Abstract
Background:
The purpose of the study was to determine the risk factors of post-surgery myasthenia crisis (PMC) among myasthenia gravis (MG) patients.
Methods:
A meta-analysis to synthesize all eligible literatures was conducted to analyze PMC predictors among MG patients.
Results:
A total of 15 trials with 2626 patients were included for the meta-analysis. As a result, patients with history of MC (RR = 3.36, 95%CI: 2.46–4.59, P < .001), generalized MG (RR = 0.39, 95%CI: 0.26–0.59, P < .001), bulbar symptom (RR = 3.59,95%CI:2.53–5.09, P < .001), thymoma (RR = 2.10, 95%CI:1.37–3.21, P = .001), post-surgery morbidity presence(RR = 2.59, 95%CI:1.90–3.54, P < .001), high-dose pyridostigmine usage (SMD = 0.480, 95%CI: 0.35–0.61 P < .001) tended to develop PMC. Large dose of steroid may reduce the incidence of PMC (RR = 0.41 95%CI: 0.18–0.94, P = .036). Regular steroid use (P = .066), immunosuppressive therapy (P = .179), gender (P = .774), and age at thymectomy (P = .212) had no impact upon PMC development.
Conclusion:
History of PMC, thymoma, generalized MG, bulbar symptom, and concomitant complication are the risk factors of PMC.
Keywords: meta-analysis, myasthenia crisis, myasthenia gravis, risk factor, thymectomy
1. Introduction
Myasthenia gravis is an auto-immune disease, characterized by muscle weakness and the synthesis of anti-Acetylcholine-Receptor Antibody (anti-AchR-Ab).[1] Most MG patients are pathologically associated with thymus abnormalities (including thymic malignancy and thymus hyperplasia), which are responsible for auto-antibodies in the circulation.[2] Thymectomy, demanding an en bloc removal of thymic tissues, has been known as the standard option to treat MG and reduces the generation of anti-AchR Ab. It is reported that the remission rate after thymectomy reached 80%.[3]
After thymectomy, a rapidly deteriorating function of neuromuscular junction may lead to postoperative myasthenia crisis (PMC), which is due to respiratory muscle paralysis and presented as prolonged mechanical ventilation or re-ventilation after extubation.[4] So it is a life-threatening complication and a major problem after thymectomy. The incidence of PMC ranges from 6.2% to 30.3%.[5,6]
Thymectomy was classified as 4 surgical ways, the most prevalent approaches of which are minimally invasive surgery (e.g., video-assisted thoracoscopic surgery, VATS) and trans-sternotomy (TS). Open surgery was considered increasing the risk of PMC compared with minimally invasive approach. Besides surgical ways, infections and other post-surgical complications, more severe MG status, and some other reasons may contribute to PMC.
However, predictive factors of PMC varied by studies and still no consensus on this problem has been reached. Thus, we made a meta-analysis to indentify the clinical indicators predicting PMC occurrence.
2. Methods
2.1. Inclusive criteria and exclusive criteria
2.1.1. Inclusive criteria
Literatures were included in the meta-analysis if they met the following criteria:
-
1.
Randomized controlled trial or cohort study.
-
2.
All subjects were about MG patients who received thymectomy.
-
3.
Outcome measure: Incidence of postoperative myasthenia crisis among MG patients after thymectomy.
-
4.
The clinical trial presented analysis of possible factors influencing the risk of PMC (age at surgery, gender, MC history, association of thymoma, MG stage, anti-AchR-Ab titer, etc) with detailed information.
-
5.
Literatures written either in English or Chinese.
2.1.2. Exclusive criteria
Literatures were excluded if any of the reasons as follows were matched
-
1.
Case report or review.
-
2.
Only description of general myasthenia crisis, but not MC after thymectomy.
-
3.
Experimental research or animal research.
-
4.
No available data was shown in the literature.
2.2. Literature retrieval
We searched databases of PubMED, EMBASE, and Cochrane to achieve related trials published before June 1, 2019. Sample search terms included “Myasthenia crisis” and “myasthenia gravis”
2.3. Filter of literature and data extraction
Two researchers screened all the literatures retrieved via reading abstracts and titles, papers cannot be removed by the criteria above were examined twice based on full texts. Articles conformed to the inclusive criteria were included in our trial. Information extracted from literatures included first author's name, publication year, mean ages of 2 groups (PMC and non-PMC), ratios of male to female of 2 groups (PMC and non-PMC), risk factors of developing PMC, and data presenting the influence of a factor upon the risk of PMC.
2.4. Statistical analysis
STATA11.0 (Stata-Corp LP, College Station, TX, USA) was applied to assess statistical significance. Between-study heterogeneity was assessed first with Q Test and inconsistency index (I2).[7] A P value >.1 combined with I2 < 50% implied heterogeneity was insignificant and Fixed Effect Model (Mantel–Haenszel method)[8] was available. Otherwise the heterogeneity was significant and we would look for clinical source of heterogeneity to try to reduce it. If the heterogeneity still existed, Random Effect Model (DerSimonian–Laird method)[9] was applied. All P values less than .05 were considered statistically significant. For binary variables, relative risk (RR) was performed to assess the significance of the difference; as to numerable variables, Standardized Mean Difference (SMD) was applied to estimate the significance of the difference. If more than 10 articles were included in 1 meta-analysis, evaluation of publication bias was necessary.[10] A P value less than .05 suggested publication bias was statistically significant.
