Abstract
To evaluate progression of skin atrophy during 8 years of complete Conus-Cauda Syndrome and its recovery after 2 years of surface Functional Electrical Stimulation a cohort study was organized and implemented.
Functional assessments, tissue biopsies, and follow-up were performed at the Wilhelminenspital, Vienna, Austria; skin histology and immunohistochemistry at the University of Padova, Italy on 13 spinal cord injury persons suffering up to 10 years of complete conus/cauda syndrome. Skin biopsies (n. 52) of both legs were analyzed before and after 2 years of home-based Functional Electrical Stimulation delivered by large anatomically shaped surface electrodes placed on the skin of the anterior thigh. Using quantitative histology we analyzed: 1. Epidermis atrophy by thickness and by area; 2. Skin flattening by computing papillae per mm and Interdigitation Index of dermal-epidermal junctions; 3. Presence of Langerhans cells.
Linear regression analyses show that epidermal atrophy and flattening worsen with increasing years post- spinal cord injury and that 2 years of skin electrostimulation by large anatomically shaped electrodes reverses skin changes (pre-functional Electrical Stimulation vs post-functional Electrical Stimulation: thickness 39%, P < .0001; area 41%, P < .0001; papillae n/mm 35%, P < 0.0014; Interdigitation index 11%, P < 0.018), producing a significant recovery to almost normal levels of epidermis thickness and of dermal papillae, with minor changes of Langerhans cells, despite 2 additional years of complete Conus-Cauda Syndrome.
In complete Conus-Cauda Syndrome patients, the well documented beneficial effects of 2 years of surface h-b Functional Electrical Stimulation on strength, bulk, and muscle fiber size of thigh muscles are extended to skin, suggesting that electrical stimulation by anatomically shaped electrodes fixed to the skin is also clinically relevant to counteract atrophy and flattening of the stimulated skin. Mechanisms, pros and cons are discussed.
Keywords: denervated-degenerating muscle, epidermis morphometry, h-bFES, long-term effects, skin biopsy, spinal cord injury
The authors disclose financial support for the research, authorship, and/or publication of this article as follows: EU Commission Shared Cost Project RISE (Contract no. QLG5-CT-2001-02191) and the Austrian Ministry of Science funds to Dr H. Kern and Prof W. Mayr, Vienna (Austria) covered the clinical costs, the production of customized devices, and the international management of the project. The support of the European Regional Development Fund-Cross Border Cooperation Program SLOVAKIA–AUSTRIA (Interreg- Iva) project ‘Mobilität im Alter’ MOBIL N_00033 and of the Ludwig Boltzmann Society (Vienna) are gratefully acknowledged. This work was also supported by institutional funds from the Department of Neuroscience, Section of Human Anatomy, University of Padova, Italy and from the Interdepartmental Research Center of Myology, Department of Biomedical Sciences of the University of Padova, Italy. U. Carraro, B. Ravara, and S. Zampieri thank the A&C M-C Foundation for Translational Myology, Padova, Italy for support. This work was funded in part by NIH NIAMS 1R03AR053706-01A2 to Amber L Pond.
1. Introduction
Spinal cord injury (SCI) can result in damage outside the nervous and musculoskeletal systems. SCI can induce an immune depression syndrome and produce increased blood flow to vital organs, shunting circulation away from the skin resulting in tissue hypoxia which may antagonize throphic processes. Thus dermatological complications may develop after SCI for reasons related to the injury or the life styles of the patients because the skin becomes more vulnerable to hypoxia, injury and infection.[1] Despite the clinical relevance of dermatological complications, information on the histological changes that occur in skin of SCI persons is scarce, mainly because biopsies are not clinically justifiable before major problems occur (specifically, pressure sore complications).[2]
Through the EU Program RISE,[3,4] we previously showed that in SCI patients suffering with complete conus and cauda equina syndrome (Conus-Cauda Syndrome), and thus with permanent denervation and degeneration of muscles (DDM), atrophic Quadriceps muscles were recovered to almost normal size by home-based Functional Electrical Stimulation (h-bFES). Both the safety and effectiveness of the electrical stimulation of permanently denervated muscles is well documented by the impressive positive changes that occur in the stimulated thigh muscles after two years of h-bFES,[3,4,5,6,7,8,9] using very large skin-contact electrodes and a new electrical stimulator designed in Vienna, Austria. The use of the equipments and of the related stimulation protocols of DDM have been validated by the successful EU Program: RISE [Use of electrical stimulation to restore standing in paraplegics with long-term DDMs (QLG5-CT-2001-02191)].[4] Thanks to the generous efforts of the Dr. Schuhfried Medizintechnik Company based in Vienna, Austria the necessary stimulators and large electrodes are now commercially available.[10]
Because tissue biopsies harvested included the skin, we had the opportunity to extend our analyses to this tissue. Recently, a pilot study composed of 12 biopsies from 3 SCI patients found that the SCI-induced changes that occurred in the skin of patients from 1 to 8 years after SCI were reversed after 2 years of electrical stimulation,[11] as it is described for other skin disorders.[12,13,14,15]
Here, those results are extended adding quantitative histologic and immunohistochemical analyses of 52 skin biopsies harvested from both legs of 13 SCI patients who were enrolled in the EU Project RISE.[4] We stress that the skin was harvested from the dorsal aspect of the thigh and that the tissue biopsies were collected only from SCI patients who did not present with any local or general dermatological complications. We hope that the present positive observations may lead to the organization of additional trials on electrical stimulation effectiveness for management of incoming pressure sores in people living with SCI, metabolic diseases, and late aging.
