression-free survival compared with that for placebo plus fulvestrant.[32] Our meta-analysis suggests that neoadjuvant buparlisib can substantially reduce the incidence of progressive disease and improve the stable disease for breast cancer, but demonstrates no obvious influence on overall response rate, and clinical benefit rate compared to placebo.
In the BELLE-3 trial, patients predominantly received study treatment as third-line therapy for advanced disease, and almost 90% of patients suffer from progression during mTOR inhibitor treatment or within 30 days from the last dose. That study aims to investigate the combination treatment of buparlisib plus fulvestrant to overcome resistance to mTOR inhibitors by targeting the PI3K pathway upstream. The results reveal higher progression-free survival in combination therapy than fulvestrant alone in patients with PIK3CA mutations.[24] These are consistent with the results in BELLE-2 trial.[32] Thus, PIK3CA status may be an important predictive biomarker to assess the benefit with neoadjuvant buparlisib treatment for breast cancer.
The safety profile of buparlisib is broadly consistent in many studies when in combination with paclitaxel, fulvestrant, or letrozole.[25,32,33] Neoadjuvant buparlisib commonly results in the increase in hyperglycaemia, elevated ALT and AST, rash, gastrointestinal disorders (nausea and diarrhoea), and psychiatric disorders.[23,32] In BELLE-2 trial, buparlisib plus fulvestrant lead to grade 3 to 4 elevations in ALT in 25% of patients and elevations in AST in 18% of patients.[32] In our meta-analysis, neoadjuvant buparlisib also causes higher incidence of elevated ALT and AST, hyperglycaemia, and serious adverse events compare to placebo for breast cancer, but there is no statistical difference of deaths between two groups.
Several limitations exist in this meta-analysis. First, our analysis is based on only four RCTs, and more RCTs with large sample size should be conducted to explore this issue. Next, although there is no significant heterogeneity, different combination and methods of neoadjuvant buparlisib may lead to some bias. Finally, it is not feasible to perform subgroup analysis based on HER2 and PIK3CA status among current studies.
5. Conclusion
Neoadjuvant buparlisib may provide some benefits to treat breast cancer, but also leads to the increase in serious adverse events.
Author contributions
Conceptualization: Qian Luo, Hui Lu.
Data curation: Qian Luo, Hui Lu.
Formal analysis: Qian Luo, Hui Lu.
Funding acquisition: Qian Luo, Hui Lu.
Investigation: Qian Luo, Hui Lu, Ying Wang.
Methodology: Qian Luo, Ying Wang.
Project administration: Qian Luo, Xian Zhou, Ying Wang.
Resources: Hui Lu, Xian Zhou, Ying Wang.
Software: Hui Lu, Xian Zhou, Ying Wang.
Supervision: Qian Luo, Hui Lu, Xian Zhou.
Validation: Qian Luo, Xian Zhou.
Visualization: Qian Luo, Xian Zhou, Ying Wang.
Writing – original draft: Qian Luo, Xian Zhou, Ying Wang.
Writing – review & editing: Qian Luo, Xian Zhou, Ying Wang.
Footnotes
Abbreviations: AKT = protein kinase B, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CI = confidence interval, HER2 = human epidermal growth factor receptor-2, mTOR = mammalian target of rapamycin, PI3K = phosphoinositide 3 kinase, PIK3CA = phosphatidylinostitol 3-kinase catalytic subunit, RCTs = randomized controlled trials, RR = risk ratios.
How to cite this article: Luo Q, Lu H, Zhou X, Wang Y. The efficacy and safety of neoadjuvant buparlisib for breast cancer. Medicine. 2019;98:44(e17614).