2.5. Ethical statement
Because this study is a meta-analysis thus approval by ethic committee was not necessary according to local regulations (Medical ethic committee of Sichuan University). In addition, informed consent from patients was also not necessary.
3. Results
3.1. Result of study selection
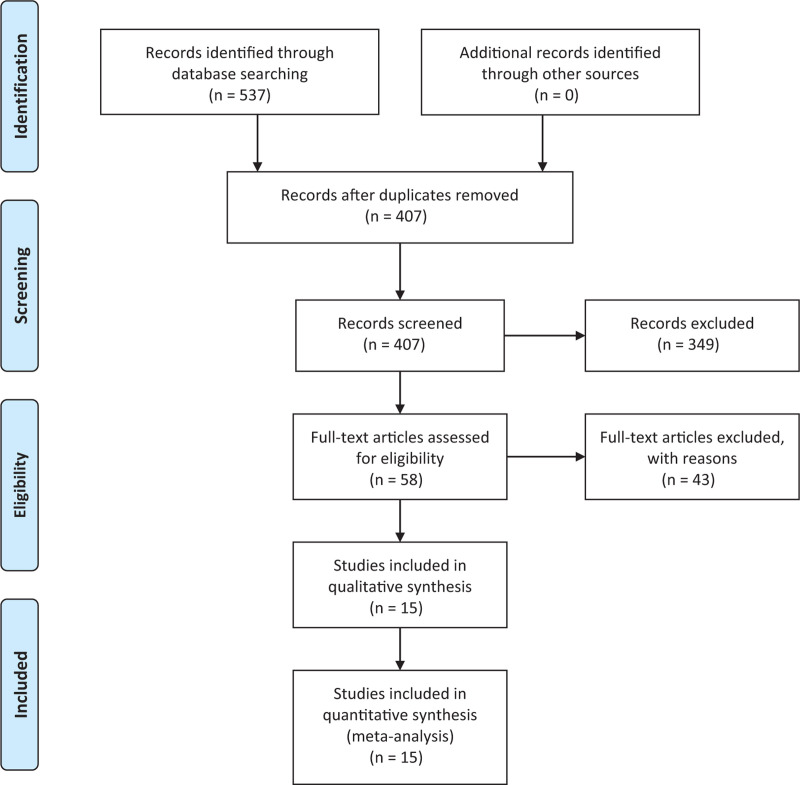
A total of 537 articles were retrieved in according with the search strategy, 130 duplicated articles were removed from the research first. Next the authors reviewed the abstracts of the remaining 407 articles according to the inclusive and exclusive criteria. Three hundred forty nine trials were further excluded during this process. Then full texts of the remaining 58 trials were examined. Finally, 15 trials were included for our research.[4–6,11–22] The reasons of eliminating articles were summarized as follows and shown in Figure 1:
Figure 1.
Process of selecting eligible articles for further research.
The article focused on other topics without description of PMC (n = 294).
Duplicated articles from different databases (n = 130).
Review articles or case reports (n = 55).
Mere introduction of myasthenia crisis, but not crisis after surgery (n = 27).
No description of risk factors for PMC, or no available data presenting risk factors for PMC in the article (n = 11).
Article written in other languages (n = 5).
3.2. Characteristics of all the studies
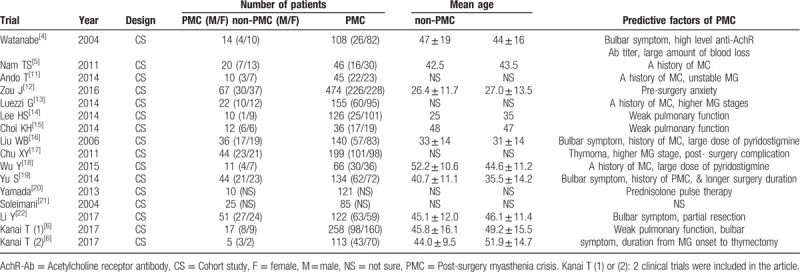
A total of 15 trials and 2626 MG patients were included in the meta-analysis (398 cases for PMC patients, 2228 cases for non-PMC). All articles were published between 2004 and 2017, and patients ranged from Asia to Europe. All included trials were cohort studies, and no randomized controlled trial on this issue has been published yet. Detailed information of each study was shown in Table 1.
Table 1.
Information of each study involved in the meta-analysis evaluating risk factors of PMC.
3.3. Results of data synthesis
3.3.1. Patients’ own characteristics
MG patients with history of myasthenia crisis had a higher risk of PMC than those without myasthenia crisis record. Heterogeneity was significant (I2 = 49.5%) and the source was not found, hence Random Effect Model was used. The outcome was, RR = 3.36 95%CI (2.46, 4.59) P < .001, shown in Figure 2a.
Figure 2.