2. Materials and methods
2.1. Enrollment of participants of the European project RISE and surface stimulation of thigh muscles
The results presented in this study are from a subset (n. 13) of the SCI patients enrolled in the EU Project RISE.[4] All subjects were suffering with complete conus-cauda syndrome (that is, up to 8 years of complete and permanent peripheral denervation) and were volunteers (aged 20–54 years), who received detailed information, and signed an informed consent agreeing to perform 2 years of h-bFES training and to offer skin and muscle biopsies from the right and left legs, both before and after h-bFES. We certify that all applicable rules concerning the ethical use of human volunteers were followed during the course of this research (approval of ethical committee, Vienna, Austria: Ethikkommission der Stadt Wien Austria: EK-02-068-0702). Inclusion and exclusion criteria for enrollment and demographic details of the enrolled subjects are listed in the original report on muscle results.[4] Clinical and functional assessments, as well as follow-up and tissue biopsies, were performed at the Wilhelminenspital, Vienna, Austria. Complete denervation of right and left quadriceps muscles was assessed before and after 2 years of h-bFES by test electrical stimulation, needle electromyography, brain motor control assessment and both transcranial and lumbosacral magnetic stimulation.[4]
2.2. Surface electrical stimulation of thigh muscles
Two large electrodes (200 cm2) were placed on the skin of the anterior aspect of the thigh to full cover the quadriceps muscle. The proximal electrode was positioned as near as possible to the inguinal fold, the distal as near as possible to the knee. The skin biopsy was harvested over the Vastus lateralis, that is, under the proximal part of the distal electrode located at the line between distal and middle 1/3 of the tight. The electrical stimulation protocol delivered to the skin and muscles of the thigh by a purpose developed stimulator and anatomically shaped large electrodes (200 cm2) that is now available thanks to the generous efforts of Schuhfried Company, Vienna, Austria (available at http://schuhfriedmed.at/stimulette-en/stimulette-den2x-) 10 started 3 weeks after skin biopsy, both at the enrolment and after 2 years of h-bFES.[4]
The progressive h-b FES protocol was personalized to the conditions of the denervated muscles, that is mainly due to the timespan between SCI and enrollment of the SCI patients. In particular, it starts with very long single electrical impulses (120 ms). As soon the denervated muscles recovered the ability to respond with sustained contractions to burst of stimuli (40 ms impulse duration, 10 ms pause = 20 Hz frequency) the h-b FES protocol was changed to repetitive tetanic stimulation. These tetanic contractions improved the force of m. quadriceps and the ability to allow for standing up and stepping-in-place training. This functional adjusted protocol is the key to obtain the astonishing increase in quality and quantity of the thigh muscle 4 and, as here demonstrated, of the skin characteristics.
2.3. Human skin biopsies
At enrollment and again after about 2 years of h-bFES, 52 skin biopsies (6 mm diameter) were performed to allow safe collection of muscle tissues in an area where electrodes were routinely applied. The location of the skin biopsies were the same in all patients (see point 1.), but the post FES biopsy was located 3 mm medial from the scar of the first biopsy. Their harvesting at the post 2-year home-based FES stimulation protocol spanned from 15 months to 32 months (mean duration 26 +/− 1.50 SE). The skin biopsies were harvested without restrictions, following prudent clinical criteria.[4,6]
2.4. Skin histology
The skin biopsies were fixed in 10% buffered formalin and embedded in paraffin. Histological sections of 5 μm thickness were then collected and stained by a standard Hematoxilin-Eosin (HE) stain protocol and with a standard immunohistochemical procedure to analyze CD1a, a specific marker of Langerhans Cells (LCs).
2.5. Immunohistochemistry to visualize CD1a-positive langerhans cells
After de-paraffinization, 5 μm thick sections were immunostained to visualize CD1a-positive structures using Dako Autostainer/Autostainer Plus (Dako, Milan, Italy) and CD1a antibodies (M3571 anti-human CD1a Dako) diluted 1:100 in EnVision buffer. Each section was incubated with peroxidase-blocking serum (EnVision FLEX Peroxidase-Blocking Reagent, Dako) for 5 minutes to quench non-specific binding and then for 30 minutes with the primary antibody. After this, sections were incubated with a labeled polymer (EnVision FLEX /HRP, Dako) for 20 minutes and 3,3’-diaminobenzidine (EnVision FLEX Substrate buffer, + DAB + Chromogen; Dako) to label positive primary antibody binding. All the sections were finally counterstained with Hematoxylin (EnVision FLEX Hematoxylin, Dako) for 5 minutes to reveal the presence of nuclei, then dehydrated with an increasing scale of alcohol solutions (70%, 95%, 99%), cleared with xylene and mounted. These steps were performed at room temperature.[16]
2.6. Thickness of the epidermis
Three sections per sample were considered for analysis using a light microscope (B-293 PLi, Optika Srl, Ponteranica, BG, Italy) interfaced with a HDMI camera (Optika 4083.13E HDMI Easy camera).[11] Images (1024 × 576 pixel size) were then captured and stored as TIFF files on the workstation associated with the instrument. Care was taken to accurately align the epidermal layer parallel to the largest side of the frame before acquiring each image. Images were analyzed using the ImageJ software freely available at https://imagej.nih.gov/ij/.[17]
By using a regular grid to systematically sample the tissue image, the distance between the outermost surface of the epidermis (excluding the stratum corneum) and the dermo–epidermal junction was obtained at regular intervals throughout the entire length of the section (vertical white lines in Fig. 1 A). The mean value of the distances obtained in each sample was considered to be an estimate of the epidermal thickness of that sample and the values were converted in μm.