Compliance with ethical standards.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. New Engl J Med 2011;365:1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Witton CJ, Reeves JR, Going JJ, et al. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol 2003;200:290–7. [DOI] [PubMed] [Google Scholar]
- [3].Cleator SJ, Ahamed E, Coombes RC, et al. A 2009 update on the treatment of patients with hormone receptor-positive breast cancer. Clin Breast Cancer 2009;9Suppl 1:S6–17. [DOI] [PubMed] [Google Scholar]
- [4].Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012;379:633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25–32. [DOI] [PubMed] [Google Scholar]
- [6].Robidoux A, Tang G, Rastogi P, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol 2013;14:1183–92. [DOI] [PubMed] [Google Scholar]
- [7].Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2–positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol 2012;30:1989–95. [DOI] [PubMed] [Google Scholar]
- [8].Untch M, Loibl S, Bischoff J, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol 2012;13:135–44. [DOI] [PubMed] [Google Scholar]
- [9].Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278–84. [DOI] [PubMed] [Google Scholar]
- [10].Wuerstlein R, Harbeck N. Neoadjuvant therapy for HER2-positive breast cancer. Rev Recent Clin Trials 2017;12:81–92. [DOI] [PubMed] [Google Scholar]
- [11].Harbeck N, Gnant M. Breast cancer. Lancet 2017;389:1134–50. [DOI] [PubMed] [Google Scholar]
- [12].Nakatsukasa K, Koyama H, Oouchi Y, et al. Docetaxel and cyclophosphamide as neoadjuvant chemotherapy in HER2-negative primary breast cancer. Breast Cancer 2017;24:63–8. [DOI] [PubMed] [Google Scholar]
- [13].Tong YW, Wang G, Wu JY, et al. Insulin-like growth factor-1, metabolic abnormalities, and pathological complete remission rate in HER2-positive breast cancer patients receiving neoadjuvant therapy. OncoTargets Ther 2019;12:3977–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mukohara T. PI3K mutations in breast cancer: prognostic and therapeutic implications. Breast Cancer 2015;7:111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Araki K, Miyoshi Y. Mechanism of resistance to endocrine therapy in breast cancer: the important role of PI3K/Akt/mTOR in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer 2018;25:392–401. [DOI] [PubMed] [Google Scholar]
- [16].Li J, Xiao Q, Bao Y, et al. HER2-L755S mutation induces hyperactive MAPK and PI3K-mTOR signaling, leading to resistance to HER2 tyrosine kinase inhibitor treatment. Cell Cycle 2019;18:1513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].O’Brien NA, Browne BC, Chow L, et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther 2010;9:1489–502. [DOI] [PubMed] [Google Scholar]
- [18].Rexer BN, Chanthaphaychith S, Dahlman K, et al. Direct inhibition of PI3K in combination with dual HER2 inhibitors is required for optimal antitumor activity in HER2+ breast cancer cells. Breast Cancer Res 2014;16:R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Eichhorn PJ, Gili M, Scaltriti M, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res 2008;68:9221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maira SM, Pecchi S, Huang A, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther 2012;11:317–28. [DOI] [PubMed] [Google Scholar]
- [21].Ando Y, Inada-Inoue M, Mitsuma A, et al. Phase I dose-escalation study of buparlisib (BKM120), an oral pan-class I PI3K inhibitor, in Japanese patients with advanced solid tumors. Cancer Sci 2014;105:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rodon J, Bendell J, Abdul RA, et al. P3-16-01: Safety Profile and Clinical Activity of Single-Agent BKM120, a Pan-Class I PI3K Inhibitor, for the Treatment of Patients with Metastatic Breast Carcinoma. AACR; 2011. [Google Scholar]
- [23].Ma CX, Luo J, Naughton M, et al. A phase I trial of BKM120 (Buparlisib) in combination with fulvestrant in postmenopausal women with estrogen receptor–positive metastatic breast cancer. Clin Cancer Res 2016;22:1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Di Leo A, Johnston S, Lee KS, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2018;19:87–100. [DOI] [PubMed] [Google Scholar]
- [25].Martin M, Chan A, Dirix L, et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4). Ann Oncol 2017;28:313–20. [DOI] [PubMed] [Google Scholar]
- [26].Loibl S, de la Pena L, Nekljudova V, et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+ primary breast cancer: a randomised, double-blind, placebo-controlled phase II trial (NeoPHOEBE). Eur J Cancer 2017;85:133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions version 5.1. 0 [updated March 2011]. The Cochrane Collaboration; 2011. [Google Scholar]
- [29].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [30].Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982–9. [DOI] [PubMed] [Google Scholar]
- [31].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [32].Baselga J, Im SA, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mayer IA, Abramson VG, Isakoff SJ, et al. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 2014;32:1202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