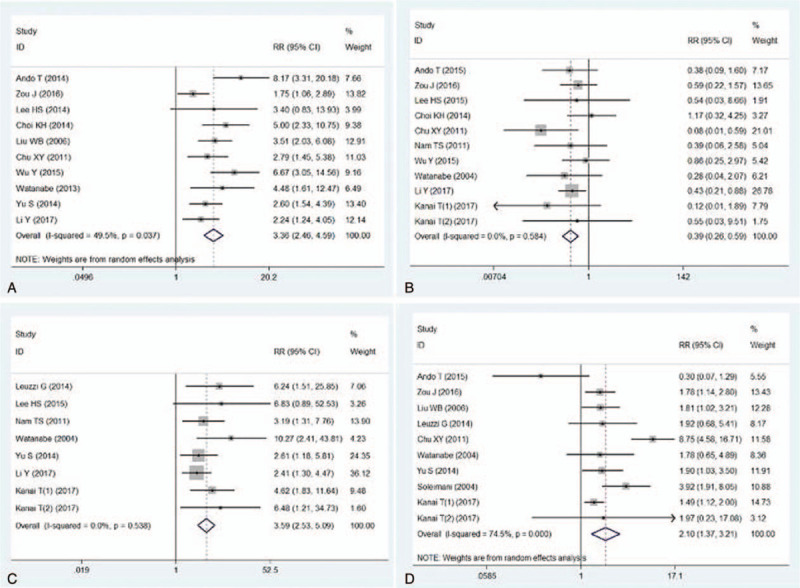
Meta-analysis evaluated the effect of MG status upon the risk of PMC. 2a: forest plot compared MG patients with history of MC and those without history of MC in terms of the risk of PMC. 2b: forest plot described generalized MG vs ocular MG with regard to the risk of PMC. 2c: forest plot evaluated the risk of PMC for MG patients with and without bulbar symptoms. 2d: Forest plot compared the incidence of PMC between thymomatous MG patients and non-thymomatous MG patients. MG: myasthenia gravis, PMC = post-operative myasthenia crisis.
Generalized MG patients presented a higher incidence of PMC than ocular patients. Heterogeneity was low (I2 = 0.0%) and Fixed Effect Model was used. The outcome was, RR = 0.39 95%CI (0.26, 0.59), P < .001, Figure 2b. The incidence of PMC was higher in patients with bulbar symptom than in those without bulbar symptom, RR = 3.59 95%CI (2.53, 5.09) P < .001, Figure 2c.
Thymomatous MG patients showed a higher incidence of PMC than non-thymomatous MG patients. Heterogeneity was high and no clinical source was found. With Random Effect Model we achieved the outcome, RR = 2.10 95%CI (1.37, 3.21) P = .001, Figure 2d.
Female patients were similar with male cohorts in terms of the risk of PMC. The outcome was, RR = 1.03 95%CI (0.85, 1.24) P = .774, Figure 3a. With regard to the comparison of mean age between PMC patients and non-PMC patients, the difference reached no statistical significance (SMD = 0.11 95% CI: −0.06 to 0.29, P = .212, Fig. 3b).
Figure 3.
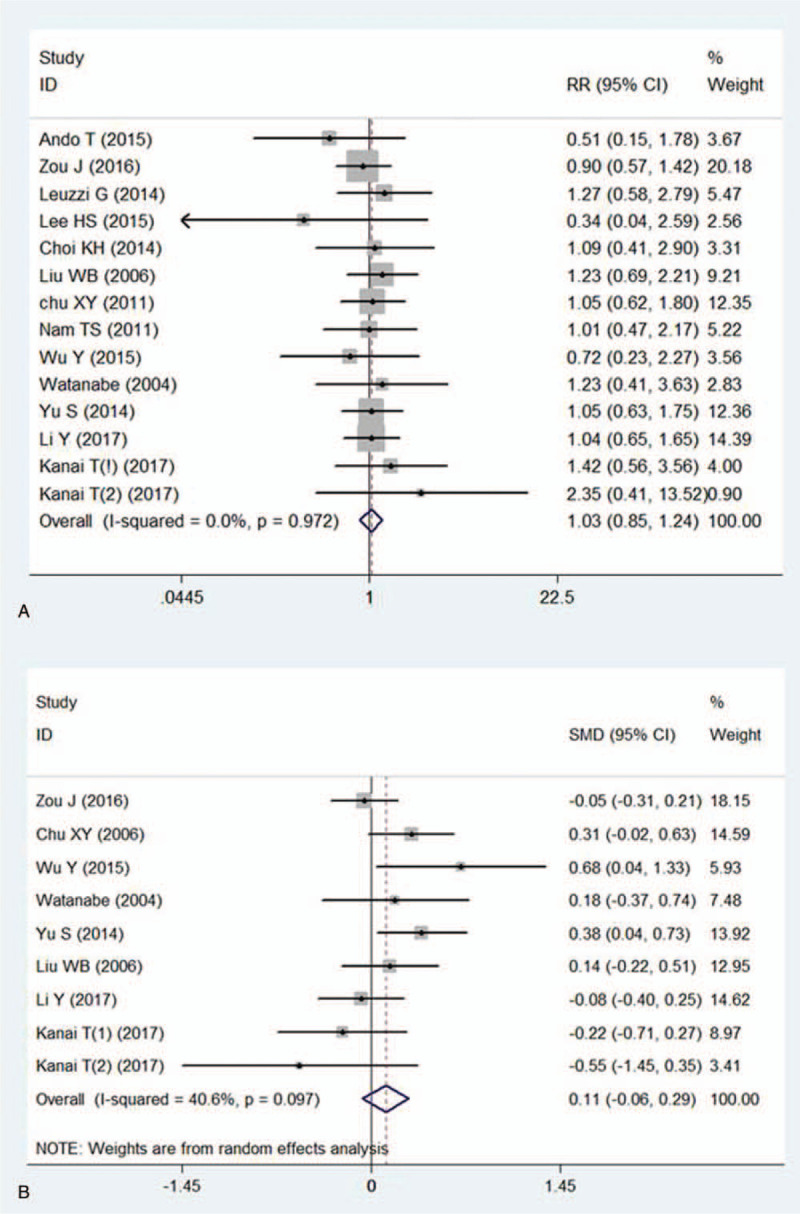
Meta-analysis evaluated the effect of basic characteristics upon the risk of PMC. Figure3a: forest plot compared the incidences of PMC between female MG patients and male MG patients. Figure3b: forest plot presented mean age for PMC patients versus non-PMC patients.