Figure 1.
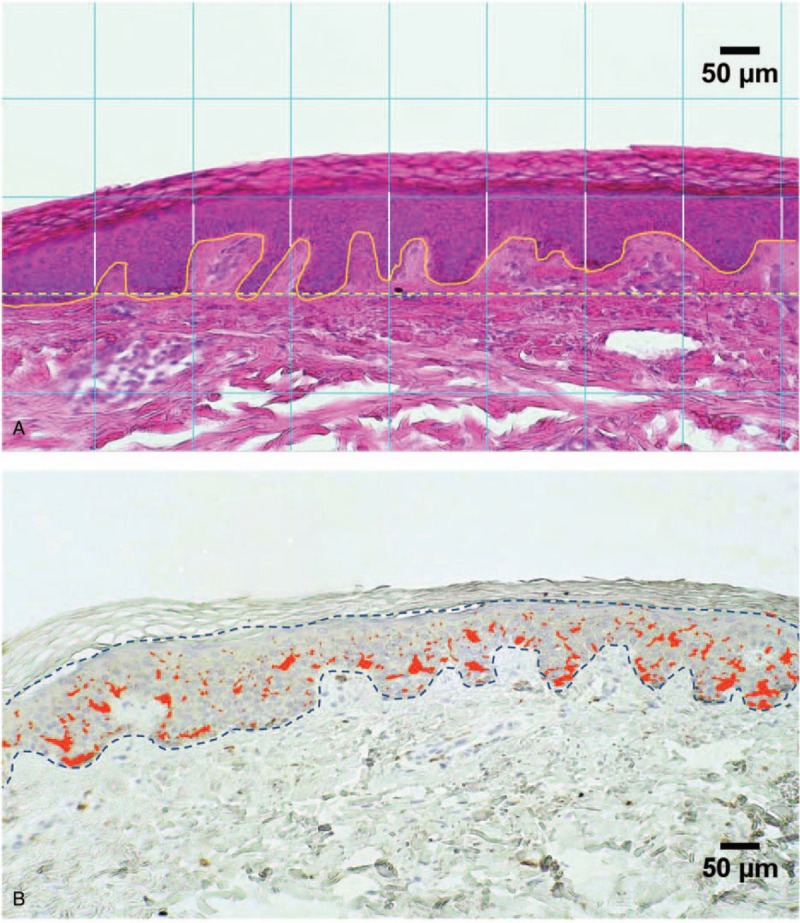
Morphometry of the epidermis. A. H-E stained section of the skin showing the epidermal region. The interdigitation index was estimated as the ratio between the length of the basal membrane in each analyzed field (white solid line) and the width of the field itself (yellow dashed line). Regular grid (white lines) is for epidermal thickness B. Immunohistochemical staining of Langerhans cells. The CD1-positive structures were identified by color thresholding (red areas) and the area they covered was computed in each field to calculate the index of the density of the cytoplasmic extensions (IR%).
2.7. Interdigitation Index and number of dermal papillae-like structures
The epidermal and dermal layers interdigitate where they meet. The Interdigitation Index is an easy measure of the complexity of the interdigitation within the epidermal-dermal junction. Here, images were analyzed using ImageJ software and the size of each captured image (on the order of 1024 × 576 pixels) was converted to μm. The cursor was used to outline the interdigitation border that corresponds to the basal lamina of the epidermis. The computer calculated the length of the basal lamina and of the distance between the starting point and the end point of the outline (i.e., the width of the image). The index is the ratio between the lengths of the basal lamina divided by the width of the image. If the interdigitation index is 1.00 the skin is flat (there are no interdigitations). The value is slightly higher than 1.00 when there are small interdigitations (dermal papillae-like structures) and increases to larger values as the dermal papillae complexity increases to higher levels; these are greater in value in young people where the normal value is 1.30.[18] The numbers of the dermal papillae-like structures were also counted and expressed per 1 mm of each image.
2.8. Image analysis of immunohistochemical CD1a-positive langerhans cells
Computer-assisted image analysis was performed to characterize CD1a immunoreactivity in the epidermis. At a primary magnification of 10×, 4 fields per section were randomly selected and their images were acquired in full color (RGB, 24-bit) and processed to correct shading, and then saved in TIFF format. All the image analysis procedures were then performed by using the ImageJ software. CD1-positive areas of the 26 skin biopsies were identified by color thresholding and then measured in each selected field as illustrated in Figure 1B. The ratio between the immunoreactive area and the total area of epidermis, estimated by interactively tracing the boundary of the epidermal layer with exclusion of the stratum corneum (dashed line in Fig. 1B), was then taken as an index of the density of the cytoplasmic extensions (IR%) of the CD1a-positive structures in the epidermis. The number of LCs was estimated by manual counting of the stained cell bodies per linear mm of the epidermis.[19] The result was then squared to provide an estimate of the number of LCs per squared mm of skin (LC/mm2).