3.3.2. Peri-operative treatment
When it came to the evaluation of steroid use, heterogeneity was significant (I2 = 46.1%). We found that regular steroid use was applied in 5 studies, while in another 3 studies MG patients were treated with steroid pulse therapy. Therefore sub-group analysis was applied in this indicator. No significant difference of PMC incidence was found between patients with regular steroid use and without steroid use (RR = 1.28 95%CI: 0.98–1.65 P = .066, Fig. 4a). However, MG patients with steroid pulse therapy or large dose steroid presented a lower PMC incidence than those without steroid therapy (RR = 0.41 95%CI: 0.18–0.94 P = .036, Fig. 4a).
Figure 4.
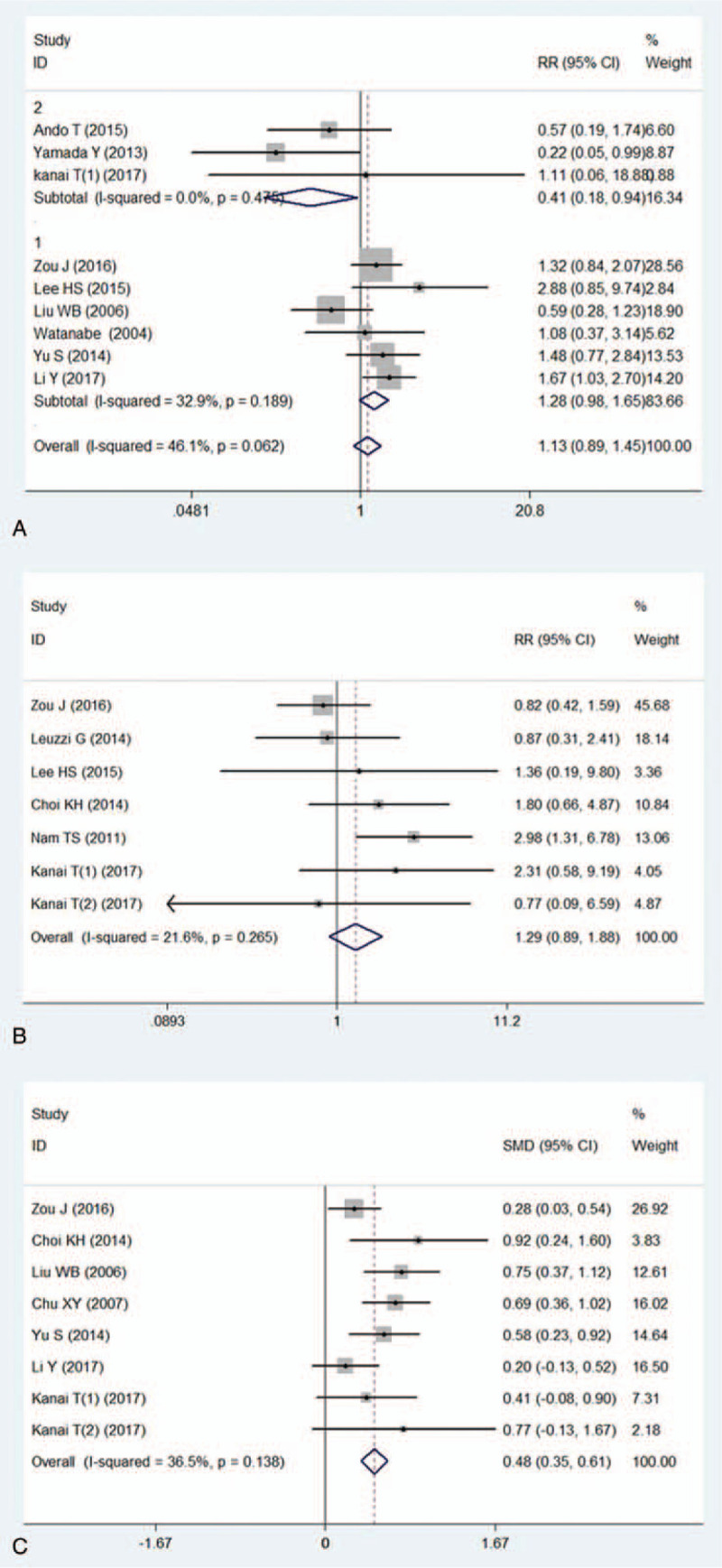
Meta-analysis evaluated the effect of peri-operative medications upon the risk of PMC. 4a: forest plot showed the difference of incidences of PMC between regular steroid use and no steroid use in peri-operative MG patients (sub-group 1), forest plot described the difference of the risk of PMC between high-dose steroid therapy and no steroid use in peri-operative MG patients (sub group 2). 4b: Forest plot presented the risk of PMC for patients treated with immunosuppressive therapy and those without such medication. 4c: forest plot compared mean dose of pyridostigmine between patients with PMC and patients without PMC.