2.9. Statistical analyses
Statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad software, La Jolla, CA). For each leg, representative values of the parameters before and after h-bFES treatment were obtained by averaging the values taken from the corresponding biopsies and a paired Student's t test was applied to compare the 2 sets of measurements leg-by-leg. To get more details on the effect of treatment with respect to the time of SCI, the same analysis was performed on sub-groups of data, obtained by grouping samples according to three SCI time intervals (<1–1.7 years; 2.9–4.1 years; 5.4–7.6 years). Furthermore, linear regression analysis was applied to evaluate the correlation between the evaluated morphological and immunohistochemical parameters and the SCI time. The limit for statistical significance was always considered P < .05.
3. Results
The 52 skin biopsies analyzed were harvested from both right and left thighs of 13 SCI persons before and after 2 years of h-bFES. After HE staining, all the 52 biopsies presented with a regular stratified squamous epithelium rich in cells. The epidermis, consisting of several layers of cells, was distinguishable from the underlying dermis and exhibited a regular and consistent basal membrane. The HE staining revealed the various layers of keratinocytes (Figs. 1 and 2). The surface layer is the stratum corneum which sloughs away naturally and is evenly colored, thus it was not included in the measured point-count thickness nor in the cross sectional area of the epidermis. Although some samples show the skin organization as having ridges and papillae, such as the sample from the SCI patient biopsied at less than 1 year from injury (Fig. 2A), in several samples the epidermis appears very atrophic, that is, thinner and flattened. In particular atrophy was observed in the skin biopsies harvested from SCI patients who had suffered their SCI 3 years prior to enrollment and had not yet received h-bFES treatment (see Fig. 2C).
Figure 2.
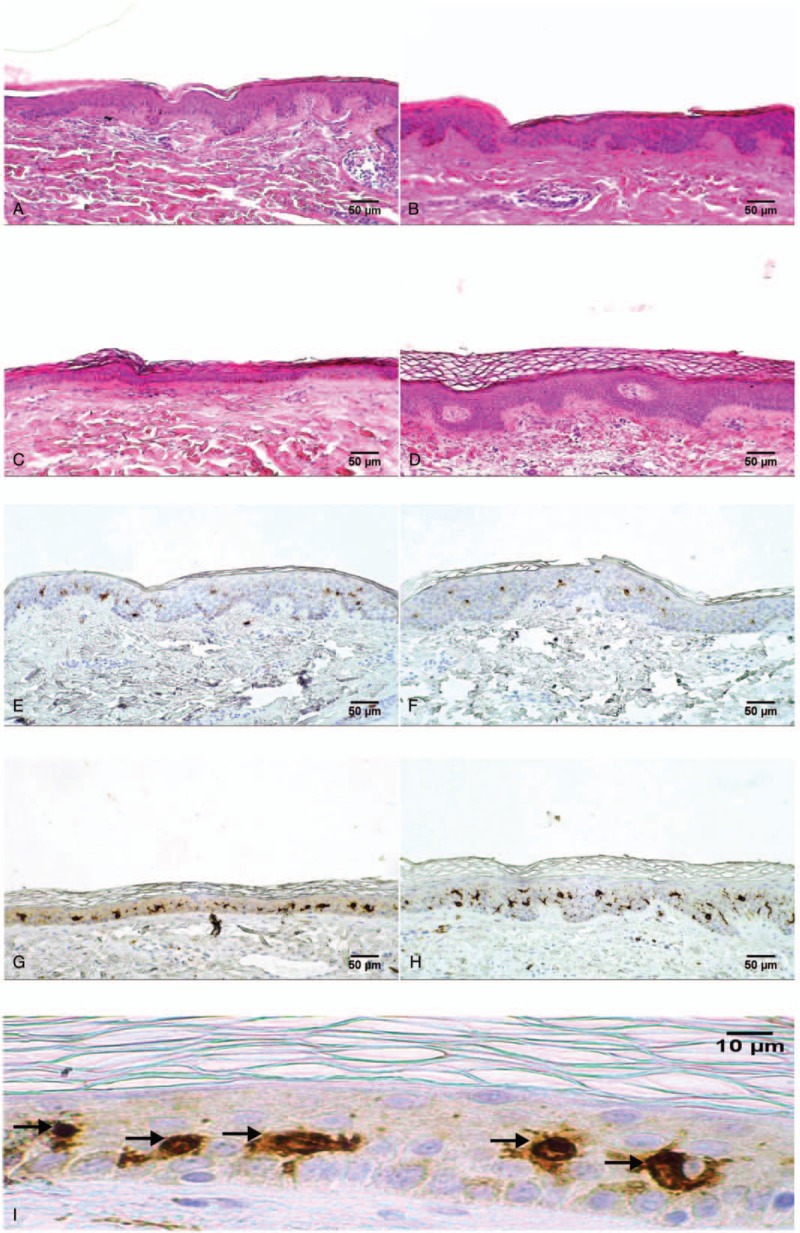
Skin sections before and after 2 years of h-bFES by anatomically shaped large electrodes. H-E staining (A–D) and anti-CD1a-immunostaining for Langerhans Cells (LCs) (E-I) in skin specimens. Panels A and E are representative skin sections from an SCI patient injured for less than 1 year biopsied before the h-bFES treatment while B and F are sections from the same individual biopsied after two years of h-bFES. Panels C and G represent skin sections taken from a patient suffering with SCI for 8 years biopsied before h-bFES while panels D and H are sections from the same individual biopsied after two years of h-bFES by anatomically shaped large electrodes. Magnification of 40× (panel I) shows LC bodies (black arrows). Scale bar = 50 μm, 10× magnification (panels A–H) and 10 μm for 40× magnification (panel I).
3.1. Extent of skin atrophy determined as epidermis thickness and flattening
Linear regression analyses of skin atrophy, against years post SCI at enrollment, show that 4 different methods for measuring thickness and flattening of the skin provide strong evidence that the epidermis progressively decreases in thickness and in dermal papillae complexity (Fig. 3A–D); that is, it becomes flattened (note numbers of papillae-like per mm of skin and Interdigitation Index). However, 2 years of h-bFES almost recovers the epidermis values to those present in skin harvested less than one year after SCI (Fig. 3A–D). Note that the after-h-bFES lines are shifted to the right to stress the timing of biopsy harvesting. Further, linear regression analysis (Fig. 3) of persons grouped according to years after SCI at biopsy harvesting, also show that there was a significant decrease in skin thickness with increased SCI time, at least from less than 1 year until almost 8 years after SCI. After 2 additional years of SCI (during which h-bFES was applied 5-days per week), epidermis thickness was restored to a level of thickness comparable to the samples harvested at less than 1 year of SCI (Fig. 3A, B).
Figure 3.
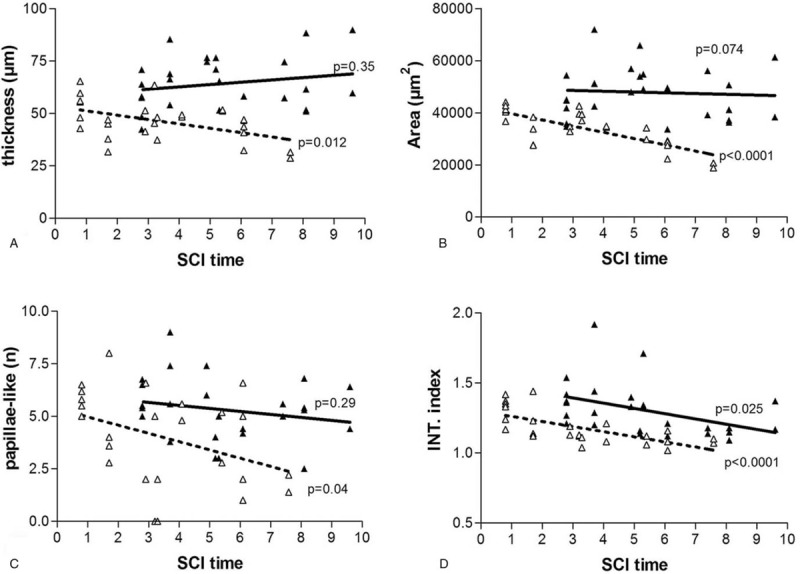
Skin deteriorates with time after SCI, but h-bFES improves the condition of this tissue. Linear regression analysis before (“pre” with dotted lines and empty triangle) and after (“post” with continuous lines and filled black triangles) 2 years of h-bFES by anatomically shaped large electrodes. Epidermal: A. thickness; B. transverse area; C. Number of papillae-like structures per 1 mm; and D. Interdigitation Index (INT.index). p < 0.05.
The number of papillae-like structures per mm of skin and the Interdigitation Index of the dermal papillae in biopsies harvested at enrollment (i.e., pre h-bFES) also significantly decrease with SCI duration, with a larger decrease in the number of papillae-like structures and an insignificant recovery of dermal papillae number after 2 years of h-bFES (Fig. 3C) but with significant recovery of interdigitation index (Fig. 3D).
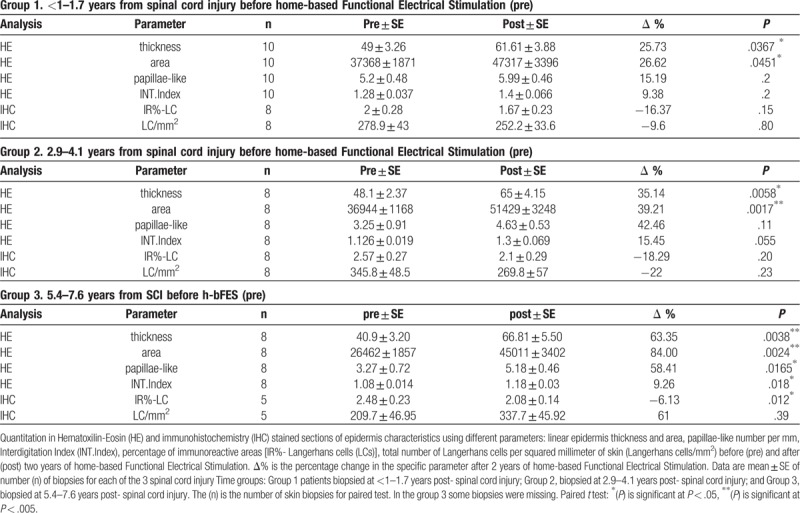
These changes are recognizable in Figure 2B, D and Figure 3C, D, and statistically confirmed by paired test analyses between pre- and post-h-bFES values (Table 1).