Also no significant difference of PMC was found between immunosuppressive therapy group and non-immunosuppressive therapy group. The outcome was, RR = 1.29 95%CI (0.89, 1.88) P = .179, Figure 4b.
Patients underwent PMC took a larger mean dose of pyridostigmine than those without PMC. The outcome was, SMD = 0.48 95%CI: 0.35–0.61 P < .001, Figure 4c.
The risk of PMC was higher in MG patients with other post-operative complications than those without other complications. (RR = 2.59 95%CI: 1.90–3.54, P < .001, Fig. 5a).
Figure 5.
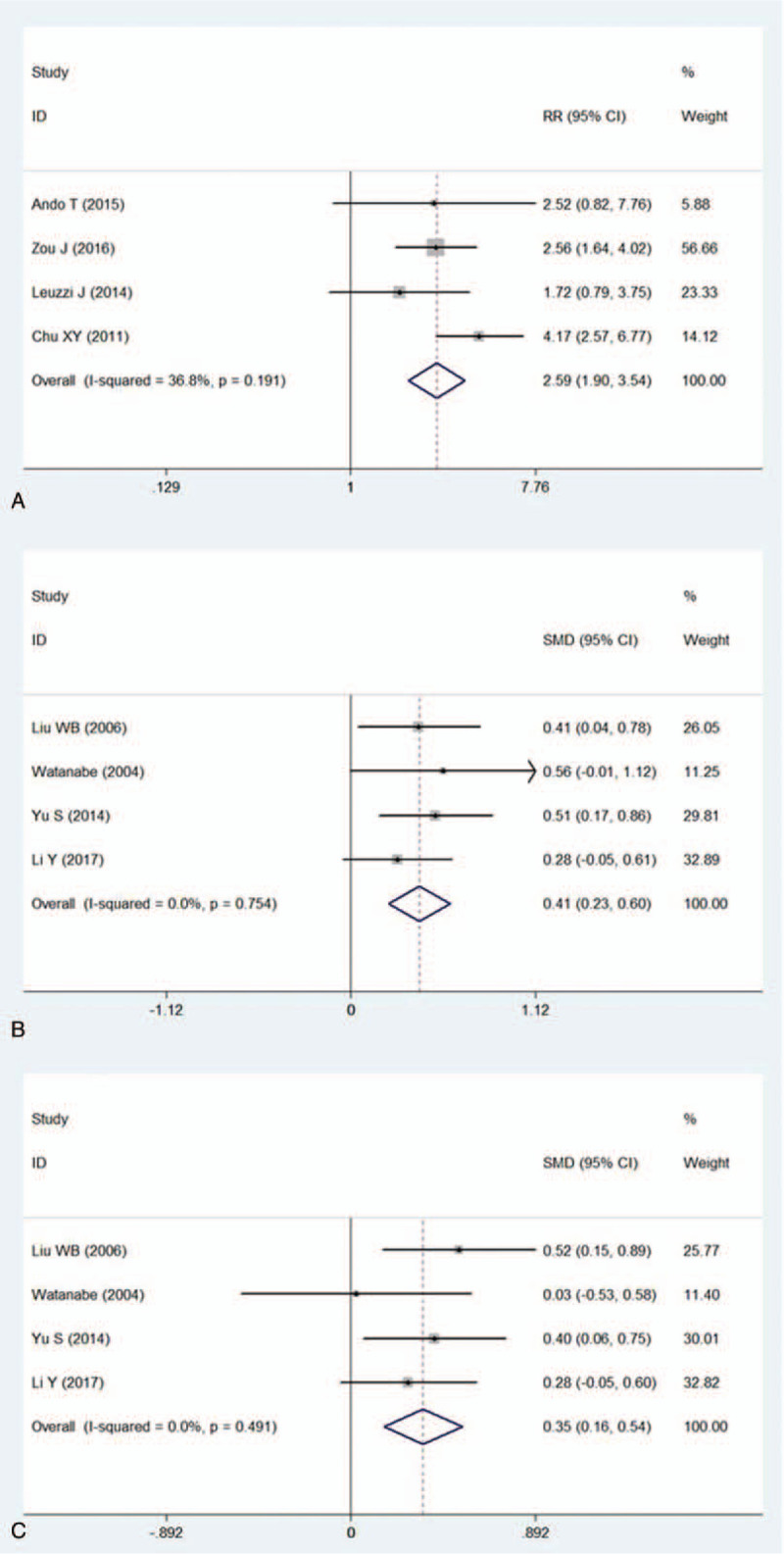
Meta-analysis evaluated the effect of surgical parameters upon the risk of PMC. 5a: forest plot compared possibilities of PMC for MG patients with post-operative complications and those without any complications. 5b: forest plot presented difference of mean surgery time between patients with PMC and patients without PMC. 5c: forest plot presented difference of mean blood loss between patients with and without PMC.