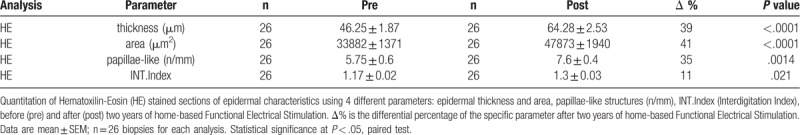
Table 1.
Morphometric analyses of epidermis and dermal-epidermal papillae.
3.2. Extent of skin recovery by h-bFES determined as epidermis thickness and flattening
Table 1 shows that the mean (+/− SE) of the 26 skin biopsies harvested after 2 years of h-bFES (post) are significantly higher than those collected at enrollment (pre) for the 4 parameters analyzed by paired test analysis with a significant 39% increase in epidermis thickness, a 41% increase on area, a 35% increase on papillae-like structures and an 11% increase in the interdigitation index after 2 years of h-bFES. The linear regression analyses presented in Figure 3A–D (compare pre h-bFES values, dotted lines and empty triangle, with 2 years post h-bFES, continuous lines and filled black triangles) clearly indicate that 2 years of h-bFES recovers epidermal thickness and dermal-epidermal complexity to the values present in skin biopsies harvested at 1 year after SCI.
Grouping the biopsies of the 13 subjects according to three periods from SCI (Table 2; each sample pre h-bFES with correspondent post h-bFES) also confirms that the effects of h-bFES are significant for all the morphological parameters in the persons belonging to the third group (5.4 to 7.6 years after SCI). Indeed, with this group the changes the (Δ%) show the highest recovery (58.41%) in dermal-epidermis properties (Table 2-group 3), while for the first and second groups of patients (enrolled from less than 1 to 1.7 years after SCI) the positive increase is present only for the epidermal thickness values.
Table 2.
Analysis of the parameters dividing the biopsies into 3 periods of spinal cord injury time.
3.3. Langerhans cells
Epidermal LCs are unique dendritic cells that are found in stratified epithelia, and are part of the skin immune system,[20,21] aging having profound influences on keratinocytes and LCs.[22] Figure 2, panels E to H show the results of immunostaining skin biopsies with an anti-epidermal CD1a antibody specific for cells bodies and cytoplasmic extensions of LCs (labeled in brown on the background of keratinocytes lightly stained with Hematoxylin). LCs are present in all biopsies. In the Tables and Figures the immunostained LC cell bodies and cytoplasmic extensions, computed as percentage of epidermis area, are stained in brown (IR% or LCs density). Figure 4A shows in a linear regression graph that, although there is an increase in IR% with increased time from SCI, this change is not statistically significant. On the other hand, when the LC bodies are computed per mm2 of epidermis (see examples in panel I of Fig. 2) the linear regression graph reveals that there is a decrease with increased time from SCI, however, this is not a statistically significant. Nonetheless, after 2 additional years of h-bFES the LCs are slightly, but not statistically, increased (Fig. 4B).
Figure 4.
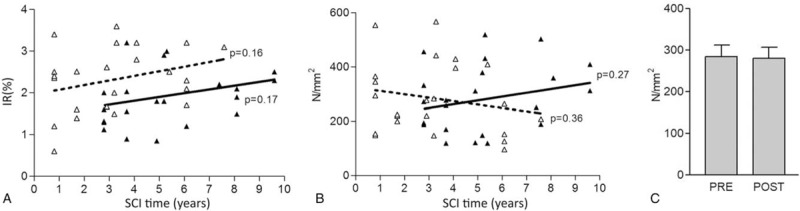
Linear regression analysis (A and B) before (“pre” with dotted lines and empty triangle) and after (“post” with continuous lines and filled black triangles) two years of h-bFES by anatomically shaped large electrodes. for: (A) density of LCs and (B) number of LC bodies per square millimeter of epidermis; (C) total number per square millimeter of epidermis. P < .05.
Consistently, the total number of LC bodies per squared millimeter of skin (LC/mm2) are very similar before and after h-bFES (Fig. 4C), confirming that electrostimulation did not have significant effects on this cell population. Only when the samples were grouped according to the 3 periods of SCI Time, some pre vs post h-bFES groups present statistically significant differences, that is, those of the longer SCI duration (Table 2-IR%-LC). It is worth noting that h-bFES produces, eventually, opposite effects than those due by SCI per se.
4. Discussion
Our skin analyses show that:
1. epidermal atrophy and flattening worsen with increasing years post-SCI; and
2. Two-years of skin electrostimulation by anatomically shaped electrodes produces a significant recovery to almost normal levels of epidermis thickness and of dermal papillae, with minor changes of Langerhans cells, despite 2 additional years of complete denervation due to SCI.