Mean surgery time was longer in patients underwent PMC than those without PMC (SMD = 0.41 95%CI: 0.23–0.60, P < .001, Fig. 5b). Patients underwent PMC suffered from more blood loss during thymectomy than non-PMC patients (SMD = 0.35 95%CI: 0.16–0.54 P < .001, Fig. 5c).
3.3.3. Publication bias
Publication bias was evaluated for the impact of thymoma (P = .283), history of MC (P = .049), MG stage (P = .533), and gender (P = .827) upon the incidence of PMC. The bias was only significant for history of MC (P = .049, detailed information was not shown).
4. Discussion
Myasthenia crisis occurs in 15% to 20% of MG patients.[23] How to prevent PMC is of great importance to the treatment of post-surgery MG patients. But until now the predictors of PMC have not been confirmed yet.
Several clinical trials included in this study suggested history of myasthenia crisis is an independent risk factor of PMC (shown in Table 1). Our meta-analysis pooled a similar result and all literatures included in data synthesis suggested MG patients with a history of MC had a higher risk of PMC than those without any MC record (shown in Fig. 2). Hence the publication bias was of statistical significance because of the consistency of the results among the literatures. It was reported that MG patients with a record of MC may experience crisis recurrence, and the relapse rate ranges from 30% to 50%.[24,25] In addition, receiving thymectomy is another inducing factor of MC.[25] Therefore, the presence of MC history should be valued before thymectomy for MG patients.
In this study we found thymoma is related to a higher risk of PMC, which may be explained by 2 possible reasons. First, about 10% to 20% of MG patients are associated with thymomas.[26] MG associated thymoma exports auto-reactive T cells to peripheral and then the auto-immunity is built in the circulation.[3,27] During thymectomy, extrusion of thymoma is unavoidable so auto-reactive T cells may be released from thymoma, which leads to deterioration of MG status. Especially when invasive thymoma was not removed completely, auto-reactive T cells may be exported to peripheral continuously. Li et al[22] suggested that incomplete resection of thymoma is related to a higher PMC incidence. Second, if invasive thymoma involves surrounding structures (e.g., lung, pericardial and pleura), complete thymectomy requires extended resection. The risk of PMC may be increased for MG patients underwent pleura resection and lung resection.[13]
Whether complete resection of thymoma can be achieved is determined not only by extent of tumor invasion, but also by surgical procedures and methods. To date, VATS has proved to be identical to TS in terms of complete resection of thymus malignancy.[28] In addition, VATS, the minimally invasive approach most widely used, was once considered as the way of less injury to chest wall, less blood loss, and therefore less PMC occurrence than TS.[29] But other studies also reported TS is similar with VATS regarding to the risk of PMC.[30] According to the outcomes of another meta-analysis we found no difference of incidence of PMC between 2 surgical ways.[31] Besides surgical methods, there are also some surgical factors affecting this complication. In this meta-analysis, the risk of myasthenia crisis increased with longer surgery time and more blood loss.
Severity of myasthenic symptoms also affects PMC occurrence. A more serious MG status may increase the risk of PMC. Depending on the present meta-outcome, generalized MG causes more PMC while ocular MG is safer. Moreover, accompanied by bulbar symptoms is another important predictor of PMC. These conclusions are consistent with findings in previous studies that MG symptoms worse than stage IIA (Osserman type) indicate a higher risk of PMC,[13] and existence of bulbar symptom is the sign of PMC after thymectomy.[32] Therefore, it should be cautious for thoracic surgeons to operate on severe generalized MG patients, especially upon those combined with bulbar symptoms.
It has been reported that post-surgery morbidities such as pulmonary infection may result in PMC.[18] The outcome of present research confirmed this founding. Besides pneumonia, weak pulmonary function also has a strong correlation with PMC.[14,15] In general, PMC was defined as the need of prolonged ventilation or re-ventilation after surgery for respiratory failure due to muscle weakness, which was brought by myasthenic exacerbation.[4] Pulmonary weakness may contribute to this process so it is a potential predictor of PMC. Apart from pulmonary infection, other complications have not yet been proved to be related with PMC occurrence. For instance, neither phrenic nerve injury nor palsy was regarded as an inducing factor.[4,14]
Peri-operative use of pyridostigmine is still controversial. The drug can be either applied for prevention of PMC[33] or suspended due to prevention of cholinergic crisis.[34] From this meta-analysis we suggest patients with PMC took a larger dose of pyridostigmine than non-PMC patients. In our opinion, the large-dose anti-cholinesterase medication was used in MG patients with worse MG situations and higher clinical stages, so large-dose pyridostigmine represents a higher risk of PMC. According to the outcome of this study, whether regular steroid and immunosuppressive therapy was applied makes no effect on the risk of PMC. Regular dose of steroid and immunosuppressive therapy are also widely used in MG patients with unstable status, thus no significant effect of preventing PMC was shown. Still no evidence suggests they are useful therapies against PMC. But steroid pulse medication or large-dose steroid use might be an effective way to prevent PMC. Besides steroid pulse therapy,[21] plasma exchange and immunoglobulin[35] are also possible options to avoid PMC.
We also found neither age of thymectomy nor gender influences PMC development. Still no evidence indicated age or sex as independent factors of PMC.
There are several limitations in this meta-analysis. Some clinical factors may affect the risk of PMC, such as anti-AchR Ab titer and concomitant disease were not involved in this research. Available articles were somehow few and no high-quality research was included in this meta-analysis.
In conclusion, myasthenia crisis history, post-surgery morbidities, generalized MG, thymoma, and bulbar symptoms are possible risk factors of PMC. Steroid pulse therapy may prevent PMC. Longer surgery duration and more blood loss may also contribute to PMC. Regular dose of steroid, application of immunosuppressive therapy, and male or female make no effect on PMC.
Author contributions
Data curation: Yingcai Geng, Hanlu Zhang.
Formal analysis: Yingcai Geng.
Investigation: Yingcai Geng.
Methodology: Yingcai Geng, Hanlu Zhang, Yun Wang.
Project administration: Yun Wang.
Software: Yingcai Geng.
Supervision: Yun Wang.
Validation: Yun Wang.
Writing – original draft: Yun Wang.
Writing – review & editing: Yun Wang.
Footnotes
Abbreviations: anti-AchR-Ab = anti-Acetylcholine-Receptor Antibody, MC = myasthenia crisis, MG = myasthenia gravis, PMC = post-operative myasthenia crisis, RR = relative risk, SMD = standardized mean difference, TS = trans-sternotomy, VATS = video-assisted thoracoscopic surgery.
How to cite this article: Geng Y, Zhang H, Wang Y. Risk factors of myasthenia crisis after thymectomy among myasthenia gravis patients: a meta-analysis . Medicine. 2020;99:1(e18622).
This research was supported by grants from Chengdu City Science and Technology Project of China (No. 0040205301E42) and the National Key Research Project of China (No. 2017YFC0113502).
We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
None of the authors has any conflict of interest to disclose.
References
- [1].Ciafaloni E, Sanders DB. Advances in myasthenia gravis. Curr Neurol Neurosci Rep 2002;2:89–95. [DOI] [PubMed] [Google Scholar]
- [2].Marx A, Willcox N, Leite MI, et al. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity 2010;43:413–27. [DOI] [PubMed] [Google Scholar]
- [3].Bachmann K, Burkhardt D, Schreiter I, et al. Thymectomy is more effective than conservative treatment for myasthenia gravis regarding outcome and clinical improvement. Surgery 2009;145:392–8. [DOI] [PubMed] [Google Scholar]
- [4].Watanabe A, Watanabe T, Obama T, et al. Prognostic factors for myasthenic crisis after transsternal thymectomy in patients with myasthenia gravis. J Thorac Cardiovasc Surg 2004;127:868–76. [DOI] [PubMed] [Google Scholar]
- [5].Nam TS, Lee SH, Kim BC, et al. Clinical characteristics and predictive factors of myasthenic crisis after thymectomy. J Clin Neurosci 2011;18:1185–8. [DOI] [PubMed] [Google Scholar]
- [6].Kanai T, Uzawa A, Sato Y, et al. A clinical predictive score for postoperative myasthenic crisis. Ann Neurol 2017;82:841–9. [DOI] [PubMed] [Google Scholar]
- [7].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [9].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [10].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [11].Ando T, Omasa M, Kondo T, et al. Predictive factors of myasthenic crisis after extended thymectomy for patients with myasthenia gravis. Eur J Cardiothorac Surg 2015;48:705–9. discussion 709. [DOI] [PubMed] [Google Scholar]
- [12].Zou J, Su C, Lun X, et al. Preoperative anxiety in patients with myasthenia gravis and risk for myasthenic crisis after extended transsternal thymectomy: a CONSORT study. Medicine (Baltimore) 2016;95:e2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Leuzzi G, Meacci E, Cusumano G, et al. Thymectomy in myasthenia gravis: proposal for a predictive score of postoperative myasthenic crisis. Eur J Cardiothorac Surg 2014;45:e76–88. discussion e88. [DOI] [PubMed] [Google Scholar]
- [14].Lee HS, Lee HS, Lee HE, et al. Predictive factors for myasthenic crisis after videoscopic thymectomy in patients with myasthenia gravis. Muscle Nerve 2015;52:216–20. [DOI] [PubMed] [Google Scholar]
- [15].Choi KH, Nam TS, Lee SH, et al. Preoperative pulmonary function is strongly related to myasthenic crisis after thymectomy. Neurol India 2014;62:164–8. [DOI] [PubMed] [Google Scholar]