The skin is a complex sensory organ that protects the internal body from chemical, physical, and biological insults despite its apparently simple structure. The skin is also the target of several physical, chemical, metabolic and genetic pathogenic agents that affect epidermis and dermis. Dermatological complications may, indeed, be present in SCI, and some of them are life-threatening and need prevention and long-term care.[2,12,15,23,24,25]
The harvested skin biopsies were not exposed to compression ischemia/hypoxia, but only to the general neurovegetative dysfunction of the leg circulation related to SCI and to a wheel-chair life style. Although a group of control normal adult skin biopsies were not available, our humble opinion is that the skin of SCI persons enrolled during the first year after trauma, may be taken as almost normal skin samples (or certainly the less exposed to atrophic changes). Our assumption finds some support by the clear time-dependent changes we observed in the group of skin biopsies harvested at enrollment and by the observation that electrical stimulation brings the recovery level to the values presented by the SCI patients who were enrolled during the first year after SCI.
As to the h-bFES mechanism(s), the general interpretation may be that the positive effects stemming from h-bFES are related to the increase of muscle fiber activation and co-activation per day, and through the increased blood perfusion of muscles and skin that directly affect cell metabolism. The increase of blood perfusion is recognized easily as skin redness after some stimulation, but it is important to mention that dynamic echomyography shows that skin perfusion is increased very early, if not immediately after application of electrical stimulation, that is, long before any potential electrical stimulation-induced skin damage.[26] Even more important is the observation that the increased perfusion in skin and muscles persists many hours after a h-bFES session.[27,28,29,30] Whether the increased perfusion is a mandatory mechanism, we do not know, because it is almost impossible to increase muscle activation and skin metabolism without modifying blood perfusion. Anyhow, even secondary to other primary cellular mechanisms, the increased perfusion is a mandatory accompanying effect of any volitional muscle hypertrophy exercise. Indeed, the positive effects are not likely a consequence of a boost in skin immunity, at least not because of any change in LCs. While the aging of skin has profound influences on keratinocytes and LCs,[22] the present results suggests that up to at least 10 years of SCI (including 2 years of h-bFES) have no significant effects on the content of LCs.
Our approach is of course not free of limitations, specifically we do not know how long the effect of electrical stimulation on skin atrophy would last after the end of the treatment and if it is extended to skin areas that are subject to chronic pressure and chronic ischemia where pressure sores usually develop. If patients and ethical committees will agree it should be very useful to demonstrate that the same beneficial effect could be obtained also in those areas.
Third and more important, skin obviously reacts to all kinds of stimuli. We cannot rule out that the manipulation of the skin necessary for electric stimulation (cleaning, applying electrodes, etc.) induces enough reaction of the skin to produce the changes seen – in other words, it is possible that simple daily application of an ointment accompanied with some rubbing for such a specific period of time could produce the same changes. Again, to obtain permission from patient and ethical committees is the bottle-neck to perform skin biopsies in non-electrically stimulated persons.
Whether or not the same mechanisms are present in the application of electrical stimulation for wound healing is discussed in recent reviews.[12,13,14,15] However, the stimulation protocols are very different from those here discussed.
Nonetheless, we stress that for complete Conus-Cauda patients h-bFES is the only option to recover their permanent denervated degenerating muscles.[4]
5. Conclusion
We definitively prove that 2 years of h-bFES reversed the process of skin atrophy and the flattening that occurs in between 1 and 8 years post-SCI. We provide evidence that h-bFES produced an increased epidermal thickness and dermal-epidermal complexity, both of which are two important mechanisms of skin resistance to physical, chemical and biological insult. If confirmed and extended by independent studies, our results will provide the preliminary evidence to justify further testing of electrical stimulation as an important contributory mechanism to manage skin disorders, in particular incoming pressure sores in SCI, metabolic diseases and late aging.
Author contributions
All authors equally contributed to designing and implementating the study and approved the final typescript.
Footnotes
Abbreviations: DDM = degeneration of muscles, h-bFES = home-based Functional Electrical Stimulation, HE = Hematoxilin-Eosin, INT.index = Interdigitation Index, LCs = Langerhans Cells, SCI = spinal cord injury.
How to cite this article: Albertin G, Ravara B, Kern H, Hofer C, Loefler S, Jurecka W, Guidolin D, Rambaldo A, Porzionato A, De Caro R, Zampieri S, Pond A, Alaibac M, Carraro U. Two-years of home based Functional Electrical Stimulation recovers epidermis from atrophy and flattening after years of complete Conus-Cauda Syndrome. Medicine. 2019;98:52(e18509).
The authors declare that they have no conflict of interest.
References
- [1]. Marbourg JM, Bratasz A, Mo X, et al. Spinal cord injury suppresses cutaneous inflammation: implications for peripheral wound healing. Neurotrauma 2017;34:1149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Hitzig SL, Eng JJ, Miller WC, et al. An evidence-based review of aging of the body systems following spinal cord injury. Spinal Cord 2011;49:684–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Kern H, Hofer C, Löfler S, et al. Atrophy, ultra-structural disorders, severe atrophy and degeneration of denervated human muscle in SCI and Aging. Implications for their recovery by Functional Electrical Stimulation, updated 2017. Neurol Res 2017;39:660–6. [DOI] [PubMed] [Google Scholar]
- [4]. Kern H, Carraro U, Adami N, et al. Home-based functional electrical stimulation rescues permanently denervated muscles in paraplegic patients with complete lower motor neuron lesion. Neurorehabil Neural Repair 2010;24:709–21. [DOI] [PubMed] [Google Scholar]
- [5]. Kern H, Carraro U, Adami N, et al. One year of home-based functional electrical stimulation (FES) in complete lower motor neuron paraplegia: recovery of tetanic contractility drives the structural improvements of denervated muscle. Neurol Res 2010;32:5–12. [DOI] [PubMed] [Google Scholar]
- [6]. Kern H, Boncompagni S, Rossini K, et al. Long-term denervation in humans causes degeneration of both contractile and excitation contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): a role for myofiber regeneration? J Neuropathol Exp Neurol 2004;63:919–31. [DOI] [PubMed] [Google Scholar]
- [7]. Boncompagni S, Kern H, Rossini K, et al. Structural differentiation of skeletal muscle fibers in the absence of innervation in humans. Proc Natl Acad Sci USA 2007;104:19339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Gargiulo P, Reynisson PJ, Helgason B, et al. Muscle, tendons, and bone: structural changes during denervation and FES treatment. Neurol Res 2011;33:750–8. [DOI] [PubMed] [Google Scholar]
- [9]. Edmunds KJ, Gíslason MK, Arnadottir ID, et al. Quantitative computed tomography and image analysis for advanced muscle assessment. Eur J Transl Myol 2016;26:6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Available at http://schuhfriedmed.at/stimulette-en/stimulette-den2x-. [Google Scholar]
- [11]. Albertin G, Hofer C, Zampieri S, et al. In complete SCI patients, long-term functional electrical stimulation of permanent denervated muscles increases epidermis thickness. Neurol Res 2018;40:277–82. [DOI] [PubMed] [Google Scholar]
- [12]. Hunckler J, de Mel A. A current affair: electrotherapy in wound healing. J Multidiscip Healthc 2017;10:179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Zhao M, Bai H, Wang E, et al. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci 2004;117 (Pt 3):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Bayat M, Asgari-Moghadam Z, Maroufi M, et al. Experimental wound healing using microamperage electrical stimulation in rabbits. J Rehabil Res Dev 2006;43:219–26. [DOI] [PubMed] [Google Scholar]
- [15]. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–46. [DOI] [PubMed] [Google Scholar]
- [16]. Porzionato A, Macchi V, Guidolin D, et al. Histopathology of carotid body in heroin addiction. Possible chemosensitive impairment. Histopathology 2005;46:296–306. [DOI] [PubMed] [Google Scholar]
- [17]. ImageJ – Image Processing and Analyses in Java is available at https://imagej.nih.gov/ij/. [Google Scholar]
- [18]. Timár F, Soós G, Szende B, et al. Interdigitation index - a parameter for differentiating between young and older skin specimens. Skin Res Technol 2000;6:17–20. [DOI] [PubMed] [Google Scholar]
- [19]. Stojadinovic O, Yin N, Lehmann J, et al. Increased number of Langerhans cells in the epidermis of diabetic foot ulcers correlates with healing outcome. Immunol Res 2013;57:222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Merad M, Manz MG, Karsunky H, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol 2002;3:1135–41. Epub 2002 Nov 4. Erratum in: Nat Immunol 2003;4:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Deckers J, Hammad H, Hoste E. Langerhans cells: sensing the environment in health and disease. Front Immunol 2018;9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Zegarska B, Pietkun K, Giemza-Kucharska P, et al. Changes of Langerhans cells during skin ageing. Postepy Dermatol Alergol 2017;34:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Mäki-Turja-Rostedt S, Stolt M, Leino-Kilpi H, et al. Preventive interventions for pressure ulcers in long-term older people care facilities: a systematic review. J Clin Nurs 2018. [DOI] [PubMed] [Google Scholar]
- [24]. Lemmer DP, Alvarado N, Henzel MK, et al. What lies beneath: Why some pressure injuries may be unpreventable for individuals with spinal cord injury. Arch Phys Med Rehabil 2018;100:1042–9. pii: S0003-9993(18)31512-0. [DOI] [PubMed] [Google Scholar]
- [25]. Groah SL, Schladen M, Pineda CG, et al. Prevention of pressure ulcers among people with spinal cord injury: a systematic review. PM R 2015;7:613–36. [DOI] [PubMed] [Google Scholar]
- [26]. Zanato R, Stramare R, Boato N, et al. Dynamic echomyography shows that FES in peripheral denervation does not hamper muscle reinnervation. Biomed Tech (Berl) 2013;58: Suppl 1: pii: /j/bmte.2013.58.issue-s1-A/bmt-2013-4034/bmt-2013-4034.xml. [DOI] [PubMed] [Google Scholar]
- [27]. Sarabon N. Effects of Munari powder on physical and sensory-motor parameters: a preliminary report. Eur J Transl Myol 2015;25:5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Barberi L, Scicchitano BM, Musaro A. Molecular and cellular mechanisms of muscle aging and sarcopenia and effects of electrical stimulation in seniors. Eur J Transl Myol 2015;25:231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Sajer S. Mobility disorders and pain, interrelations that need new research concepts and advanced clinical commitments. Eur J Transl Myol 2017;27:7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Sajer S, Guardiero GS, Scicchitano BM. Myokines in home-based functional electrical stimulation-induced recovery of skeletal muscle in elderly and permanent denervation. Eur J Transl Myol 2018;28:7905. [DOI] [PMC free article] [PubMed] [Google Scholar]