- [16].Liu WB, Men LN, Tang BY, et al. [Prognostic factors of myasthenic crisis after extended thymectomy in patients with generalized myasthenia gravis] Zhonghua Yi Xue Za Zhi 2006;86:2737–40. [PubMed] [Google Scholar]
- [17].Chu XY, Xue ZQ, Wang RW, et al. Predictors of postoperative myasthenic crisis in patients with myasthenia gravis after thymectomy. Chin Med J (Engl) 2011;124:1246–50. [PubMed] [Google Scholar]
- [18].Wu Y, Chen Y, Liu H, et al. Risk factors for developing postthymectomy myasthenic crisis in Thymoma Patients. J Cancer Res Ther 2015;11 Suppl 1:C115–117. [DOI] [PubMed] [Google Scholar]
- [19].Yu S, Lin J, Fu X, et al. Risk factors of myasthenic crisis after thymectomy in 178 generalized myasthenia gravis patients in a five-year follow-up study. Int J Neurosci 2014;124:792–8. [DOI] [PubMed] [Google Scholar]
- [20].Soleimani A, Moayyeri A, Akhondzadeh S, et al. Frequency of myasthenic crisis in relation to thymectomy in generalized myasthenia gravis: a 17-year experience. BMC Neurol 2004;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yamada Y, Yoshida S, Suzuki H, et al. Efficacy of perioperative high-dose prednisolone therapy during thymectomy in myasthenia gravis patients. J Cardiothorac Surg 2013;8:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li Y, Wang H, Chen P, et al. Clinical outcome and predictive factors of postoperative myasthenic crisis in 173 thymomatous myasthenia gravis patients. Int J Neurosci 2018;128:103–9. [DOI] [PubMed] [Google Scholar]
- [23].Maggi L, Andreetta F, Antozzi C, et al. Thymoma-associated myasthenia gravis: outcome, clinical and pathological correlations in 197 patients on a 20-year experience. J Neuroimmunol 2008;201-202:237–44. [DOI] [PubMed] [Google Scholar]
- [24].Thomas CE, Mayer SA, Gungor Y, et al. Myasthenic crisis: clinical features, mortality, complications, and risk factors for prolonged intubation. Neurology 1997;48:1253–60. [DOI] [PubMed] [Google Scholar]
- [25].Kalita J, Kohat AK, Misra UK. Predictors of outcome of myasthenic crisis. Neurol Sci 2014;35:1109–14. [DOI] [PubMed] [Google Scholar]
- [26].Kondo K, Monden Y. Thymoma and myasthenia gravis: a clinical study of 1,089 patients from Japan. Ann Thorac Surg 2005;79:219–24. [DOI] [PubMed] [Google Scholar]
- [27].Hoffacker V, Schultz A, Tiesinga JJ, et al. Thymomas alter the T-cell subset composition in the blood: a potential mechanism for thymoma-associated autoimmune disease. Blood 2000;96:3872–9. [PubMed] [Google Scholar]
- [28].Burt BM, Yao X, Shrager J, et al. Determinants of complete resection of thymoma by minimally invasive and open thymectomy: analysis of an international registry. J Thorac Oncol 2017;12:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee CY, Kim DJ, Lee JG, et al. Bilateral video-assisted thoracoscopic thymectomy has a surgical extent similar to that of transsternal extended thymectomy with more favorable early surgical outcomes for myasthenia gravis patients. Surg Endosc 2011;25:849–54. [DOI] [PubMed] [Google Scholar]
- [30].He Z, Zhu Q, Wen W, et al. Surgical approaches for stage I and II thymoma-associated myasthenia gravis: feasibility of complete video-assisted thoracoscopic surgery (VATS) thymectomy in comparison with trans-sternal resection. J Biomed Res 2013;27:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gung Y, Zhang H, Li S. Sternotomy versus video-assisted thoracoscopic surgery for thymectomy of myasthenia gravis patients: a meta-analysis 2016;9:285–94. [DOI] [PubMed] [Google Scholar]
- [32].Gracey DR, Divertie MB, Howard FM, Jr, et al. Postoperative respiratory care after transsternal thymectomy in myasthenia gravis. A 3-year experience in 53 patients. Chest 1984;86:67–71. [DOI] [PubMed] [Google Scholar]
- [33].Pego-Fernandes PM, de Campos JR, Jatene FB, et al. Thymectomy by partial sternotomy for the treatment of myasthenia gravis. Ann Thorac Surg 2002;74:204–8. [DOI] [PubMed] [Google Scholar]
- [34].Kas J, Kiss D, Simon V, et al. Decade-long experience with surgical therapy of myasthenia gravis: early complications of 324 transsternal thymectomies. Ann Thorac Surg 2001;72:1691–7. [DOI] [PubMed] [Google Scholar]
- [35].Jensen P, Bril V. A comparison of the effectiveness of intravenous immunoglobulin and plasma exchange as preoperative therapy of myasthenia gravis. J Clin Neuromuscul Dis 2008;9:352–5. [DOI] [PubMed] [Google Scholar]